Abstract
Sensorimotor adaptation occurs when there is a discrepancy between the expected and actual sensory consequences of a movement. This learning can be precisely measured, but its source has been hard to pin down because standard adaptation tasks introduce two potential learning signals: task performance errors and sensory prediction errors. Here we employed a new method that induces sensory prediction errors without task performance errors. This method combines the use of clamped visual feedback that is angularly offset from the target and independent of the direction of motion, along with instructions to ignore this feedback while reaching to targets. Despite these instructions, participants unknowingly showed robust adaptation of their movements. This adaptation was similar to that observed with standard methods, showing sign dependence, local generalization, and cerebellar dependency. Surprisingly, adaptation rate and magnitude were invariant across a large range of offsets. Collectively, our results challenge current models of adaptation and demonstrate that behavior observed in many studies of adaptation reflect the composite effects of task performance and sensory prediction errors.
INTRODUCTION
Sensorimotor learning is often described as a process of error reduction. By this view, the motor system generates a movement that it judges most likely to achieve the desired sensory outcome (Jordan & Rumelhart, 1992). Subsequently, the difference between the desired feedback and actual feedback arising from the motor command indicates the form and magnitude of the error. Learning occurs when this information is used to modify future performance. The preeminent theoretical framework for this form of learning involves a process where internal models are retuned to minimize errors via gradient descent (Cheng & Sabes, 2006; Pouget & Snyder, 2000; Thoroughman & Shadmehr, 2000; Miall & Wolpert, 1996; Jordan & Rumelhart, 1992).
The empirical base for this theoretical framework primarily comes from studies of short-term motor learning where a perturbation is introduced to disrupt the sensory feedback and/or alter the limb dynamics during reaching movements (Shadmehr, Smith, & Krakauer, 2010; Krakauer, Pine, Ghilardi, & Ghez, 2000; Scheidt et al., 2000; Thoroughman & Shadmehr, 2000; Held & Hein, 1958). Perturbations such as these introduce a sensory prediction error, the discrepancy between the expected and observed sensory consequences of a given motor command (Wolpert, Ghahramani, & Jordan, 1995a). For example, in visuomotor adaptation tasks, participants are trained to reach to visually defined targets, with feedback limited to a cursor that is, at least initially, veridical with hand position. A perturbation here might involve rotating the feedback cursor by 45°, thus creating a difference between the predicted location of the cursor and its actual position, the sensory prediction error. Models in which learning is driven solely by the magnitude and direction of these errors have provided an excellent account of short-term sensorimotor adaptation.
Sensory prediction error is distinct from task performance error. The latter refers to any source of information that signals task success or failure. Task performance error can be presented symbolically; for example, the pitch of a tone might indicate if the movement resulted in a hit or miss, or the participant might see a point value, indicating the distance from the target. In most studies of sensorimotor adaptation, task performance feedback is provided. However, the most common form of task performance feedback in adaptation tasks is the distance between the cursor and target given the standard experimental instructions to “hit the target with the visual cursor.” As such, task performance errors are confounded with sensory prediction errors. When feedback is perturbed, the participant no longer achieves their intended goal. As adaptation proceeds and sensory prediction errors become smaller, task performance also improves. This situation makes it difficult to determine if sensory prediction errors, task performance errors, or a combination of these signals drives adaptation.
Behavioral changes have been observed in response to either error type, and historically, it has been argued that greater changes occur when both types of error are present (Schaefer, Shelly, & Thoroughman, 2012; Wolpert, Ghahramani, & Jordan, 1995b; Howard, 1968; Held & Freedman, 1963). However, these early studies did not use methods to clearly discriminate between different kinds of learning processes. Recent work has shown that task performance errors drive both explicit and implicit changes in behavior and that these changes are distinct from adaptation driven by sensory prediction error (Miyamoto, Wang, Brennan, & Smith, 2014; Taylor & Ivry, 2011).
The use of feedback that is contingent upon the participant’s behavior has made it difficult to dissociate the impact of task performance errors and sensory prediction errors. For instance, participants can be given a specific aiming strategy to counter a feedback perturbation, a manipulation that immediately reduces the large task performance error that typically accompanies the abrupt introduction of a perturbation (Mazzoni & Krakauer, 2006; Welch, 1969). However, this strategy does not eliminate the imposed sensory prediction error, which will drive implicit adaptation. Moreover, the implicit response to this error will reintroduce a task performance error after only a few reaches, forcing the sign of the two error signals into opposition. This results in interesting interactions between explicit aiming and implicit adaptation, but the dual operation of both processes makes it difficult to characterize their individual features (Taylor, Krakauer, & Ivry, 2014; Taylor & Ivry, 2011). Without characterizing each process in isolation, we cannot know whether their combined operation is simply the sum of the two independent processes, or whether there is modulation of either process by the other.
Here we set out to better characterize the sensory prediction error learning process by measuring its operation when isolated from task performance errors. To bridge the gap between traditional techniques and our new method, we first demonstrate how task instructions can dramatically affect the behavioral response to a sensorimotor perturbation without changing a primary hallmark of implicit adaptation: the aftereffect observed when the perturbation is removed.
We then employ a new approach that combines two existing techniques to selectively engage the implicit learning process that produces these aftereffects. Specifically, we used time-locked visual feedback that followed an invariant trajectory, independent of the participant’s direction of movement (Shmuelof et al., 2012). This eliminated the typical contingency between angular hand position and visual feedback and provided a constant sensorimotor discrepancy over the course of the entire perturbation block. Critically, participants were fully informed of the nature of the manipulation and were instructed to ignore the feedback (Welch, 1969). By emphasizing that the task goal was to move the hand to the target and informing the participants of the nature of the visual manipulation, we eliminated signals that conveyed task performance error. Over a series of experiments, we exploit this method to reveal behavioral changes that result purely from sensory prediction errors.
METHODS
Participants
Undergraduate students (n = 160, 115 women, age = 22 ± 2 years) were recruited from the University of California, Berkeley, Department of Psychology research participant pool. Cerebellar degeneration patients and age-matched controls (n = 10/group) were recruited from the Ivry Lab neurological patient database and communities around the San Francisco Bay Area (see Table 1). Patients with ataxia were scored on the International Cooperative Ataxia Rating Scale (ICARS), and a formal medical history was taken to confirm the etiology of each patient’s disease. When available, the outcome of genetic tests for spino-cerebellar ataxia (SCA) subtypes is provided. Only right-handed participants (Oldfield, 1971) were recruited. The institutional review board at the University of California, Berkeley, approved the research protocol. We did not perform a power analysis to predetermine our group sizes for each condition, as our experiments used a novel technique to induce the adaptation we measured. We did not know the magnitude of the group level effect it would have nor how it would affect the trial-by-trial variability of reaching. We did not, however, expect a wildly different outcome relative to standard techniques, leading us to choose a standard group size for the field, with 10 participants per condition. The only exception was Experiment 2, where we increased the group sizes to 20 participants because of high variance at generalization probe targets.
Table 1.
Ataxia Patient Details
| Patient No. | Age | Sex | ICARS | Diagnosis |
|---|---|---|---|---|
| 1 | 34 | Male | 17 | SCA3 |
| 2 | 74 | Male | 20 | Family history |
| 3 | 53 | Male | 39 | Family history |
| 4 | 68 | Female | 31 | Unknown |
| 5 | 51 | Male | 22 | Family history |
| 6 | 44 | Male | 10 | SCA5 |
| 7 | 44 | Male | 31 | Unknown |
| 8 | 77 | Male | N/A | Unknown |
| 9 | 63 | Male | N/A | SCA10 |
| 10 | 55 | Male | 48 | SCA6 |
ICARS = International Cooperative Ataxia Rating Scale; SCA = spino-cerebellar ataxia.
Experimental Apparatus
All participants performed center-out reaches on a horizontal surface while seated at a custom-made tabletop. Participants held a modified air hockey “paddle” that contained a stylus, and moved this device across a digitizing tablet (49.3 cm × 32.7 cm; Intuos 4XL; Wacom, Vancouver, WA). An LCD screen, 53.2 cm × 30 cm (ASUS, Taipei, Taiwan), was suspended 27 cm above the tablet. The experimental software was custom written in Python 2.7 with Pygame 1.9 modules (Beaverton, OR).
