Abstract
Seizures commonly occur in a variety of serious neurological illnesses, and lead to additional morbidity and worsened outcomes. Recently, it has become clear that not all seizures in the acute brain injury setting are evident on scalp EEG. To address this, we have developed a protocol for depth electrode placement in the neuro-intensive care unit for patients in whom the clinical suspicion of occult seizures is high. In the current manuscript, we review the literature on depth EEG monitoring for ictal events in critically-ill, unconscious patients, focusing on the incidence of seizures not detected with scalp EEG in various conditions. We critically discuss evidence in support of and against treating these events that are only detectable on depth recordings. We describe additional specific scenarios in which depth EEG recordings may be helpful, including for the detection of delayed cerebral ischemia following subarachnoid hemorrhage. We then describe current techniques for bedside electrode placement. Finally, we outline potential avenues for future investigations, including the use of depth electrodes to describe circuit abnormalities in acute brain injury.
Keywords: Electroencephalography, Intracortical electroencephalography, Invasive electroencephalography, Depth electroencephalography, Neuromonitoring, NICU
1. Introduction
1.1. Seizures in acute brain injury
Seizures are common sequelae of a variety of neurological injuries, including trauma [1], infection [2], neoplasia [3], as well as intracerebral [4], subdural [5], and subarachnoid hemorrhage (SAH) [6]. It is now appreciated that many seizures in acutely ill, comatose patients are not clinically evident; nonconvulsive seizures have been observed in approximately 8% of all subarachnoid hemorrhage patients [7] and approximately 10% of comatose traumatic brain injury patients [8]. Because of this, continuous electroencephalographic monitoring (cEEG) has been proposed as the standard of care for unresponsive patients after acute brain injury (ABI) [9,10].
In ABI there is accumulating evidence supporting additional harm from uncontrolled seizure activity, including nonconvulsive status epilepticus [11], hippocampal atrophy ipsilateral to seizure onset [11], and nonconvulsive seizure burden following ABI, such as SAH, has been associated with worse functional and cognitive outcomes [12]. Seizures after TBI were associated with increases in intracranial pressure and metabolic crisis [8], and status epilepticus strongly correlated with mortality in the same report. Epilepsy (i.e., unprovoked seizures) commonly occurs after both TBI [13] and subarachnoid hemorrhage [14], although the contribution of early seizures to risk of epilepsy remains an open question.
1.2. Historical use of scalp and depth EEG for neuromonitoring
Seizure activity without obvious clinical manifestations, such as non-convulsive seizures, cannot be detected by direct observation, and thus necessitates the use of other neuromonitoring techniques. As early as the 1930s, EEG monitoring was used in epilepsy [15] and being evaluated as a method for detecting intracranial lesions [16]. Since that time, techniques with greater capacity for seizure detection than discrete monitoring with scalp EEG have emerged. In 1991, Hilz and colleagues described the first case reports of using continuous EEG (cEEG) to monitor patients in the neurological intensive care unit [17] (NICU), and subsequent work demonstrated cEEG could be used to detect non-convulsive seizures [18,19]and to prognosticate [20,21].Similarly, depth EEG, pioneered in 1961, offered another useful technique for seizure detection and significantly improved enumeration, lateralization, and localization of seizure foci compared to the use of clinical features and scalp EEG [22]. Since that time, depth EEG has been used increasingly for localization of seizure foci in refractory epilepsy [23].
The surgical epilepsy group at Yale advanced depth EEG technology by combining it with microdiaylsis [24]. Although their early work focused on adenosine and glutamate [25], a subsequent study characterized increases in local extracellular lactate which accompanied temporal lobe seizures [26]. Lactate concentrations increased on average by 90% following seizures, and persisted for 60–90 min after seizure onset. This suggested that seizures caused a period of regional metabolic distress later termned metabolic crisis, a condition that neurologists were beginning to appreciate as a contributor to poor outcomes in ABI patients [27]. This combination of microdialysis and depth EEG thus had both theoretical and practical appeal as a technique for monitoring in the NICU.
