Abstract
The basal forebrain (BF) controls sleep-wake cycles, attention and reward processing. Compared to cholinergic and GABAergic neurons, BF glutamatergic neurons are less well understood, due to difficulties in identification. Here, we use vesicular glutamate transporter 2 (vGluT2)-tdTomato mice, expressing a red fluorescent protein (tdTomato) in the major group of BF glutamatergic neurons (vGluT2+) to characterize their intrinsic electrical properties and cholinergic modulation. Whole-cell, patch-clamp recordings were made from vGluT2+ neurons in coronal BF slices. Most BF vGluT2+ neurons were small/medium sized (<20 μm), exhibited moderately sized H-currents and had a maximal firing frequency of ∼50 Hz. However, vGluT2+ neurons in dorsal BF (ventral pallidum) had larger H-currents and a higher maximal firing rate (83 Hz). A subset of BF vGluT2+ neurons exhibited burst/cluster firing. Most vGluT2+ neurons had low-threshold calcium spikes/currents. vGluT2+ neurons located in ventromedial regions of BF (in or adjacent to the horizontal limb of the diagonal band) were strongly hyperpolarized by the cholinergic agonist, carbachol, a finding apparently in conflict with their increased discharge during wakefulness/REM sleep and hypothesized role in wake-promotion. In contrast, most vGluT2+ neurons located in lateral BF (magnocellular preoptic area) or dorsal BF did not respond to carbachol. Our results suggest that BF glutamatergic neurons are heterogeneous and have morphological, electrical and pharmacological properties which distinguish them from BF cholinergic and GABAergic neurons. A subset of vGluT2+ neurons, possibly those neurons which project to reward-related areas such as the habenula, are hyperpolarized by cholinergic inputs, which may cause phasic inhibition during reward-related events.
Keywords: Vesicular glutamate transporter, sleep, cortical activation, whole-cell, Alzheimer's disease, patch-clamp
The basal forebrain (BF) is a subcortical brain region involved in the control of sleep-wake cycles, cortical activation, attention and reward processing (Brown et al., 2012; Zaborszky et al., 2012; Brown and McKenna, 2015; Lin et al., 2015). BF integrity and function is impaired in diverse neuropsychiatric conditions such as coma (Edlow et al., 2013), dementia (Whitehouse et al., 1982) and narcolepsy (Nishino et al., 1995; Reid et al., 1998). In particular, atrophy of BF cholinergic neurons is a prominent feature of dementia associated with Alzheimer's disease, mild cognitive impairment and Parkinson's disease (Teipel et al., 2011; Grothe et al., 2012; Muller and Bohnen, 2013). Thus, a clear understanding of the cellular properties of BF neurons and the effects of cholinergic neurons on other BF neurons is important to understand the functional consequences of loss of cholinergic neurons in these disorders.
The BF contains three, largely non-overlapping groups of neurons which release the neurotransmitters acetylcholine, GABA and glutamate (Zaborszky et al., 2012). Subsets of all three of these groups project to the neocortex (Freund and Meskenaite, 1992; Gritti et al., 2003; Henny and Jones, 2008; Kim et al., 2015; Zaborszky et al., 2015; Do et al., 2016) and have been implicated in promoting wakefulness and fast cortical rhythms (Anaclet et al., 2015; Kim et al., 2015; Xu et al., 2015; Zant et al., 2016). Other subsets project caudally to areas involved in reward processing and sleep-wake control such as the lateral hypothalamus and lateral habenula and/or locally within the BF (Zaborszky et al., 2012; McKenna et al., 2015a; Xu et al., 2015; Agostinelli et al., 2016; Brown et al., 2016; Do et al., 2016; Golden et al., 2016).
Glutamatergic neurons are the least well understood of the three main neurotransmitter phenotypes within BF. The discovery of vesicular glutamate transporters (vGluT) allowed the first definitive identification of glutamate neurons throughout the brain (Fremeau et al., 2004), including BF. In the BF, vGluT2 is the predominant isoform (Hur and Zaborszky, 2005), including in BF glutamatergic neurons which project outside the BF to the cortex, lateral habenula, hypothalamus and other subcortical regions (Hur and Zaborszky, 2005; Henny and Jones, 2006, 2008; McKenna et al., 2015a; Agostinelli et al., 2016; Do et al., 2016). Strong optogenetic excitation of BF vGluT2+ neurons increased wakefulness (Xu et al., 2015), whereas weaker chemogenetic activation reduced cortical delta activity (Anaclet et al., 2015) suggesting that BF vGluT2+ neurons promote arousal. Furthermore, the caudal projections of BF vGluT2+ neurons to the lateral habenula and lateral hypothalamus are suggestive of a role in reward processing (Henny and Jones, 2006; McKenna et al., 2015a; Agostinelli et al., 2016; Brown et al., 2016; Do et al., 2016; Golden et al., 2016). However, the ionic and neurotransmitter mechanisms which regulate their activity remain to be elucidated.
Single-unit recordings in vivo from identified BF vGluT2+ neurons revealed that their discharge rate exhibits state-dependent modulation, being slightly increased during wakefulness and REM sleep when compated to NREM sleep (Xu et al., 2015). However, the discharge of BF putative glutamatergic neurons is heterogeneous (Manns et al., 2003a; Hassani et al., 2009). In particular, phasic firing is a notable feature of the discharge of a subset of putative BF glutamatergic neurons (Manns et al., 2003a; Hassani et al., 2009), whereas others discharge tonically. The mechanisms underlying these state-dependent discharge patterns are unknown at present, since the intrinsic electrical properties of identified vGluT2+ neurons in regions of the BF containing neurons projecting to the neocortex have not been reported. Our results here suggest that the heterogeneity of discharge patterns of BF glutamatergic neurons observed in vivo (Manns et al., 2003a; Hassani et al., 2009) is matched by a diversity of intrinsic electrical properties in vitro. Thus, it is likely that several functionally distinct groups of BF vGluT2+ neurons exist.
Little is known about the neurotransmitter modulation of BF vGluT2+ neurons. Recent optogenetic studies in vitro, reported as we were conducting this study, suggested that the predominant effect of cholinergic neurons on BF vGluT2+ neurons is a hyperpolarization (Xu et al., 2015). This result is apparently in conflict with the increased discharge of vGluT2+ neurons during wakefulness/REM sleep and with a recent model of BF control of sleep and wakefulness which proposed that these neurons promote wakefulness. However, we find here that only a subset of BF vGluT2+ neurons, possibly projection neurons involved in reward processing, are inhibited by the cholinergic receptor agonist, carbachol. Other BF vGluT2+ neurons, possibly interneurons involved in maintaining the activity of BF cholinergic and GABAergic neurons during wakefulness/REM sleep, are unaffected by carbachol. Parts of these results have been presented in abstract form (Yang et al., 2015; Brown et al., 2016; Yang et al., 2016).
Experimental Procedures
Animals
Mice expressing the enzyme Cre Recombinase under the control of the vGluT2 promoter i.e. vGluT2-Cre mice (strain 016963; Jackson Laboratories, Bar Harbor, ME, USA), were crossed with a reporter strain expressing the red fluorescent marker, tdTomato strain in the presence of Cre recombinase (strain 007905; Jackson Laboratories, Bar Harbor, ME, USA) to generate mice which express red fluorescence in the major subset of BF glutamatergic neurons (vGluT2-tdTomato mice). Previous work has validated the selective expression of Cre Recombinase in vGluT2 neurons using in situ hybridization (Vong et al., 2011), including in BF (Anaclet et al., 2015; Xu et al., 2015). In our own work we have found that there is virtually no overlap between tdTomato and markers of cholinergic and GABAergic neurons in BF (McKenna et al., 2015a; McKenna et al., 2015b). Thus, vGluT2-tdTomato mice represent a valid model to investigate glutamatergic BF neurons.
vGluT2-tdTomato mice aged 13-22 d were used for in vitro electrophysiological recordings, as in our previous in vitro studies of the intrinsic properties and cholinergic modulation of BF neurons (McKenna et al., 2013; Yang et al., 2014). Mice were housed in a temperature controlled facility with lights on at 0700 and lights off at 1900 hrs. Food and water were available to the animals ad libitum. The animal experiments described herein were approved by the IACUC committee of the VA Boston Healthcare System. All experiments were performed in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) and were consistent with US Veterans Administration, US National Institutes of Health and Harvard University guidelines. All efforts were made to minimize the number of animals used and their suffering.
Preparation of BF slices for electrophysiological recordings
vGluT2-tdTomato mice were deeply anesthetized with isoflurane, decapitated and the brain was removed from the skull. The brain was affixed to the stage of a Vibratome 3000 or VT1200S vibrating-blade microtome (Leica Biosystems, Nussloch, Germany) and two 300 μm, coronal BF slices were cut centered on the region between 0.26 and -0.22 mm from bregma (Franklin and Paxinos, 2008). Following slicing, the brain sections were stored at room temperature in artificial cerebrospinal fluid (ACSF) with the following composition (in mM): 124 NaCl, 1.8 KCl, 25.6 NaHCO3, 1.2 KH2PO4, 2 CaCl2, 1.3 MgSO4, and 10 glucose (osmolarity, 300 mOsm) and saturated with 95% O2/5% CO2. The brain slices were allowed to recover from the slicing procedure for >1 h and were then transferred individually to the recording chamber where they were superfused with warmed ACSF (32°C) at a flow-rate of 2–3 ml/min.