Reaching Task
Participants made reaches from the center of the workspace to targets positioned at a radial distance of 8 cm. The center location and target location were indicated by a white circle (1.2 cm diameter) and filled blue circle (1.6 cm), respectively. Direct vision of the hand was occluded by the monitor, and the lights were extinguished in the room to minimize peripheral vision of the arm. Position of the hand was indicated by a filled white cursor (4-mm circle). Participants were instructed to rapidly “slice” through the blue target to hit it with the white dot. If movements were not fast enough (300 msec), the words “too slow” were generated by the sound system of the computer. Visual feedback of the cursor was provided during the reach until the movement amplitude exceeded 8 cm. The cursor then froze in position for 1 sec to provide feedback concerning the spatial accuracy of the movement. The participant was free to begin moving back to the center during this time. After the spatial feedback period, the cursor disappeared and a white ring appeared, indicating the radial distance between the hand and center start position. The ring was displayed to aid the participant in returning to the center, without providing angular information about hand position. Once the participant maintained the digitizing stylus within the central start position for 200 msec, the target for the next trial was displayed.
Experimental Feedback Conditions and Procedure
Depending on the specific experimental condition and block, there were four types of visual feedback: no feedback, veridical feedback, rotated feedback, and clamped visual feedback. The cursor was not visible on no-feedback trials, whereas on veridical feedback trials, the cursor accurately showed the position of the hand. Rotated feedback involved an angular rotation of the cursor from the true hand position about the central start position. For clamped visual feedback trials, the feedback followed a trajectory that was fixed along a specific heading angle (Shmuelof et al., 2012). The radial position of the cursor was based on the radial extent of the participant’s hand (up to 8 cm amplitude) but was independent of the angular position of the hand.
Before the introduction of clamped visual feedback trials, participants were fully briefed on what they would see and were informed that they could not control the direction of the cursor movement. They were instructed to ignore the cursor and reach directly to the visual location of the target. These same instructions (“ignore the cursor and move your hand directly to the target location”) were employed in the rotated feedback conditions of Experiment 5. Note that in the Ignore conditions, the visuomotor rotation was explicitly described to participants.
Experiment 1
This experiment had two goals. First, we sought to assess how task instructions affected implicit adaptation. Second, we introduce the clamped visual feedback method to examine changes in performance in the absence of task performance errors. There were three groups of participants, Compensate, Ignore, and Clamp. All groups were briefed on the nature of the perturbation, whether it was a 45° rotation or clamped feedback offset by 45°. Both the Ignore and Clamp groups were told to ignore the feedback while moving their hands directly to the target. The Compensate group was told to compensate for the imposed rotation to make the cursor hit the target. The Compensate participants were not given instructions on how, specifically, they should compensate for the rotation. Our primary measure of implicit adaptation was the aftereffect participants exhibited following removal of the perturbation. In this block, the participants were instructed to move their hands directly to the target in the absence of visual feedback.
There were eight targets, spaced in 45° increments around 360° of the workspace. One target was presented on each trial, and the order was constrained such that all eight locations were tested in cycles of eight trials. The session started with two baseline blocks of 40 trials without visual feedback, and then 40 more trials with veridical feedback. The experimenter then informed the participant that the visual feedback would no longer be veridical, either rotated by 45° (Compensate, Ignore) or clamped 45° from the target location (Clamp). The perturbation block was composed of 240 trials, 30 to each of the eight target locations. This block was followed by two additional blocks: first, an eight-trial aftereffect block (1 reach/location) in which there was no visual feedback and then a 40-trial block with veridical visual feedback.
Experiment 2
Experiment 2 was designed to examine performance changes from extended exposure to task-irrelevant clamped visual feedback, comparing performance in this condition to that observed in a standard visuomotor rotation condition. “Learning” and generalization functions were obtained during and following exposure to clamped visual feedback or a visuomotor rotation. The visual target appeared at 1 of 24 locations, spaced in 15° increments around 360°. The test session began with a no-feedback block (96 trials) to measure intrinsic angular biases when reaching to each of the 24 target locations, followed by a block of trials with veridical feedback (96 trials). These two baseline blocks were followed by the perturbation block in which all of the reaches were to the 90° location (50 trials). For participants in the Rotation group (n = 20), visual feedback of the cursor was rotated in the counter-clockwise direction by 22.5° during this block. For participants in the clamped feedback group (n = 20), the visual feedback was invariant across trials, restricted to a trajectory that was offset from the reach target by 22.5°. The Rotation group was not informed of the perturbation; they were instructed to “do your best to hit the target.” The clamped feedback group was fully informed that the direction of the feedback cursor would be invariant and independent of their movement direction. These participants were instructed to move their hand to the visual location of the target and ignore the cursor. Following the perturbation block, the participants completed a no-feedback block (24 trials, 1 reach to each of 24 locations), with the instructions emphasizing for both groups that they should move directly to the visual target. This was followed by a veridical feedback block (96 trials) and, finally, a no-feedback block (96 trials).
Experiment 3
This experiment examined how the adaptation to task-irrelevant clamped visual feedback is affected by cerebellar degeneration, a neurological disorder that is consistently associated with sensorimotor adaptation deficits. A group of patients with varying severity of cerebellar ataxia (see Table 1 for details) and age-matched controls (n = 10/group) were exposed to a 45°-offset clamped visual feedback while reaching to four targets spaced in 90° increments around 360°. The trial structure began with a no-feedback and veridical feedback baseline blocks (40 trials each). The program was then paused, and the experimenter described the task-irrelevant clamped visual feedback, instructing the participants to always reach directly to the target while ignoring the feedback. The participants then completed a clamped visual feedback block (160 trials), following by a washout block (40 trials) with veridical feedback.
Experiment 4
This set of experiments examined how adaptation to task-irrelevant clamped visual feedback varies as a function of the magnitude of the angular offset of feedback from the target. Participants (n = 90, 10/group) were randomly assigned to one of nine groups that differed in terms of the size of the counterclockwise clamped visual feedback: 0°, 7.5°, 15°, 30°, 45°, 60°, 95°, 135°, and 175°. To ensure that adaptation was sign dependent, half of the participants in the 30° and 60° groups were exposed to clockwise clamped visual feedback offsets.
The procedure was the same for all nine groups. On each trial, a visual target appeared at one of eight locations, spaced in 45° increments around 360°. The session started with two baseline blocks: first, 40 trials without visual feedback and then 40 more trials with veridical feedback. The experimenter then informed the participant that the visual feedback would no longer be veridical but would be clamped at a fixed angle from the target location and that this feedback should be ignored. The clamped visual feedback block was composed of 240 trials, 30 to each of the eight target locations. This block was followed by two washout blocks: first, an eight-trial block (1 reach/location) in which there was no visual feedback and then a 40-trial block with veridical visual feedback.
Experiment 5
This task was designed to assess whether the invariant response to clamped visual feedback of varying size (Experiment 4) was specific to conditions in which the feedback was not contingent on hand position or whether it was a general property of adaptation. To evaluate these hypotheses, we employed standard visuomotor rotation feedback (feedback direction based on reach direction) but informed the participants about the feedback perturbation. The participants were instructed to ignore the cursor and move their hand directly to the target location, similar to the instructions provided in task-irrelevant clamped visual feedback conditions.
Participants (n = 30, 10/group) were randomly assigned to one of three rotation sizes: 7.5°, 45°, or 95°. These rotations were selected to test the range over which we observed performance changes from clamped visual feedback in Experiment 4. The statistical comparisons for this experiment included three groups from Experiment 4 that were exposed to clamped visual feedback of the same magnitude of angular offset. The test session was organized in an identical manner, consisting of 40 no-feedback baseline trials, 40 feedback baseline trials, a 240-trial perturbation block, and two washout blocks, one with no visual feedback (8 trials) and one with veridical feedback (40 trials). Target number and spacing were the same as Experiment 4.
Data Analysis
The primary dependent variable in these experiments was the heading direction, defined by the position of the hand with respect to the target when the radial amplitude of the movement reached 8 cm. We describe this position in terms of “hand angle,” taking the difference between the heading direction and target position. Reaches with endpoint hand angles ±90° from the target location were excluded from analysis (less than 1% of trials on average, maximum 7%). Separate analyses were performed in which heading direction was identified at peak radial speed. There were no meaningful differences in the two behavioral measures, and thus, we only present the data from the 8-cm endpoint measure. All t tests were two-tailed unless otherwise noted. Statistical calculations were made with MATLAB 2009b (t tests) (The MathWorks, Natick, MA) and SPSS 17 (ANOVAs) (IBM, Armonk, NY).
