Abstract
Theoretical models of binge eating and eating disorders include both transdiagnostic and eating disorder-specific risk factors. Negative urgency (i.e., the tendency to act impulsively when distressed) is a critical transdiagnostic risk factor for binge eating, but limited research has examined interactions between negative urgency and disorder-specific variables. Investigating these interactions can help identify the circumstances under which negative urgency is most strongly associated with binge eating. We examined whether prominent risk factors (i.e., appearance pressures, thin-ideal internalization, body dissatisfaction, dietary restraint) specified in well-established etiologic models of eating disorders moderate negative urgency-binge eating associations. Further, we investigated whether phenotypic moderation effects were due to genetic and/or environmental associations between negative urgency and binge eating. Participants were 988 female twins aged 11–25 years from the Michigan State University Twin Registry. Appearance pressures, thin-ideal internalization, and body dissatisfaction, but not dietary restraint, significantly moderated negative urgency-binge eating associations, with high levels of these risk factors and high negative urgency associated with the greatest binge eating. Twin moderation models revealed that genetic, but not environmental, sharing between negative urgency and binge eating was enhanced at higher levels of these eating disorder-specific variables. Future longitudinal research should investigate whether eating disorder risk factors shape genetic influences on negative urgency into manifesting as binge eating.
Keywords: binge eating, negative urgency, transdiagnostic, moderation, twin study
Etiologic and maintenance models of binge eating and associated eating disorders (EDs) specify a role for both transdiagnostic and ED-specific risk factors. For example, the dual-pathway model of bulimic symptom development includes key transdiagnostic (i.e., negative affectivity) and ED-specific (i.e., dietary restraint) proximal risk factors that may act independently or in concert to increase binge eating (Stice, 2001). Similarly, the cognitive-behavioral model of bulimic symptom maintenance has recently been extended to include not only ED-specific factors like weight and shape concerns, but also the personality trait of impulsivity (Schnitlzer, von Ranson, & Wallace, 2012). Models that include both transdiagnostic and disorder-specific risk factors are critical for elucidating interactive pathways that lead to the emergence and continuance of ED symptoms.
Among transdiagnostic risk factors for binge eating, negative urgency has garnered considerable attention. Negative urgency is defined as the tendency to act impulsively in response to negative emotions (Whiteside & Lynam, 2001). Negative urgency-binge eating associations have been detected in both at-risk samples and in individuals with clinical pathology (Fischer, Settles, Collins, Gunn, & Smith, 2012; Racine et al., 2015). Further, negative urgency prospectively predicted increases in binge eating in college females (Fischer, Peterson, & McCarthy, 2013) and early adolescent girls (Pearson, Combs, Zapolski, & Smith, 2012). Importantly, negative urgency relates to binge eating over and above general negative affect (Racine et al., 2013), with negative urgency and negative affect representing independent risk pathways for binge eating onset (Pearson, Zapolski, & Smith, 2015).
Despite the well-replicated association between negative urgency and binge eating, few studies have investigated whether negative urgency interacts with ED-specific risk factors to lead to binge eating. Identifying factors that strengthen (i.e., moderate) negative urgency-binge eating associations is critical for understanding the circumstances under which negative urgency may be most likely to predict binge eating. This is particularly important given that negative urgency is associated with numerous forms of psychopathology (e.g., alcohol/substance use, depression; Fischer et al., 2012; Smith, Guller, & Zapolski, 2013), suggesting that not all individuals with negative urgency will develop binge eating. Investigating interactions among ED-specific risk factors and negative urgency can increase the specificity of etiologic models and may have prevention and intervention implications. For example, prevention programs that aim to reduce negative urgency (e.g., through mindfulness instruction) could be supplemented with modules that target disorder-specific moderators of negative urgency-binge eating associations.
Prominent disorder-specific risk factors featured in well-established etiologic models of eating disorders (e.g., Tripartite Model, Restraint Theory; Dual-Pathway model; Polivy & Herman, 1985; Stice, 2001; Thompson, Heinberg, Altabe, & Tantleff-Dunn, 1999) include appearance pressures, thin-ideal internalization, body dissatisfaction, and dietary restraint. Indeed, recent reviews have highlighted the significance of these risk factors for predicting disordered eating symptoms, including binge eating, and eating disorder diagnoses (Culbert, Racine, & Klump, 2015; Pearson, Wonderlich, & Smith, 2015; Stice, 2016). In a 2-year longitudinal study of adolescent girls, all four of these risk factors predicted binge eating onset (Stice, Presnell, & Spangler, 2002). Further, findings from a large 5-year prospective study of diverse adolescents suggested that both body dissatisfaction and dieting predict later binge eating (Neumark-Sztainer et al., 2006; Neumark-Sztainer, Paxton, Hannan, Haines, & Story, 2006).