2. Discussion
2.1. Depth EEG placement in NICU patients
Depth EEG electrodes are typically placed in stuporous or comatose patients as part of a so-called “bundle” of other invasive monitoring modalities. At our institution, five modalities are typically placed via two cranial bolts. The larger bolt is an Integra three-port bolt (IM3.ST: Plainsboro, NJ) which has fittings for a Camino™ intracranial pressure monitor, a Licox™ brain-tissue oxygenation monitor, and a microdialysis catheter. The depth electrode is placed through a Codman (Synthes-Depuy Codman Neuro: Raynham, MA). dual-port bolt (the other port is used for Hemedex Bowman Perfusion Monitor™ [Hemedex Inc.: Cambridge, MA] blood flow monitoring). Of note, many similar bolt options are available from several companies; some Hemedex bolts, in particular, have the advantage of MRI compatibility and some offer up to four ports. General techniques for multimodality monitoring have been recently reviewed [28]. The focus of the current manuscript will be on depth electrode placement and recordings, while other monitoring techniques including subdural strip electrodes will not be discussed.
Prior to placement, the patient's family is asked about medications that interfere with hemostasis, as well as a history of easy bleeding or bruising, and routine blood tests are ordered for platelet count and coagulation parameters. The imaging is then reviewed by the primary team and the neurosurgeon, and an appropriate place for electrode placement is identified. Typically this is near Kocher's point, but may be somewhat more rostral, if craniotomy or ventriculostomy incisions need to be avoided. Pathology typically dictates the side of the procedure; all things being equal, we prefer right-sided procedures.
Reference and ground electrodes are placed on the patient. The operative site is marked and shaved, and the patient is prepped and draped in the usual sterile fashion. Cranial Access Kit such as that by Codman™ are available including circular drapes that adhere to the patient's skin. For the depth electrode, skin is incised, and a small self-retaining retractor is placed on the skull. The coronal suture may be identified and used as a landmark, if desired. The drill bit accompanying the Codman™ bolt kit is fitted to the drill in the Cranial Access Kit. At this point, the surgeon may place the electrode freehand, or if desired under electrophysiological guidance. If he or she plans to place it freehand, the electrode is placed inside the Codman™ dual-lumen bolt assembly and measured to a depth of 2.5 or 3 cm from the tip of the bolt fitting, depending on preoperative imaging (Fig. 1). We use a 2-0 silk tie to mark the appropriate depth. If placement under electrophysiological guidance is desired, this step is not needed. Using a freehand technique, the skull is drilled. An 18-gauge needle is used to create a circumferential durotomy. The bolt fitting is placed, and the electrode is inserted to depth. If using electrophysiological guidance, the end of the electrode is attached to a Cabrio connector (Ad-Tech Medical™: Racine, WI). The electrode is then passed through the bolt, and potentials for all eight channels are monitored. The electrode is optimally placed when signal is detected on all channels. The bolt fittings are tightened, and a strain relief loop is created using a Tegaderm bandage (3M™: Two Harbors, MN) to prevent inadvertent removal. The wound is dressed with Xeroform gauze (Covidien™: Jersey City, NJ).
Fig. 1.
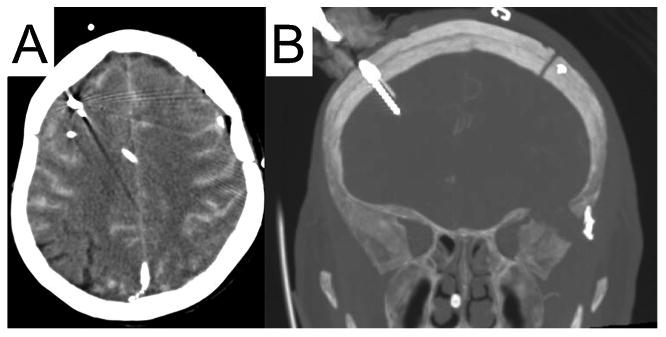
Typical bedside placement of a depth electrode. Panel A: Patient with diffuse SAH has an EVD, a brain tissue oxygenation monitor, a depth electrode (red arrow), and several other probes placed in the right frontal lobe. Panel B: Coronal image reveals an eight-contact depth electrode in the frontal cortex.
Optimal placement of the depth has not been determined in the present published literature. In general, we have attempted to place the electrode in perilesional tissue, with a strong bias towards the area around Kocher's point, but it is not clear that this is the best location. Moreover, freehand techniques we utilize are not optimized for precise placement; notably, frameless stereotactic techniques have been used for depth electrode placement in the past [29] and may be useful in this setting as well.