RESULTS
Experiment 1
In many visuomotor adaptation studies, participants are aware, at least initially, of the perturbation (Werner et al., 2015; Hwang, Smith, & Shadmehr, 2006; Kagerer, Contreras-Vidal, & Stelmach, 1997). For example, with a 45° visuomotor rotation, participants recognize that the feedback has been manipulated and may adjust their movement goals by implementing an aiming strategy to compensate for the error (Taylor et al., 2014). To regulate the use of aiming strategies, we borrowed a technique that has been used in the prism adaptation literature to experimentally control for both awareness and changes in movement intent (Welch, 1969). We informed two groups of participants that there would be a 45° visuomotor rotation of their feedback. One group was instructed to compensate for the perturbation, with a goal to make the cursor hit the target (Compensate). The other group was told to ignore the cursor and continue moving their hand directly to the target (Ignore). Immediately following the perturbation block, both groups were instructed to aim directly to the target and no visual feedback was provided. These two conditions represent extremes in how naive participants might employ an aiming strategy in a visuomotor rotation task, allowing us to explore how this affects behavior when the perturbation is present and, more importantly, after it has been removed (Figure 1B).
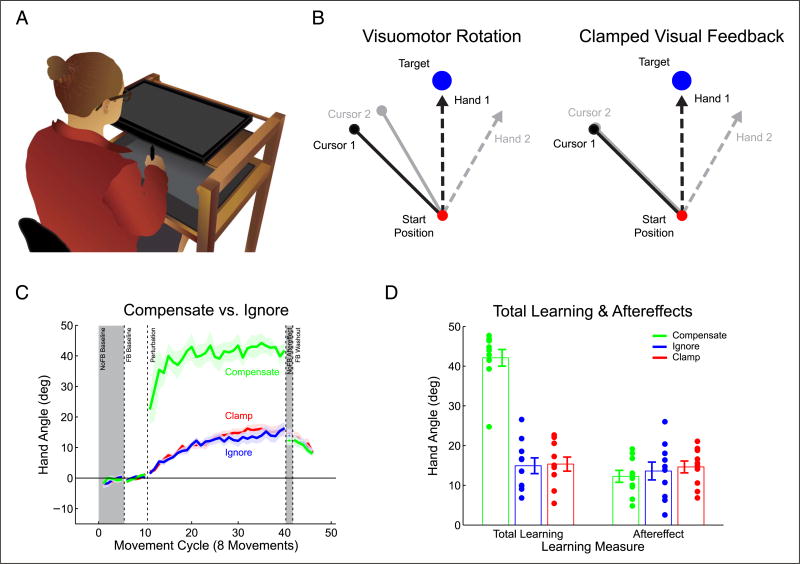
Figure 1.
A new method to induce implicit visuomotor adaptation. (A) Participants made center-out reaches on a digitizing tablet while grasping a stylus. (B) Illustration of the two visual feedback manipulations. For visuomotor rotations, the visual feedback is contingent on the hand movement. Clamped visual feedback breaks this contingency, with the feedback the same for all hand movements. Both types of feedback can be made irrelevant to the task with explicit instructions, but the error signal remains invariant with clamped visual feedback. (C) Behavior over all movement cycles for the Compensate, Ignore, and Clamp groups. (D) Baseline-adjusted mean hand angle over the last cycle of the perturbation block and the no-feedback aftereffect cycle. Dots are individual means; lines indicate group mean ± SEM. Gray shading denotes cycles without visual feedback.
Both groups showed a significant change in hand angle between the feedback baseline and the last five cycles of the perturbation block (Figure 1C; Compensate = 42.3°, t(9) = 16.7, p < .0001; Ignore = 14.1°, t(9) = 7.4, p < .0001). The change was much greater for the Compensate group compared with the Ignore group (95% CIs [21°, 34°], t(9) = 8.9, p < .0001). Following the perturbation block, both groups showed significant aftereffects when instructed to move their hands directly to the target (Figure 1D; Compensate = 12.3°, t(9) = 8.2, p < .0001; Ignore = 14.1°, t(9) = 6.1, p < .0002). Strikingly, the magnitude of the aftereffect was not different between the two groups despite the large difference in behavior during the perturbation block (95% CIs [−7°, 4.3°], t(9) = −0.4, p = .62). The large decrease in hand angle for the Compensate group could be expected, given that the participants likely used aiming strategies to compensate for the rotation, and were instructed to move their hands directly to the target when the aftereffect was measured. Assuming that the aftereffect provides a probe of implicit adaptation, these results suggest that an implicit aftereffect is minimally affected by aiming strategies.
The similarity of the aftereffect for the Compensate and Ignore groups is particularly interesting given the difference in performance errors between the two groups. Both groups saw their feedback change over the course of the perturbation block as implicit adaptation progressed. For the Compensate group, continuous changes arising from implicit adaptation would actually lead to poorer performance if the participant’s initial aiming strategy sufficiently countered the perturbation. The implicit learning would therefore necessitate adjustments of the aiming strategy to maintain good performance (Taylor et al., 2014; Taylor & Ivry, 2011). Things are not as clear for the Ignore group, but the contingency between the movement and cursor position may have induced participants, either explicitly or implicitly, to modify their behavior because of perceived performance errors (they were instructed that the feedback would always be rotated by 45°). This is especially problematic as mechanisms associated with reward and punishment have been hypothesized to modulate the rate of learning and retention in visuomotor adaptation tasks (Galea, Mallia, Rothwell, & Diedrichsen, 2015; Nikooyan & Ahmed, 2015). Such a modulation of learning or retention could have affected our measures of implicit adaptation in either group. We therefore sought to experimentally control task performance errors while simultaneously measuring implicit adaptation to putative sensory prediction errors.
To isolate implicit adaptation from task performance errors, we tested a third group in a condition in which we clamped the direction of the cursor’s motion to an invariant angular offset (45°) from the target, while locking the motion of the cursor to the radial extent of the hand (Figure 1B). Participants were fully informed about the nature of this manipulation and instructed to ignore the cursor on all trials, always moving their hand directly to the target location. We refer to this method as task-irrelevant clamped visual feedback (Clamp). Note that, although both the Clamp and Ignore groups were instructed to ignore the feedback, the contingency between behavior and the direction of motion was only broken for the Clamp group, making it the only group that is fully isolated from task performance errors.
The Clamp group showed an adaptation profile that was remarkably similar to the Ignore group (Figure 1C), with a 15.5° change in hand angle between baseline and the last five cycles of the perturbation (t(9) = 9.5, p < .0001). These participants also showed a significant aftereffect when asked to reach directly to the target without any visual feedback (14.6°, t(9) = 9.8, p < .0001). Interestingly, all of the Clamp participants changed their behavior implicitly, reporting surprise that their hands were not traveling directly to the target when veridical cursor feedback was reinstated in a final washout phase.
When the Clamp, Ignore, and Compensate groups were directly compared in a Condition × Block mixed ANOVA, there was an effect of Condition (Figure 1D; F(2, 27) = 29.49, p < .001), block (F(2, 54) = 262.5, p < .001) and a significant interaction (F(4, 54) = 52.0, p < .001). The interaction was driven by the difference between the groups during the perturbation block (F(2, 27) = 82.3, p < .001). There was no difference between the groups in the aftereffect block (F(2, 27) = 0.16, p = .852).
These results suggest that the differences in task performance between the three groups had a negligible effect on implicit adaptation. The aftereffect was similar regardless of whether or not the participants compensated for a perturbation in which the feedback was contingent on task performance (Compensate vs. Ignore) and was also similar when the feedback was non-contingent (Clamp). Indeed, the rate of adaptation during the perturbation block was similar for the Ignore and Clamp groups, providing novel, trial-by-trial evidence that the sensorimotor system automatically adapts in response to sensory prediction errors, independent of task goals.
Characterizing the Adaptive Response from Sensory Prediction Errors
In the next two experiments, we further examined the changes in performance induced by task-irrelevant clamped visual feedback. In particular, we sought evidence that the learning process engaged by this type of feedback exhibits features associated with the adaptation of an internal model from sensory prediction errors.
