Abstract
We demonstrate a chemically-detachable cell-glue system based on linkers containing disulfide bonds as well as functional groups for metabolic glycoengineering and bioorthogonal click chemistry. The artificial cell-cell adhesion can be broken by the administration of glutathione (5 mM), which triggers the degradation of disulfide bonds. Both the gluing and detachment processes are rapid (< 10 min) and minimally cytotoxic.
Adhesion between cells is a fundamental process enabling direct cell-cell interaction and tissue formation.1, 2 Cell-cell adhesion in vivo involves complex cell signaling and specific adhesion molecules.3 Recently, there have been interests in developing artificial methods to form cell-to-cell adhesion for biomedical applications. These methods are based on various artificial binding molecules on cell surface, such as avidin-biotin pairs,4 nucleotide with complementary sequences,5 aptamers,6 and antibody dimers.7 Recently, we have developed a robust cell-gluing system based on click chemistry and metabolic glycoengineering.8 The bioorthogonality, specificity, and fast reaction rate of click chemistry based on tetrazine (Tz) and trans-cyclooctene (TCO) allowed the gluing process to be completed in vitro in 10 min and remain stable in vivo in mice. Here, we describe an extension of this method to incorporate a mechanism to break the bonds between glued cells by simple administration of a chemical agent.
Two different approaches for detachable cell glue systems have been previously reported. Yousaf group used oxime-hydroquinone chemistry that can reversibly degrade via electrochemical stimuli.9 Although this technique is useful for two-dimensional cell patterning, electronic signal requires special plates, such as gold surface, and cannot be easily applied in vivo. Wagner group developed a genetic engineering-based method that forms cell-cell assembly by recombinant fusion protein of dihydrofolate reductase inhibitor methotrexate and disintegrates by treatment of trimethoprim, a bacterial dihydrofolate reductase inhibitor.10 By comparison, our chemical-based technique is simpler and applicable to general situations including in vivo environments.
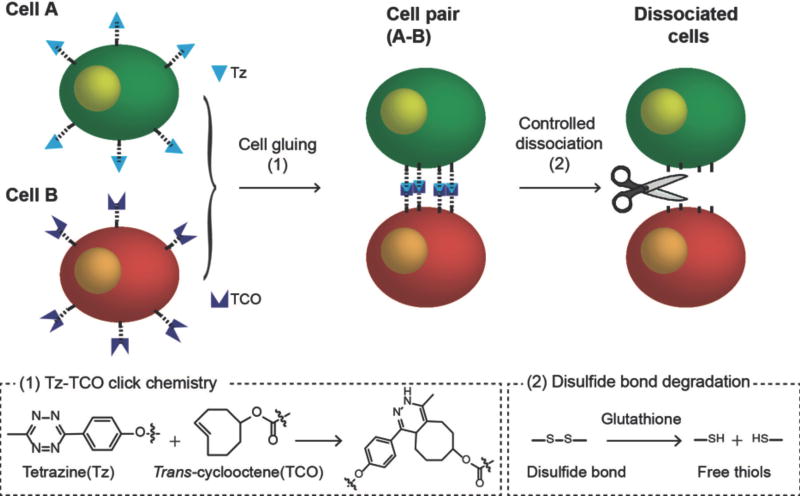
As before,8 we used metabolic glycoengineering to introduce chemical functional groups to the cell surface with minimum perturbation on cellular viability and functions.11 After treatment of tetraacetylated N-azidoacetyl-d-mannosamine (Ac4ManNAz) to A549 cells, azide groups are generated on the cell surface (Fig. S3 in ESI).12 In this work, we used novel rationally-designed crosslinkers, dibenzocyclootyne disulfide tetrazine (DBCO-SS-Tz) and dibenzocyclootyne disulfide trans-cyclooctene (DBCO-SS-TCO), which have two functional groups for click chemistry—Tz and TCO—and degradable disulfide (SS) bonds in their backbone (Fig. S1 and S2 in ESI). The azide-modified cells are treated with these crosslinkers, so that the crosslinkers are conjugated to the azide groups by azide-DBCO click chemistry, and Tz- or TCO-modified cells are prepared;13 When the Tz-modified and TCO-modified cells are mixed together, cell gluing between these two cell groups is established by Tz-TCO click chemistry (Scheme 1). The disulfide bonds in the backbone are cleaved by glutathione (GSH).14 Therefore, the detachment of the glued cells can be achieved simply by administration of GSH (Scheme 1).
Scheme 1.
Illustration of the chemically-detachable cellular glue system based on click chemistry linkers with degradable disulfide bonds.
