Abstract
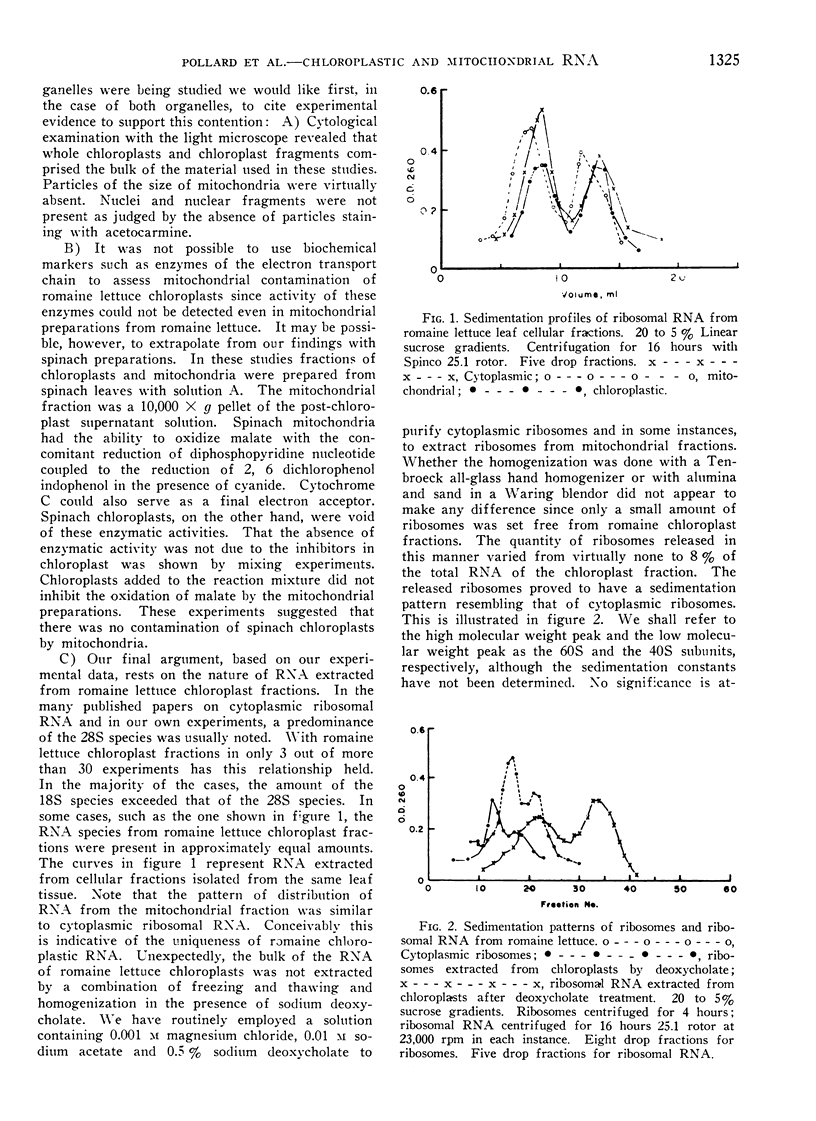
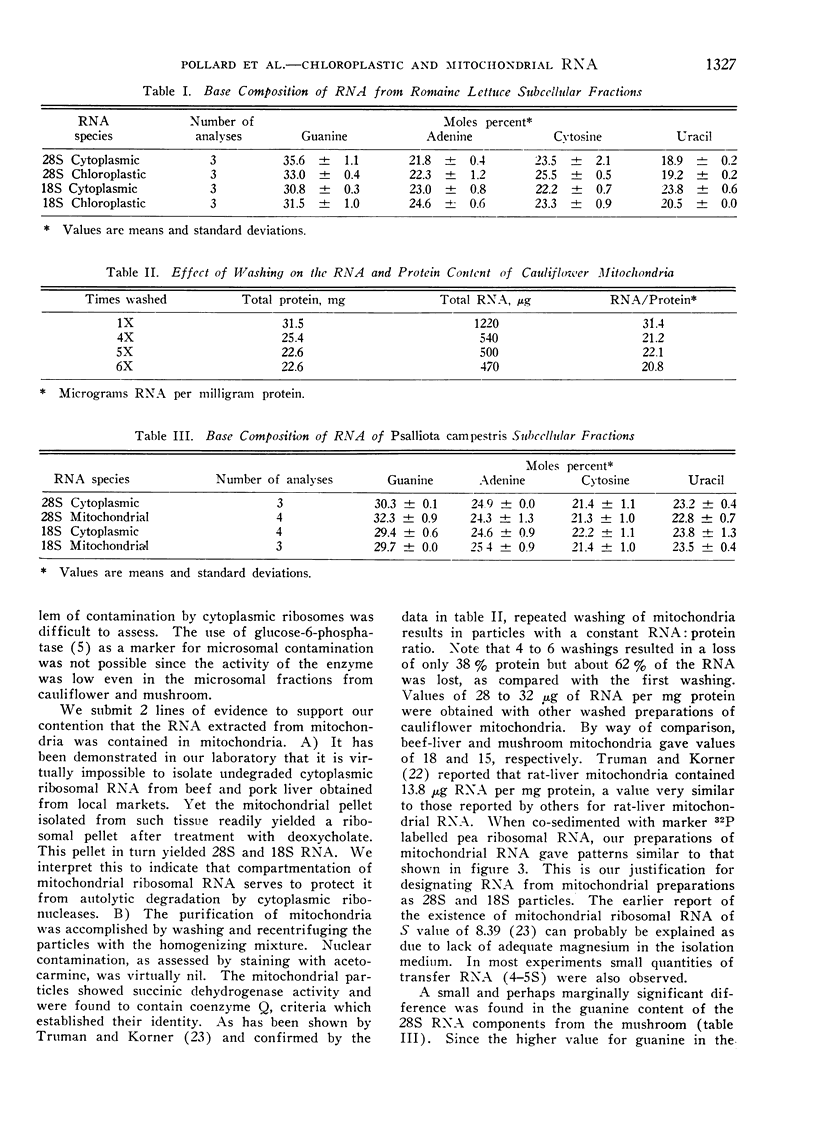
RNA prepared from fractions of chloroplasts and mitochondria sedimented at rates characteristic of ribosomal RNA. A predominance of the 18S species was frequently observed in preparations from chloroplasts from romaine lettuce and endive. The usual distribution, a preponderance of the 28S species, was observed in studies on tomato and spinach chloroplasts and mitochondria from mushroom and cauliflower. Comparisons of the base composition of RNA from organelles with their cytoplasmic ribosomal counterparts revealed that the 18S component from romaine lettuce chloroplast was different. A marginally significant difference was observed in the 28S particle from mushroom mitochondria preparations whereas distinct differences, reflected in all the bases, were seen when the 18S component of cauliflower mitochondria preparations was compared with cytoplasmic RNA.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APP A. A., JAGENDORF A. T. INCORPORATION OF LABELLED AMINO ACIDS BY CHLOROPLAST RIBOSOMES. Biochim Biophys Acta. 1963 Oct 15;76:286–292. [PubMed] [Google Scholar]
- BRAWERMAN G. The isolation of a specific species of ribosomes associated with chloroplast development in Euglena gracilis. Biochim Biophys Acta. 1963 Jun 25;72:317–331. [PubMed] [Google Scholar]
- Baltus E., Quertier J. A method for the extraction and characterization of RNA from subcellular fractions of Acetabularia. Biochim Biophys Acta. 1966 Apr 18;119(1):192–194. doi: 10.1016/0005-2787(66)90050-5. [DOI] [PubMed] [Google Scholar]
- GAUSE G. G., LOSHKAREVA N. P., ZBARSKY I. B., GAUSE G. F. DEOXYRIBONUCLEIC ACID BASE COMPOSITION IN CERTAIN BACTERIA AND THEIR MUTANTS WITH IMPAIRED RESPIRATION. Nature. 1964 Aug 8;203:598–599. doi: 10.1038/203598a0. [DOI] [PubMed] [Google Scholar]
- Joklik W. K., Becker Y. Studies on the genesis of polyribosomes. I. Origin and significance of the subribosomal particles. J Mol Biol. 1965 Sep;13(2):496–510. doi: 10.1016/s0022-2836(65)80112-7. [DOI] [PubMed] [Google Scholar]