Experiment 2
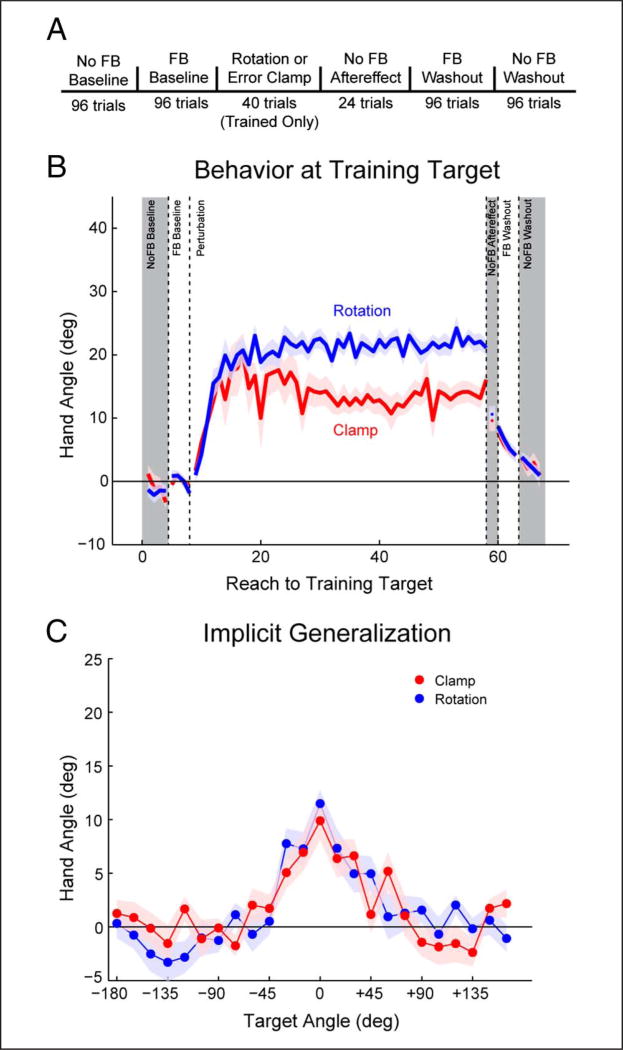
One hallmark of sensorimotor adaptation is that generalization is primarily local (Krakauer et al., 2000; Ghahramani, Wolpert, & Jordan, 1996; Imamizu, Uno, & Kawato, 1995). After adapting to targets at one location, the change in hand angle when reaching to other locations (without feedback) decreases as a function of the distance between the training location and probe locations, reaching a near-zero asymptote for targets that are more than 60° from the training location. We compared the generalization function following training with a 22.5° visual error clamp to that observed following training with a 22.5° visuomotor rotation. During training, all reaches were directed at a single location, with the feedback either non-contingent (Clamp) or contingent (Standard) on the participant’s hand position (Figure 2A). The Standard group was naive to the perturbation, whereas participants in the Clamp group were briefed on the perturbation and instructed to ignore the feedback. In the subsequent generalization block, participants performed one reach to each of 24 locations, spaced at 15° around the workspace (Figure 2A). No feedback was provided in this block, and the participants in the Standard group were explicitly instructed to reach directly to the target, thus providing them with the same instructions as that given to the Clamp group for this phase of the task.
Figure 2.
Aftereffects and generalization are similar following exposure to a standard visuomotor rotation and a clamped visual feedback. (A) Task trial design. Participants reached to all targets for every phase of the experiment, except for the perturbation block in which all reaches were to a single target. (B) Behavior at the training target during each phase of the experiment. Both groups show a change in heading angle in response to the visual feedback perturbation, with the rotation group changing hand angle by an additional 8°. However, there was no difference once the perturbation was removed. (C) Baseline subtracted aftereffect at all targets. The generalization functions are remarkably similar for the two groups around the workspace. Values are group means; shading and error bars denote SEM. Gray shading denotes cycles without visual feedback.
Both groups showed a shift in hand angle across the training block (Figure 2B; F(1, 38) = 400.8, p < .001). There was a reliable interaction between feedback condition and training block (F(1, 38) = 19.4, p < .001), with the Standard group exhibiting a larger shift in hand angle over the block relative to the Clamp group (t(38) = 4.41, p < .001). Despite these differences in performance during the perturbation block, the generalization functions for the two groups were remarkably similar: There was no difference between the two groups across the data from all probe locations (Figure 2C, F(1, 38) = .09, p = .76). Interestingly, even at the training location, the change in hand angle during the generalization block was not different for the two groups (t(38) = .73, p = .47). Similar to Experiment 1, the drop in hand angle at the training target for the Standard group, relative to the perturbation block, suggests that participants were aiming to compensate for errors during the perturbation block.
Experiment 3
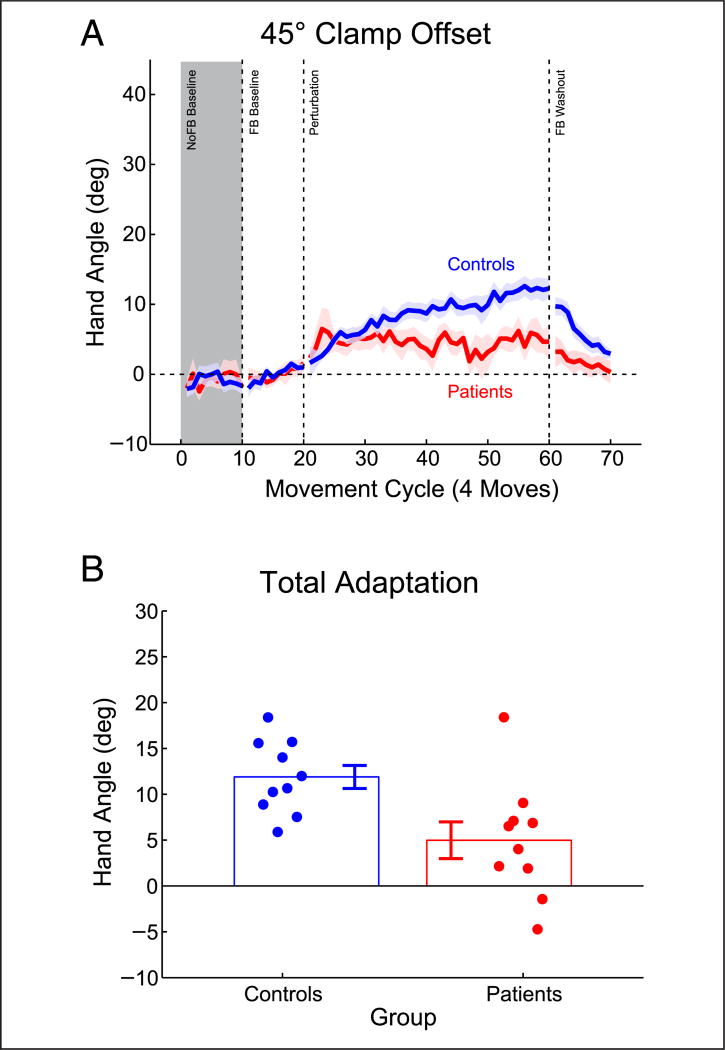
Adaptation to sensory prediction errors is dependent on the integrity of the cerebellum (Schlerf, Xu, Klemfuss, Griffiths, & Ivry, 2013; Izawa, Criscimagna-Hemminger, & Shadmehr, 2012; Criscimagna-Hemminger, Bastian, & Shadmehr, 2010; Taylor, Klemfuss, & Ivry, 2010; Tseng, Diedrichsen, Krakauer, Shadmehr, & Bastian, 2007; Smith & Shadmehr, 2005; Martin, Keating, Goodkin, Bastian, & Thach, 1996; Weiner, Hallett, & Funkenstein, 1983). As such, if the behavioral change induced by clamped feedback is driven by sensory prediction errors, these changes should be attenuated in individuals with cerebellar pathology. To test this prediction, we compared the behavior of individuals with cerebellar degeneration and matched controls in response to a 45° visual clamp. As in the other experiments, all participants were fully briefed on the nature of the perturbation and told to ignore the visual feedback while reaching directly to the target.
On average, the patients took approximately 100 msec longer than the controls to complete the reaching movements, although this effect was not reliable at the group level (t(18) = −1.44, p = .19). Both groups responded to the visual clamp (Figure 3A), exhibiting a significant change in hand angle relative to baseline over the last 10 movement cycles or 40 reaches (Figure 3B; controls: t(9) = 9.43, p < .001; patients: t(8) = 2.4, p = .04). However, the cerebellar group showed a smaller change (approximately 5° shift) than the controls (approximately 12° shift) (t(18) = 2.58, p = .02). Movement time was not correlated with the magnitude of the change in hand angle in either the controls (r8 = −.11, p = .77) or patients (r7 = −.55, p = .12).
Figure 3.
Adaptation to a task-irrelevant clamped visual feedback is attenuated in patients with cerebellar degeneration. (A) Behavior for patients and controls over all movement cycles (4 reaches/cycle). (B) Adaptation over the last 10 cycles of the perturbation block showing both group lines indicate group mean ± SEM and individual hand angle values (dots, horizontally displaced for visualization). Gray shading denotes cycles without visual feedback.
Experiments 2 and 3 provide converging evidence that the behavioral changes induced by noncontingent, clamped feedback are similar to those which occur from adaptation to sensory prediction errors in standard tasks. Generalization following noncontingent feedback was local and essentially identical to that observed with the standard method that involved contingent feedback and performance errors. The similarity in generalization between the two groups suggests that the clamped feedback method induces a similar change in an internal model (Krakauer et al., 2000; Ghahramani et al., 1996), rather than inducing a more idiosyncratic form of learning at the training location. Moreover, the behavioral response to the clamped feedback was attenuated in patients with cerebellar degeneration. Taken together, the results of the first three experiments suggest that clamped feedback provides a powerful tool to examine implicit adaptation of an internal model that is driven by sensory prediction errors, without contamination from task performance error learning.
