SUMMARY
Replicating Lgr5+ stem cells and quiescent Bmi1+ cells behave as intestinal stem cells (ISCs) in vivo. Disrupting Lgr5+ ISCs triggers epithelial renewal from Bmi1+ cells, from secretory or absorptive progenitors, and from Paneth cell precursors, revealing a high degree of plasticity within intestinal crypts. Here we show that GFP+ cells from Bmi1GFP mice are pre-terminal enteroendocrine cells and identify CD69+CD274+ cells as related goblet cell precursors. Upon loss of native Lgr5+ ISCs, both populations revert towards an Lgr5+ cell identity. While active histone marks are distributed similarly between Lgr5+ ISCs and progenitors of both major lineages, thousands of cis-elements that control expression of lineage-restricted genes are selectively open in secretory cells. This accessibility signature dynamically converts to that of Lgr5+ ISCs during crypt regeneration. Beyond establishing the nature of Bmi1GFP+ cells, these findings reveal how chromatin status underlies intestinal cell diversity and dedifferentiation to restore ISC function and intestinal homeostasis.
Keywords: Intestinal stem cells, facultative stem cells, accessible chromatin, cell plasticity
Graphical Abstract

INTRODUCTION
Self-renewal of the intestinal epithelium is sustained principally by Lgr5+ stem cells that lie at the bottom of intestinal crypts and replicate daily (Barker et al., 2007). It is unclear if a separate pool of quiescent stem cells is dedicated to replenishing Lgr5+ intestinal stem cells (ISC) when necessary (Barker, 2014; Richmond et al., 2016; Sangiorgi and Capecchi, 2008; Takeda et al., 2011). Ambiguous expression domains of Cre recombinase in lineage-tracing studies and of putative molecular markers fuel the uncertainty. Chromatin states can shed useful light on cell populations and on the basis for ISC recovery after ablative injuries.
Ideas about a facultative pool of reserve ISC originated in observations on rare cells that reside mainly in crypt tier 4 and retain S-phase labels for long periods, indicating replicative quiescence (Potten, 1998). These label-retaining cells (LRC) share many features – small numbers, locations near tier 4, and infrequent cell division (Sangiorgi and Capecchi, 2008; Yan et al., 2012) – with GFP+ cells in mice that express GFP from the Bmi1 locus, Bmi1Gfp (Hosen et al., 2007). Attesting to their stem cell potential, BmiGfp cells generate colonies in culture and increase in number when Lgr5+ ISC are ablated in vivo (Sangiorgi and Capecchi, 2008; Yan et al., 2012), a circumstance in which a Bmi1-expressing population is the source of new ISC (Tian et al., 2011). One problem in interpreting these data is that Bmi1 is expressed throughout intestinal crypts, including Lgr5+ ISC and transit-amplifying (TA) cells (Itzkovitz et al., 2012; Munoz et al., 2012), in sharp contrast to the few GFP+ cells found in BmiGfp mice (Li et al., 2014; Yan et al., 2012). Therefore, although Bmi1 is often regarded as a specific marker of quiescent ISC, GFP+ cells in BmiGfp mouse intestines represent a subset of Bmi1-expressing cells (Itzkovitz et al., 2012; Li et al., 2014), and because TA cells may express Cre recombinase in BmiCre mice, it is difficult to pinpoint the source of replenished Lgr5+ ISC by lineage tracing (Tian et al., 2011). Identification of crypt cells by chromatin states and new molecular markers may help resolve these open questions.
Damage to Lgr5+ ISC triggers crypt regeneration not only from Bmi1+ cells, but also from specified enterocytes (Ent) and secretory (Sec) progenitors (Pro) (Tetteh et al., 2016; van Es et al., 2012). Even LRC – which were previously thought to represent quiescent ISC (Potten, 1998) but are now recognized as precursors of terminal Paneth and some enteroendocrine (EE) cells – contribute to the salvage (Buczacki et al., 2013; Li et al., 2016). This considerable plasticity implies that diverse crypt cells either have similar chromatin states or efficiently overcome a chromatin barrier when recruited to dedifferentiate into ISC. Judging by the active histone modifications H3K4me2 and H3K27ac, the cis-element profiles of Lgr5+ ISC, Sec-Pro and Ent-Pro are strikingly similar (Kim et al., 2014). This similarity may explain divergence of the two intestinal lineages by lateral inhibition, but it is unclear how it engenders crypt cell diversity, including Bmi1Gfp cells.
Here we show that BmiGfp cells are pre-terminal EE cells and identify related goblet cell precursors that express CD69 and CD274. Neither population seems to represent a dedicated pool of quiescent ISC, but both dedifferentiate rapidly in response to ablation of native ISC. We identify thousands of genome sites where chromatin is selectively open in Sec-Pro, BmiGfp and CD69+CD274+ cells, but not in ISC or Ent-Pro. Although these sites lack strong histone activation marks, they are bona fide enhancers for Sec-restricted genes. Upon loss of native ISC, the distinctive chromatin signature of Bmi1Gfp and CD69+CD274+ cells reverts dynamically toward that of Lgr5+ cells. This transition reflects the rapid breach of a chromatin barrier when specified crypt cells are enlisted to restore ISC function.
RESULTS
Bmi1Gfp cells are mature EE cells and not a dedicated pool of ‘reserve’ ISC
In duodenal crypts in BmiGfp mice (Hosen et al., 2007), GFP+ cells lie just above the Lgr5+ ISC and Paneth cell zone (Fig. 1A). To determine the lineage of these GFP+ cells, we isolated them by flow cytometry (Fig. S1A) and compared their RNA-seq profiles to those of Lgr5+ ISC (Barker et al., 2007), Sec-Pro isolated from wild-type crypts after pharmacologic inhibition of Notch signaling (van Es et al., 2005), and Ent-Pro from Atoh1−/− crypts (Kim et al., 2014) (Table S1, Fig. S1B); high Epcam mRNA levels confirmed the epithelial origin of each population (Fig. S1B). We also profiled Lgr5+ ISC harvested from another mouse strain, Lgr5Dtr-Gfp (Tian et al., 2011), and Sec-Pro isolated after genetic disruption of Notch activity (Kim et al., 2014) (Fig. S1C). Hundreds of transcripts were enriched or present exclusively in Bmi1Gfp cells, such as the Serpina1 cluster, TFs of the Ets/Fli/Fev family, and especially genes known for EE-cell expression (Figs. 1B and S1D). In principal component analysis (PCA), Bmi1Gfp cells accounted for the bulk of variation (Fig. 1C). Compared to the other populations, transcripts present selectively (>3-fold, q <0.05) in Bmi1Gfp cells were depleted in the ‘Cell cycle’ category and enriched for ‘Quiescence’ and ‘Targets of fusion transcription factor EWSR1-FLI1’ (Fig. S1E). Thus, Bmi1Gfp cells have an mRNA profile distinct from Lgr5+ ISC and crypt progenitors.
Figure 1. Bmi1Gfp cells belong to the EE lineage.
(A) Confocal microscopy on optically cleared tissue confirms the distinct locations of Lgr5+ ISC (crypt base) and Bmi1Gfp cells (higher crypt tiers). Scale bar, 10 μm. (B) RNA-seq tracks illustrating Bmi1Gfp cell-restricted genes, including the Serpina1 cluster and EE marker Chga. (C) Principal component analysis (PCA) of mRNA differences among crypt cells. Bmi1Gfp cells are distinct from others along PC1. Three ISC samples were derived from Lgr5Gfp and two from Lgr5Dtr mice. 2 SP samples each were isolated after pharmacologic or genetic Notch inhibition. SP: Sec-Pro, EP: Ent-Pro. (D) Cell trajectory models based on global mRNA levels impute Bmi1Gfp cells as descendants of Sec-Pro. Scores reflect monotonic expression changes for each trajectory. (E) Flow cytometry plots showing lack of Bmi1Gfp cells in Atoh1−/− crypts. (F) Gene Set Enrichment Analysis (GSEA) of the 500 highest expressed genes in Bmi1Gfp cells in relation to EE, Paneth, and goblet cell transcriptomes. (G) Co-expression of GFP and CHGA in Bmi1Gfp duodenal cells (N=165 cells). Representative individual and merged fluorescence images are displayed. Scale bar, 20 μm.
See also Figures S1 and S2.