Whole-cell recordings from BF vGluT2+ neurons
Brain slices were held in place in a rectangular bath chamber (model R-27, Warner Instruments, CT, USA) on the stage of an upright microscope (model Axioskop2, Carl Zeiss Microscopy LLC, NY, USA). BF vGluT2+ neurons were targeted for recording if they expressed the red fluorescent marker, tdTomato (excitation: emission wavelengths: 560 nm: 630 nm). vGluT2+ (tdTomato+) neurons were photographed under infra-red differential interference contrast optics before recording, using a black-and-white Hamamatsu ORCA-AR CCD camera (Hamamatsu, Japan) attached to the microscope. The diameter of the neurons along their long-axis was measured from these images which were calibrated using a standard grid. Glass patch pipettes had an initial resistance of 3-6 M(Ω) when filled with an intracellular solution with the following composition: (in mM): 130 potassium gluconate, 5 NaCl, 2 MgCl2, 10 HEPES, 0.1 EGTA, 2 Na2ATP, 0.5 NaGTP, 4 MgATP, 1 spermine, and 0.5% biocytin, pH 7.25 with KOH (280 mOsm). A -15 mV liquid junction potential between pipette and bath solutions was calculated for these intracellular and extracellular solutions using pClamp 10.3 software and the membrane potential measurements were adjusted accordingly. Membrane potential/current were recorded using a Multiclamp 700A amplifier and pClamp 10.3 software (Molecular Devices) with a sampling rate of 20 kHz and a low-pass (Bessel) filter frequency of 10 kHz (current clamp) or 1 kHz (voltage clamp). Bridge balance was adjusted and maintained in current-clamp recordings throughout the experiment. Current-clamp experiments were accepted for analysis if action potentials were overshooting and the series resistance changed by less than 10% during the experiment. In voltage-clamp recordings, series resistance was <20 M(Ω) and was not compensated.
Characterization of the intrinsic electrical properties of BF vGluT2+ neurons
The intrinsic electrical properties of BF vGluT2+ neurons were characterized using the same methodology we used to characterize those of BF cholinergic and GABAergic neurons (McKenna et al., 2013). A series of 1 s long hyperpolarizing and depolarizing current pulses were applied in current-clamp beginning from the resting membrane potential (RMP). We adjusted the size of the first hyperpolarizing step according to the input resistance of the neuron so that the negative peak of the membrane potential during the initial part of the step would reach approximately the same membrane potential, -120 mV. Subsequently, increasingly positive current steps (in increments one-fifth the size of the initial step) were applied until the steps became depolarizing. The series of steps was ended when the firing rate did not increase further.
For descriptive purposes and to facilitate the interpretation of in vivo optogenetic, chemogenetic and pharmacological experiments, neurons were grouped together according to their location within the BF (Fig. 1A). Neurons in the ventromedial BF were located mainly in the horizontal limb of the diagonal band (HDB) as defined by Franklin and Paxinos (2008). Neurons located in dorsal BF were located mainly in the ventral pallidum (VP), whereas those in lateral BF were located in the magnocellular preoptic area (MCPO). However, not all anatomists agree on these subdivisions of BF and it is not yet clear whether different functional groups of BF vGluT2+ neurons follow these subdivisions. Furthermore, the boundaries between BF subnuclei are not readily apparent when searching for fluorescent neurons in 300 μm brain slices under high-power magnification (x40). For instance, some neurons we recorded in ventromedial BF may have been located in lateral regions of the lateral preoptic area (LPO) or the ventral part of substantia innominata (SI) rather than HDB. For these reasons, in the manuscript we use the terms ventromedial, dorsal and lateral BF to describe the approximate location of the recorded neurons rather than the terms HDB, MCPO, VP and SI.
Figure 1. Subregional location and intrinsic membrane properties of BF vGluT2+ neurons recorded in this study.
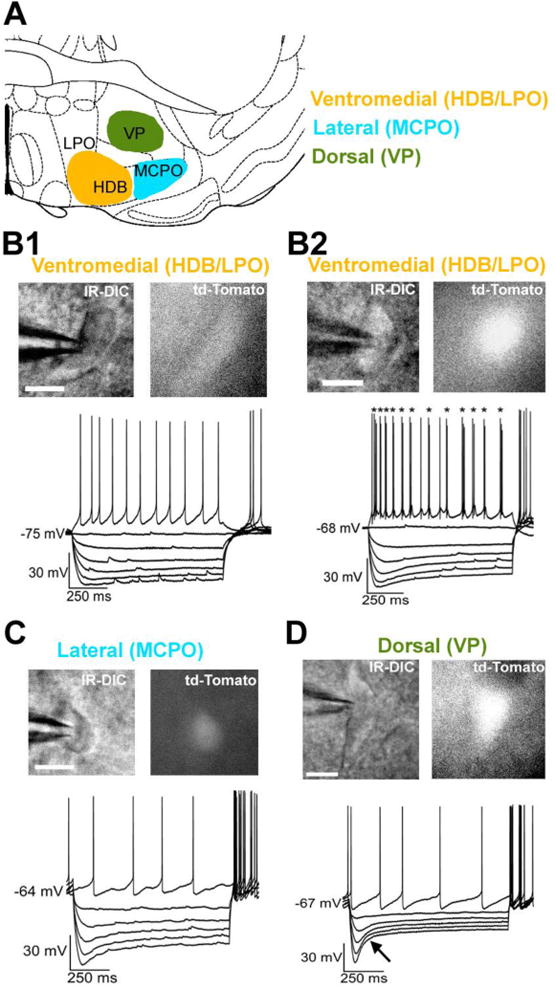
A and C Schematic diagram showing the BF subregions where electrophysiological recordings were made (Bregma 0.14 mm. Adapted from (Franklin and Paxinos, 2008). HDB: horizontal limb of the diagonal band. LPO: lateral preoptic area. MCPO: magnocellular preoptic nucleus. VP: ventral pallidum. B-D. Top, Black and white infrared differential interference contrast (IR-DIC) and fluorescent (tdTomato) images, scale bar: 10 μm. Bottom, recordings of the voltage response to hyperpolarizing and depolarizing steps. B1 and B2. Examples of two different types of vGluT2+ neurons in the ventromedial BF. Both types of neurons showed a small depolarizing sag during hyperpolarizing steps. Neurons showed either tonic (B1) or burst firing (labeled with asterisks in B2) during depolarizing steps. C and D. Images and intrinsic membrane properties of vGluT2 neurons in lateral and dorsal BF respectively. Note that dorsal vGluT2+ neurons (D) showed a larger sag (black arrow) during hyperpolarizing steps when compared to others types of BF vGluT2+ neurons (B and C).
Protocols used to investigate hyperpolarization-activated cation (H) currents and low-threshold (T-type) calcium spikes/currents
Inward rectification during hyperpolarizing current pulses (% depolarizing sag) was determined from the largest hyperpolarizing step according to the following formula [ (steady-state voltage at the end of the step - RMP)/(peak voltage - RMP) ] × 100. To characterize hyperpolarization-activated inward currents (Ih) under voltage-clamp, neurons were held at -60 mV and the voltage was stepped to potentials from -135 mV to -75 mV with a 10 mV increment and a 10 s interval between steps. To confirm the involvement of hyperpolarization-activated cyclic nucleotide-gated (HCN) cation channels in the slowly developing inward current observed under voltage-clamp, we bath-applied a specific HCN channel blocker, ZD7288 (50 μM; Ascent Scientific, Cambridge, MA, USA).
To characterize low-threshold spikes under current-clamp, current was applied to adjust the membrane potential to ∼-70 mV and then we applied 1 s long, hyperpolarizing current steps, as described above. The sizes of the hyperpolarizing current steps were titrated according to the input resistance of the neuron so that the negative peak of the membrane potential during the largest of the steps reached -120 mV. With this protocol, neurons were first recorded in ACSF to observe the low-threshold spikes following the removal of hyperpolarizing steps, and then recorded in the presence of the voltage-gated sodium channel blocker, 500 nM tetrodotoxin (TTX) (Abcam, Cambridge, MA) to observe TTX-independent spikes. To characterize the low threshold currents under voltage clamp, neurons were pre-incubated in TTX for 5 min and then stepped to potentials from -125 mV to -55 mV (10 mV increment) for 1 s from a holding potential of -55 mV. The amplitudes of inward currents immediately following the removal of hyperpolarizing steps were measured. To test the involvement of T-type calcium channels in generating these inward currents, we added a calcium channel blocker specific to T-type calcium channels, TTA-P2 (Alomone Labs, Israel)(Dreyfus et al., 2010; Choe et al., 2011) to the bathing solution surrounding the brain slices.
Protocol to test the effect of carbachol on membrane potential and input resistance
The cholinergic agonist, carbachol (50 μM), was applied to the recording chamber via the perfusion line for 2-3 min and the membrane response was recorded under current clamp in the presence of TTX (0.5 μM) to block sodium-dependent action potentials. Membrane voltage and current were recorded continuously using a MiniDigi 1B system and Axoscope 10.3 software (Axon instruments) using a sampling frequency of 10 kHz and a low pass (Bessel) filter frequency of 10 kHz. To monitor input resistance a small 200 ms hyperpolarizing current (-50 pA) step was applied every 15 s and the membrane potential response to this current step was recorded using pClamp10.3 software. In order to minimize the effect of voltage-dependent conductances on the measurement of the input resistance, current was briefly injected during the peak of the carbachol response to return the membrane potential to baseline. The effect of carbachol on membrane potential or input resistance were assessed by comparing the membrane potential or input resistance immediately before the carbachol application and at the peak of the effect.