2.2. Safety
Bedside depth electrode placement appears to have a safety profile similar to that of other intracranial monitoring probes. One series of sixty-one patients undergoing multimodality monitoring reported malfunction or dislodgement as the most common complication (43%), while infection and hemorrhage were considerably less frequent (5% and 3%, respectively) [30]. The three patients with infections each had a CSF culture that grew a different organism (Candida albicans, Escheria coli, and Acinetobacter), and all three had their intracranial hardware removed. The most serious complication was a large intracerebral hemorrhage in one patient; however, the patient was considered at very high risk as he was taking aspirin and clopidogrel at admission, and the treatment plan was discussed extensively with the patient's family who desired an aggressive treatment paradigm. The overall hemorrhage rate is notably in line with hemorrhage rates reported for Camino intracranial pressure monitors (2–4%). However, larger, multicenter studies will be needed to confirm these findings.
2.3. Findings on depth versus surface EEG
There is evidence that depth EEG has superior sensitivity to cEEG for non-convulsive seizures. A study examining fourteen patients with brain injury who were subsequently implanted with an eight-contact depth electrode demonstrated that depth EEG detected seizures in eight patients who demonstrated no ictal activity on cEEG, and detected seizures in two additional patients whose seizures showed only intermittent corresponding ictal activity on surface electrodes [31]. In a subsequent study of 48 comatose subarachnoid hemorrhage patients, researchers found that eighteen patients (38%) showed seizure activity on intracortical EEG, while only four (8%) showed seizure activity on continuous scalp EEG [32] (Fig. 2, left panel). Recently, this finding was reproduced in traumatc brain injury patients; in a cohort of 34 patients, 21 had seizures or periodic discharges, and nine of these had ictal phenomena observable only on the depth electrode [33]. Depth EEG recordings also have the advantage (over scalp electrodes) of high signal-to-noise ratio and sensitivity to gamma band oscillations [34].
Fig. 2.
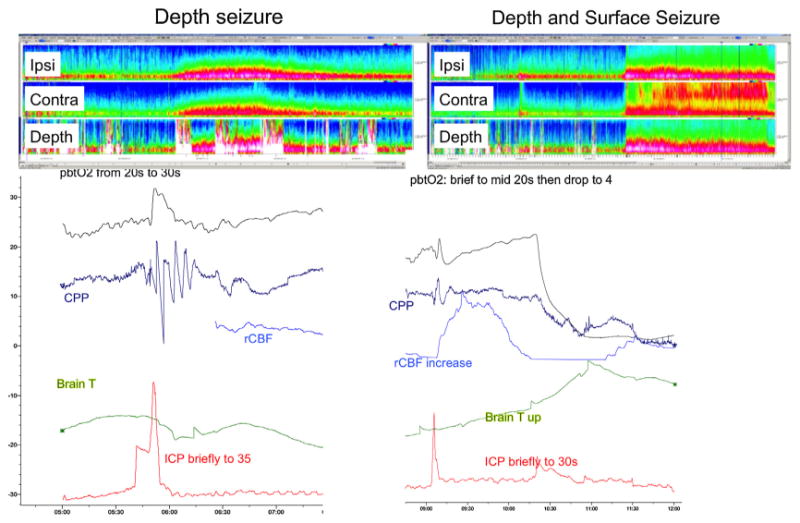
Depth electrodes detect seizures invisible to scalp electrodes. Top left panel: Compressed spectral array (CSA) of EEG data recorded from electrodes ipsilateral to the depth (top row), contralateral electrodes (middle row), and the depth electrode (bottom row). CSA reveals a depth-only seizure associated with an increase in broadband amplitude in scalp contacts, without clear ictal activity. Bottom left panel: There is evidence of hyperemia with an increased pbtO2 and CPP,as well as a spike in ICP. Top right panel: CSA of EEG data reflecting a seizure detectable on both scalp and depth contacts. Bottom right panel: pbtO2 collapses, and there is a spike in ICP, brain temperature, and cerebral blood flow.