Because one prevalent view is that BmiGfp cells represent quiescent ISC that periodically replenish the pool of Lgr5+ ISC (Li et al., 2014; Richmond et al., 2016; Yan et al., 2012), we sought to determine their position within the crypt hierarchy. We used successive changes in mRNA expression to derive quantitative scores for every possible trajectory among stem, progenitor, and the abundant differentiated villus cell types: enterocytes and goblet cells (Experimental Procedures and Fig. S1F). Among every possible model, those in which BmiGfp cells spawn Lgr5+ ISC or progenitors gave unfavorable scores; only a model where they originate in Sec-Pro gave a high positive score (Fig. 1D). In line with this result, crypts lacking ATOH1, a TF required for Sec specification (Shroyer et al., 2007; Yang et al., 2001), were largely devoid of Bmi1Gfp cells (Figs. 1E and S1G).
Bmi1Gfp cells lacked transcripts classically ascribed to goblet or Paneth cells; rather, they were enriched for canonical EE genes, with the hormone Ghrelin being particularly abundant and specific (Fig. S2A). We therefore purified crypt EE and Paneth cells using flow cytometry for CD24 and Ulex europaeus agglutinin (Wong et al., 2012), verified the purity of various cell isolates by RT-PCR analysis of established lineage markers (Fig. S2B), and profiled transcripts using RNA-seq (Table S1). Gene set enrichment (GSEA) (Subramanian et al., 2005) and trajectory analyses indicated that Bmi1Gfp are related most closely to EE cells (Figs. 1F and S2C). Moreover, 91% of BmiGfp cells showed high expression of the EE-specific marker CHGA (Fig. 1G) and the population is enriched for RNAs encoding many hormones and TFs known to control EE cells (Fig. S2D–E). These data reveal Bmi1Gfp cells as non-replicating ChgA+ EE cells, with high expression of hormone RNAs and Neurod1 (compared to Neurog3) suggesting advanced differentiation (Li et al., 2012); our trajectory data suggest pre-terminal EE maturity (Fig. S2C). Bmi1Gfp cells thus seem not to represent a dedicated pool of reserve ISC, though they may when necessary dedifferentiate into Lgr5+ ISC, like other precursors (Buczacki et al., 2013; Schwitalla et al., 2013; Tetteh et al., 2016; van Es et al., 2012; Westphalen et al., 2014). Notably, no crypt cells have been captured in the act of dedifferentiation.
Identification of goblet-cell precursors in wild-type mouse crypts
Because our goal was to capture such cells and examine transitional chromatin states, we sought next to identify potentially labile crypt populations in wild-type mice. Noting that some surface marker RNAs express at considerably higher levels in Bmi1Gfp than in Lgr5+ ISC (Fig. S3A), we used flow cytometry to test expression of selected such proteins. CD69 and CD274 reproducibly marked ~9% (N=7) of viable wild-type EPCAM+ crypt epithelial cells (Fig. S3B). These proteins were rarely present alone, and both flow cytometry and immunofluorescence robustly identified a CD69+CD274+ cell population that localized in crypt positions above the Paneth-ISC zone (Figs. 2A–B and S3B). Although Cd69 and Cd274 mRNAs are absent from Lgr5+ ISC (Fig. S3A), to exclude any potential overlap of cell types we treated Lgr5Dtr-Gfp mice with 4 doses of Diphtheria toxin (Tian et al., 2011) and harvested crypt cells the following day. Lgr5+ ISC were lost after toxin treatment as expected (Fig. S3C), whereas CD69+CD274+ cells remained (Fig. 2B), indicating that the populations are distinct. Moreover, few CD69+CD274+ cells took up bromodeoxyuridine (Fig. 2C), revealing that they are largely quiescent. CD69+CD274+ cells were absent from Atoh1−/− intestines and their mRNA profile matched that of Sec-Pro (Fig. 2D), indicating that they belong to the Sec lineage.
Figure 2. CD69 and CD274 mark a crypt Sec population of goblet cell precursors.
(A) Wild-type CD69+CD274+ cell fractions detected by flow cytometry with both Ab (N=5) and by immunofluorescence (IF), which showed co-expression in cells lying just above the crypt base. Scale bar, 10 μm. (B) Elimination of Lgr5+ ISC in Lgr5Dtr-Gfp mouse intestines by Diphtheria toxin (DT) did not remove CD69+CD274+ cells (N=4), and IF in untreated Lgr5Dtr-Gfp mice shows their location just above the Lgr5+ ISC zone. (C) Representative IF of serial tissue sections showing non-overlapping signals from CD69+CD274+ cells and bromodeoxyuridine (BrdU)+ cells. Scale bar, 10 μm. (D) Absence of CD69+CD274+ cells by flow cytometry in Atoh1−/− intestines (N=3), and GSEA of the 500 highest expressed genes in wild-type mice reveals a closer match with Sec-Pro (SP) than with Ent-Pro (EP). (E) Relative mRNA levels of 8,953 genes differentially expressed (>3-fold, q <0.05) among secretory cell types in duplicate, grouped by unsupervised k-means clustering (k=6, as determined by the gap statistic). (F) RNA-seq tracks showing expression of goblet cell-specific Fcgbp. (G) Comparative cell trajectory modeling based on global RNA expression imputes CD69+CD274+ cells as goblet-cell precursors.
See also Figure S3.
Although Cd69 and Cd274 are expressed in Bmi1Gfp cells (Fig. S3A), analysis of all mRNAs present at different levels in various Sec populations suggested that FACS-sorted Bmi1Gfp and CD69+CD274+ cells are distinct entities. K-means clustering of differential mRNAs confirmed the close relation of Bmi1Gfp to EE cells and revealed that CD69+CD274+ cells most resemble goblet cells (Fig. 2E). Indeed, the CD69+CD274+ population lacks Chga, Cck, Ghrl, and other abundant EE transcripts but selectively expresses known goblet-cell genes (Pelaseyed et al., 2014; Zhu et al., 2016) (Figs. 2E–F and S3D). mRNA flux in the Sec lineage also gave the best score for the trajectory Sec-Pro → CD69+CD274+ cells → goblet cells (Fig. 2G). Thus, CD69+CD274+ crypt cells represent a wild-type Sec population that is anatomically close to the ISC zone and strongly biased toward goblet cell differentiation.
mRNA changes identify Sec cells in the act of dedifferentiation
Lineage tracing studies have elegantly revealed the origins of new Lgr5+ ISC, generally days or weeks after injury to the native ISC pool (Barker, 2014). To detect such a transition in Bmi1Gfp cells, we confirmed that 10 Gy whole-body γ-irradiation eliminates Lgr5+ ISC, as reported (Metcalfe et al., 2014), then delivered this dose to Bmi1Gfp mice, and isolated GFP+ cells 36 h later. Because the Lgr5 locus is unmodified in these mice, GFP+ cells originate exclusively in the Bmi1Gfp population and the short interval enabled capture of cells during their possible conversion to GFP− ISC. Irradiated Bmi1Gfp mice yielded 3 to 4 times more GFP+ cells than unirradiated controls, as reported (Tian et al., 2011; Yan et al., 2012). After irradiation, Bmi1Gfp cells showed mRNA features that normally signify either Bmi1Gfp cells or Lgr5+ ISC (but not both); reduced levels of otherwise abundant and specific transcripts, such as the Serpina1 cluster, were particularly evident (Fig. 3A). RT-PCR analysis of changes in Bmi1Gfp cells, compared to bulk crypt cells isolated before and after irradiation, demonstrated the specificity of this response (Figs. 3B and S3E). Thus, radiation-induced loss of native ISC forces Bmi1Gfp cells into a genuine transition.
Figure 3. Modulation of gene expression in BmiGfp and CD69+CD274+ cells upon Lgr5+ ISC loss.