Given that both negative urgency and these ED-specific variables are robustly related to binge eating, we may expect binge eating symptoms to be more prevalent in individuals with high levels of both sets of risk factors. Specifically, the combination of high negative urgency and ED-specific risk factors may be associated with the greatest likelihood of impulsive binge eating when experiencing a negative mood. According to Pearson and colleagues (2015), negative urgency is thought to be associated with weaker self-control capacities because, over time, these individuals learn to use impulsive behavior when distressed. Individuals with ED risk factors may have additional difficulty engaging in self-regulation as a result of repetitive negative thoughts about one’s body and/or efforts to maintain one’s diet. Thus, individuals with high negative urgency and high ED risk factors may be most likely to binge eat when upset because of a focus on short-term (i.e., alleviating distress) versus long-term (i.e., weight loss) goals.
Although two prior studies investigated negative urgency-dietary restraint interactions (Emery, King, Fischer, & Davis, 2013; Wenzel, Weinstock, Vander Wal, & Weaver, 2014), only one study to date has considered interactions among negative urgency and several disorder-specific risk factors in the prediction of binge eating-related phenotypes (Racine & Martin, 2016). In a sample of college women, interactions among negative urgency and appearance pressures, thin-ideal internalization, body dissatisfaction, and dietary restraint were uniformly significant in predicting a composite measure of dysregulated eating. Moreover, these interactive effects were specific to dysregulated eating, in that interactions were not significantly associated with depressive symptoms or problematic alcohol use. These findings suggest that individuals with high negative urgency may be most likely to develop binge eating (versus another form of psychopathology) if they have high levels of one or more ED-specific risk factors (Racine & Martin, 2016). However, these findings need to be replicated, as this is the only study that has considered multiple ED-specific risk factors as moderators of negative urgency-binge eating associations.
More importantly, testing interactions solely at the phenotypic level of analysis limits our understanding of the mechanisms that may underlie interactive relationships. Both additive genetic factors (i.e., genetic effects that add across genes) and non-shared environmental factors (i.e., factors that make members of a twin pair different from one another, e.g., different peer groups) contribute to individual differences in negative urgency and binge eating (Bulik, Sullivan, & Kendler, 1998; Klump, McGue, & Iacono, 2002; Racine et al., 2013). Moreover, negative urgency and binge eating share a significant proportion of their genetic and non-shared environmental risk factors; a recent study reported that the genetic and environmental influences on negative urgency and binge eating were correlated at magnitudes of .77 and .29, respectively (Racine et al., 2013). If ED risk factors moderate negative urgency-binge eating associations, it is important to understand whether phenotypic moderation occurs due to differences in genetic and/or environmental associations. In other words, the proportion of shared genetic and/or environmental risk factors for negative urgency and binge eating may vary depending on one’s level of the disorder-specific moderator.
One possibility is that ED-specific risk factors enhance genetic associations between negative urgency and binge eating, such that the magnitude of shared genetic influences between negative urgency and binge eating is greater at higher levels of the ED-specific risk factor. In other words, in the presence of ED-specific variables, individual differences in binge eating may be more likely to be explained by genetic risk factors that also influence negative urgency, rather than genetic risk factors for binge eating that relate to other mechanisms (e.g., obesity; Bulik, Sullivan, & Kendler, 2003). This pattern of moderation may reflect the fact that ED risk factors enhance a genetically mediated tendency towards rash action in the face of negative affect that then leads to binge eating. If confirmed, these findings would point to a biologically-based mechanism by which ED variables increase risk for binge eating.
Another possibility is that ED-specific risk factors enhance non-shared environmental associations between negative urgency and binge eating, such that non-shared environmental influences on negative urgency and binge eating are shared to a larger degree at higher levels of ED variables. For example, environmental contexts that could theoretically lead to both negative urgency and binge eating (e.g., abusive or traumatic experiences, poor parental modeling, deviant peer groups; Arens, Gaher, Simons, 2012; Zhu et al., 2016) may be more likely to influence binge eating in the presence of ED-specific risk factors. Investigating whether ED-specific risk factors moderate the relative contribution of genetic/environmental factors to negative urgency-binge eating associations can significantly enhance current biopsychosocial theories of risk, as this research will provide insight into the mechanisms underlying interactions among transdiagnostic and disorder-specific risk factors for binge eating. Further, this research represents a first step in the development of targeted prevention and intervention programs that aim to avert risk processes in individuals most vulnerable to binge eating. Specifically, if we can identify the precise combination of genetic, environmental, personality, and disorder-specific risk factors, we may be able to enhance current programs to modify risk factors across multiple levels of analysis and to “match” treatments to individual profiles of risk.
The aim of the current study was to examine whether appearance pressures, thin-ideal internalization, body dissatisfaction, and dietary restraint moderate phenotypic and etiologic (i.e., genetic and environmental) associations between negative urgency and binge eating in adolescent and young adult female twins. We hypothesized that binge eating symptoms would be greatest in individuals who endorsed high levels of negative urgency and who reported ED risk factors. Further, given the relative predominance of genetic influences in explaining the negative urgency-binge eating relationship (Racine et al., 2013), we predicted that negative urgency and binge eating would share a greater proportion of their genetic risk factors in individuals who endorsed appearance pressures, thin-ideal internalization, body dissatisfaction, and/or dietary restraint. In other words, we expected that these ED risk factors would impact the phenotypic negative urgency-binge eating relationship through an increase in shared genetic variance.