Chemicals and statistical analysis
Chemicals used in this study were purchased from Sigma-Aldrich (St. Louis, MD, USA) unless otherwise specified and prepared/stored per company instructions. Mean data are presented ± standard error of the mean (SEM). Non-repeated measures one-way Analysis of variance (ANOVA) was used to compare the intrinsic electrical properties of different types of BF neurons. Kruskal-Wallis was used for data without normal distribution. If the main effect in the ANOVA was found to be significant, and more than two groups were compared, a pair-wise comparison between groups was then made, using Bonferroni post-hoc test for one-way ANOVA and Dunn's post-hoc-test for Kruskal-Wallis test. To determine the pharmacological effects, paired t tests or Wilcoxon matched-paired signed rank test was used for statistical analysis of significance for data with or without normal distribution respectively. Statistical analysis was conducted using GraphPad Prism software (La Jolla, CA). Differences were considered significant if p < 0.05.
Results
Morphology and intrinsic membrane properties of BF vGluT2+ neurons
In general, BF vGluT2 neurons were small/medium-sized (15.4 ± 0.6 μm, n=53) and ovoid, spindle or triangular-shaped (Fig. 1). The intrinsic electrical properties and pharmacology of mouse BF vGluT2+ neurons are summarized in Table 1. On average, vGluT2+ neurons had a relatively hyperpolarized resting membrane potential of -70.0 ± 0.8 mV, a maximum firing frequency of 56.1 ± 4.0 Hz, and a moderately-sized afterhyperpolarization (AHP) following individual action potentials (AP) (-16.1 ± 0.7 mV). Half of the vGluT2+ population was spontaneously active in the absence of injected current (11.0 ± 1.4 Hz, n=27/53), the other half was silent in the absence of injected current. When tested with depolarizing current injection, most (51/53) BF vGluT2+ neurons discharged a train of single action potentials with little adaptation during the step (Fig. 1: B1, C, D). However, a small subset (2/53) exhibited an unusual pattern of cluster firing consisting of doublets or triplets of action potentials separated by brief, shallow AHPs (Fig. 1B2). When tested with hyperpolarizing current injection, most BF vGluT2+ neurons demonstrated a moderately-sized, slowly-developing rectification (depolarizing sag) during the current step (Fig. 1B-C), although a subset (Fig. 1D) exhibited a much stronger sag (see next section).
Table 1. Intrinsic membrane properties and cholinergic modulation of BF glutamatergic, GABAergic and cholinergic neurons in the mouse.
| Neuronal Property | vGlut2+ neurons (red fluorescent in vGlut2-tdTomato mice) |
Large (>20 μm) GABA neurons (GFP+ in GAD67-GFP mice) |
Small (<20 μm) GABA neurons (GFP+ in GAD67- GFP mice) (no Ih, N = 19) |
Cholinergic (GFP negative in GAD67- GFP mice) (N=26, ChAT+ n=13)) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| All (N=53) |
Ventromedial (HDB/LPO) (N=25) |
Lateral (MCPO) (N=20) |
Dorsal (VP) (N=8) |
All (N=166) |
Large Ih (N=89) |
Small Ih (N=77) |
|||
| Long diameter (μm) | 15.4±0.6 | 15.8±0.8 | 14.4±0.7 | 16.9±2.0 | 24.4±0.4 | 24.3±0.5 | 24.5±0.8 | 14.2±0.5 | 24.4±0.8 |
| Short diameter (μm) | 9.3±0.2 | 9.6±0.3 | 8.9±0.3 | 9.5±0.4 | 13.7±0.2 | 13.9±0.3 | 13.6±0.3 | 9.7±0.5 | 15.9±0.7 |
| RMP (mV) | -70.0±0.8 | -70.1±1.3 | -70.6±1.2 | -68.3±1.4 | -63.3±0.6 | -61.5±0.7 | -65.4±0.8 | -77.7±1.5 | -71.5±1.0 |
| Rin (MO) | 558.1±56.9 | 605.3±98.3 | 522.2±80.5 | 505.8±100.0 | 236±18 | 181±23 | 300±28 | 238±34 | 370±32 |
| Sag % | 26.0±1.8 | 20.1±2.0 | 26.0±2.4 | 44.4±2.9 | 41.4±1.2 | 52.9±1.1 | 28.1±1.0 | - | - |
| Spontaneous firing (Hz) | 11.0±1.4(n=27/53) | 13.1± 2.8 (n= 10/25) | 10.1±2.3 (n= 10/20) | 9.4±2.3 (n=7/8) | 12.9±0.8 (n=153/166) | 15.0±1.2 (n=89/89) | 10.3±1.1 (n=64/77) | 0 | 1.1±0.5 (n=11/26) |
| Maximum firing (Hz) | 56.1±4.0 | 51.9±6.9 | 50.7±3.9 | 83.3±7.6 | 87±5 | 94±7 | 76±9 | 36±10 | 13.7±0.8 |
| AP half width (ms) | 0.77±0.05 | 0.81±0.08 | 0.81±0.08 | 0.54±0.05 | 0.61±0.02 | 0.64±0.03 | 0.56±0.03 | 0.92±0.07 | 0.8±0.06 |
| AP threshold (mV) | -55.6±0.8 | -53.7±1.4 | -58.1±1.2 | -54.9±1.2 | -53.8±1.1 | -52.6±2.0 | -55.4±0.4 | -56.1±0.8 | -52.1±0.5 |
| AP amplitude (mV) | 72.1±1.4 | 69.9±2.1 | 74.6±2.0 | 72.5±4.3 | 76.1±0.9 | 76.5±1.0 | 75.7±1.6 | 74.0±1.7 | 74.4±1.2 |
| AHP (mV) | -16.1±0.7 | -17.2±1.2 | -14.4±1.1 | -17.1±1.0 | -15.3±0.3 | -14.3±0.4 | -16.6±0.5 | -8.4±1.0 | -26.4±1.0 |
| Postsynaptic response to 50 μM carbachol (mV) | -15.6±2.9 (n=6/7) | -13.7±3.8 (n=2/6) | 0 (n=3/3) | 8.1±1.1 | 11.3±1.6 | ||||
Data are presented as mean ± SEM. Data from mouse BF GABAergic (GAD67+) and cholinergic (ChAT+) neurons are from McKenna et al., 2013 and are re-presented here to allow easy comparison with the properties of BF vGluT2+ neurons.
To provide a basis for comparison with other types of BF neurons, we also included in Table 1 the properties of mouse BF cholinergic and GABAergic neurons recorded in our previous study under the same conditions (McKenna et al., 2013). Significance differences in size (F(3,258)=59.17, p<0.0001, one-way ANOVA test), maximal firing rates (H(3)=77.58, p<0.0001, Kruskal-Wallis test), afterhyperpolarization amplitudes (F(3,236)=65.56, p<0.0001, one-way ANOVA test), resting membrane potentials (F(3, 169)=42.98, p<0.0001, one-way ANOVA test), input resistances (H(3)=70.25, p<0.0001, Kruskal-Wallis test) and action potential half-width (H(3)=25.43, p<0.0001, Kruskal-Wallis test) were found when comparing BF vGluT2+ neurons (present study), cholinergic neurons, large-sized GABAergic neurons and small-sized GABAergic neurons (McKenna et al., 2013). When compared to mouse BF cholinergic neurons, as a group, BF vGluT2+ neurons were significantly smaller (p<0.0001, Bonferroni post-hoc-test), had a higher maximal firing frequency (p<0.0001, Dunn's post-hoc-test) and exhibited smaller afterhyperpolarizations (p<0.0001, Bonferroni post-hoc-test). When compared to large-sized (>20 μm), putative cortically-projecting GABAergic neurons (McKenna et al., 2013), vGluT2+neurons had a more hyperpolarized RMP (p<0.0001, Bonferroni post-hoc-test), higher input resistance (p<0.001, Dunn's post-hoc-test), broader action potentials (p<0.05, Dunn's post-hoc-test) and a lower maximal firing frequency (p<0.01, Dunn's post-hoc-test). When compared to smaller ‘striatal-like’ BF GABAergic neurons (McKenna et al., 2013), BF vGluT2+ neurons could be distinguished by the presence of time-dependent inward rectification (depolarizing sag mediated by H-current – see below), whereas the ‘striatal-like’ GABAergic neurons exhibited instantaneous inward rectification (McKenna et al., 2013). In addition, when compared to this group of neurons, BF vGluT2+ neurons had a less hyperpolarized RMP (p<0.0001, Bonferroni post-hoc-test), higher input resistance (p<0.001, Dunn's post-hoc-test) and larger AHPs (p<0.0001, Bonferroni post-hoc-test).