(A) mRNA changes in BmiGfp cells before and after γ-irradiation. The 4,393 genes differentially expressed (>2-fold, q <0.05) in Lgr5+ and BmiGfp cells are arranged in descending order of baseline differences; RNA levels in post-irradiated BmiGfp cells are shown alongside. Representative RNA-seq tracks showing reduced levels of BmiGfp cell-specific Serpina1 transcripts. (B) qRT-PCR analysis showing that changes in gene expression triggered by ISC loss occur in BmiGfp and not all crypt cells. (C) mRNA changes in CD69+CD274+ cells (CD++) before and after ISC loss. The 4,432 genes differentially expressed (>2-fold, q <0.05) in Lgr5+ and CD++ cells are arranged in descending order of differences, and RNA levels in CD++ cells after Diphtheria toxin (DT) are shown in the middle. (D) PCA of mRNA modulation showing that, upon DT-induced ISC loss, CD++ cells cluster away from native CD++, and closer to ISC. (E) Representative RNA-seq data showing increase of ISC-specific (Cdca7) and reduction of goblet-specific (Aqp3) transcripts. (F) Heatmap representation of 12 stringently-defined ISC marker genes (Munoz et al., 2012). (G) qRT-PCR analysis showing selective gain of ISC maker genes in CD++ cells compared to bulk populations of crypt cells (N=3 each, error bars are standard deviations from biological triplicates).
See also Figure S3.
To determine whether CD69+CD274+ cells also can revert into ISC, we were sensitive to the possibility that, beyond eliminating Lgr5+ cells, γ-irradiation might directly affect other cell types. We therefore treated Lgr5Dtr-Gfp mice with Diphtheria toxin, which ablates Lgr5+ ISC selectively (Tian et al., 2011). We verified that Lgr5+ ISC begin to return 2 days after the last dose of toxin and harvested CD69+CD274+ cells 1 day after this final dose, i.e., while GFP+ cells are absent from the crypt (Fig. S3C). We observed attenuation of CD69+CD274+ cell-specific genes and clear activation of genes ordinarily expressed only in ISC (Fig. 3C). PCA placed post-toxin cells in a discrete group, related more closely than parental CD69+CD274+ cells to Lgr5+ ISC (Fig. 3D), and at least half among a panel of 12 stringently defined ISC-specific genes (Munoz et al., 2012) were reproducibly activated in CD69+CD274+ cells during ISC recovery (Fig. 3E–F). Similar gain of ISC-specific transcripts was not evident in bulk crypt cells isolated before and after toxin treatment (Figs. 3G and S3F). Thus, after ISC loss, mRNA changes in CD69+CD274+ cells represent another authentic and active transition toward ISC. Of note, only 5 and 33 of the hundreds of transcripts altered after ISC loss in CD69+CD274+ and Bmi1Gfp cells, respectively, correspond to the 212 genes linked to ‘Cell Proliferation’ in Gene Ontology.
Selectively open chromatin distinguishes Sec cell populations from Lgr5+ ISC
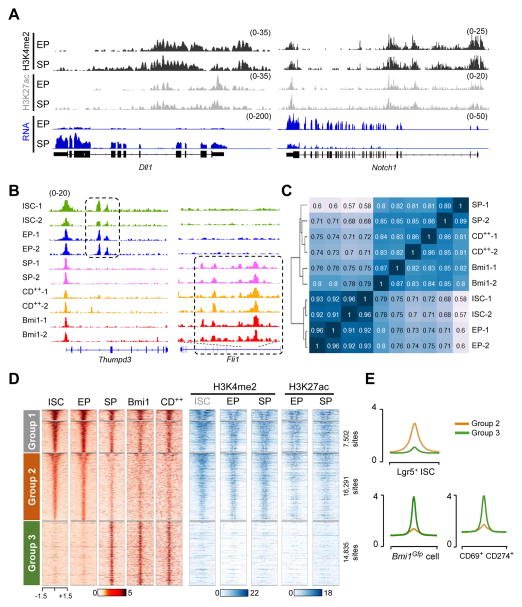
These changes in gene expression and cell identity must have a basis in chromatin. We have reported, however, that the active histone marks H3K4me2 and H3K27ac appear at the same genome sites in specified Sec- and Ent-Pro (Kim et al., 2014). To confirm these findings at nucleosome resolution, we digested chromatin from Pro cells with micrococcal nuclease and repeated ChIP-seq for these histone marks (Table S1). Global analysis of the data confirmed near identity of Sec- and Ent-Pro profiles (Fig. S4A), as reflected in the findings at the lineage-restricted Notch1 and Dll1 loci (Fig. 4A). These data underscore the need to identify a basis other than active histone marks for differentiation and dediferentiation of intestinal crypt cells. Because the numbers of Bmi1Gfp or CD69+CD274+ cells are too small for robust ChIP, we used the Assay for Transposase-Accessible Chromatin (ATAC-seq) (Buenrostro et al., 2013) to identify areas of open chromatin (Table S1). Replicate samples gave concordant ATAC signals (Fig. S4B) that coincided with promoters and putative enhancers and readily distinguished cell types (Fig. 4B). Unsupervised hierarchical clustering of the ~50,000 ATAC sites we identified in one or more populations classified Bmi1Gfp and CD69+CD274+ cells as distinct from Lgr5+ ISC or Ent-Pro and most similar to Sec-Pro (Fig. 4C). Because promoters vary little across cell types (Heintzman et al., 2009), we focused on the ~41,000 putative enhancers (sites >1 kb from transcription start sites). K-means clustering (see Fig. S4C–D) revealed 3 main groups: sites with similar ATAC signals in all 5 cell types (Group 1, ~10,000 sites), regions that gave stronger signals in ISC and Ent-Pro (Group 2, ~16,000 sites), and those that were only apparent – or strongest – in the three Sec populations (Group 3, ~14,800 sites) (Fig. 4D).
Figure 4. Distinctive profile of chromatin access separates Sec cells, including BmiGfp and CD69+CD274+, from ISC and Ent cells.
(A) ChIP- and RNA-seq data tracks showing similar locations and strength of active enhancer marks H3K4me2 and H3K27ac along loci specifically expressed in Sec- (SP, Dll1) and Ent-Pro (EP, Notch1). (B) ATAC signal tracks from duplicates of each crypt cell type at loci that illustrate differential chromatin access. (C) Matrix of Spearman correlation coefficients for global chromatin access derived from ATAC-seq data. Sec-Pro, Bmi1Gfp and CD++ cells cluster separately from ISC and Ent-Pro. (D) Heatmaps showing K-means-derived grouping of 41,167 regions (>1 kb from TSSs) of ATAC- identified open chromatin alongside active histone marks (Chip-seq) at the same sites. ChIP data on ISC were reported previously from sonicated chromatin (Kim et al., 2014); MNase-ChIP data on EP and SP are new to this study. (E) Average ATAC signals at Group 2 and Group 3 enhancers in ISC, Bmi1Gfp and CD++ cells.
See also Figure S4.
Nearly all the candidate enhancers in Groups 1 and 2 carry H3K4me2 and/or H3K27ac in both progenitors and Lgr5+ ISC (Fig. 4D) and correspond to sites previously identified (Kim et al., 2014) by ChIP-seq for these active histone marks (Fig. S4D). In contrast, regions in Group 3 lack these marks and had therefore eluded detection previously. Sites open in Ent-Pro gave ATAC signals of comparable strength in Lgr5+ ISC, while chromatin access in CD69+CD274+ and Bmi1Gfp cells resembled Sec-Pro (Figs. 4D–E). This dominance of Group 2 enhancers in Lgr5+ ISC and Ent-Pro, and of Group 3 enhancers in the Sec lineage, is striking.