In acute brain injury, nonconvulsive seizures may result in metabolic distress and intracranial pressure changes which may be detectable using invasive neuromonitoring [32,35–39]. During a seizure, the brain metabolism may increase briefly [32], brain tissue partial pressure of oxygen in the brain's extracellular fluid drops [32,35], and the intracranial pressure increases [36,39,38] (Fig. 2, bottom right panel). The brain endures a “metabolic crisis,” characterized by an elevated lactate/pyruvate ratio and decreased extracellular glucose; findings thought to indicate increased glucose consumption, reduced oxidative metabolism, and an impaired redox state [37].
Emerging evidence suggests seizures isolated to the depth electrode may be associated with a variety of deleterious findings on multimodality monitoring [31–33]. In subarachnoid hemorrhage, depth EEG seizures are associated with increases in heart rate and blood pressure, and there was a trend towards increased ICP and CPP (Fig.2,bottom left panel) [32]. Some evidence suggests that in patients acute brain injury regional cerebral blood flow increases only minutes after the onset of seizure possibly due to the fact that vasoreactivity is impaired following acute brain injury [32]. In trauma, depth seizures were associated with local metabolic derangement, as indicated by increases in the lactate-pyruvate ratio and decreases in glucose [33]. These associations do not prove unequivocally that isolated findings on depth EEG themselves indicate a deleterious process; however, depth only seizures are more strongly associated with poor outcome than surface seizures. The current does suggest that additional study is warranted.
2.4. Developing applications
As described above, depth EEG is used for seizure detection in tandem with other neuromonitoring modalities, including scalp EEG. Much of neurocritical care is focused on detection and minimizing the effects of secondary complications of ABI. Depth EEG may have a role for early detection of secondary neurological complications commonly seen in NICU patients, although the available data is sparse. In particular, its heightened signal-to-noise ratio may be an advantage in the detection of ischemia. In one series, researchers reported two patients who experienced secondary neurological complications (one patient with SAH complicated by widespread cerebral infarct in the setting of sepsis, and one patient with a large ischemic infarct complicated by hemorrhagic conversion), both of whom showed isolated changes on depth EEG between two and six hours before detection on other invasive neuromonitoring devices, and eight hours prior to clinical detection by physical exam [31]. Similarly, in a report of five patients with poor-grade subarachnoid hemorrhage who underwent intracortical EEG placement, researchers reported that alpha-delta ratio calculated every20 s was able to correctly predict the presence or absence of vasospasm in all five patients [40]. This may offer the added opportunity of automated and reliable detection of ischemia from vasospasm in patients with subarachnoid hemorrhage. Thus, while further studies with larger cohorts are necessary, the evidence for depth EEG as a tool for early detection of secondary neurological complications is encouraging.
The interpretation of physiological signals recorded from invasive brain monitoring is challenging at times [41]. One opportunity to more comprehensively gain insights into underlying brain physiology and evolving pathophysiology is a more comprehensively assessment of brain physiology. Intracortical EEG recordings may offer a major step in this direction. For example a drop in partial brain oxygenation may suggest both ischemia or a seizure. The EEG recording may show depressed or discontinuous background activity in the former and seizure activity inn the later. In other words intracortical EEG recordings may further support interpretation of other invasive brain monitoring signals.
3. Future outlook
Bedside depth electrode placement appears to be safe and effective for the detection of occult seizures in unconscious patients in the NICU. It is technically straightforward, and requires no specialized equipment, beyond the electrode itself, EEG monitoring equipment, and some basic neurosurgical supplies. However, the technique itself is ripe for improvement, which includes both the technical aspects of electrode placement as well as the analysis of the data. Targeted placement of the electrode using stereotactic neuronavigation may allow for recordings of specific brain regions. Depth electrodes have been used in patients undergoing evaluation for epilepsy surgery to describe brain networks required in a number of cognitive functions, including memory [42], reward-based decision making [43], and consciousness [44]. In particular, high gamma band EEG activity (>70 Hz) is thought to reflect local action potentials [45]; this suggests that depth electrodes in the ICU could potentially be used to study frontal brain regions compromised in unconscious patient with acute brain injury.
4. Conclusion
Depth electrodes allow detection of seizures not visible on scalp recordings. Further research is needed to confirm the clinical relevance of depth-only seizures. Additionally, isolated cases suggest that depth electrodes may aid in the detection of evolving ischemia. In the near term, we depth EEG has the potential to be integrated as a tool in the neurointensivist's armamentarium.