Subregional differences in the intrinsic electrical properties of BF vGluT2+ neurons
Based on their location, intrinsic electrical properties and response to carbachol, we characterized the BF vGluT2+ neurons into three groups (see methods), ventromedial, lateral and dorsal (Fig. 1). Ventromedial (n=25) and lateral (n=20) BF vGluT2+ neurons had very similar intrinsic electrical properties (Fig. 1B and 1C, Table 1; No significant difference found). For example, both groups had a RMP of ∼-70 mV, a maximum firing frequency of ∼50 Hz, and a small depolarizing sag during the hyperpolarizing-current steps (sag % ∼25%) whose time-course was well-fit with a single-term exponential function. About half of the neurons in each region was spontaneously active (∼10 Hz). The action potential (AP) parameters were also similar for both groups (AP threshold ∼-55 mV, AP amplitude ∼70mV, AP duration at half-width ∼0.8 ms, and AHP amplitude ∼-15 mV). However, differences in the response to carbachol were found (described below). Furthermore, neurons with cluster-type discharge (n=2) were only found in ventromedial BF.
vGluT2+ neurons located in dorsal BF (n=8) showed properties which distinguished them from the ventromedial/lateral neurons. Dorsal vGluT2+ neurons had a significantly larger depolarizing sag during the hyperpolarizing-current steps (44.4 ± 2.9%, p<0.0001, unpaired-t-test) whose time course required a double- exponential function to obtain an adequate fit (Fig. 1D). Almost all dorsal vGluT2+ neurons were spontaneously active (9.4 ± 2.3 Hz, n=7/8) and had a significantly higher maximum firing frequency (83.3±7.6 Hz, p=0.002, Mann Whitney test). Dorsal vGluT2+ neurons had a slightly shorter AP duration at half-width (0.54±0.05 ms) than the other two groups (p=0.0479, unpaired-t-test). The intrinsic properties of this group of BF vGluT2+ neurons were reminiscent of one group of BF GABAergic neurons which also exhibited a large depolarizing sag (McKenna et al., 2013). However, when compared to this group of GABAergic neurons, dorsal vGluT2+ neurons had a more hyperpolarized RMP (p=0.0003, unpaired-t-test), a higher input resistance (p=0.0004, Mann Whitney test), and a different response to carbachol i.e. they were not depolarized (Yang et al., 2014).
BF vGluT2+ neurons exhibit hyperpolarization-activated inward (H) currents and T-type calcium currents
The time-dependent inward rectification we observed in vGluT2+ neurons during hyperpolarizing current pulses is normally due to inward currents mediated by activation of hyperpolarization-activated, cyclic-AMP modulated cation (HCN) channels. To test for the presence of HCN channels in BF vGluT2+ neurons, we examined the effect of a specific HCN channel blocker, ZD7288, on these inward currents in ventromedial/lateral BF vGluT2+ neurons, in the presence of tetrodotoxin (TTX) to block voltage-gated sodium channels. As shown in Fig. 2, hyperpolarizing steps induced inward currents in BF vGluT2 neurons during the step (inward current amplitude at Vhold=-135 mV: -49.6 ± 8.5 pA). These inward currents were well fit with a standard exponential equation (time constant=243 ± 46.9 ms, Amplitude=50.5±9.0 pA). ZD7288 (50 μM) significantly blocked these hyperpolarization-induced inward currents (inward current amplitude at Vhold=-135 mV, -10.6±3.1 pA in the presence of ZD7288, p=0.0008 compared to without ZD7288, n=7, paired-t-test).
Figure 2. A selective blocker for hyperpolarization-activated cyclic nucleotide-gated (HCN) channels, ZD7288 (50 μM), blocked hyperpolarization activated inward currents in BF vGluT2+ neurons.
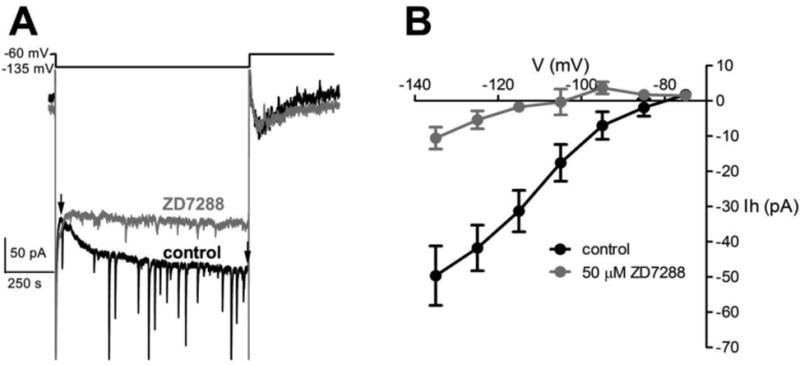
A. A representative neuron was stepped to -135 mV for 1s to induce an inward current (Ih) (black trace) which was blocked by ZD7288 (grey trace). The amplitude of the current was measured as the difference between the two points labeled with arrows. B. I-V plot with the voltage step values as the x-axis and Ih amplitudes shown on the y axis. Data were plotted as mean ± SEM (n=7).
Most BF vGluT2 neurons displayed rebound spikes at the offset of hyperpolarizing current steps (n=36/53) in current-clamp. We analyzed the patterns of the rebound spikes for neurons which were silent at rest (n=23). The majority (16/23) of these neurons showed single spikes (Nine of these neurons were in ventromedial BF, six of them were in lateral BF and one was in dorsal BF), while the remainder (7/23) discharged multiple action potentials (2.4±0.3). Five of them were in ventromedial BF and 2 were in lateral BF. One neuron in ventromedial BF exhibited a burst during depolarizing steps as well as following hyperpolarizing steps.
Rebound spikes following hyperpolarization could be due to activation of low-threshold calcium channels whose inactivation is removed by the hyperpolarizing step. To test this, we used the following protocol to uniformly induce rebound spikes in BF vGluT2 neurons. Neurons were initially held at ∼-70 mV with DC current injections and then were injected with negative currents to hyperpolarize the membrane potential to -130 mV. At the offset of the hyperpolarizing current steps, all neurons displayed rebound spikes (n=7/7) (representative example shown in Fig. 3A). We then applied a selective T-type calcium channel blocker, TTA-P2 (Dreyfus et al., 2010; Choe et al., 2011). TTA-P2 (1, 3 or 5 μM) blocked the rebound spikes (n=7; 1 μM, n=3; 3 μM, n=1; 5 μM n=3), suggesting that T-type calcium channels are responsible for the rebound spikes. In the presence of 500 nM TTX, TTA-P2 (1 & 3 μM) significantly inhibited the rebound depolarization induced by the same protocol used in the previous experiment (amplitude of rebound depolarization: In TTX alone, 7.8±1.9 mV vs. In TTX+TTA-P2, 2.9±0.7 mV, p=0.0313, Wilcoxon matched-paired signed rank test, n=7. 1 μM, n=6; 3 μM, n=1. Fig. 3B).
Figure 3. The selective T-type calcium channel inhibitor, TTA-P2, blocked low-threshold spikes/currents in BF vGluT2+ neurons.
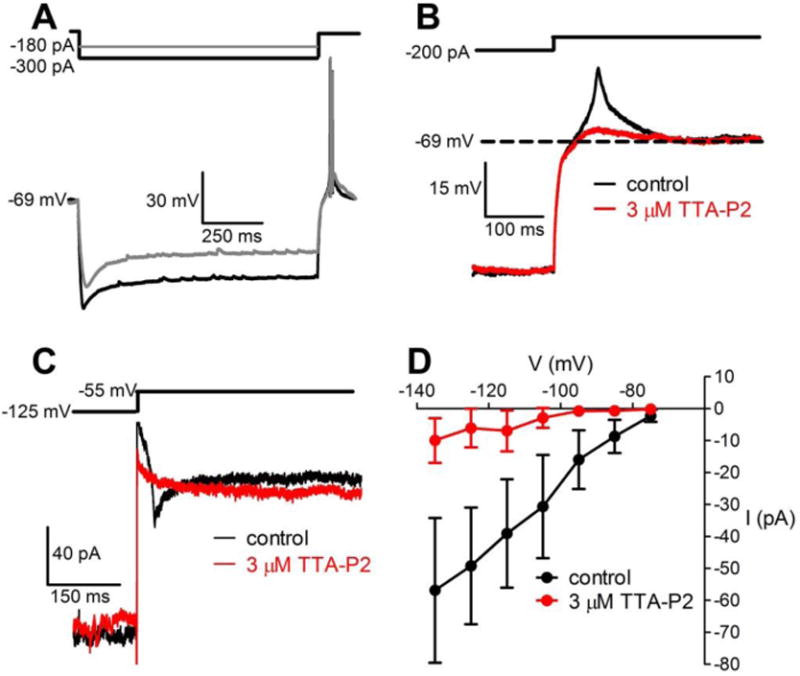
A. In current clamp, a representative vGluT2+ neuron showed rebound spikes following 1 s hyperpolarizing current injections (-180 or -300 pA). The neuron was initially held at ∼-70 mV. B. The same neuron shown in A was incubated with 500 nM TTX to block sodium-dependent action potentials. A hyperpolarizing current injection induced a low-threshold spike (control: black trace) which was blocked by TTA-P2 (red trace). C. In voltage clamp and in the presence of TTX, a representative vGluT2+ neuron showed an inward current at the removal of a 1s voltage step to -125 mV (control: black trace). TTA-P2 also blocked this rebound inward current (red trace). D. I-V plot with the voltage steps shown on the x-axis and the amplitude of rebound inward currents shown on the y-axis. Data were plotted as mean± SEM (n=6).
To further examine the involvement of T-type calcium currents, we recorded the current changes in the presence of 500 nM TTX under voltage-clamp. Neurons were held at -55 mV and stepped to more negative potentials (-135 mV to -75 mV). At the offset of the hyperpolarizing step, BF vGluT2+ neurons exhibited inward currents (Fig. 3C) which reached peak amplitude in ∼20 ms (time to peak 19.1 ± 7.3 ms), with an amplitude of -56.9 ± 22.7 pA and a duration of 214 ± 66 ms (n=6). TTA-P2 (3 μM) virtually abolished these inward currents (Fig. 3D; -135 mV step, inward current amplitude in TTA-P2: -9.9±7.0 pA, p=0.0372 compared to that without TTA-P2, paired-t-test, n=6). Taken together, these data suggest that BF vGluT2 neurons express HCN and T-type calcium channels which play roles in modulating their firing.