Regions of selectively open chromatin in Sec cells represent bona fide active cis-elements
Many active ES cell enhancers have open chromatin but lack H3K27ac (Pradeepa et al., 2016), similar to Group 3 sites in Sec cells (Figs. 4D and S4D). Because such discrepancy between open chromatin and active histone marks is not appreciated widely in adult tissues, we asked if Group 3 sites correspond to functional enhancers. ChIP-seq for the poised enhancer feature H3K4me1 (Fig. S5A) (Shlyueva et al., 2014) gave stronger signals than the other marks, revealing H3K4me1 at many Group 3 sites (Figs. 5A–B). Of note, this mark also appeared at the same sites in Sec- and Ent-Pro; thus, the 3 histone marks associated with enhancer activity showed high concordance with ATAC signals across all cell types (Fig. S5B). In further support of the bona fides of Group 3 regions, ATAC signals are comparable to those in Group 2 (Fig. 4D–E); many of the sites are also accessible in lymphocytes (Lara-Astiaso et al., 2014); and nearby genes are as or more enriched for distinctive biological processes than those near well-marked enhancers in Group 2 (Fig. S5C). Moreover, while Group 2 regions are enriched in motifs for known enterocyte-active factors, HNF4A and CDX2 (Verzi et al., 2010), those in Group 3 are equally enriched for distinct TF sequence motifs (ETS, FLI, FEV, RUNX – Fig. S5C). Related to the latter motifs, RNA expression and ATAC signals for several genes in the FLI1/FEV family are specific to Sec cells (Fig. 5C), and FEV in particular is present in rare crypt cells that lie just above the ISC zone, coincident with CD69+ cells (Fig. 5D). Finally, many loci other than Ets1 showed ATAC- and RNA-seq signals restricted to the same cells, e.g., Lgr5 introns 6 and 8 showed significant chromatin access only in ISC (Fig. S5D). To assess global trends in cell-restricted gene control, we applied a GSEA approach (Subramanian et al., 2005) to relate cell type-specific transcripts (Fig. 2E) to sites of open chromatin. While no set of specific genes was preferentially located near Group 2 regions (Fig. S5E), those enriched in Bmi1Gfp cells and Sec-Pro were preferentially located near Group 3 sites (Fig. 5E). Thus, these areas of selective chromatin access are authentic enhancers for Sec-specific genes, associated with distinct TFs and biologic functions.
Figure 5. Chromatin selectively open in the Sec lineage controls Sec-restricted genes.
(A) The 14,835 sites in Group 3 show discernible H3K4me1, which is weaker than signals in Group 3, but present at similar levels in Ent- and Sec-Pro. (B) ATAC- and ChIP-seq data from each cell type at a representative locus, demonstrating co-localization of open chromatin and H3K4me1 in non-promoter regions within Group3. (C) Illustrative ATAC- and RNA-seq tracks showing Ets1 mRNA and open chromatin in a 3′ intron, both restricted to the Sec cell types SP, Bmi1Gfp and CD++. (D) Location of rare FEV+ and CD69+ cells in cells near crypt tier 4 (top – stained separately) and co-expression of both markers (bottom – stained simultaneously). Scale bar, 10 μm. (E) GSEA of genes located <25 kb from Group 3 enhancers shows robust association with transcripts highly enriched in Bmi1Gfp cells and Sec-Pro (SP), relative to Lgr5+ ISC. NES, normalized enrichment score.
See also Figure S5.
Dynamic transitions of open chromatin in dedifferentiating crypt Sec cells
The nearly identical profiles of chromatin access in Lgr5+ ISC and Ent-Pro (Fig. 4D) imply a low barrier for the latter cells to replace lost Lgr5+ ISC, as occurs after γ-irradiation (Tetteh et al., 2016). However, Dll1+ Sec-Pro (van Es et al., 2012) and label-retaining cells (Buczacki et al., 2013) also can replace lost Lgr5+ ISC, and Bmi1Gfp and CD69+CD274+ cells both transition toward Lgr5+ ISC (Fig. 3). The abundance of additional enhancers (Group 3) in the Sec lineage implies that these cells must traverse this chromatin barrier when they dedifferentiate.
To witness chromatin dynamics during dedifferentiation, first we harvested Bmi1Gfp cells 24 h and 36 h after 10 Gy γ-irradiation and compared the ATAC profiles to those of Bmi1Gfp cells from unirradiated mice. Unsupervised clustering based on ATAC signals in all populations placed the post-radiation cells closer to Lgr5+ ISC than to the parental cells (Fig. 6A). Post-radiation cells showed low ATAC signal at many Group 3 regions that are unequivocally accessible in unirradiated Bmi1Gfp cells and stronger signals at many ISC-specific sites (Fig. 6B); the latter effect may be dampened by the inability to capture fully reverted cells, whose diminished Bmi1 expression would reduce GFP to sub-threshold levels. We then compared chromatin from CD69+CD274+ cells isolated before and after ablation of ISC in Lgr5Dtr mice. Again, the baseline profile of accessible chromatin shifted dramatically to that of Lgr5+ ISC, showing loss of Group 3 and gain of Group 2 enhancers (Fig. 6C–D). Although we isolated cells based on CD69 and CD274, which are restricted to Lgr5− cells (Figs. 2B and S3A), the gain of Group 2 enhancers was extensive, in agreement with activation of genes usually restricted to Lgr5+ ISC (Fig. 3D–F). Global analyses confirmed significant adaptive modulation of chromatin state during Sec cell dedifferentiation, irrespective of the cell source (Bmi1Gfp or CD69+274+) or the mode of ISC attrition (Figs. 6E and S6).
Figure 6. Dynamic modulation of chromatin access in BmiGfp and CD++ cells upon loss of Lgr5+ ISC.
(A, C) Similarity matrices (Spearman correlation coefficients) for global chromatin access (ATAC-seq) in BmiGfp cells (A) before and 24 h and 36 h after γ-irradiation, and in CD++ cells (C) 24 h after the last dose of Diphtheria toxin (DT), in relation to other crypt populations. Both post-radiation GFP+ cells and post-DT CD++ cells cluster with Lgr5+ ISC, away from their respective parental populations. (B, D) Widespread loss of ATAC signal at Group 3 sites, with commensurate relative gain at many Group 2 regions, in duplicate samples of BmiGfp cells after γ-irradiation (B) and post-DT CD++ cells (D). ATAC-seq tracks in each case illustrate losses and gains of open chromatin. (E) Relative strengths of ATAC signals averaged across all Group 2 and Group 3 enhancers upon ISC loss in BmiGfp and CD++ cells. Aggregate profiles from wild-type cells (Fig. 4E) are shown again for comparison.
See also Figure S6.
DISCUSSION
At least two mutually exclusive intestinal crypt cell types manifest stem cell behavior in vivo: cycling Lgr5+ cells (Barker et al., 2007) and quiescent Bmi1hi cells (Sangiorgi and Capecchi, 2008; Yan et al., 2012). In addition, loss of Lgr5+ ISC induces dedifferentiation of both Sec and Ent precursors (Buczacki et al., 2013; Tetteh et al., 2016; van Es et al., 2012), which probably underlies injury-dependent conversion of Bmi1-expressing TA cells into ISC (Tian et al., 2011). The basis for this exceptional plasticity among specialized crypt cells is unclear. Using open chromatin to shed light on this question, we show that ~15,000 selectively accessible genomic sites constitute a Sec lineage signature. These Group 3 enhancers open when Sec cells differentiate from Lgr5+ ISC and they rapidly relinquish chromatin access when Bmi1Gfp or wild-type CD69+CD274+ cells dedifferentiate in the face of ISC loss. The reversible ATAC profile in Sec cells reveals dynamic modulation of lineage-specific chromatin access in vivo.
The locations and strength of active histone marks H3K4me1/2 and H3K27ac are strikingly similar in Sec- and Ent-Pro (Kim et al., 2014). These enhancer marks generally coincide with sites identified in assays for open chromatin, such as DNaseI hypersensitivity or ATAC (Buenrostro et al., 2013; Mercer et al., 2013). Thus, while the scant histone marks at Sec-specific enhancers explain why they were not detected previously, the disparity between chromatin access and active histones is atypical, especially in light of their regulatory activity. One possibility is that the sites acquire canonical histone marks in terminally mature cells. However, we failed to detect H3K27ac-marked regions in villus goblet cells or CD24hi crypt EE cells, beyond those present in Sec-Pro. Alternatively, enhancers might be active without the histone modifications we tested, so long as TFs can bind, especially in short-lived post-mitotic cells where bookmarking of enhancers (Kadauke and Blobel, 2013) may be unnecessary. Many active ES cell enhancers also have open chromatin but lack H3K27ac (Pradeepa et al., 2016). A third, intriguing possibility is that weak histone marks at Group 3 enhancers account for their instability and lower the barrier for specified Sec cells to dedifferentiate.
Transcripts for FEV, FLI1, RUNX, and other Ets-family TFs are selectively expressed and abundant in Sec-Pro, Bmi1Gfp and CD69+CD274+ cells, and sequence motifs for these TFs are enriched at Group 3 enhancers. This convergence of information from chromatin and mRNA profiles implicates these TFs in Sec differentiation. The same TFs are implicated in pancreatic endocrine cell differentiation (Gross et al., 2016) and control related processes in secretory blood cells (Ciau-Uitz et al., 2013). Thus, intestinal Sec cells may have evolved by co-opting functional modules from an ancestral anti-microbial cell, and Ets-family TFs may enable the necessary lineage-restricted chromatin access.