- KISLEV N., SWIFT H., BOGORAD L. NUCLEIC ACIDS OF CHLOROPLASTS AND MITOCHONDRIA IN SWISS CHARD. J Cell Biol. 1965 May;25:327–344. doi: 10.1083/jcb.25.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LYTTLETON J. W. Isolation of ribosomes from spinach chloroplasts. Exp Cell Res. 1962 Mar;26:312–317. doi: 10.1016/0014-4827(62)90183-0. [DOI] [PubMed] [Google Scholar]
- MCCARTHY B. J., HOYER B. H. IDENTITY OF DNA AND DIVERSITY OF MESSENGER RNA MOLECULES IN NORMAL MOUSE TISSUES. Proc Natl Acad Sci U S A. 1964 Oct;52:915–922. doi: 10.1073/pnas.52.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIDGLEY J. E. EFFECTS OF DIFFERENT EXTRACTION PROCEDURES ON THE MOLECULAR CHARACTERISTICS OF BACTERIAL RIBOSOMAL RIBONUCLEIC ACID. Biochim Biophys Acta. 1965 Feb 8;95:232–243. doi: 10.1016/0005-2787(65)90488-0. [DOI] [PubMed] [Google Scholar]
- ROODYN D. B., FREEMAN K. B., TATA J. R. THE STIMULATION BY TREATMENT IN VIVO WITH TRI-IODOTHYRONINE OF AMINO ACID INCORPORATION INTO PROTEIN BY ISOLATED RAT-LIVER MITOCHONDRIA. Biochem J. 1965 Mar;94:628–641. doi: 10.1042/bj0940628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz M., Sinclair J., DeSalle L., Haselkorn R., Swift H. H. Isolation of deoxyribonucleic acid from mitochondria of chick embryo heart and liver. Proc Natl Acad Sci U S A. 1965 May;53(5):1126–1133. doi: 10.1073/pnas.53.5.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer D. Protein synthesis by isolated spinach chloroplasts. Arch Biochem Biophys. 1965 Aug;111(2):381–390. doi: 10.1016/0003-9861(65)90200-6. [DOI] [PubMed] [Google Scholar]
- TRUMAN D. E., KORNER A. Incorporation of amino acids into the protein of isolated mitochondria. A search for optimum conditions and a relationship to oxidative phosphorylation. Biochem J. 1962 Jun;83:588–596. doi: 10.1042/bj0830588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANKOFSKY S. A., SPIEGELMAN S. Distinct cistrons for the two ribosomal RNA components. Proc Natl Acad Sci U S A. 1963 Apr;49:538–544. doi: 10.1073/pnas.49.4.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YUDKIN M. D., DAVIS B. NATURE OF THE RNA ASSOCIATED WITH THE PROTOPLAST MEMBRANE OF BACILLUS MEGATERIUM. J Mol Biol. 1965 May;12:193–204. doi: 10.1016/s0022-2836(65)80293-5. [DOI] [PubMed] [Google Scholar]