A subset of BF vGluT2+ neurons are inhibited by the acetylcholine receptor agonist carbachol
To investigate the interaction between BF cholinergic neurons and vGluT2+ neurons, we bath applied an acetylcholine receptor agonist, carbachol, to BF brain slices and recorded the responses from vGluT2+ neurons in current-clamp. In the presence of TTX (500 nM), carbachol (50 μM) strongly hyperpolarized vGluT2 neurons located in the ventromedial BF (HDB/LPO) (-15.6 ± 2.9 mV, n=6/7. Fig. 4), suggesting a postsynaptic inhibitory modulation by cholinergic inputs to BF glutamatergic neurons. The input resistance was significantly decreased with carbachol application in these neurons (control vs. carbachol: 542.1 ± 170.4 M(Ω) vs. 318.9 ± 124.1 M(Ω), p=0.0313, Wilcoxon matched-pairs signed rank test), suggesting carbachol may induce opening of potassium channels in ventromedial BF vGluT2 neurons. Most vGluT2+neurons located in lateral or dorsolateral BF (MCPO and VP) did not respond to carbachol (n=7/9; Table 1). No depolarization by carbachol was observed in BF vGluT2+ neurons, in marked contrast to the effect of carbachol on BF GABAergic neurons (Yang et al., 2014).
Figure 4. The cholinergic agonist, carbachol, hyperpolarized vGluT2+ neurons located in the ventromedial basal forebrain.
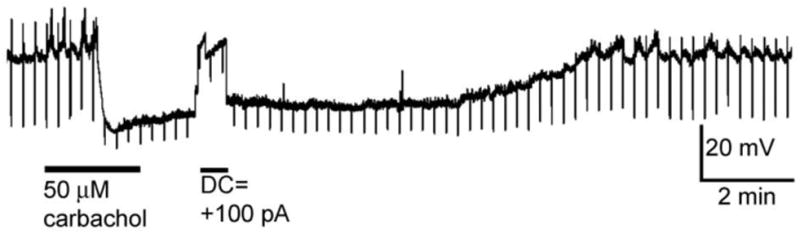
Carbachol (50 μM) was bath applied for 2 min in the presence of TTX (500 nM). A current step of -50 pA was applied every 15 s to test for changes in input resistance. Hyperpolarizations induced by carbachol were associated with a decrease in input resistance, indicating opening of ion channels. Abbreviation: DC – direct current.
Discussion
The main advances reported in this study are as follows: 1) We have used vGluT2-tdTomato mice to identify the intrinsic membrane properties and pharmacology of the major subgroup of glutamatergic neurons (vGluT2+) in the intermediate region of the BF which contains glutamatergic neurons projecting to the neocortex (Hur and Zaborszky, 2005); 2) Most BF vGluT2+ neurons had moderately-sized hyperpolarization-activated cation currents and a maximal firing rate intermediate between that of cholinergic and GABAergic neurons. Many neurons exhibited low-threshold calcium currents and a subset discharged multiple action potential in a doublet, burst or cluster pattern when depolarized from a hyperpolarized membrane potential or from rest; 3) BF vGluT2+ neurons exhibited a combination of size, intrinsic electrical properties and pharmacology which distinguish them from neighboring cholinergic and GABAergic neurons (Table 1); 4) There was heterogeneity in the intrinsic membrane properties of BF vGluT2+ neurons and in the response to the cholinergic agonist, carbachol, suggesting that several functionally distinct types of BF vGluT2 neurons likely exist; 5) The predominant response to the cholinergic receptor agonist, carbachol, is a hyperpolarization, largely restricted to vGluT2+ neurons located in ventromedial BF.
Limitations of this study
There are several potential limitations of this study. Firstly, this study was conducted in young (13-22d) mice, due to technical difficulties in detecting and recording from fluorescent neurons in the BF of adult mice. Thus, it is possible that the electrical or pharmacological properties we describe here could change with the age of the animal. However, we note that we found no significant differences in the properties of GABAergic neurons between mice of this age and those recorded in older animals (1.5-3 months) in our previous study (McKenna et al., 2013). Secondly, it is possible that tdTomato does not label absolutely all BF vGluT2 neurons i.e. although tdTomato expression is selective for vGluT2 neurons (Anaclet et al., 2015; Xu et al., 2015), it may not be sensitive enough to detect all of them, so a small subpopulation might have been missed. Thirdly, the properties of BF vGluT2 neurons could be different in other species, including humans. Such cross-species comparisons require methods to selectively identify BF vGluT2 neurons in species other than mice. Finally, we acknowledge that we have not studied BF glutamatergic neurons which express vGluT1 or vGluT3. Study of these neurons awaits the development of new transgenic mice which express fluorescent proteins in these minor subsets of BF glutamatergic neurons.
Intrinsic membrane properties of BF vGluT2+ neurons and comparison with cholinergic and GABAergic neurons
Characterization of the intrinsic electrical properties of BF neurons is an important way to classify and distinguish them. Comparisons of the properties of neurochemically distinct neurons addresses the question of whether individual electrical properties e.g. action potential width/shape are sufficient to distinguish different neuronal phenotypes in the absence of fluorescent markers in vitro or post-hoc labeling in vivo. Furthermore, knowledge of the maximal firing rate constrains hypotheses of the role of these neurons in control of cortical EEG. For instance, neurons with a high maximal firing rate, such as the subset of GABAergic neurons which contain parvalbumin, are more likely to play a role in directly entraining high-frequency EEG oscillations than slow-firing cholinergic neurons with long-lasting afterhyperpolarizations (McKenna et al., 2013; Kim et al., 2015). Measurement of the electrical properties of individual neurons and their kinetics is essential for constructing computational models of neuronal discharge. Finally, identification of particular ionic currents facilitates the analysis of drug actions and potential actions of neuromodulators.
In our studies, as a group vGluT2 neurons exhibited a unique constellation of features which set them apart (Table 1), although in individual neurons there was some overlap in individual properties between vGluT2+ neurons and other types of BF neurons. When compared to mouse cholinergic neurons (Hedrick and Waters, 2010; Hawryluk et al., 2012; McKenna et al., 2013), vGluT2+ neurons were much smaller, had narrower action potentials, smaller afterhyperpolarizations and had a much higher maximal firing frequency (56 Hz for vGluT2+ vs 14 Hz for cholinergic; (McKenna et al., 2013)). BF vGluT2+ neurons exhibited time-dependent inward rectification mediated by activation of HCN channels whereas cholinergic neurons exhibit a different type of rectification mediated by potassium channels (Hedrick and Waters, 2010; Hawryluk et al., 2012; McKenna et al., 2013). A small subset of BF vGluT2+ neurons exhibited an unusual pattern of cluster firing we have not observed in BF cholinergic or GABAergic neurons. This type of discharge was originally observed previously in a group of unidentified non-cholinergic neurons recorded using sharp-electrodes in guinea pigs (Alonso et al., 1996) and subsequently in identified vGluT2+ neurons in rostral hippocampal-projecting BF (Sotty et al., 2003) but had not heretofore been described in vGluT2+ neurons in more caudal BF regions with neocortically-projecting neurons. This pattern of firing is also like the firing pattern observed in a subset of glutamatergic thalamic lateral geniculate neurons involved in generating EEG alpha rhythms (Lorincz et al., 2008).
When compared to cortically-projecting GABAergic/parvalbumin neurons (McKenna et al., 2013), vGluT2+ neurons were smaller, had a more hyperpolarized RMP and lower maximal firing rates (56 Hz for vGluT2+ neurons, 87 Hz for large GABAergic neurons and 162 Hz for parvalbumin GABAergic positive neurons). Most BF vGluT2+ neurons exhibited low-threshold calcium currents, a feature which was not observed in large GABAergic neurons (although occasional smaller GABAergic neurons show this feature – unpublished observations). Notably, BF vGluT2+ neurons were either hyperpolarized or unaffected by carbachol (present results & (Xu et al., 2015)) whereas large, putative cortically-projecting GABAergic/parvalbumin neurons were uniformly and strongly excited (Yang et al., 2014; Xu et al., 2015).
We previously reported another group of BF GABAergic neurons with a similar size as BF vGluT2 neurons, which were unaffected by carbachol (McKenna et al., 2013). However, in contrast to this group of GABAergic neurons which exhibited an instantaneous rectification when hyperpolarized (McKenna et al., 2013), most, if not all vGluT2+ neurons recorded in this study exhibited a time-dependent depolarizing sag during hyperpolarizing current pulses which was blocked by ZD7288, suggesting that it was due to hyperpolarization-activated cyclic nucleotide-gated cation (HCN) channels. Furthermore, the resting membrane potential of vGluT2+ neurons was more depolarized than this group of GABAergic neurons, which had a very hyperpolarized RMP and other properties like those reported for striatal medium spiny neurons (Blomeley and Bracci, 2008).
To summarize, our analysis of the intrinsic electrical properties of the whole group of BF vGluT2 neurons suggest that they have properties intermediate between slow-firing cholinergic neurons and fast-discharging, cortically-projecting GABAergic neurons. However, the overlap of these properties are such that the intrinsic electrical properties of individual neurons cannot be used to conclusively identify the neurochemical phenotype in the absence of selective fluorescent markers, optical tagging or post-hoc labeling. The presence of moderately-sized H-currents and low-threshold calcium currents suggests that cluster or burst discharge is likely to be a feature of these neurons in vivo in mice (Xu et al., 2015), as previously proposed in rats (Manns et al., 2003a).