Acknowledgments
We thank the nurses, attendings, fellows, and neurology and neurosurgery residents of the Neuroscience ICU for their overall support of this project. This publication was supported by the NLM of the NIH under Award Number R01LM011826. Additional support for this work included a grant from the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number UL1 TR000040, formerly the National Center for Research Resources, Grant Number UL1 RR024156. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflict of interest: Drs Charles B. Mikell and Timothy G. Dyster report no conflicts of interest.
Dr Claassen reports no conflicts of interest relevant to the current publication. However, he serves on the Advisory Board for planning of a study for SAGE pharmaceuticals and Actelion. He is also the site-PI for several NIH sponsored clinical trials.
References
- 1.Temkin NR. Preventing and treating posttraumatic seizures: the human experience. Epilepsia. 2009;50:10–3. doi: 10.1111/j.1528-1167.2008.02005.x. http://dx.doi.org/10.1111/j.1528-1167.2008.02005.x. [DOI] [PubMed] [Google Scholar]
- 2.Kramer U, Shorer Z, Ben-Zeev B, Lerman-Sagie T, Goldberg-Stern H, Lahat E. Severe refractory status epilepticus owing to presumed encephalitis. J Child Neurol. 2005;20:184–7. doi: 10.1177/08830738050200030301. [DOI] [PubMed] [Google Scholar]
- 3.Schaller B, Rüegg SJ. Brain tumor and seizures: pathophysiology and its implications for treatment revisited. Epilepsia. 2003;44:1223–32. doi: 10.1046/j.1528-1157.2003.05203.x. http://dx.doi.org/10.1046/j.1528-1157.2003.05203.x. [DOI] [PubMed] [Google Scholar]
- 4.Claassen J, Jeete N, Chum F, Green R, Schmidt M, Choi H, et al. Electrographic seizures and periodic discharges after intracerebral hemorrhage. Neurology. 2007;69:1356–65. doi: 10.1212/01.wnl.0000281664.02615.6c. http://dx.doi.org/10.1212/01.wnl.0000281664.02615.6c. [DOI] [PubMed] [Google Scholar]
- 5.Garrett MC, Komotar RJ, Starke RM, Merkow MB, Otten ML, Connolly ES. Predictors of seizure onset after intracerebral hemorrhage and the role of long-term antiepileptic therapy. J Crit Care. 2009;24:335–9. doi: 10.1016/j.jcrc.2008.10.015. http://dx.doi.org/10.1016/j.jcrc.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 6.Rhoney DH, Tipps LB, Murry KR, Basham MC, Michael DB, Coplin WM. Anticon-vulsant prophylaxis and timing of seizures after aneurysmal subarachnoid hemorrhage. Neurology. 2000;55:258–65. doi: 10.1212/wnl.55.2.258. http://dx.doi.org/10.1212/WNL.55.2.258. [DOI] [PubMed] [Google Scholar]
- 7.Dennis LJ, Claassen J, Hirsch LJ, Emerson RG, Connolly ES, Mayer SA, et al. Nonconvulsive status epilepticus after subarachnoid hemorrhage. Neurosurgery. 2002;51:1136–44. doi: 10.1097/00006123-200211000-00006. http://dx.doi.org/10.1097/00006123-200211000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Vespa PM, Nuwer MR, Nenov V, Ronne-Engstrom E, Hovda DA, Bergsneider M, et al. Increased incidence and impact of nonconvulsive and convulsive seizures after traumatic brain injury as detected by continous electroencephalographic monitoring. J Neurosurg. 1999;91:750–60. doi: 10.3171/jns.1999.91.5.0750. http://dx.doi.org/10.3171/jns.1999.91.5.0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herman ST, Abend NS, Bleck TP, Chapman KE, Drislane FW, Emerson RG, et al. Consensus statement on continuous EEG in critically ill adults and children, part II: personnel, technical specifications, and clinical practice. J Clin Neurophysiol. 2015;32:96–108. doi: 10.1097/WNP.0000000000000165. http://dx.doi.org/10.1097/WNP.0000000000000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Claassen J, Vespa P. Participants in the International Multi-disciplinary Consensus Conference on Multimodality Monitoring, Electrophysiologic Monitoring in Acute Brain Injury. Neurocrit Care. 2014;21:129–47. doi: 10.1007/s12028-014-0049-x. http://dx.doi.org/10.1007/s12028-014-0022-8. [DOI] [PubMed] [Google Scholar]