Sensitivity of the Adaptive Response to Sensory Prediction Error Size
In the final two experiments, we employ clamped visual feedback to assess constraints on sensorimotor adaptation. In standard visuomotor rotation studies, there is usually a positive correlation or a dose-dependent response between the size of the perturbation and the magnitude of compensation, at least when the perturbation is consistent during training (Abeele & Bock, 2001). However, in these tasks the amount of behavioral change required to reinstate good task performance is confounded with the size of the perturbation. As such, it is difficult to assess if dose dependency is a feature of adaptation from sensory prediction errors, a feature of learning from task performance errors, or both. Our clamped feedback method, by eliminating task performance errors, allows us to reexamine the impact of error size on adaptation from sensory prediction errors.
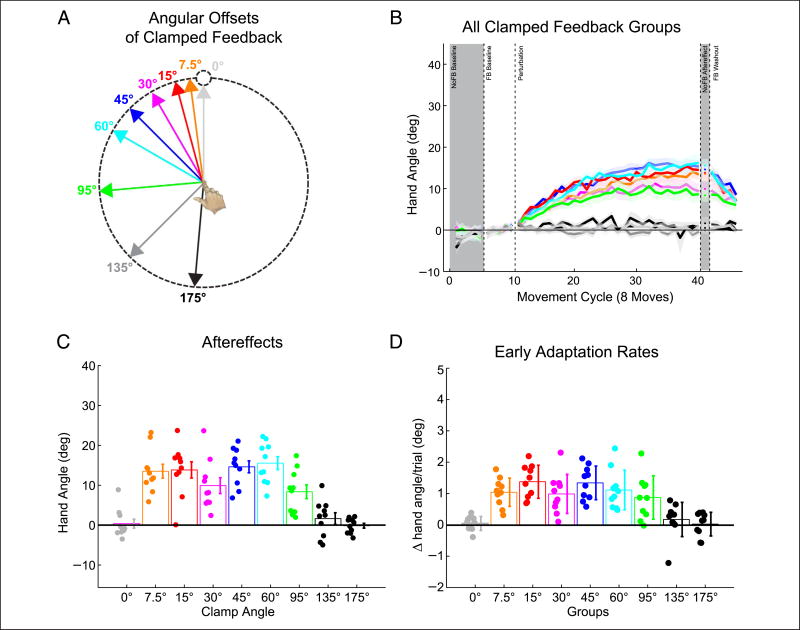
Experiment 4
We used a between-subject design, testing different groups of participants with clamped visual feedback in which the angular offset between the target and feedback ranged from 0° to 175° (Figure 4A). As expected, no change in hand angle was observed for the group exposed to a 0° clamp (i.e., where the cursor always went directly to the target). Surprisingly, we failed to observe dose-dependent adaptation for clamped feedback offsets between 7.5° and 95° (Figure 4B). For these groups, the change in hand angle during the perturbation block was similar, and the size of the aftereffect was largely invariant. For the two larger clamps, 135° and 175°, the presence of the clamp had no effect on hand angle.
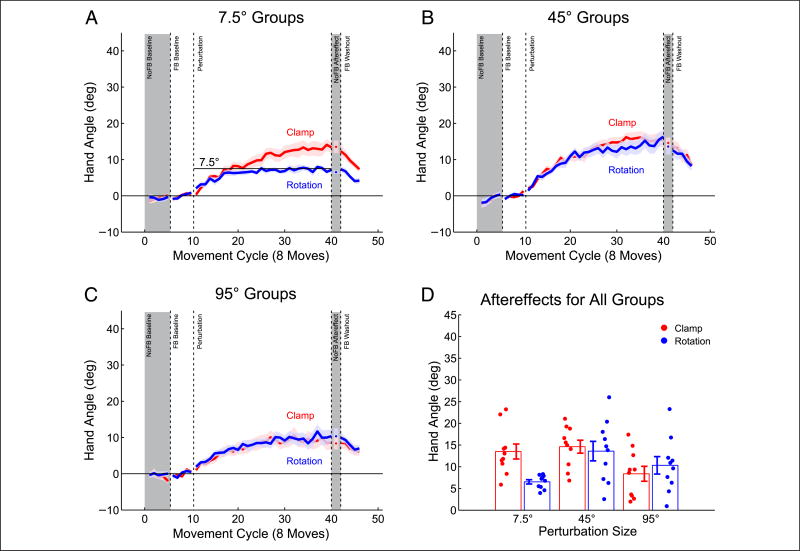
Figure 4.
Adaptation to clamped visual feedback does not scale with error size. (A) Color coding for clamped visual feedback of different offsets. Note that targets appeared at one of eight positions on a given trial. (B) Behavior for all groups, with data averaged over movements to all eight targets for each cycle. (C) Sensorimotor aftereffects, measured immediately following the perturbation block. (D) Per-trial adaptation rate over the first 10 cycles (80 trials) of the clamped visual feedback block. Dots are individual means; lines indicate group mean ± SEM. Gray shading denotes cycles without visual feedback.
These observations were confirmed by a single factor ANOVA of the aftereffect data. There was a significant effect of Offset size (Figure 3C; F(8, 81) = 16.8, p < .001), with the change in hand angle reliably different from baseline for clamped feedback offsets between 7.5° and 95° (paired t tests, ps ≤ .0001). In an analysis restricted to these groups, there was no correlation between the magnitude of the visual clamp and the size of the aftereffect (r58 = −.15, p = .26). Moreover, the per-trial rate of change in hand angle over the initial 10 movement cycles (80 trials) was not different between the groups that responded to the clamp (Figure 4D; F(5, 54) = 1.17, p = .336). Thus, we found no evidence of error size dependence in adaptation when task performance error was absent, suggesting that this is not a property of adaptation from sensory prediction errors.
Experiment 5
In the final experiment, we asked whether the absence of a dose-dependent response in Experiment 4 was idiosyncratic to clamped visual feedback or indicative of a more general property of implicit adaptation from sensory prediction errors. As emphasized above, clamped visual feedback breaks the spatial contingency between the direction of the hand movement and the angular movement of the feedback cursor. It is possible that the motor system learns that the feedback is not contingent upon behavior, resulting in a habituation of learning from the clamped visual feedback and perhaps a lack of dose dependence.
To examine whether the lack of contingency caused the absence of a dose-dependent response, we again employed the Ignore Rotation (Rotation) condition from Experiment 1, where the direction of the feedback cursor was rotated and contingent on the angular position of the hand. Participants were informed of the size and direction of the feedback perturbation and instructed to actively ignore the feedback, attempting to reach directly to the visual location of the target. Three groups of participants were tested, with the size of the perturbation set to rotations of 7.5°, 45°, or 95°. We compared their performance to the groups instructed to ignore visual clamps of the same magnitude in Experiment 4, thus creating a data set of six groups (Rotation vs. Clamp, with three magnitudes) in the statistical analyses. (The data for both 45° groups were previously shown in Experiment 1.)
All groups showed a shift in hand angle during the training block (Figure 5A–C) and a positive aftereffect, relative to baseline (Figure 5D, F(1, 54) = 271.9, p < .001). The magnitude of the aftereffect was modulated by perturbation size (F(2, 54) = 3.44, p = .04), but not by feedback contingency (F(1, 54) = 2.05, p = .16). There was, however, a Size × Feedback contingency interaction (F(2, 54) = 5.04, p = .01). This interaction was driven by a simple effect of Feedback contingency in the 7.5° groups (F(1, 56) = 10.7, p = .002), with the Clamp group showing a larger change in hand angle than the Rotation group. This is to be expected given that, for the 7.5° Rotation group, full cancellation of the perturbation has been achieved when the hand angle has shifted by 7.5°. In contrast, the visual discrepancy persists throughout the training block for the Clamp group. Interestingly, the magnitude of the change in hand angle was the same for the Rotation and Clamp groups who were exposed to either a 45° or 95° perturbation (F(1, 56) = .13, p = .71; F(1, 56) = .29, p = .59).
Figure 5.
Visual prediction error, contingent or not, drives adaptation. (A–C) Behavior over all movement cycles for each group with rotated feedback, presented with the corresponding clamped data from Experiment 3. (D) Sensorimotor aftereffects for all groups. Only the 7.5° group shows a difference between perturbation types. Dots are individual means; lines indicate group mean ± SEM. Gray shading denotes cycles without visual feedback.
Taken together, the results of Experiments 4 and 5 indicate that the absence of a dose-dependent response to clamped visual feedback is not due to the lack of a contingency between the participants’ behavior and the visual feedback. We surmise that the invariant magnitude of change in response to clamped feedback of varying size reflects a fundamental property of implicit adaptation to sensory prediction errors, with the dose dependency effects in standard tasks arising from the contribution of other learning processes that are sensitive to task performance errors (see Discussion).