Heterogeneity of BF vGluT2+ neurons
One important conclusion of this study is that BF vGluT2+ neurons do not comprise one homogeneous group. We found that there was heterogeneity in the RMP/spontaneous firing rate, maximal firing rate, inward rectification (H-currents), firing pattern, low-threshold calcium currents and response to the cholinergic agonist carbachol. The location of the vGluT2+ neurons influenced these properties. Thus, only those vGluT2+ neurons located in the ventromedial BF exhibited cluster/burst firing and most were inhibited by carbachol whereas those in dorsal BF had stronger H-currents with different kinetics, faster maximal firing and were unaffected by carbachol. A similar population of fast-firing vGluT2+ neurons with prominent H-currents was previously described in rostral BF (Huh et al., 2010). The in vivo discharge properties and function of these fast-firing neurons in the dorsal BF area is unknown although a role in motivated behavior seems likely, through their projections to reward-related regions such as the ventral tegmental area (Geisler et al., 2007; Root et al., 2015).
Although to the best of our knowledge this is the first study to report the intrinsic properties of vGluT2+ neurons in the intermediate region of the BF containing neurons projecting to the neocortex (Hur and Zaborszky, 2005), other studies have investigated identified vGluT2+ neurons in rostral (septal) BF regions projecting to the hippocampus, using either a transgenic mouse strategy similar to the one we have employed here or single-cell PCR to identify vGluT2+ neurons (Sotty et al., 2003; Manseau et al., 2005; Huh et al., 2010). These studies suggest that, as in our study in more caudal BF regions, several functionally distinct groups of vGluT2+ neurons exist in the rostral BF which differ in their discharge pattern (tonic, cluster, burst), amplitude of H-current and role in generating rhythmic activity (Huh et al., 2010; Robinson et al., 2016).
What is the significance of the heterogeneity of vGluT2+ neurons discharge patterns and pharmacology? One explanation could be that different groups correspond to projection neurons and local circuit neurons. Optogenetic (Xu et al., 2015; Robinson et al., 2016) and anatomical studies (Wu et al., 2003; Hajszan et al., 2004; McKenna et al., 2015a) have shown that BF vGluT2+ neurons provide local excitatory inputs to cholinergic and GABAergic neurons in both rostral and intermediate BF regions. Anatomical tracing studies in rat and mice have shown that subsets of BF vGluT2+ neurons project to the neocortex (Hur and Zaborszky, 2005; Henny and Jones, 2008; Do et al., 2016), areas involved in motivated behavior such as the lateral habenula (McKenna et al., 2015a; Brown et al., 2016; Do et al., 2016), ventral tegmental area (Geisler et al., 2007), lateral hypothalamus (Henny and Jones, 2006) and other subcortical areas (McKenna et al., 2015a; Brown et al., 2016; Do et al., 2016). Thus, the intrinsic electrical properties and pharmacology of BF vGluT2+ neurons may segregate according to their postsynaptic target.
What mechanism could account for the different discharge patterns of vGluT2+ neurons? A possible contributing factor could the presence of different calcium binding proteins in subsets of vGluT2+ neurons (Gritti et al., 2003; Zaborszky et al., 2012). Calcium binding proteins present in BF neurons such as parvalbumin, calbindin, calretinin and secretagogin (Zaborszky et al., 2012) can affect intracellular calcium dynamics and thereby alter the effect of calcium on various membrane channels/pumps. For instance parvalbumin expression in a subset of cortically-projecting BF GABAergic neurons leads to narrow action potentials and a very high maximal firing rates (McKenna et al., 2013), likely by limiting calcium-activated potassium conductances. In the rat, it has been suggested that calbindin may be a marker for cortically-projecting BF glutamate neurons, whereas calretinin is a marker for local circuit glutamatergic neurons (Gritti et al., 2003). Our preliminary immunohistochemical studies in vGluT2-tdTomato mice also suggest that substantial proportions of vGluT2+ neurons express calbindin or calretinin (Brown et al., 2016; McKenna et al., 2016). Interestingly, a cluster of calbindin+/vGluT2+ neurons is present in the ventromedial BF (Brown et al., 2016; McKenna et al., 2016), in the region which contains cortically-projecting vGluT2+ neurons in the rat (Hur and Zaborszky, 2005). Conversely, calretinin neurons are more prevalent in lateral and dorsal BF regions. In the future, it will be interesting to investigate the whether the presence of these calcium binding proteins in vGluT2+ neurons is a marker for particular neuronal properties. In a preliminary investigation, we attempted to use calretinin-tdTomato mice (calretinin-Cre mice, stock number 010774, Jackson Labs crossed with the same tdTomato reporter strain 007905 we use here) but we found that tdTomato expression in these mice showed very low overlap with calretinin immunohistochemistry in the BF. Thus, further investigation of this topic will require new genetic tools.
Functional implications of cholinergic inhibition of BF vGluT2 neurons
The inhibitory effects of carbachol on vGluT2+ neurons we observed here are broadly consistent with the optogenetic results of Xu and colleagues (2015) who found that optical stimulation of BF cholinergic neurons led to a short-lasting nicotinic excitation followed by a much longer lasting and pronounced muscarinic hyperpolarization (Xu et al., 2015). In the current study our mode of bath application of carbachol likely precluded the observation of fast-desensitizing nicotinic responses. Our study expanded upon the work of Xu et al., (2015) in that we found that the inhibitory effect of carbachol was largely restricted to the ventromedial BF (HDB/LPO). Furthermore, we found that it was associated with a decrease in input resistance suggesting that the effect of carbachol is likely mediated via activation of muscarinic M2/M4 receptors and subsequent opening of inwardly-rectifying potassium channels, as in other brain areas (Nicoll et al., 1990). Unfortunately, currently available pharmacological and immunohistochemical tools to distinguish between M2 and M4 receptors have limited selectively. Thus, we did not attempt to further dissect out the receptor subtype involved.
In a recent in vivo optodialysis study (Zant et al., 2016) we found that the wake-promoting effects of selective optical stimulation of BF cholinergic neurons could be abolished by local BF perfusion of cholinergic receptor antagonists, suggesting that the effect required interaction with other local non-cholinergic neurons. Selective optical stimulation of BF vGluT2+ neurons can elicit a strong wake promoting effect (Xu et al., 2015). Thus, BF vGluT2+ neurons are one possible mediator of the wake-promoting effect of stimulating BF cholinergic neurons. However, based on the results presented here and those of Xu and colleagues (2015) the predominant cholinergic effect on BF vGluT2+ neurons is an inhibitory one. Thus, it appears unlikely that increased activity of vGluT2+ neurons can explain the wake-promoting effect of BF cholinergic stimulation. Rather, the strong cholinergic excitation of cortically BF GABAergic neurons (Yang et al., 2014; Xu et al., 2015), appears a more likely explanation for the intra-BF wake-promoting action of cholinergic stimulation (Zant et al., 2016). Similarly, the promotion of wakefulness induced by intra-BF infusions of carbachol in cats (Baghdoyan et al., 1993) seems most likely to be due to activation of GABAergic neurons since both cholinergic neurons and glutamatergic neurons (present results) would be inhibited.
The predominant inhibitory effect of cholinergic neurons on BF vGluT2+ neurons observed by both our group and Xu et al., (2015) was surprising considering the wake/REM-on profile of both cholinergic and vGluT2+ BF neurons (Lee et al., 2004; Xu et al., 2015) and appears inconsistent with a primary role for BF vGluT2+ neurons in wake promotion (Xu et al., 2015). What role then does cholinergic modulation of BF vGluT2+neurons play in vivo? Based on our in vitro results and the prevailing literature we can only speculate but, two possibilities come to mind. Firstly, if the cholinergic inhibition is relatively mild then it might serve to de-inactivate the low-threshold calcium channels that we have shown here are present in BF vGluT2+ neurons. Such an action would promote rhythmic firing when these neurons receive excitatory inputs, a pattern of discharge observed in vivo in putative BF glutamatergic neurons (Manns et al., 2003b; Hassani et al., 2009). Another possibility is that the cholinergic inhibition only occurs in behavioral circumstances when cholinergic neurons are highly active or show highly synchronized activity. Recent in vivo single-unit recording studies by Hangya and colleagues revealed that cholinergic neurons are rapidly activated by stimuli predicting reward (Hangya et al., 2015), whereas another population of non-cholinergic BF neurons are inhibited by the same stimuli with a longer delay. Interestingly, recent anatomical tracing studies (McKenna et al., 2015a; Brown et al., 2016; Do et al., 2016; Golden et al., 2016) indicated that BF vGluT2+ neurons have prominent projections to brain areas involved in reward processing such as the lateral habenula. These direct excitatory projections to the habenula suggest BF vGluT2+ neurons would encode a negative reward prediction. Thus, one possibility is that vGluT2+ neurons inhibited by cholinergic activation correspond to the subpopulation of neurons reported by Hangya and colleagues (Hangya et al., 2015) which are inhibited at long-latency by rewarding stimuli, and cholinergic neurons mediate this inhibition via activation of muscarinic receptors and opening of potassium channels. Such a cholinergic inhibition of BF vGluT2+ neurons signaling negative reward predictions might also explain the promotion of cataplexy induced by carbachol infusions into the BF in narcoleptic dogs (Nishino et al., 1995; Reid et al., 1998) since cataplexy is most commonly induced by stimuli related to positive rewards. In contrast, we propose that those vGluT2+ neurons which are unaffected by carbachol likely represent local interneurons which help maintain the increased discharge of cholinergic and cortically-projecting GABAergic neurons during wakefulness and REM sleep (Xu et al., 2015). In the future, it will be interesting to test these possibilities in vivo using single-unit recordings combined with selective excitation/inhibition of cholinergic systems.