- 11.Vespa PM, McArthur DL, Xu Y, Eliseo M, Etchepare M, Dinov I, et al. Non-convulsive seizures after traumatic brain injury are associated with hippocampal atrophy. Neurology. 2010;75:792–8. doi: 10.1212/WNL.0b013e3181f07334. http://dx.doi.org/10.1212/WNL.0b013e3181f07334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Marchis GM, Pugin D, Meyers E, Velasquez A, Suwatcharangkoon S, Park S, et al. Seizure burden in subarachnoid hemorrhage associated with functional and cognitive outcome. Neurol. 2016;86:253–60. doi: 10.1212/WNL.0000000000002281. http://dx.doi.org/10.1212/WNL.0000000000002281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Temkin NR, Dikmen SS, Wilensky AJ, Keihm J, Chabal S, Winn HR, Randomized A. Double-blind study of phenytoin for the prevention of post-traumatic seizures. N Engl J Med. 1990;323:497–502. doi: 10.1056/NEJM199008233230801. [DOI] [PubMed] [Google Scholar]
- 14.Claassen J, Peery S, Kreiter KT, Hirsch LJ, Du EY, Connolly ES, et al. Predictors and clinical impact of epilepsy after subarachnoid hemorrhage. Neurology. 2003;60:208–14. doi: 10.1212/01.wnl.0000038906.71394.de. [DOI] [PubMed] [Google Scholar]
- 15.Gibbs FA. Electroencephalography in epilepsy. J Pediatr. 1939;15:749–62. http://dx.doi.org/10.1016/S0022-3476(39)80076-2. [Google Scholar]
- 16.Williams D, Gibbs FA. The localization of intracranial lesions by electroencephalography. N Engl J Med. 1938;218:998–1002. [Google Scholar]
- 17.Hilz MJ, Litscher G, Weis M, Claus D, Druschky KF, Pfurtscheller G, et al. Continuous multivariable monitoring in neurological intensive care patientspreliminary reports on four cases. Intensive Care Med. 1991;17:87–93. doi: 10.1007/BF01691429. http://dx.doi.org/10.1007/BF01691429. [DOI] [PubMed] [Google Scholar]
- 18.Jordan KG. Continuous EEG and evoked potential monitoring in the neuroscience intensive care unit. J Clin Neurophysiol. 1993;10:445–75. doi: 10.1097/00004691-199310000-00006. http://dx.doi.org/10.1097/00004691-199310000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Claassen J, Mayer Sa, Kowalski RG, Emerson RG, Hirsch LJ. Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. Neurology. 2004;62:1743–8. doi: 10.1212/01.wnl.0000125184.88797.62. http://dx.doi.org/10.1212/01.WNL.0000125184.88797.62. [DOI] [PubMed] [Google Scholar]
- 20.Vespa PM, Boscardin WJ, Hovda DA, McArthur DL, Nuwer MR, Martin NA, et al. Early and persistent impaired percent alpha variability on continuous electroencephalography monitoring as predictive of poor outcome after traumatic brain injury. J Neurosurg. 2002;97:84–92. doi: 10.3171/jns.2002.97.1.0084. http://dx.doi.org/10.3171/jns.2002.97.1.0084. [DOI] [PubMed] [Google Scholar]
- 21.Young GB, Jordan KG, Doig GS. An assessment of nonconvulsive seizures in the intensive care unit using continuous EEG monitoring: an investigation of variables associated with mortality. Neurology. 1996;47:83–9. doi: 10.1212/wnl.47.1.83. http://dx.doi.org/10.1212/WNL.47.1.83. [DOI] [PubMed] [Google Scholar]