The RNA profile of Bmi1Gfp cells, coupled with their absence in Atoh1−/− intestines, identifies them as EE, rather than cells dedicated to replenishing ISC. Several hormone mRNA levels are nearly as high as in CD24+ EE cells, further implying that they constitute a mature, pre-terminal EE population. Carcinoid tumors in humans are indolent EE malignancies often associated with clinical syndromes of hormone excess. The cells we identify with high expression of Bmi1, EE-restricted TFs, and gut hormones may represent the murine equivalent of crypt cells that give rise to carcinoid tumors. This idea matches with evidence that human carcinoid tumors arise in cells with BMI1 levels modestly higher than in surrounding crypt cells (Sei et al., 2016).
Bmi1Gfp cells in mice resemble – and may be a subset of – the label-retaining cells that differentiate predominantly into Paneth, but also into EE, cells (Buczacki et al., 2013; Li et al., 2016). Surface expression of CD69 and CD274 marks a third Sec population, which may include some Bmihi cells and LRC, but mainly contains immature goblet cells. Thus, different approaches have identified precursors for all 3 major Sec cell types: goblet, EE, and Paneth. It is likely significant that each of these precursors resides close to ISC, in crypt tiers where the Ent and Sec lineages diverge as cells exit the ISC compartment. On the one hand, chromatin profiles suggest that Ent-Pro might face a lower barrier to dedifferentiate into ISC than Sec cells. On the other hand, the magnitude of mRNA (Fig. 3) and chromatin (Fig. 6) shifts in dedifferentiating Bmi1Gfp and CD69+CD274+ cells suggests adaptation of a large fraction of cells. Taken together, the observations suggest that Ent or Sec cells closest to ISC may be best positioned to perceive ISC deficiency and dedifferentiate in response.
In summary, we show that Bmi1Gfp and CD69+CD274+ cells are pre-terminal EE and goblet cells, respectively, that revert to the ISC state when native ISC are depleted. Our findings also reveal a coherent basis for efficient conversion of lineage-specified crypt cells into ISC. The distinctive properties of Lgr5+ ISC and the Sec lineage may largely reflect differential chromatin access at Group 3 enhancers, which relinquish this access under conditions of ISC loss.
STAR METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Requests should be directed to, and will be fulfilled by, the Lead Contact, Ramesh Shivdasani (ramesh_shivdasani@dfci.harvard.edu) after execution of a suitable Materials Transfer Agreement.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mice
All mouse strains used in this study (BmiGfp, Lgr5Cre-Gfp, Lgr5Dtr-Gfp, RbpjFl/Fl;VillinCreER-T2, Atoh1Fl/Fl;VillinCreER-T2) were maintained on a mixed C57Bl/6 and 129/Sv background. Animals were housed in a Specific Pathogen-Free environment in 12-hour light/dark cycles with room temperature at 23 ± 1° C and humidity at 55 ± 15%. Food and water were available ad libitum. Animals were weaned 21 days after birth and handled and euthanized according to protocols approved by the Animal Care and Use Committee of the Dana-Farber Cancer Institute. Mice were at least 8 weeks old at the time of experimental treatments and cell isolations. Mice of both sexes were used in all experiments and littermates were used as controls.
METHOD DETAILS
Mouse treatments
BmiGfp mice received 10 Gy whole-body γ-irradiation 24 h or 36 h before euthanasia. To ablate ISC in Lgr5Dtr-Gfp mice, we administered intraperitoneal (i.p.) Diphtheria toxin (Sigma-Aldrich, 50 μg/kg) every other day and harvested intestines 24 h after the 4th dose. To enrich Ent-Pro, we injected Atoh1Fl/Fl;VillinCreER-T2 mice daily with 1 mg tamoxifen for 5 days and waited >4 weeks to eliminate Sec cells. Mice were genotyped by PCR before weaning and the genotypes of all experimental animals were confirmed by repeat PCR.
To enrich Sec-Pro and goblet cells, we treated wild-type mice twice 12 h apart with the Notch inhibitor dibenzazepine (DBZ, 48 mg/kg). About 38 h after the first dose, we harvested intestines and scraped off villi using glass slides. The crypt-enriched tissue was rotated in 5 mM EDTA (pH 8) in phosphate-buffered saline (PBS) at 4°C for 45 min. The tissue was manually shaken every 10 minutes and the EDTA solution was changed once after 30 min. Remaining villi were filtered out using 70-μm filter and crypts enriched for Sec-Pro were pelleted by centrifugation at 130g at 4° C. To isolate goblet cells, we collected villi 115 h after wild-type mice were treated with DBZ as above, by shaking intestines gently in 5 mM EDTA for 30 min at 4°C, followed by elimination of crypts by passing through 70 um filters.
For an alternative means to enrich Sec-Pro, we injected RbpjFl/Fl;VillinCreER-T2 mice 4 times with 2 mg tamoxifen over 2 days, harvested crypts on day 3, and processed the tissue as described above for DBZ-treated animals. To detect proliferating cells, we collected intestines 1 h after injecting mice i.p. with 1 mg BrdU in PBS.
Purification of intestinal epithelial cells
Cells were isolated from the proximal 1/3 small intestine. After scraping villi using a glass slide, intestines were rinsed, then rotated in 5 mM EDTA (pH 8) in PBS at 4°C for 45 min, with manual shaking every 10 min and a change of solution after 30 min. Released crypts were passed through a 70-μm strainer to eliminate villi and dissociated into single cells by rotating in 4% TrypLE solution (TermoFisher) at 37°C for 30–45 min. GFP+ cells from Lgr5Cre-Gfp, Lgr5Dtr-Gfp, and BmiGfp mice were isolated on a FACSAria II SORP flow cytometer. To isolate CD69+CD274+ cells, single-cell suspensions were labelled with BV421-conjugated CD69 Ab (BD Biosciences 562920) and APC-conjugated CD274 Ab (BD Biosciences 564715). Dead cells were eliminated using DAPI for all flow cytometry isolations. Live epithelial cells staining with FITC-conjugated EPCAM (eBiosciences, 11-5791-82) and CD69 or CD274 Ab were used to identify the signal range and to gate for FACS sorting of live CD69+CD274+ cells.
To isolate CD24hiUEAhi Paneth cells and CD24hiUEA− EE cells, crypts were dissociated by rotating in DMEM containing 0.5 U/ml Dispase (Stem Cell Technologies) at 37°C for 30 min (Wong et al., 2012). Cells were labelled with Alexa Fluor 647-conjugated CD24 antibody (Biolegend) and Atto 488-conjugated Ulex europaeus lectin (Sigma-Aldrich) at 4°C for 30 min, followed by flow cytometry.
Detection of proteins
To detect GFP+ cells, Lgr5Gfp and Bmi1Gfp mouse duodeni were fixed in 4% paraformaldehyde for 40 min, washed in PBS, and cleared by shaking in ScaleA2 solution (4 M Urea, 0.1% Triton X-100 and 10% glycerol) (Hama et al., 2011) for 45 min. Crypts were visualized using a Zeiss LSM710 laser scanning microscope, and data were analyzed using Zen2009 (Zeiss) and Fiji software (Schindelin et al., 2012). For immunohistochemistry, 5 μm wax sections of paraformaldehyde-fixed intestines were deparaffinized, rehydrated, treated with 10 mM sodium citrate buffer (pH 6) to retrieve antigens, and incubated overnight at 4°C with Ab against FEV (Santa Cruz sc-6530X, 1:200), CD69 (eBioscience 14-0691-81, 1:500) or CD274 (Bio-Rad MCA2626, 1:500), all diluted in PBS. FEV, CD69, and CD274 were detected using biotin-conjugated anti-goat, anti-hamster or anti-rat IgG (Jackson Laboratories, 1:1000). Reactions were completed using Vectastain Elite ABC Kit (Vector) and 3,3′ diaminobenzidine tetrahydrochloride (Sigma P8375, brown stain) or Vectastain ABC-AP Kit (Vector, blue stain). To co-localize markers, antigen-retrieved tissue sections were exposed to CD69 (R&D Systems AF2386, 1:500), CD274 (Bio-Rad MCA2626, 1:500), BrdU (Bio-Rad OBT0030CX), and/or GFP (Santa Cruz, SC9996) Ab in various combinations, followed by anti-goat, -rat or -mouse secondary Ab conjugated with Alexa Fluor 488, 546 or 647. Serial sections were used to identify BrdU+ replicating CD69+CD274+ cells. To localize CHGA in Bmi1Gfp mice, fixed intestines were flash-frozen in OCT compound (Tissue-Tek, 4583) and 5 μm sections were stained with CHGA Ab (Abcam ab15160, 1:100) and Cy3-conjugated anti-rabbit IgG.