In summary, our results suggest that BF glutamatergic neurons are heterogeneous and have morphological, electrical and pharmacological properties which distinguish them from BF cholinergic and GABAergic neurons. A subset of vGluT2+ neurons, possibly those neurons which project to reward-related areas such as the habenula, are hyperpolarized by cholinergic inputs, which may cause phasic inhibition during reward-related events.
Highlights.
vGluT2-tdTomato mice were used to study the properties of the major group of basal forebrain glutamatergic neurons in vitro
Intrinsic electrical properties of BF vGluT2+ neurons were heterogeneous suggesting several functional groups
Many BF vGluT2+ neurons exhibited low-threshold calcium currents
The cholinergic receptor agonist, carbachol, hyperpolarized vGluT2+ neurons located in ventromedial BF
BF cholinergic neurons may cause a phasic inhibition of ventromedial BF vGluT2+ neurons during reward-related behaviors
Acknowledgments
Funding: This work was supported by the US Department of Veterans Affairs [Merit Awards I01BX001404 and I01BX001356] and by the US National Institutes of Health [NINDS R21 NS093000 to REB, NIMH R01 MH039683 & NHLBI HL095491 to RW McCarley & NIMH R03 MH107650 to CY]. The contents of this article do not represent the views of the US Department of Veterans Affairs or the US government. The sponsors of this work played no role in the design, conception, execution or writing of this report. JTM received partial salary compensation and funding from Merck MISP, but has no conflict of interest with this work.
List of Abbreviations
- ACSF
Artificial Cerebrospinal Fluid
- AHP
Afterhyperpolarization
- AP
Action Potential
- BF
Basal Forebrain
- Cre
Cre Recombinase
- GABA
Gamma-Amino-Butyric-Acid
- HCN
Hyperpolarization-activated Cyclic Nucleotide-gated cation channels
- HDB
Horizontal limb of the Diagonal Band
- LPO
Lateral Preoptic Area
- MCPO
Magnocellular Preoptic Area
- REM
Rapid-Eye-Movement
- RMP
Resting Membrane Potential
- SEM
Standard Error of the Mean
- TTA-P2
3,5-dichloro-N-[1-(2,2-dimethyl-tetrahydro-pyran-4-ylmethyl)-4-fluoro-piperidin-4-ylmethyl]-benzamide
- TTX
Tetrodotoxin
- vGluT2
vesicular Glutamate Transporter subtype 2
- VP
Ventral Pallidum
- ZD7288
4-Ethylphenylamino-1,2-dimethyl-6-methylaminopyrimidinium chloride
Footnotes
Author contributions: CY bred the mice, prepared the brain slices, performed and analyzed the electrophysiological recordings and drafted the manuscript. JTM validated the transgenic mice and edited the manuscript. REB conceived the experiments, analyzed the data and wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agostinelli LJ, Ferrari LL, Mahoney CE, Mochizuki T, Lowell BB, Arrigoni E, Scammell TE. Descending Projections from the Basal Forebrain to the Orexin Neurons in Mice. J Comp Neurol. 2016 doi: 10.1002/cne.24158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso A, Khateb A, Fort P, Jones BE, Muhlethaler M. Differential oscillatory properties of cholinergic and noncholinergic nucleus basalis neurons in guinea pig brain slice. Eur J Neurosci. 1996;8:169–182. doi: 10.1111/j.1460-9568.1996.tb01178.x. [DOI] [PubMed] [Google Scholar]
- Anaclet C, Pedersen NP, Ferrari LL, Venner A, Bass CE, Arrigoni E, Fuller PM. Basal forebrain control of wakefulness and cortical rhythms. Nature communications. 2015;6:8744. doi: 10.1038/ncomms9744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baghdoyan HA, Spotts JL, Snyder SG. Simultaneous pontine and basal forebrain microinjections of carbachol suppress REM sleep. J Neurosci. 1993;13:229–242. doi: 10.1523/JNEUROSCI.13-01-00229.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomeley C, Bracci E. Substance P depolarizes striatal projection neurons and facilitates their glutamatergic inputs. J Physiol. 2008;586:2143–2155. doi: 10.1113/jphysiol.2007.148965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RE, McKenna JT. Turning a Negative into a Positive: Ascending GABAergic Control of Cortical Activation and Arousal. Frontiers in neurology. 2015;6:135. doi: 10.3389/fneur.2015.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW. Control of sleep and wakefulness. Physiol Rev. 2012;92:1087–1187. doi: 10.1152/physrev.00032.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RE, Yang C, McKenna JT, McNally JM, Gamble M, Hulverson A, Bellio T, McCoy J, Anderson-Chernisof M, Winston S, Deisseroth K, Thankachan S, Basheer R, McCarley RW. Basal forebrain glutamate neurons studied using vGluT2-tdTomato mice: intrinsic membrane properties, cholinergic sensitivity, calcium binding protein content and projections. Soc Neurosci Abs. 2016;83:11. [Google Scholar]
- Choe W, Messinger RB, Leach E, Eckle VS, Obradovic A, Salajegheh R, Jevtovic-Todorovic V, Todorovic SM. TTA-P2 is a potent and selective blocker of T-type calcium channels in rat sensory neurons and a novel antinociceptive agent. Molecular pharmacology. 2011;80:900–910. doi: 10.1124/mol.111.073205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do JP, Xu M, Lee SH, Chang WC, Zhang S, Chung S, Yung TJ, Fan JL, Miyamichi K, Luo L, Dan Y. Cell type-specific long-range connections of basal forebrain circuit. eLife. 2016;5:e13214. doi: 10.7554/eLife.13214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfus FM, Tscherter A, Errington AC, Renger JJ, Shin HS, Uebele VN, Crunelli V, Lambert RC, Leresche N. Selective T-type calcium channel block in thalamic neurons reveals channel redundancy and physiological impact of I(T)window. J Neurosci. 2010;30:99–109. doi: 10.1523/JNEUROSCI.4305-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlow BL, Haynes RL, Takahashi E, Klein JP, Cummings P, Benner T, Greer DM, Greenberg SM, Wu O, Kinney HC, Folkerth RD. Disconnection of the ascending arousal system in traumatic coma. J Neuropathol Experimental Neurol. 2013;72:505–523. doi: 10.1097/NEN.0b013e3182945bf6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. 3rd. New York: Academic Press; 2008. [Google Scholar]
- Fremeau RT, Jr, Voglmaier S, Seal RP, Edwards RH. VGLUTs define subsets of excitatory neurons and suggest novel roles for glutamate. Trends Neurosci. 2004;27:98–103. doi: 10.1016/j.tins.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Freund TF, Meskenaite V. gamma-Aminobutyric acid-containing basal forebrain neurons innervate inhibitory interneurons in the neocortex. Proc Natl Acad Sci USA. 1992;89:738–742. doi: 10.1073/pnas.89.2.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Derst C, Veh RW, Zahm DS. Glutamatergic afferents of the ventral tegmental area in the rat. J Neurosci. 2007;27:5730–5743. doi: 10.1523/JNEUROSCI.0012-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden SA, Heshmati M, Flanigan M, Christoffel DJ, Guise K, Pfau ML, Aleyasin H, Menard C, Zhang H, Hodes GE, Bregman D, Khibnik L, Tai J, Rebusi N, Krawitz B, Chaudhury D, Walsh JJ, Han MH, Shapiro ML, Russo SJ. Basal forebrain projections to the lateral habenula modulate aggression reward. Nature. 2016;534:688–692. doi: 10.1038/nature18601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritti I, Manns ID, Mainville L, Jones BE. Parvalbumin, calbindin, or calretinin in cortically projecting and GABAergic, cholinergic, or glutamatergic basal forebrain neurons of the rat. J Comp Neurol. 2003;458:11–31. doi: 10.1002/cne.10505. [DOI] [PubMed] [Google Scholar]
- Grothe M, Heinsen H, Teipel SJ. Atrophy of the cholinergic Basal forebrain over the adult age range and in early stages of Alzheimer's disease. Biol Psychiatry. 2012;71:805–813. doi: 10.1016/j.biopsych.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajszan T, Alreja M, Leranth C. Intrinsic vesicular glutamate transporter 2-immunoreactive input to septohippocampal parvalbumin-containing neurons: novel glutamatergic local circuit cells. Hippocampus. 2004;14:499–509. doi: 10.1002/hipo.10195. [DOI] [PubMed] [Google Scholar]