- 22.Crandall PH, Walter RD, Rand RW. Clinical applications of studies on stereotactically implanted electrodes in temporal-lobe epilepsy. J Neurosurg. 1963;10:827–40. doi: 10.3171/jns.1963.20.10.0827. http://dx.doi.org/10.3171/jns.1963.20.10.0827. [DOI] [PubMed] [Google Scholar]
- 23.Lhatoo S, Lacuey N, Ryvlin P. Principles of stereotactic electroencephalography in epilepsy surgery. J Clin Neurophysiol. 2016 doi: 10.1097/WNP.0000000000000264. [DOI] [PubMed] [Google Scholar]
- 24.During MJ, Spencer DD. Adenosine: a potential mediator of seizure arrest and postictal refractoriness. Ann Neurol. 1992;32:618–24. doi: 10.1002/ana.410320504. http://dx.doi.org/10.1002/ana.410320504. [DOI] [PubMed] [Google Scholar]
- 25.During MJ, Spencer DD. Extracellular hippocampal glutamate and spontaneous seizure in the conscious human brain. Lancet. 1993;341:1607–10. doi: 10.1016/0140-6736(93)90754-5. http://dx.doi.org/10.1016/0140-6736(93)90754-5. [DOI] [PubMed] [Google Scholar]
- 26.During MJ, Fried I, Leone P, Katz A, Spencer DD. Direct measurement of extracellular lactate in the human hippocampus during spontaneous seizures. J Neurochem. 1994;62:2356–61. doi: 10.1046/j.1471-4159.1994.62062356.x. [DOI] [PubMed] [Google Scholar]
- 27.Hovda DA, Lee SM, Smith ML, Von Stuck S, Bergsneider M, Kelly D, et al. The neurochemical and metabolic cascade following brain injury: moving from animal models to man. J Neurotrauma. 1995;12:903–6. doi: 10.1089/neu.1995.12.903. http://dx.doi.org/10.1089/neu.1995.12.903. [DOI] [PubMed] [Google Scholar]
- 28.Frontera J, Ziai W, O'Phelan K, Leroux PD, Kirkpatrick PJ, Diringer MN, et al. Regional brain monitoring in the neurocritical care unit. Neurocrit Care. 2015;22:348–59. doi: 10.1007/s12028-015-0133-x. http://dx.doi.org/10.1007/s12028-015-0133-x. [DOI] [PubMed] [Google Scholar]
- 29.Mehta AD, Labar D, Dean A, Harden C, Hosain S, Pak J, et al. Frameless stereotactic placement of depth electrodes in epilepsy surgery. J Neurosurg. 2005;102:1040–5. doi: 10.3171/jns.2005.102.6.1040. http://dx.doi.org/10.3171/jns.2005.102.6.1040. [DOI] [PubMed] [Google Scholar]
- 30.Stuart RM, Schmidt M, Kurtz P, Waziri A, Helbok R, Mayer SA, et al. Intracranial multimodal monitoring for acute brain injury: a single institution review of current practices. Neurocrit Care. 2010;12:188–98. doi: 10.1007/s12028-010-9330-9. http://dx.doi.org/10.1007/s12028-010-9330-9. [DOI] [PubMed] [Google Scholar]
- 31.Waziri A, Claassen J, Morgan Stuart R, Arif H, Schmidt MJ, Mayer SA, et al. Intracortical electroencephalography in acute brain injury. Ann Neurol. 2009;66:366–77. doi: 10.1002/ana.21721. http://dx.doi.org/10.1002/ana.410320504. [DOI] [PubMed] [Google Scholar]
- 32.Claassen J, Perotte A, Albers D, Kleinberg S, Schmidt JM, Tu B, et al. Non-convulsive seizures after subarachnoid hemorrhage: multimodal detection and outcomes. Ann Neurol. 2013;74:53–64. doi: 10.1002/ana.23859. http://dx.doi.org/10.1002/ana.410320504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vespa P, Tubi M, Claassen J, Buitrago-Blanco M, McArthur D, Velazquez AG, et al. Metabolic crisis occurs with seizures and periodic discharges after brain trauma. Ann Neurol. 2016 doi: 10.1002/ana.24606. [DOI] [PubMed] [Google Scholar]