The data from the 7.5° groups in Experiment 5 provide further evidence that our clamped visual feedback manipulation induces a sensory prediction error: The adaptation functions in the Clamp and Rotation conditions were nearly identical until the behavioral change for the Rotation group canceled the visual perturbation. Note that, per our instructions, in both conditions the feedback was not relevant to the participant’s task performance. Nonetheless, the error was sufficient to drive a change in hand angle. Furthermore, the 7.5° group shows the limitations of the Rotation condition. In this case, the full adaptive response to a given error size may not be observed because the error is canceled by the participant’s behavior. The performance of the 7.5° Clamp group indicates that adaptation would have continued unabated if the error had not been canceled.
DISCUSSION
We examined the behavioral response to task-irrelevant clamped visual feedback, introducing a method to measure adaptation in the absence of contamination from processes that are sensitive to task performance error. Previous studies have used clamped visual feedback to induce adaptation, but their participants were not informed that feedback would be clamped; as such, the participants likely believed that changes in their behavior could affect this feedback to reinstate good task performance (Vaswani et al., 2015; Scheidt, Conditt, Secco, & Mussa-Ivaldi, 2005). A critical difference between previous clamp studies and our method is that we fully informed participants of the nature of the manipulation and asked them to ignore it. Because our participants knew what to expect, the feedback was task-irrelevant, in addition to being clamped.
In our clamped feedback conditions spanning 7.5°–95° of angular offset, participants showed a robust change in reach direction. These changes in behavior appear to engage the same learning process that underlies implicit adaptation in standard visuomotor rotation paradigms: The change in reach direction was opposite in sign to the visual feedback, generalized locally, and was dependent on the cerebellum. Our method also provides the unique ability to directly measure implicit adaptation over an unlimited number of trials without changing the size of the imposed perturbation or relying on estimation techniques (e.g., subtracting out changes due to aiming strategies or using a model to estimate different components of learning). Taken together, our results provide a compelling demonstration of the automatic and obligatory nature of adaptation to sensory prediction errors and illuminate constraints on this process that are often obscured with traditional methods.
Invariant Adaptation across a Large Range of Error Sizes
Adaptation to the clamped visual feedback was relatively stereotyped, proceeding at a similar rate and reaching a similar final magnitude across a wide range of angular offsets (7.5°–95°). The absence of a dose-dependent response here appears, at least superficially, at odds with the behavioral changes observed in standard sensorimotor adaptation studies. In those studies, the rate of change in behavior, as well as the final magnitude of that change, increases with the size of the perturbation (Abeele & Bock, 2001).
However, several studies have reported a breakdown in the linear relationship between the size of the error and the response to that error (Kasuga, Hirashima, & Nozaki, 2013; Marko, Haith, Harran, & Shadmehr, 2012; Wei & Körding, 2009; Fine & Thoroughman, 2006). Rather than use a constant perturbation, these studies have employed a method in which the size and sign of the perturbation varied from trial to trial, with an overall mean of zero (but see Robinson, Noto, & Bevans, 2003). One study (Wei & Körding, 2009) reported that the rate of adaptation was linearly proportional for small perturbation values and saturated somewhere between 5° and 15°. The current results do not bear on the question of proportionality for small errors, a question relevant for future work. Nevertheless, the results are consistent with the finding that learning rates saturate for larger errors. The learning rate, as revealed by our clamped feedback method, indicates that implicit adaptation was invariant for errors between 7.5° and 95° and only dropped off for larger errors.
An important similarity between our clamped feedback method and zero-mean random studies is that both perturb feedback in a way that makes an adaptive response irrelevant to task performance, albeit for different reasons. With a zero-mean random perturbation, a change in behavior from one trial to the next is as likely to increase or decrease performance, forcing only “obligatory” adaptation to take place (Srimal, Diedrichsen, Ryklin, & Curtis, 2008). Tellingly, if the mean of the random distribution is shifted away from zero, a dose-dependent response is restored (Fine & Thoroughman, 2007; Scheidt, Dingwell, & Mussa-Ivaldi, 2001). We propose this is because ideal performance in this context requires compensation equal to the mean of the distribution and that dose-dependent adaptation emerges because corrections in response to the perturbation are relevant for task performance. That is, zero-mean perturbations engage only adaptation from sensory prediction errors, whereas nonzero mean perturbations also involve adaptation to sensory prediction and task performance errors.
Both our method and zero-mean random task designs provide converging evidence of a lack of error size dependence for implicit adaptation. We note that random perturbations have limited utility because the size and sign of the perturbation are constantly changing. This means that the method cannot be used to measure the full adaptive response to a given error over many continuous trials but rather is limited to the response to the error over just a few trials. Moreover, the measurement of adaptation to a given perturbation may be influenced by the context created by randomly varying perturbations (Castro, Hadjiosif, Hemphill, & Smith, 2014; Herzfeld, Vaswani, Marko, & Shadmehr, 2014; Wei & Körding, 2009; Scheidt et al., 2001). A strength of our method is that it allows the adaptive response to a fixed perturbation to be measured directly over a great number of trials, until learning from this specific error reaches an asymptote. In our experiments, implicit adaptation continued to increase over 40 reaches to each target, and the magnitude of this change was invariant to error sizes between 7.5° and 95°. We believe this invariance would continue until learning becomes asymptotic, although our experiments were not long enough to assess whether learning had fully achieved this state. It is important to emphasize that previous studies reporting insensitivity to error size focused solely on the rate of learning; the methods used in those studies could not address whether a similar insensitivity to error size also holds for learning that accumulates over many trials.
The Limits of Implicit Adaptation
Most theoretical frameworks of motor learning assume that implicit adaptation will compensate for the majority of a sensorimotor discrepancy if given a sufficient amount of training, even for very large errors (Cheng & Sabes, 2006; Pouget & Snyder, 2000; Thoroughman & Shadmehr, 2000; Jordan & Rumelhart, 1992). Our results suggest that this assumption is incorrect, at least with respect to implicit adaptation that arises from sensory prediction errors. Regardless of the size of the perturbation, the average change in hand angle was only around 12° across our various conditions. It is possible that performance had not reached asymptote, but this angular shift is substantially less than the raw change in hand angle observed in standard visuomotor rotation tasks of the same length. Interestingly, similar values have been observed for implicit adaptation in studies that have employed methods designed to dissociate implicit and explicit components of adaptation (Bond & Taylor, 2015; Miyamoto et al., 2014). In light of these convergent results from very different task contexts, we believe it unlikely that the limit of implicit adaptation is related to our instructions to ignore the feedback in either the visual clamp or rotation conditions tested here.
At present, we can suggest possible hypotheses for this constraint on the extent of adaptation. It may be that this upper bound reflects an interaction between proprioception and vision: As adaptation from the clamped visual feedback produces a change in heading angle with respect to the target, the difference between expected and observed proprioception increases. Total learning could reflect the point that these two sensory prediction errors reach a state of dynamic tension. However, as traditionally described, such a process would be sensitive to the magnitude of visual and proprioceptive errors (Zaidel, Ma, & Angelaki, 2013; Henriques & Cressman, 2012; Ghahramani, Wolpert, & Jordan, 1997). This predicts differences in learning rate based on the magnitude of the visual error and, as such, is unlikely to account for our data given the invariant response to error magnitude. Furthermore, visuomotor adaptation is unaffected when visual feedback conflicts with proprioception (Marko et al., 2012). Nonetheless, it is possible that magnitude of total learning is limited by proprioception.
Another possibility for the invariant amount of total adaptation is that learning and forgetting have reached a state of dynamic tension (Cheng & Sabes, 2006; Thoroughman & Shadmehr, 2000). The present work suggests that the learning rate saturates for sensory prediction errors above 7.5°. This would also mandate that the forgetting term is independent of error size. This seems a reasonable assumption, although we cannot evaluate this quantitatively given that our experiments were not designed to make reliable measurements of forgetting. It will be important in future work to further assess the constraints on implicit adaptation; at present, our method has revealed important limitations with current theoretical models of this process.
Implications for Models of Sensorimotor Adaptation
Many models of adaptation have been developed to characterize learning from sensorimotor perturbations across extended blocks of trials. A core property of most of these models is a delta learning rule, whereby the amount of trial-by-trial change in behavior is the product of the error multiplied by a scalar learning rate, with the latter assumed to be constant across a range of perturbations (Cheng & Sabes, 2006; Thoroughman & Shadmehr, 2000). Such models fail to describe our data, as they predict an adaptive response that scales linearly with the size of the perturbation. Notably, models featuring fixed scalar learning rates also fail to predict behavior in tasks with random perturbations that flip sign and magnitude from trial to trial (Marko et al., 2012; Wei & Körding, 2009; Fine & Thoroughman, 2006). We propose that linear state-space models, and indeed any model that features error size multiplied by a scalar learning rate, will provide an inappropriate characterization of adaptation from sensory prediction errors.