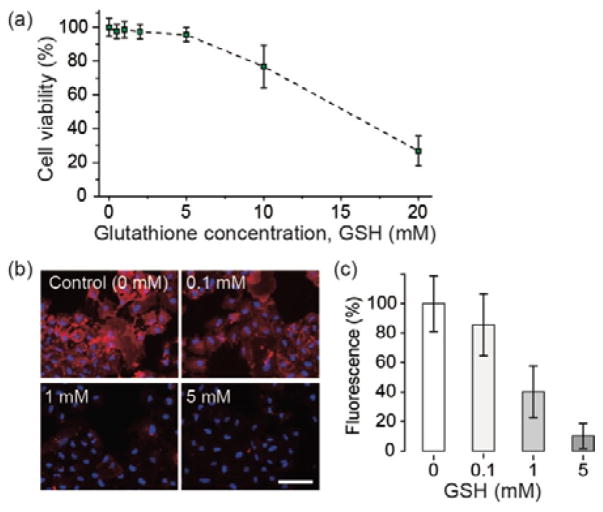
To determine the optimum concentration of GSH, we first evaluated the intrinsic toxicity of GSH on cells. A549 cells were treated with various concentrations of GSH and a cell viability assay (Prestoblue™) was performed. We found GSH at concentrations of 10 and 20 mM decreased cell viability significantly, but concentrations below 5 mM had negligible effects on cell viability (Fig 1a). Based on this result, we determine 5 mM to be the maximum concentration for non-toxic detachment.
Figure 1.
Chemical modification of cells for gluing and detachment. (a) Cell viability after treatment of glutathione (GSH) using PrestoblueTM assay. (b) Detachment of Tz groups from cell surface after treatment of GSH. Tz-modified A549 cells were labeled with TCO-Cy3 (red), and treated with GSH (blue = Hoechst 33342). Scale bar, 50 μm. (c) The magnitude of fluorescence from the data in (b).
To evaluate the efficiency for cleaving linkers, we added Cy3-TCO conjugates to the culture media of Tz-modified cells. The fluorescent probes are bound to the Tz groups on the cell surface, and the fluorescence intensity from Cy3 bound on the cell surface indicates the amount of Tz groups. We treated these cells with GSH at three different concentrations, 0.1, 1, and 5 mM. After washing the cells, the Cy3 fluorescence was measured. As expected, the fluorescence intensity decreased with the concentration of GSH (Fig. 1b). This supports the mechanism that the administered GSH breaks disulfide bonds and thus reduce the total amount of linkers on the cell surface. At a concentration of 5 mM, the Cy3 fluorescence intensity decreased to about 16.4 % compared to the control group without GSH treatment (0 mM). This indicates that at this condition the glue strength between cells would be degraded by a factor of 6. We obtained similar results with TCO-modified cells by using DBCO-SS-TCO crosslinkers and Cy3-Tz as fluorescent probes (Fig. S4 in ESI).
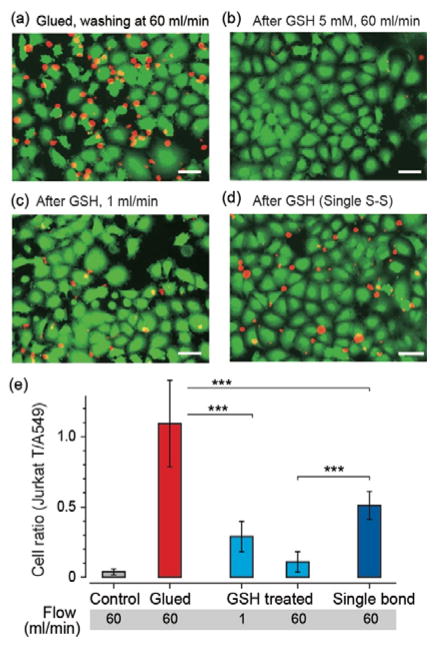
To analyse cell gluing strength, we cultured GFP-expressing A549 cells in a monolayer in a microfluidic chamber, and treated them to Tz-modified cells with the protocol described above. To produce glued cells, we added RFP-expressing TCO-modified Jurkat T cells in suspension into the chamber and incubated for 10 min. We injected PBS into the fluidic channels at different flow speeds and measured the number of TCO-modified Jurkat T cells that remained bound to the Tz-modified A549 cells adherent on the chamber under fluorescent microscopy. After applying flow at a speed of 60 ml/min for 10 min, we found about equal number of T cells to be attached on the A549 cells (Fig 2a), whereas almost all non-modified Jurkat T cells in a control group were washed away by the flow (Fig 2e).
Figure 2.