RNA-seq
Lgr5+ ISC, Bmi1Gfp, EE, Paneth, goblet, and CD69+CD274+ cells were collected by FACS directly in Trizol reagent (ThermoFisher); RNA from Sec- and Ent-Pro was also purified using Trizol. RNA isolates were treated with DNAse (Qiagen) to remove contaminating DNA. Total RNA (5 to 10 ng) was used to prepare libraries with the SMART-Seq v4 Ultra Low Input RNA Kit (Clontech) following the manufacturer’s protocol.
ATAC-seq
We performed ATAC-seq (Buenrostro et al., 2013) on replicate samples of 8,000 to 35,000 cells washed twice in ice-cold PBS. Cells were resuspended in 50 μl ice-cold ATAC lysis buffer (10 mM Tris·Cl, pH 7.4, 10 mM NaCl, 3 mM MgCl2, 0.1% (v/v) Igepal CA-630) and centrifuged at 500 g at 4°C to isolate nuclear pellets that we treated in 50 μl reactions with Nextera Tn5 Transposase (Illumina, FC-121-1030) for 30 min at 37°C. Column-purified DNA (Qiagen) was stored at −20°C or amplified immediately in 50 μl r eactions with high-fidelity 2X PCR Master Mix (New England Biolabs) using a common forward primer and different reverse primers with unique barcodes for each sample. From the reaction mix, 45 μl was kept on ice after 5 cycles of PCR, while 5 μl was amplified by qPCR for 20 additional cycles; the remaining 45 μl was then amplified for the 5–7 cycles required to achieve 1/3 of the maximum qPCR fluorescence intensity. Amplified DNA was purified over columns and primer dimers (<100 bp) were removed using AMPure beads (Beckman Coulter). Size distribution of the amplified DNA was analysed using High-sensitivity Qubit dsDNA Assay Kit (ThermoFisher).
ChIP-seq
For H3K4me1 ChIP-seq, Ent-Pro and Sec-Pro crypts were fixed immediately after isolation by rotating in in 1% formaldehyde for 25 min at room temperature. Fixed crypts were lysed in buffer containing 1% SDS, 10 mM EDTA, 30 mM Tris-HCl pH 8, with protease inhibitors (Roche). Lysates were sonicated using a Covaris E210 sonicator for 50 min with 5 min on/off cycles at 4°C and debris were removed by centrifugation. The resulting chromatin was flash frozen and preserved at −80°C or used immediately for ChIP. For H3K4me2 and H3K27ac, cells were treated with 0.2 U micrococcal nuclease (Sigma, N3755) in buffer containing 50 mM Tris-HCl (pH7.6), 1 mM CaCl2, 0.2% Triton X-100, protease inhibitors (Roche), and 0.5 mM phenyl methyl sulfonyl fluoride (PMSF) at 37°C for 6 min, followed by dialysis against RIPA buffer (50 mM HEPES (pH 7.6), 1 mM EDTA, 0.7% Na deoxycholate, 1% NP-40, 0.5 M LiCl) for 3 h at 4°C. Chromatin was isolated by centrifugation and i ncubated overnight at 4°C with well-validated ChIP-grade Ab against H3K4me1 (Diagenode, C15410194), H3K4me2 (Millipore, 07-030) or H3K27Ac (Active Motif, 39135), followed by capture with magnetic beads (Dynal) that we washed 4 times in RIPA buffer and twice in 1 mM EDTA in 10 mM Tris-HCL, pH 8. Cross-links were reversed using 1% SDS and 0.1 M NaHCO3 for 6 h at 65°C. DNA was purified using a kit (Qiagen) or by isolating the mononucleosome fraction in 2% E-gels (Invitrogen). Libraries were prepared using ThruPLEX kits (Rubicon, R400427), and DNA size distribution was confirmed using High-sensitivity Qubit dsDNA Assay Kit (ThermoFisher).
All libraries (RNA-seq, ATAC-seq, and ChIP-seq) were sequenced on a NextSeq 500 instrument (Illumina) to obtain 75 bp single-end reads.
QUANTIFICATION AND STATISTICAL ANALYSIS
Given the potential variability among animals and low abundance of Bmi1Gfp and CD69+CD274+ cells, all immunohistochemistry (Fig. 5C) and immunofluorescence (Figs. 2A and 3B) studies used tissue from at least 3 independent animals (N=3). Similarly, in co-localization studies to illustrate the endocrine phenotype of Bmi1Gfp cells (Fig. 1G) we interrogated large number of cells (N=165). To ensure reproducibility of purification methods for Bmi1Gfp and CD69+CD274+ cells by flow cytometry, all experiments involving FACS occurred on at least 3 and as many as 9 individual animals (N=3 to 9, Figs. 2A, 2B, 2D, S1G, S2B, S3B). For consistency in analysis and representation, FACS data from 50,000 cells were analysed in every experiment using FlowJo v10 software (FlowJo LLC).
To confirm cell purity achieved in our isolation approaches, and to exclude potential biases introduced by RNA-seq library preparation, sequencing and data processing, we conducted semi-quantitative RT-PCR analysis on mRNAs from three independent animal samples (N=3) using primers for cell-specific markers (Fig. S2B) and confirmed relative enrichment of known lineage markers. Similarly, we confirmed expression changes revealed by RNA-seq in Bmi1Gfp and CD69+CD274+ cells upon Lgr5+ ISC ablation by RT-PCR analysis of RNA samples from at least three independent (N=3) animals, using whole crypts (Figs. 3B and S3E) or ISC (Figs. 3G and S3F) to determine basal expression.
Computational analyses
Raw reads from mRNA-, ChIP-, and ATAC-seq were aligned to the mouse genome (Mm10, Genome Reference Consortium GRCm38) using TopHat v2.0.6 (Trapnell et al., 2012) or Bowtie2 (Langmead and Salzberg, 2012). For RNA-seq, transcript levels were expressed as read counts using HTSeq (Anders et al., 2015). Data were normalized and sample variability assessed by principal component analysis in DEseq2 (Love et al., 2014). Differential expression was defined using the indicated fold-changes and false-discovery rate (FDR) 0.05 using DEseq2. We used GENE-E software (Broad Institute) to generate heatmaps of RNA levels, averaged from replicate samples in reads per kb of transcript per 1M mapped reads (RPKM).
For ATAC- and ChIP-seq, aligned signals in raw (bam) files were filtered to remove PCR duplicates and reads that aligned to multiple locations; the remainder is listed in Table S1. Peaks were identified using MACS v1.4 (Zhang et al., 2008) with the p-value cut-off 10−5. ATAC peaks from Lgr5+ ISC, Sec-Pro, Ent-Pro, Bmi1Gfp, and CD69+CD274+ cells were pooled and the 50,347 unique peaks were used in sample correlations (Figs. 4C, S4B, 6A–B). Promoters (regions <1 kb from TSSs) were discarded, leaving 41,167 sites as candidate enhancers. Raw signals from individual samples or merged, highly correlated replicates (Fig. S4B) from a given cell type were converted to signal files (bigWig) using DeepTools v2.1.0 (Ramirez et al., 2014). To compare open chromatin across cell types, signals were quantile-normalized using Haystack (Pinello et al., 2014), with 50-bp windows. Normalized signals ±1.5 kb from centre of ATAC peaks were used for unsupervised k-means clustering in DeepTools v2.1.0. The optimum number of clusters was determined by gap statistics (Fig. S4C) and the 8 resulting clusters were merged, according to similarity of signal patterns, into 3 distinct groups (Fig. S4D).