Alternative models of adaptation have been developed to account for the behavior observed in response to random perturbations. One class of these models discounts errors based on their size, because of either the discrepancy between proprioception and visual feedback (Wei & Körding, 2009) or a subquadratic loss function for human sensorimotor errors (Marko et al., 2012; Körding & Wolpert, 2004). In their current form, both the proprioceptive and loss function models predict that the rate of learning will decline to zero, well below 95° of error. As such, these discounting models are unable to account for the absence of dose dependency observed in our results. Notably, the invariance was not an artifact of clamped visual feedback; it was also found when we used the ignore instructions to look at behavioral changes to task-irrelevant visuomotor rotations (Experiment 5).
Another type of model has been proposed in which the rate of adaptation is modulated over time, based on the history of experienced errors (Herzfeld et al., 2014). Of greatest relevance to the current study, this model predicts a sensitization to previously experienced errors. With clamped visual feedback, the angular offset remains invariant, ostensibly presenting the same sensory prediction error on every trial. We saw no sensitization of the adaptation rate in the clamped visual feedback experiments relative to rotation experiments where the error size decreased over trials. It may well be that this model better describes learning processes related to task performance errors, rather than sensory prediction errors.
As noted above, we suspect that a major limitation with current models of learning from sensory prediction errors is that, though assumed to provide a characterization of adaptation, they actually model data reflecting behavioral changes that arise from a composite of sensory prediction errors and task performance errors (Huberdeau, Krakauer, & Haith, 2015; McDougle, Bond, & Taylor, 2015; Wolpert, Diedrichsen, & Flanagan, 2011; Redding, Rossetti, & Wallace, 2005; Weiner et al., 1983). In this context, processes other than error-based updating of internal models could dominate performance, especially in response to large perturbations. Indeed, recent work decomposing visuomotor learning into implicit and explicit components converges with our finding that implicit adaptation is invariant, as the dose-dependent learning was solely attributed to variation in the use of explicit aiming (Bond & Taylor, 2015). Even when performance errors are removed (as in aftereffect blocks without feedback), the effect of these other learning processes may persist, further obscuring the contribution of adaptation from sensory prediction errors (Diedrichsen, White, Newman, & Lally, 2010).
Conclusions
The current experiments introduce a method designed to measure implicit adaptation in the absence of task performance errors. The behavioral change observed in response to clamped visual feedback had many of the features that are historically associated with adaptation to sensory prediction errors: The response was monotonic, generalized in a limited manner, and was attenuated in individuals with cerebellar degeneration. By isolating learning from sensory prediction errors, we found that adaptation was invariant across a large range of perturbations, both in terms of the initial learning rate and total accumulated learning. Our results suggest that this lack of a dose dependency is a fundamental characteristic of implicit “obligatory” adaptation. Moreover, the results underscore the importance of carefully considering the source of behavioral changes in studies of sensorimotor adaptation, as different types of errors may lead to different types of learning. The task-irrelevant clamped feedback method can serve as a powerful tool for future work on the role implicit adaptation in various motor learning phenomena.
Acknowledgments
We would like to thank Elizabeth Marrone, Dashel Thompson, Shabnam Bonyadi, and Giana Cirolia for their tireless assistance in keeping J. R. M. organized and helping collect experimental data. This work was supported by NIH grants NS092079 and NS074917 to R. B. I. and NS084948 to J. A. T. J. R. M., J. A. T., and R. B. I. designed all experiments. J. R. M. and D. E. P. directed collection of experimental data. J. R. M. analyzed data. J. R. M., J. A. T., D. E. P., and R. B. I. interpreted the results. J. R. M., J. A. T., and R. B. I. wrote the manuscript.
References
- Abeele S, Bock O. Sensorimotor adaptation to rotated visual input: Different mechanisms for small versus large rotations. Experimental Brain Research. 2001;140:407–410. doi: 10.1007/s002210100846. [DOI] [PubMed] [Google Scholar]
- Bond KM, Taylor JA. Flexible explicit but rigid implicit learning in a visuomotor adaptation task. Journal of Neurophysiology. 2015;113:3836–3849. doi: 10.1152/jn.00009.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro LNG, Hadjiosif AM, Hemphill MA, Smith MA. Environmental consistency determines the rate of motor adaptation. Current Biology. 2014;24:1050–1061. doi: 10.1016/j.cub.2014.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S, Sabes PN. Modeling sensorimotor learning with linear dynamical systems. Neural Computation. 2006;18:760–793. doi: 10.1162/089976606775774651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criscimagna-Hemminger SE, Bastian AJ, Shadmehr R. Size of error affects cerebellar contributions to motor learning. Journal of Neurophysiology. 2010;103:2275–2284. doi: 10.1152/jn.00822.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrichsen J, White O, Newman D, Lally N. Use-dependent and error-based learning of motor behaviors. Journal of Neuroscience. 2010;30:5159–5166. doi: 10.1523/JNEUROSCI.5406-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine MS, Thoroughman KA. Motor adaptation to single force pulses: Sensitive to direction but insensitive to within-movement pulse placement and magnitude. Journal of Neurophysiology. 2006;96:710–720. doi: 10.1152/jn.00215.2006. [DOI] [PubMed] [Google Scholar]
- Fine MS, Thoroughman KA. Trial-by-trial transformation of error into sensorimotor adaptation changes with environmental dynamics. Journal of Neurophysiology. 2007;98:1392–1404. doi: 10.1152/jn.00196.2007. [DOI] [PubMed] [Google Scholar]
- Galea JM, Mallia E, Rothwell J, Diedrichsen J. The dissociable effects of punishment and reward on motor learning. Nature Neuroscience. 2015;18:597–602. doi: 10.1038/nn.3956. [DOI] [PubMed] [Google Scholar]
- Ghahramani Z, Wolpert DM, Jordan MI. Generalization to local remappings of the visuomotor coordinate transformation. Journal of Neuroscience. 1996;16:7085–7096. doi: 10.1523/JNEUROSCI.16-21-07085.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghahramani Z, Wolpert DM, Jordan MI. Computational models of sensorimotor integration. Advances in Psychology. 1997;119:117–147. [Google Scholar]
- Held R, Freedman SJ. Plasticity in human sensorimotor control. Science. 1963;142:455–462. doi: 10.1126/science.142.3591.455. [DOI] [PubMed] [Google Scholar]
- Held R, Hein AV. Adaptation of disarranged hand-eye coordination contingent upon re-afferent stimulation. Perceptual and Motor Skills. 1958;8:87–90. [Google Scholar]
- Henriques DY, Cressman EK. Visuomotor adaptation and proprioceptive recalibration. Journal of Motor Behavior. 2012;44:435–444. doi: 10.1080/00222895.2012.659232. [DOI] [PubMed] [Google Scholar]
- Herzfeld DJ, Vaswani PA, Marko MK, Shadmehr R. A memory of errors in sensorimotor learning. Science. 2014;345:1349–1353. doi: 10.1126/science.1253138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard IP. Displacing the optical array. In: Freedman SJ, editor. The neuropsychology of spatially oriented behavior. Homewood, IL: Dorsey Press; 1968. pp. 19–36. [Google Scholar]
- Huberdeau DM, Krakauer JW, Haith AM. Dual-process decomposition in human sensorimotor adaptation. Current Opinion in Neurobiology. 2015;33:71–77. doi: 10.1016/j.conb.2015.03.003. [DOI] [PubMed] [Google Scholar]
- Hwang EJ, Smith MA, Shadmehr R. Dissociable effects of the implicit and explicit memory systems on learning control of reaching. Experimental Brain Research. 2006;173:425–437. doi: 10.1007/s00221-006-0391-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamizu H, Uno Y, Kawato M. Internal representations of the motor apparatus: Implications from generalization in visuomotor learning. Journal of Experimental Psychology: Human Perception and Performance. 1995;21:1174. doi: 10.1037//0096-1523.21.5.1174. [DOI] [PubMed] [Google Scholar]