- Hangya B, Ranade SP, Lorenc M, Kepecs A. Central Cholinergic Neurons Are Rapidly Recruited by Reinforcement Feedback. Cell. 2015;162:1155–1168. doi: 10.1016/j.cell.2015.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassani OK, Lee MG, Henny P, Jones BE. Discharge profiles of identified GABAergic in comparison to cholinergic and putative glutamatergic basal forebrain neurons across the sleep-wake cycle. J Neurosci. 2009;29:11828–11840. doi: 10.1523/JNEUROSCI.1259-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawryluk JM, Ferrari LL, Keating SA, Arrigoni E. Adenosine inhibits glutamatergic input to basal forebrain cholinergic neurons. J Neurophysiol. 2012;107:2769–2781. doi: 10.1152/jn.00528.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick T, Waters J. Physiological properties of cholinergic and non-cholinergic magnocellular neurons in acute slices from adult mouse nucleus basalis. PLoSOne. 2010;5:e11046. doi: 10.1371/journal.pone.0011046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henny P, Jones BE. Vesicular glutamate (VGlut), GABA (VGAT), and acetylcholine (VACht) transporters in basal forebrain axon terminals innervating the lateral hypothalamus. J Comp Neurol. 2006;496:453–467. doi: 10.1002/cne.20928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henny P, Jones BE. Projections from basal forebrain to prefrontal cortex comprise cholinergic, GABAergic and glutamatergic inputs to pyramidal cells or interneurons. Eur J Neurosci. 2008;27:654–670. doi: 10.1111/j.1460-9568.2008.06029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh CY, Goutagny R, Williams S. Glutamatergic neurons of the mouse medial septum and diagonal band of Broca synaptically drive hippocampal pyramidal cells: relevance for hippocampal theta rhythm. J Neurosci. 2010;30:15951–15961. doi: 10.1523/JNEUROSCI.3663-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur EE, Zaborszky L. Vglut2 afferents to the medial prefrontal and primary somatosensory cortices: a combined retrograde tracing in situ hybridization. J Comp Neurol. 2005;483:351–373. doi: 10.1002/cne.20444. [DOI] [PubMed] [Google Scholar]
- Kim T, Thankachan S, McKenna JT, McNally JM, Yang C, Choi JH, Chen L, Kocsis B, Deisseroth K, Strecker RE, Basheer R, Brown RE, McCarley RW. Cortically projecting basal forebrain parvalbumin neurons regulate cortical gamma band oscillations. Proc Natl Acad Sci USA. 2015;112:3535–3540. doi: 10.1073/pnas.1413625112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MG, Manns ID, Alonso A, Jones BE. Sleep-wake related discharge properties of basal forebrain neurons recorded with micropipettes in head-fixed rats. J Neurophysiol. 2004;92:1182–1198. doi: 10.1152/jn.01003.2003. [DOI] [PubMed] [Google Scholar]
- Lin SC, Brown RE, Hussain Shuler MG, Petersen CC, Kepecs A. Optogenetic Dissection of the Basal Forebrain Neuromodulatory Control of Cortical Activation, Plasticity, and Cognition. J Neurosci. 2015;35:13896–13903. doi: 10.1523/JNEUROSCI.2590-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorincz ML, Crunelli V, Hughes SW. Cellular dynamics of cholinergically induced alpha (8-13 Hz) rhythms in sensory thalamic nuclei in vitro. J Neurosci. 2008;28:660–671. doi: 10.1523/JNEUROSCI.4468-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns ID, Alonso A, Jones BE. Rhythmically discharging basal forebrain units comprise cholinergic, GABAergic, and putative glutamatergic cells. J Neurophysiol. 2003a;89:1057–1066. doi: 10.1152/jn.00938.2002. [DOI] [PubMed] [Google Scholar]
- Manns ID, Alonso A, Jones BE. Rhythmically discharging basal forebrain units comprise cholinergic, GABAergic, and putative glutamatergic cells. J Neurophysiol. 2003b;89:1057–1066. doi: 10.1152/jn.00938.2002. [DOI] [PubMed] [Google Scholar]
- Manseau F, Danik M, Williams S. A functional glutamatergic neurone network in the medial septum and diagonal band area. J Physiol. 2005;566:865–884. doi: 10.1113/jphysiol.2005.089664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna JT, Yang C, Franciosi S, Winston S, Abarr KK, Rigby MS, Yanagawa Y, McCarley RW, Brown RE. Distribution and intrinsic membrane properties of basal forebrain GABAergic and parvalbumin neurons in the mouse. J Comp Neurol. 2013;521:1225–1250. doi: 10.1002/cne.23290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna JT, Gamble M, Yang C, McNally JM, Hulverson A, Winston S, Thankachan S, McCarley RW, Brown RE. Neuroanatomical investigation of basal forebrain glutamatergic neurons using vGluT2-tdTomato mice. Soc Neurosci Abs. 2015a;730:13. [Google Scholar]
- McKenna JT, McNally JM, Yang C, Gamble M, Hulverson A, Winston S, Thankachan S, McCarley RW, Brown RE. vGluT2-tdTomato transgenic mice as a model system for investigation of basal forebrain glutamatergic neurons. Sleep. 2015b;38:A0124. [Google Scholar]
- McKenna JT, Gamble M, Hulverson A, McCoy J, Yang C, Winston S, Thankachan S, McCarley RW, Brown RE. Calcium binding protein profile of basal forebrain glutamatergic neurons in the vGluT2-tdTomato mouse. Sleep. 2016;39:A67. [Google Scholar]
- Muller ML, Bohnen NI. Cholinergic dysfunction in Parkinson's disease. Curr Neurol Neurosci Rep. 2013;13:377. doi: 10.1007/s11910-013-0377-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll RA, Malenka RC, Kauer JA. Functional comparison of neurotransmitter receptor subtypes in mammalian central nervous system. Physiol Rev. 1990;70:513–565. doi: 10.1152/physrev.1990.70.2.513. [DOI] [PubMed] [Google Scholar]
- Nishino S, Tafti M, Reid MS, Shelton J, Siegel JM, Dement WC, Mignot E. Muscle atonia is triggered by cholinergic stimulation of the basal forebrain: implication for the pathophysiology of canine narcolepsy. J Neurosci. 1995;15:4806–4814. doi: 10.1523/JNEUROSCI.15-07-04806.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid MS, Nishino S, Tafti M, Siegel JM, Dement WC, Mignot E. Neuropharmacological characterization of basal forebrain cholinergic stimulated cataplexy in narcoleptic canines. Exp Neurol. 1998;151:89–104. doi: 10.1006/exnr.1998.6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J, Manseau F, Ducharme G, Amilhon B, Vigneault E, El Mestikawy S, Williams S. Optogenetic Activation of Septal Glutamatergic Neurons Drive Hippocampal Theta Rhythms. J Neurosci. 2016;36:3016–3023. doi: 10.1523/JNEUROSCI.2141-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotty F, Danik M, Manseau F, Laplante F, Quirion R, Williams S. Distinct electrophysiological properties of glutamatergic, cholinergic and GABAergic rat septohippocampal neurons: novel implications for hippocampal rhythmicity. J Physiol. 2003;551:927–943. doi: 10.1113/jphysiol.2003.046847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teipel SJ, Meindl T, Grinberg L, Grothe M, Cantero JL, Reiser MF, Moller HJ, Heinsen H, Hampel H. The cholinergic system in mild cognitive impairment and Alzheimer's disease: an in vivo MRI and DTI study. Hum Brain Mapp. 2011;32:1349–1362. doi: 10.1002/hbm.21111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vong L, Ye C, Yang Z, Choi B, Chua S, Jr, Lowell BB. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron. 2011;71:142–154. doi: 10.1016/j.neuron.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse PJ, Price DL, Struble RG, Clark AW, Coyle JT, Delon MR. Alzheimer's disease and senile dementia: loss of neurons in the basal forebrain. Science. 1982;215:1237–1239. doi: 10.1126/science.7058341. [DOI] [PubMed] [Google Scholar]
- Wu M, Hajszan T, Leranth C, Alreja M. Nicotine recruits a local glutamatergic circuit to excite septohippocampal GABAergic neurons. Eur J Neurosci. 2003;18:1155–1168. doi: 10.1046/j.1460-9568.2003.02847.x. [DOI] [PubMed] [Google Scholar]
- Xu M, Chung S, Zhang S, Zhong P, Ma C, Chang WC, Weissbourd B, Sakai N, Luo L, Nishino S, Dan Y. Basal forebrain circuit for sleep-wake control. Nature Neurosci. 2015;18:1641–1647. doi: 10.1038/nn.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, McKenna JT, Brown RE. Basal forebrain vGluT2-positive neurons: electrophysiological properties and cholinergic modulation. Soc Neurosci Abs. 2015;730:14. [Google Scholar]
- Yang C, McKenna JT, Brown RE. Basal Forebrain glutamatergic and GABAergic neurons: intrinsic properties and modulation by cholinergic inputs and hypnotic agents. Sleep. 2016;39:A0075. [Google Scholar]
- Yang C, McKenna JT, Zant JC, Winston S, Basheer R, Brown RE. Cholinergic neurons excite cortically projecting basal forebrain GABAergic neurons. J Neurosci. 2014;34:2832–2844. doi: 10.1523/JNEUROSCI.3235-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborszky L, Van den Pol A, Gyengesi E. The Basal Forebrain Cholinergic Projection System in Mice. In: Watson C, Paxinos G, Puelles L, editors. The Mouse Nervous System. London: Elsevier; 2012. pp. 684–718. [Google Scholar]
- Zaborszky L, Csordas A, Mosca K, Kim J, Gielow MR, Vadasz C, Nadasdy Z. Neurons in the basal forebrain project to the cortex in a complex topographic organization that reflects corticocortical connectivity patterns: an experimental study based on retrograde tracing and 3D reconstruction. Cerebral cortex. 2015;25:118–137. doi: 10.1093/cercor/bht210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zant JC, Kim T, Prokai L, Szarka S, McNally J, McKenna JT, Shukla C, Yang C, Kalinchuk AV, McCarley RW, Brown RE, Basheer R. Cholinergic Neurons in the Basal Forebrain Promote Wakefulness by Actions on Neighboring Non-Cholinergic Neurons: An Opto-Dialysis Study. J Neurosci. 2016;36:2057–2067. doi: 10.1523/JNEUROSCI.3318-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]