To determine correlations among multiple ATAC-seq samples, read counts from all samples under consideration were summarized over pooled ATAC peaks into a matrix using DeepTools. Samples were clustered using hierarchical clustering and heatmap scales reflect the full range of sample-to-sample Spearman correlation coefficients (Figs. 4C, 6A, 6C, and S6). Enhancer ATAC-seq signals were determined using 50 bp bins over ±1.5 kb or ±3.5 kb from the center of the regions and plotted as heatmaps using DeepTools in descending order of the mean signal over ±1.5 kb, as determined for each cluster in Fig. 4D. The same order of sites was maintained for plotting regions from Groups 1, 2 and 3 in Figs. 5A, 6B, 6D, S4D, and S5C. The same signal values were used to generate aggregate profile plots in DeepTools.
Ontologies associated with genes located within 25 kb of ATAC regions were identified using GREAT analysis v3.0 (McLean et al., 2010). To identify biological processes enriched in cell populations, we applied Gene Set Enrichment Analysis (GSEA) (Subramanian et al., 2005) to curated Biocarta, Kegg, and chemical and genetic perturbation sets from the molecular signature database (MSigDB), using default parameters. Genes within 25 kb of enhancers in Groups 2 and 3 were identified using Bedtools (Quinlan and Hall, 2010). Enriched expression of these gene sets was determined in different populations using the GSEA approach (Subramanian et al., 2005); significance of the enrichment scores (FDR) was determined using 1,000 permutations of random gene sets of similar size. Similarly, 500 transcripts with the highest enrichment in Bmi1Gfp or CD69+CD274+ cells were analyzed to determine specificity for Ent- or Sec-Pro and EE, Paneth or goblet cells (Figs. 1F and 2D). Loci were visualized using the Integrated Genomics Viewer v2.3 (Robinson et al., 2011). Motifs enriched in ATAC regions were identified using the HOMER de novo algorithm v4.7.2 (Heinz et al., 2010) based on the cumulative binomial distribution.
Modeling of lineage trajectories
To define cell relationships, we constructed a mathematical model based on the simplifying assumption of monotonic changes, i.e., directions of change in mRNA expression are consistent from mother to daughter cells along a trajectory. For any trajectory A→B→C, where A, B and C represent different cell populations, we defined a coherence score based on the direction of change of differentially expressed (q<0.05) genes. For any gene g ∈ N = diff (A, B)∩diff (B, C)∩diff (A, C), where diff denotes the gene set differentially expressed in two cell types, the coherence score δg = +1 if transcript levels increase or decrease consistently along the trajectory, and as δg = −1 otherwise. The weighted sum of δg’s, where each gene is weighted by the magnitude of log fold-change | |, then gives an aggregated coherence . High positive scores for a trajectory indicate consistency of monotonic gene expression, and negative scores reflect opposing changes in many genes, along the trajectory, e.g., the ISC→EP→Enterocyte and ISC→SP→Goblet cells trajectories gave the expected high positive scores (Fig. S1F).
DATA AND SOFTWARE AVAILABILITY
Data from the study (Table S1) are deposited in the Gene Expression Omnibus (GSE83394).
Supplementary Material
Acknowledgments
Supported by the Intestinal Stem Cell Consortium (grant U01DK103152) of the NIDDK and NIAID; NIH awards R01DK081113 (RAS and G-CY), F32DK103453 (UJ), and P50CA127003; and gifts from the Neuroendocrine Tumor Research Foundation and Pan-Mass Challenge. We thank F. de Sauvage, T. Honjo, and S. Robine for generously sharing Lgr5Dtr-Gfp, RbpjFl, and Villin-CreER-T2 mice; and Myles Brown, Manasvi Shah, Calvin Kuo, and David Breault for valuable discussions. The authors declare no conflicts of interest.
Footnotes
Author contributions
UJ and RAS designed the study; UJ, MS, ZH and KM acquired data; UJ, NKO, AS and G-CY performed computational analyses; RAS interpreted data and supervised the study; UJ and RAS wrote the paper, with input from all authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anders S, Pyl PT, Huber W. HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31:166–169. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol. 2014;15:19–33. doi: 10.1038/nrm3721. [DOI] [PubMed] [Google Scholar]
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- Buczacki SJ, Zecchini HI, Nicholson AM, Russell R, Vermeulen L, Kemp R, Winton DJ. Intestinal label-retaining cells are secretory precursors expressing Lgr5. Nature. 2013;495:65–69. doi: 10.1038/nature11965. [DOI] [PubMed] [Google Scholar]
- Buenrostro JD, Giresi PG, Zaba LC, Chang HY, Greenleaf WJ. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat Methods. 2013;10:1213–1218. doi: 10.1038/nmeth.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciau-Uitz A, Wang L, Patient R, Liu F. ETS transcription factors in hematopoietic stem cell development. Blood Cells Mol Dis. 2013;51:248–255. doi: 10.1016/j.bcmd.2013.07.010. [DOI] [PubMed] [Google Scholar]
- el Marjou F, Janssen KP, Chang BH, Li M, Hindie V, Chan L, Louvard D, Chambon P, Metzger D, Robine S. Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis. 2004;39:186–193. doi: 10.1002/gene.20042. [DOI] [PubMed] [Google Scholar]
- Gross S, Garofalo DC, Balderes DA, Mastracci TL, Dias JM, Perlmann T, Ericson J, Sussel L. The novel enterochromaffin marker Lmx1a regulates serotonin biosynthesis in enteroendocrine cell lineages downstream of Nkx2.2. Development. 2016;143:2616–2628. doi: 10.1242/dev.130682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama H, Kurokawa H, Kawano H, Ando R, Shimogori T, Noda H, Fukami K, Sakaue-Sawano A, Miyawaki A. Scale: a chemical approach for fluorescence imaging and reconstruction of transparent mouse brain. Nat Neurosci. 2011;14:1481–1488. doi: 10.1038/nn.2928. [DOI] [PubMed] [Google Scholar]
- Han H, Tanigaki K, Yamamoto N, Kuroda K, Yoshimoto M, Nakahata T, Ikuta K, Honjo T. Inducible gene knockout of transcription factor recombination signal binding protein-J reveals its essential role in T versus B lineage decision. Int Immunol. 2002;14:637–645. doi: 10.1093/intimm/dxf030. [DOI] [PubMed] [Google Scholar]
- Heintzman ND, Hon GC, Hawkins RD, Kheradpour P, Stark A, Harp LF, Ye Z, Lee LK, Stuart RK, Ching CW, et al. Histone modifications at human enhancers reflect global cell-type-specific gene expression. Nature. 2009;459:108–112. doi: 10.1038/nature07829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P, Cheng JX, Murre C, Singh H, Glass CK. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell. 2010;38:576–589. doi: 10.1016/j.molcel.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosen N, Yamane T, Muijtjens M, Pham K, Clarke MF, Weissman IL. Bmi-1-green fluorescent protein-knock-in mice reveal the dynamic regulation of bmi-1 expression in normal and leukemic hematopoietic cells. Stem Cells. 2007;25:1635–1644. doi: 10.1634/stemcells.2006-0229. [DOI] [PubMed] [Google Scholar]