- Izawa J, Criscimagna-Hemminger SE, Shadmehr R. Cerebellar contributions to reach adaptation and learning sensory consequences of action. Journal of Neuroscience. 2012;32:4230–4239. doi: 10.1523/JNEUROSCI.6353-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan MI, Rumelhart DE. Forward models: Supervised learning with a distal teacher. Cognitive Science. 1992;16:307–354. [Google Scholar]
- Kagerer FA, Contreras-Vidal JL, Stelmach GE. Adaptation to gradual as compared with sudden visuo-motor distortions. Experimental Brain Research. 1997;115:557–561. doi: 10.1007/pl00005727. [DOI] [PubMed] [Google Scholar]
- Kasuga S, Hirashima M, Nozaki D. Simultaneous processing of information on multiple errors in visuomotor learning. PLoS One. 2013;8:e72741. doi: 10.1371/journal.pone.0072741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Körding KP, Wolpert DM. The loss function of sensorimotor learning. Proceedings of National Academy of Sciences U.S.A. 2004;101:9839–9842. doi: 10.1073/pnas.0308394101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer JW, Pine ZM, Ghilardi MF, Ghez C. Learning of visuomotor transformations for vectorial planning of reaching trajectories. Journal of Neuroscience. 2000;20:8916–8924. doi: 10.1523/JNEUROSCI.20-23-08916.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marko MK, Haith AM, Harran MD, Shadmehr R. Sensitivity to prediction error in reach adaptation. Journal of Neurophysiology. 2012;108:1752–1763. doi: 10.1152/jn.00177.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TA, Keating JG, Goodkin HP, Bastian AJ, Thach WT. Throwing while looking through prisms. II. Specificity and storage of multiple gaze-throw calibrations. Brain. 1996;119:1199–1212. doi: 10.1093/brain/119.4.1199. [DOI] [PubMed] [Google Scholar]
- Mazzoni P, Krakauer JW. An implicit plan overrides an explicit strategy during visuomotor adaptation. Journal of Neuroscience. 2006;26:3642–3645. doi: 10.1523/JNEUROSCI.5317-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougle SD, Bond KM, Taylor JA. Explicit and implicit processes constitute the fast and slow processes of sensorimotor learning. Journal of Neuroscience. 2015;35:9568–9579. doi: 10.1523/JNEUROSCI.5061-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miall RC, Wolpert DM. Forward models for physiological motor control. Neural Networks. 1996;9:1265–1279. doi: 10.1016/s0893-6080(96)00035-4. [DOI] [PubMed] [Google Scholar]
- Miyamoto YR, Wang SX, Brennan AE, Smith MA. Distinct forms of implicit learning that respond differentially to performance errors and sensory prediction errors. Translational and Computational Motor Control. 2014;13 [Google Scholar]
- Nikooyan AA, Ahmed AA. Reward feedback accelerates motor learning. Journal of Neurophysiology. 2015;113:633–646. doi: 10.1152/jn.00032.2014. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pouget A, Snyder LH. Computational approaches to sensorimotor transformations. Nature Neuroscience. 2000;3:1192–1198. doi: 10.1038/81469. [DOI] [PubMed] [Google Scholar]
- Redding GM, Rossetti Y, Wallace B. Applications of prism adaptation: A tutorial in theory and method. Neuroscience & Biobehavioral Reviews. 2005;29:431–444. doi: 10.1016/j.neubiorev.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Robinson FR, Noto CT, Bevans SE. Effect of visual error size on saccade adaptation in monkey. Journal of Neurophysiology. 2003;90:1235–1244. doi: 10.1152/jn.00656.2002. [DOI] [PubMed] [Google Scholar]
- Schaefer SY, Shelly IL, Thoroughman KA. Beside the point: Motor adaptation without feedback-based error correction in task-irrelevant conditions. Journal of Neurophysiology. 2012;107:1247–1256. doi: 10.1152/jn.00273.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidt RA, Conditt MA, Secco EL, Mussa-Ivaldi FA. Interaction of visual and proprioceptive feedback during adaptation of human reaching movements. Journal of Neurophysiology. 2005;93:3200–3213. doi: 10.1152/jn.00947.2004. [DOI] [PubMed] [Google Scholar]
- Scheidt RA, Dingwell JB, Mussa-Ivaldi FA. Learning to move amid uncertainty. Journal of Neurophysiology. 2001;86:971–985. doi: 10.1152/jn.2001.86.2.971. [DOI] [PubMed] [Google Scholar]
- Scheidt RA, Reinkensmeyer DJ, Conditt MA, Rymer WZ, Mussa-Ivaldi FA. Persistence of motor adaptation during constrained, multi-joint, arm Movements. Journal of Neurophysiology. 2000;84:853–862. doi: 10.1152/jn.2000.84.2.853. [DOI] [PubMed] [Google Scholar]
- Schlerf JE, Xu J, Klemfuss NM, Griffiths TL, Ivry RB. Individuals with cerebellar degeneration show similar adaptation deficits with large and small visuomotor errors. Journal of Neurophysiology. 2013;109:1164–1173. doi: 10.1152/jn.00654.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Smith MA, Krakauer JW. Error correction, sensory prediction, and adaptation in motor control. Annual Review of Neuroscience. 2010;33:89–108. doi: 10.1146/annurev-neuro-060909-153135. [DOI] [PubMed] [Google Scholar]
- Shmuelof L, Huang VS, Haith AM, Delnicki RJ, Mazzoni P, Krakauer JW. Overcoming motor “forgetting” through reinforcement of learned actions. Journal of Neuroscience. 2012;32:14617–14621. doi: 10.1523/JNEUROSCI.2184-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Shadmehr R. Intact ability to learn internal models of arm dynamics in Huntington’s disease but not cerebellar degeneration. Journal of Neurophysiology. 2005;93:2809–2821. doi: 10.1152/jn.00943.2004. [DOI] [PubMed] [Google Scholar]
- Srimal R, Diedrichsen J, Ryklin EB, Curtis CE. Obligatory adaptation of saccade gains. Journal of Neurophysiology. 2008;99:1554–1558. doi: 10.1152/jn.01024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JA, Ivry RB. Flexible cognitive strategies during motor learning. PLoS Computational Biology. 2011;7:e1001096. doi: 10.1371/journal.pcbi.1001096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JA, Klemfuss NM, Ivry RB. An explicit strategy prevails when the cerebellum fails to compute movement errors. The Cerebellum. 2010;9:580–586. doi: 10.1007/s12311-010-0201-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JA, Krakauer JW, Ivry RB. Explicit and implicit contributions to learning in a sensorimotor adaptation task. Journal of Neuroscience. 2014;34:3023–3032. doi: 10.1523/JNEUROSCI.3619-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoroughman KA, Shadmehr R. Learning of action through adaptive combination of motor primitives. Nature. 2000;407:742–747. doi: 10.1038/35037588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng YW, Diedrichsen J, Krakauer JW, Shadmehr R, Bastian AJ. Sensory prediction errors drive cerebellum-dependent adaptation of reaching. Journal of Neurophysiology. 2007;98:54–62. doi: 10.1152/jn.00266.2007. [DOI] [PubMed] [Google Scholar]
- Vaswani PA, Shmuelof L, Haith AM, Delnicki RJ, Huang VS, Mazzoni P, et al. Persistent residual errors in motor adaptation tasks: Reversion to baseline and exploratory escape. Journal of Neuroscience. 2015;35:6969–6977. doi: 10.1523/JNEUROSCI.2656-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei K, Körding K. Relevance of error: What drives motor adaptation? Journal of Neurophysiology. 2009;101:655–664. doi: 10.1152/jn.90545.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner MJ, Hallett M, Funkenstein HH. Adaptation to lateral displacement of vision in patients with lesions of the central nervous system. Neurology. 1983;33:766–772. doi: 10.1212/wnl.33.6.766. [DOI] [PubMed] [Google Scholar]
- Welch RB. Adaptation to prism-displaced vision: The importance of target-pointing. Perception & Psychophysics. 1969;5:305–309. [Google Scholar]
- Werner S, van Aken BC, Hulst T, Frens MA, van der Geest JN, Strüder HK, et al. Awareness of sensorimotor adaptation to visual rotations of different size. PLoS One. 2015;10:e0123321. doi: 10.1371/journal.pone.0123321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert DM, Diedrichsen J, Flanagan JR. Principles of sensorimotor learning. Nature Reviews Neuroscience. 2011;12:739–751. doi: 10.1038/nrn3112. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Ghahramani Z, Jordan MI. An internal model for sensorimotor integration. Science. 1995a;269:1880–1882. doi: 10.1126/science.7569931. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Ghahramani Z, Jordan MI. Are arm trajectories planned in kinematic or dynamic coordinates? An adaptation study. Experimental Brain Research. 1995b;103:460–470. doi: 10.1007/BF00241505. [DOI] [PubMed] [Google Scholar]
- Zaidel A, Ma WJ, Angelaki DE. Supervised calibration relies on the multisensory percept. Neuron. 2013;80:1544–1557. doi: 10.1016/j.neuron.2013.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]