- 34.Buzsáki G, Anastassiou CA, Koch C. The origin of extracellular fields and currents—EEG, ECoG, LFP and spikes. Nat Rev Neurosci. 2012;13:407–20. doi: 10.1038/nrn3241. http://dx.doi.org/10.1038/nrn3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valadka AB, Gopinath SP, Contant CF, Uzura M, Robertson CS. Relationship of brain tissue PO2 to outcome after severe head injury. Crit Care Med. 1998;26:1576–81. doi: 10.1097/00003246-199809000-00029. http://dx.doi.org/10.1097/00003246-199809000-00029. [DOI] [PubMed] [Google Scholar]
- 36.Ko SB, Ortega-Gutierrez S, Choi HA, Claassen J, Presciutti M, Schmidt JM, et al. Status epilepticus-induced hyperemia and brain tissue hypoxia after cardiac arrest. Arch Neurol. 2011;68:1323–6. doi: 10.1001/archneurol.2011.240. http://dx.doi.org/10.1001/archneurol.2011.240. [DOI] [PubMed] [Google Scholar]
- 37.Stein NR, McArthur DL, Etchepare M, Vespa PM. Early cerebral metabolic crisis after TBI influences outcome despite adequate hemodynamic resuscitation. Neurocrit Care. 2012;17:49–57. doi: 10.1007/s12028-012-9708-y. http://dx.doi.org/10.1007/s12028-012-9708-y. [DOI] [PubMed] [Google Scholar]
- 38.Gabor AJ, Brooks AG, Scobey RP, Parsons GH. Intracranial pressure during epileptic seizures. Electroencephalogr Clin Neurophysiol. 1984;57:497–506. doi: 10.1016/0013-4694(84)90085-3. [DOI] [PubMed] [Google Scholar]
- 39.Vespa PM, Miller C, McArthur D, Eliseo M, Etchepare M, Hirt D, et al. Non-convulsive electrographic seizures after traumatic brain injury result in a delayed, prolonged increase in intracranial pressure and metabolic crisis. Crit Care Med. 2007;35:2830–6. http://dx.doi.org/10.1097/01.CCM.0000295667.66853.BC. [PMC free article] [PubMed] [Google Scholar]
- 40.Stuart RM, Waziri A, Weintraub D, Schmidt MJ, Fernandez L, Helbok R, et al. Intracortical eeg for the detection of vasospasm in patients with poor-grade subarachnoid hemorrhage. Neurocrit Care. 2010;13:355–8. doi: 10.1007/s12028-010-9414-6. http://dx.doi.org/10.1007/s12028-010-9330-9. [DOI] [PubMed] [Google Scholar]
- 41.Le Roux P, Menon DK, Citerio G, Vespa P, Bader MK, Brophy GM, et al. Consensus Summary Statement of the International Multidisciplinary Consensus Conference on Multimodality Monitoring in Neurocritical Care. Neurocrit Care. 2014:1189–209. doi: 10.1007/s12028-014-0041-5. http://dx.doi.org/10.1007/s12028-014-0022-8. [DOI] [PMC free article] [PubMed]
- 42.Burke JF, Zaghloul Ka, Jacobs J, Williams RB, Sperling MR, Sharan AD, et al. Synchronous and asynchronous theta and gamma activity during episodic memory formation. J Neurosci. 2013;33:292–304. doi: 10.1523/JNEUROSCI.2057-12.2013. http://dx.doi.org/10.1523/JNEUROSCI.2057-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cohen MX, Axmacher N, Lenartz D, Elger CE, Sturm V, Schlaepfer TE. Neuroelectric signatures of reward learning and decision-making in the human nucleus accumbens. Neuropsychopharmacology. 2009;34:1649–58. doi: 10.1038/npp.2008.222. http://dx.doi.org/10.1038/npp.2008.222. [DOI] [PubMed] [Google Scholar]
- 44.Koubeissi MZ, Bartolomei F, Beltagy A, Picard F. Electrical stimulation of a small brain area reversibly disrupts consciousness. Epilepsy Behav. 2014;37:32–5. doi: 10.1016/j.yebeh.2014.05.027. \ http://dx.doi.org/10.1016/j.yebeh.2014.05.027. [DOI] [PubMed] [Google Scholar]
- 45.Ray S, Crone NE, Niebur E, Franaszczuk PJ, Hsiao SS. Neural correlates of high-gamma oscillations (60-200 Hz) in macaque local field potentials and their potential implications in electrocorticography. J Neurosci. 2008;28:11526–36. doi: 10.1523/JNEUROSCI.2848-08.2008. http://dx.doi.org/10.1523/JNEUROSCI.2848-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]