- Itzkovitz S, Lyubimova A, Blat IC, Maynard M, van Es J, Lees J, Jacks T, Clevers H, van Oudenaarden A. Single-molecule transcript counting of stem-cell markers in the mouse intestine. Nat Cell Biol. 2012;14:106–114. doi: 10.1038/ncb2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadauke S, Blobel GA. Mitotic bookmarking by transcription factors. Epigenetics Chromatin. 2013;6:6. doi: 10.1186/1756-8935-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TH, Li F, Ferreiro-Neira I, Ho LL, Luyten A, Nalapareddy K, Long H, Verzi M, Shivdasani RA. Broadly permissive intestinal chromatin underlies lateral inhibition and cell plasticity. Nature. 2014;506:511–515. doi: 10.1038/nature12903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara-Astiaso D, Weiner A, Lorenzo-Vivas E, Zaretsky I, Jaitin DA, David E, Keren-Shaul H, Mildner A, Winter D, Jung S, et al. Chromatin state dynamics during blood formation. Science. 2014;345:943–949. doi: 10.1126/science.1256271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HJ, Kapoor A, Giel-Moloney M, Rindi G, Leiter AB. Notch signaling differentially regulates the cell fate of early endocrine precursor cells and their maturing descendants in the mouse pancreas and intestine. Dev Biol. 2012;371:156–169. doi: 10.1016/j.ydbio.2012.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Nakauka-Ddamba A, Tobias J, Jensen ST, Lengner CJ. Mouse Label-Retaining Cells Are Molecularly and Functionally Distinct From Reserve Intestinal Stem Cells. Gastroenterology. 2016;151:298–310. e297. doi: 10.1053/j.gastro.2016.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Yousefi M, Nakauka-Ddamba A, Jain R, Tobias J, Epstein JA, Jensen ST, Lengner CJ. Single-cell analysis of proxy reporter allele-marked epithelial cells establishes intestinal stem cell hierarchy. Stem Cell Reports. 2014;3:876–891. doi: 10.1016/j.stemcr.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean CY, Bristor D, Hiller M, Clarke SL, Schaar BT, Lowe CB, Wenger AM, Bejerano G. GREAT improves functional interpretation of cis-regulatory regions. Nat Biotechnol. 2010;28:495–501. doi: 10.1038/nbt.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer TR, Edwards SL, Clark MB, Neph SJ, Wang H, Stergachis AB, John S, Sandstrom R, Li G, Sandhu KS, et al. DNase I-hypersensitive exons colocalize with promoters and distal regulatory elements. Nat Genet. 2013;45:852–859. doi: 10.1038/ng.2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe C, Kljavin NM, Ybarra R, de Sauvage FJ. Lgr5+ stem cells are indispensable for radiation-induced intestinal regeneration. Cell Stem Cell. 2014;14:149–159. doi: 10.1016/j.stem.2013.11.008. [DOI] [PubMed] [Google Scholar]
- Munoz J, Stange DE, Schepers AG, van de Wetering M, Koo BK, Itzkovitz S, Volckmann R, Kung KS, Koster J, Radulescu S, et al. The Lgr5 intestinal stem cell signature: robust expression of proposed quiescent ‘+4’ cell markers. EMBO J. 2012;31:3079–3091. doi: 10.1038/emboj.2012.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelaseyed T, Bergstrom JH, Gustafsson JK, Ermund A, Birchenough GM, Schutte A, van der Post S, Svensson F, Rodriguez-Pineiro AM, Nystrom EE, et al. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol Rev. 2014;260:8–20. doi: 10.1111/imr.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinello L, Xu J, Orkin SH, Yuan GC. Analysis of chromatin-state plasticity identifies cell-type-specific regulators of H3K27me3 patterns. Proc Natl Acad Sci USA. 2014;111:E344–353. doi: 10.1073/pnas.1322570111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten CS. Stem cells in gastrointestinal epithelium: numbers, characteristics and death. Philos Trans R Soc Lond B Biol Sci. 1998;353:821–830. doi: 10.1098/rstb.1998.0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradeepa MM, Grimes GR, Kumar Y, Olley G, Taylor GC, Schneider R, Bickmore WA. Histone H3 globular domain acetylation identifies a new class of enhancers. Nat Genet. 2016;48:681–686. doi: 10.1038/ng.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan AR, Hall IM. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics. 2010;26:841–842. doi: 10.1093/bioinformatics/btq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez F, Dundar F, Diehl S, Gruning BA, Manke T. deepTools: a flexible platform for exploring deep-sequencing data. Nucleic Acids Res. 2014;42:W187–191. doi: 10.1093/nar/gku365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond CA, Shah MS, Carlone DL, Breault DT. An enduring role for quiescent stem cells. Dev Dyn. 2016;245:718–726. doi: 10.1002/dvdy.24416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JT, Thorvaldsdottir H, Winckler W, Guttman M, Lander ES, Getz G, Mesirov JP. Integrative genomics viewer. Nat Biotechnol. 2011;29:24–26. doi: 10.1038/nbt.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Roman AK, Tovaglieri A, Breault DT, Shivdasani RA. Distinct Processes and Transcriptional Targets Underlie CDX2 Requirements in Intestinal Stem Cells and Differentiated Villus Cells. Stem Cell Reports. 2015;5:673–681. doi: 10.1016/j.stemcr.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet. 2008;40:915–920. doi: 10.1038/ng.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwitalla S, Fingerle AA, Cammareri P, Nebelsiek T, Goktuna SI, Ziegler PK, Canli O, Heijmans J, Huels DJ, Moreaux G, et al. Intestinal tumorigenesis initiated by dedifferentiation and acquisition of stem-cell-like properties. Cell. 2013;152:25–38. doi: 10.1016/j.cell.2012.12.012. [DOI] [PubMed] [Google Scholar]
- Sei Y, Feng J, Zhao X, Forbes J, Tang D, Nagashima K, Hanson J, Quezado MM, Hughes MS, Wank SA. Polyclonal Crypt Genesis and Development of Familial Small Intestinal Neuroendocrine Tumors. Gastroenterology. 2016;151:140–151. doi: 10.1053/j.gastro.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlyueva D, Stampfel G, Stark A. Transcriptional enhancers: from properties to genome-wide predictions. Nat Rev Genet. 2014;15:272–286. doi: 10.1038/nrg3682. [DOI] [PubMed] [Google Scholar]
- Shroyer NF, Helmrath MA, Wang VY, Antalffy B, Henning SJ, Zoghbi HY. Intestine-specific ablation of mouse atonal homolog 1 (Math1) reveals a role in cellular homeostasis. Gastroenterology. 2007;132:2478–2488. doi: 10.1053/j.gastro.2007.03.047. [DOI] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda N, Jain R, LeBoeuf MR, Wang Q, Lu MM, Epstein JA. Interconversion between intestinal stem cell populations in distinct niches. Science. 2011;334:1420–1424. doi: 10.1126/science.1213214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetteh PW, Basak O, Farin HF, Wiebrands K, Kretzschmar K, Begthel H, van den Born M, Korving J, de Sauvage F, van Es JH, et al. Replacement of Lost Lgr5-Positive Stem Cells through Plasticity of Their Enterocyte-Lineage Daughters. Cell Stem Cell. 2016;18:203–213. doi: 10.1016/j.stem.2016.01.001. [DOI] [PubMed] [Google Scholar]
- Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD, de Sauvage FJ. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011;478:255–259. doi: 10.1038/nature10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc. 2012;7:562–578. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Es JH, Sato T, van de Wetering M, Lyubimova A, Nee AN, Gregorieff A, Sasaki N, Zeinstra L, van den Born M, Korving J, et al. Dll1+ secretory progenitor cells revert to stem cells upon crypt damage. Nat Cell Biol. 2012;14:1099–1104. doi: 10.1038/ncb2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, Begthel H, Cozijnsen M, Robine S, Winton DJ, Radtke F, et al. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–963. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- Verzi MP, Shin H, He HH, Sulahian R, Meyer CA, Montgomery RK, Fleet JC, Brown M, Liu XS, Shivdasani RA. Differentiation-specific histone modifications reveal dynamic chromatin interactions and partners for the intestinal transcription factor CDX2. Dev Cell. 2010;19:713–726. doi: 10.1016/j.devcel.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphalen CB, Asfaha S, Hayakawa Y, Takemoto Y, Lukin DJ, Nuber AH, Brandtner A, Setlik W, Remotti H, Muley A, et al. Long-lived intestinal tuft cells serve as colon cancer-initiating cells. J Clin Invest. 2014;124:1283–1295. doi: 10.1172/JCI73434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong VW, Stange DE, Page ME, Buczacki S, Wabik A, Itami S, van de Wetering M, Poulsom R, Wright NA, Trotter MW, et al. Lrig1 controls intestinal stem-cell homeostasis by negative regulation of ErbB signalling. Nat Cell Biol. 2012;14:401–408. doi: 10.1038/ncb2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan KS, Chia LA, Li X, Ootani A, Su J, Lee JY, Su N, Luo Y, Heilshorn SC, Amieva MR, et al. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc Natl Acad Sci USA. 2012;109:466–471. doi: 10.1073/pnas.1118857109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Bermingham NA, Finegold MJ, Zoghbi HY. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science. 2001;294:2155–2158. doi: 10.1126/science.1065718. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, Nusbaum C, Myers RM, Brown M, Li W, et al. Model-based analysis of ChIP-Seq (MACS) Genome Biol. 2008;9:R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Chen Z, Jiang Z. Expression, Distribution and Role of Aquaporin Water Channels in Human and Animal Stomach and Intestines. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17091399. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.