Gluing and detachment of cells in a microfluidic system. (a) Fluorescence images of Jurkat T cells (red) on A549 cells (green) in a microfluidic chamber after washing. Sham control sample without GSH treatment, flow rate = 60 ml/min. (b) The ratio of glued cells (Jurkat-A549 pairs) remaining after flow tests. (c) Fluorescence image after a GSH treatment (5 mM) and washing at a flow rate of 60 ml/min. (d) GSH treatment and washing at a flow rate of 1 ml/min. (e) A control sample after gluing using DBCO-TCO instead of DBCO-SS-TCO so that each cell-connecting linker contains only a single S-S bond, GSH treatment, and washing at a flow rate of 60 ml/min. Scale bars, 50 μm.
We performed this flow assay on glued cells after GSH treatment at a concentration of 5 mM and found that the ratio of remaining Jurkat T cells to adherent A569 cells decreased significantly to about 10%; that is, about 90% of initially glued T cells were detached and washed away by the flow at a speed of 60 ml/min (Fig. 2b). At a reduced flow speed of 1 ml/min, 70% of T cells were washed away (Fig. 2c). These data show the effect of GSH on the degradation of the bonding strength of the glued cells.
To confirm that the mechanism of detachment is due to the GSH-induced breakage of the disulfide (S-S) bonds in the linkers, we prepared TCO-modified Jurkat T cells with DBCO-TCO (no disulfide bond) and glue the cells with A549 cells modified with DBCO-SS-Tz (Fig. S5 and S6 in ESI). In this case, there is only one S-S cleavage site in each cell-cell linker, as opposed to the double S-S bonds in the previous linkers. After GSH treatment and flow at 60 ml/min, we measured a remaining cell ratio of 45%, indicating only ~55% of the glued Jurkat T cells were washed away (Fig. 2d). It shows that two S-S bonds are advantageous for cleavage of the linkage and detachment of cells compared to one S-S bond. These results also evidence that the detachment involves the degradation of S-S bonds by GSH.
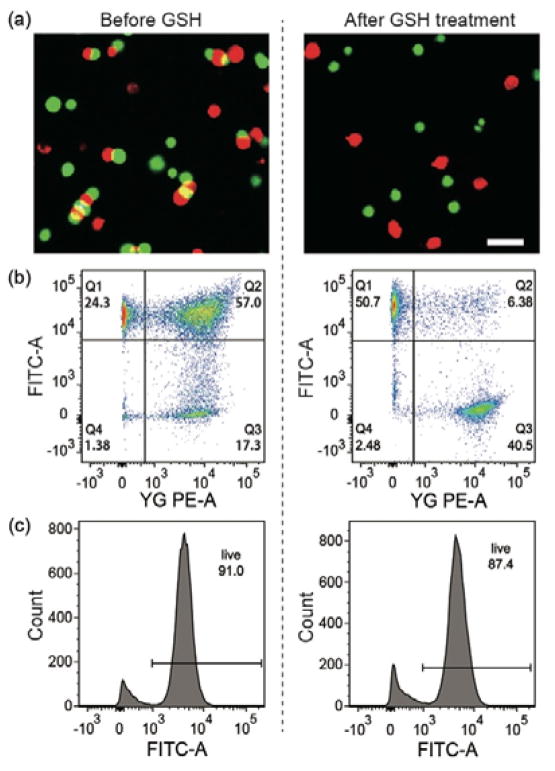
To evaluate our system for suspension cells, we prepared two groups of Jurkat T cells and modified them with DBCO-SS-Tz and DBCO-SS-TCO, respectively. After 10 min of incubation, fluorescence microscopy was used to confirm selective attachment of Tz- and TCO-modified Jurkat T cells (Fig. 3a). In flow cytometry, the ratio of double-positive counts was measured to be 57% (Fig. 3b). After incubating with GSH (5 mM), the ratio of glued cells decreased to 6.4% (Fig. 3b), indicating that nearly 90% (6.4/58) of the glued cells were dissociated by the GSH treatment.
Figure 3.
The efficiency and viability of cell gluing and detachment. (a) Fluorescence images of the glued and detached Jurkat T cells in suspension. Scale bar, 30 μm. (b) Flow cytometry data of the glued and detached Jurkat T cells. (c) Cell viability data after gluing and detachment (calcein AM staining and flow cytometry).
After incubating with a cell viability probe, calcein AM, a cytometry analysis showed that 91% cells were alive after gluing, and that 87% cells remained vital after the gluing and de-gluing processes (Fig. 3c). This data confirms the low cytotoxicity of the detachable cell glue system.
In summary, we have demonstrated a rapid, efficient, non-toxic, artificial gluing and controlled detachment of cells by click chemistry and chemically-degradable bonds. In this study, we used disulfide bonds as the cleavable site in the cell-cell linkers and GSH as the chemical triggering agent. There are many other chemical bonds developed for degradation15 by various stimuli, such as chemicals,16 light,17 pH,18 or enzymes.19 Our scheme may be extended to different embodiments optimized for specific applications.
Supplementary Material
Acknowledgments
This work was supported in part by the U.S. National Institutes of Health (R01-CA192878, P41-EB015903), National Science Foundation (ECCS-1562863), Department of Defense (FA9550-11-1-0331), the Future Planning (NRF-2011-0031644), Basic Science Research Program by the Ministry of Education (2016R1C1B3013951), and Mid-career Researcher Program by the Ministry of Science through the National Research Foundation of Korea, and National IT Industry Promotion Agency (IT Consilience Creative Program, NIPA-2014-H0201-14-1001).
Notes and references
- 1.Ahrends R, Ota A, Kovary KM, Kudo T, Park BO, Teruel MN. Science. 2014;344:1384–1389. doi: 10.1126/science.1252079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meshel AS, Wei Q, Adelstein RS, Sheetz MP. Nat Cell Biol. 2005;7:157–164. doi: 10.1038/ncb1216. [DOI] [PubMed] [Google Scholar]
- 3.Lee S, Mandic J, Van Vliet KJ. Proceedings of the National Academy of Sciences. 2007;104:9609–9614. doi: 10.1073/pnas.0702668104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang B, Song J, Yuan H, Nie C, Lv F, Liu L, Wang S. Adv Mater. 2014;26:2371–2375. doi: 10.1002/adma.201304593. [DOI] [PubMed] [Google Scholar]
- 5.Gartner ZJ, Bertozzi CR. Proceedings of the National Academy of Sciences. 2009;106:4606–4610. doi: 10.1073/pnas.0900717106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiong X, Liu H, Zhao Z, Altman MB, Lopez-Colon D, Yang CJ, Chang LJ, Liu C, Tan W. Angew Chem Int Ed. 2013;52:1472–1476. doi: 10.1002/anie.201207063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim CH, Axup JY, Dubrovska A, Kazane SA, Hutchins BA, Wold ED, Smider VV, Schultz PG. J Am Chem Soc. 2012;134:9918–9921. doi: 10.1021/ja303904e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koo H, Choi M, Kim E, Hahn SK, Weissleder R, Yun SH. Small. 2015;11:6458–6466. doi: 10.1002/smll.201502972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pulsipher A, Dutta D, Luo W, Yousaf MN. Angew Chem Int Ed. 2014;53:9487–9492. doi: 10.1002/anie.201404099. [DOI] [PubMed] [Google Scholar]
- 10.Gabrielse K, Gangar A, Kumar N, Lee JC, Fegan A, Shen JJ, Li Q, Vallera D, Wagner CR. Angew Chem Int Ed. 2014;53:5112–5116. doi: 10.1002/anie.201310645. [DOI] [PubMed] [Google Scholar]
- 11.Saxon E, Bertozzi CR. Science. 2000;287:2007–2010. doi: 10.1126/science.287.5460.2007. [DOI] [PubMed] [Google Scholar]
- 12.Koo H, Lee S, Na JH, Kim SH, Hahn SK, Choi K, Kwon IC, Jeong SY, Kim K. Angew Chem Int Ed. 2012;51:11836–11840. doi: 10.1002/anie.201206703. [DOI] [PubMed] [Google Scholar]
- 13.Neves AA, Stöckmann H, Wainman YA, Kuo JCH, Fawcett S, Leeper FJ, Brindle KM. Bioconj Chem. 2013;24:934–941. doi: 10.1021/bc300621n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee MH, Kim JY, Han JH, Bhuniya S, Sessler JL, Kang C, Kim JS. J Am Chem Soc. 2012;134:12668–12674. doi: 10.1021/ja303998y. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Q, Re Ko N, Kwon Oh J. Chem Commun. 2012;48:7542–7552. doi: 10.1039/c2cc32408c. [DOI] [PubMed] [Google Scholar]
- 16.Koo H, Jin G-w, Kang H, Lee Y, Nam K, Zhe Bai C, Park J-S. Biomaterials. 2010;31:988–997. doi: 10.1016/j.biomaterials.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Choi SK, Thomas T, Li M-H, Kotlyar A, Desai A, Baker JR., Jr Chem Commun. 2010;46:2632–2634. doi: 10.1039/b927215c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koo H, Lee H, Lee S, Min KH, Kim MS, Lee DS, Choi Y, Kwon IC, Kim K, Jeong SY. Chem Commun. 2010;46:5668–5670. doi: 10.1039/c0cc01413c. [DOI] [PubMed] [Google Scholar]
- 19.Reents R, Jeyaraj DA, Waldmann H. Drug Discov Today. 2002;7:71–76. doi: 10.1016/s1359-6446(01)02088-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.