Methods
Participants
Participants were 988 female twins (544 monozygotic [MZ]; 444 dizygotic [DZ]) between the ages of 11–25 years (M (SD) = 16.88 (2.60)) from the Michigan State University Twin Registry (MSUTR).1 The MSUTR is a population-based twin registry that recruits twins born in Michigan using birth record methods described previously (Burt & Klump, 2013). Both MSUTR twins (Burt & Klump, 2013) and participants in the current study (80% Caucasian; 13% African American, 6% Multiracial, <1% Asian; <1% American Indian/Alaskan Native) are demographically representative of the recruitment region.
Participants were drawn from one of two MSUTR projects: the Twin Study of Hormones and Behavior across the Menstrual Cycle (HBMC study; female twins ages 16–25) and the Twin Study of Mood, Behavior, and Hormones during Puberty (MBHP study; female twins ages 9–15). The HMBC and MBHP studies were designed to investigate phenotypic and etiologic effects of ovarian hormones on disordered eating. Thus, several inclusion/exclusion criteria were necessary to capture natural hormonal variation, including no psychotropic or steroid medication use in past 4 weeks, no pregnancy or lactation in past 6 months, and no history of genetic/medical conditions known to influence hormone functioning or appetite/weight. Regular menstruation for the past 6 months also was an inclusion criterion for HBMC participants. The majority of our sample (94%) was required to be free from hormonal contraceptive use over the past 3 months, although a subset of HBMC participants (n = 60) were drawn from a related pilot study that recruited for current contraceptive use. As reported previously (Racine et al., 2013), these inclusion/exclusion criteria do not significantly impact general impulsivity or binge eating scores. Given known increases in genetic influences on disordered eating during puberty in females (Klump, Perkins, Burt, McGue, & Iacono, 2007), MBHP twins were required to be in mid-puberty or beyond for the current study (score > 2.5 on the Pubertal Development Scale; Petersen, Crockett, Richards, & Boxer, 1988).
Measures
Zygosity
A physical similarity questionnaire was used as the primary determinant of zygosity (Peeters, Van Gestel, Vlietinck, Derom, & Derom, 1998). This questionnaire has demonstrated over 95% accuracy when compared with genotyping (Lykken, Bouchard, McGue, & Tellegen, 1990). Physical similarity questionnaires were completed by research assistants, the twins’ parent (usually mother), and the twins (HBMC study only). When results across raters were discrepant, questionnaire responses, twin photographs, and DNA (i.e., twin concordance across several single-nucleotide polymorphisms) were examined by the principal investigator (KLK) and graduate students to determine final zygosity.
The UPPS-P Impulsive Behavior Scale
The adult (Lynam, Smith, Whiteside, & Cyders, 2006) and child (Zapolski, Stairs, Settles, Combs, & Smith, 2010) versions of the UPPS-P Impulsive Behavior Scale were used to assess negative urgency in the HBMC and MBCP studies, respectively. The adult and child Negative Urgency subscales contain 12 and 8 items, respectively (e.g., “When I am upset, I often act without thinking”), that are rated on a 4-point scale. Internal consistency estimates for these subscales were high in the current study (adult α = .86 ; child α = .88) and in previous work (Smith et al., 2007; Zapolski et al., 2010). Adequate test-retest reliability has been reported in college students over 1 month (r = .73; Anestis, Selby, & Joiner, 2007) and middle school students over 6 months-1 year (rs = .53–.66; Pearson et al., 2012). Finally, convergent and discriminant validity have been established; negative urgency scores measured via self-report and interview assessments are highly correlated, and negative urgency exhibits lower correlations with other UPPS-P impulsive traits (Smith et al., 2007; Zapolski et al., 2010).
Minnesota Eating Behaviors Survey (MEBS; von Ranson, Klump, Iacono, & McGue, 2005).2
The MEBS is a 30-item true/false questionnaire that was developed to assess disordered eating symptoms in children as young as 10 years and was used to measure binge eating and body dissatisfaction. The Binge Eating subscale includes 7 items that assess thoughts of binge eating (e.g., “I think a lot about overeating (eating a really large amount of food)”) and engagement in binge eating behaviors (e.g., “Sometimes I eat lots and lots of food and feel like I can’t stop”). The Body Dissatisfaction subscale is comprised of 6 items that assess discontent with body shape and size (e.g., “My stomach is too big”). Internal consistency estimates for the Binge Eating and Body Dissatisfaction subscales were adequate-to-good in the current study (αs = .70 and .82, respectively) and in previous work (von Ranson et al., 2005). Concurrent validity of the MEBS Binge Eating and Body Dissatisfaction subscales has been established through correlations with similar scales on established ED measures (von Ranson et al. 2005). Finally, women with BN or any ED have higher scores on the Binge Eating and Body Dissatisfaction subscales, respectively, compared to unaffected control women (von Ranson et al., 2005).
Notably, the current study employed a continuous binge eating measure that assesses binge eating tendencies, as other methods (i.e., objective binge eating episode [OBE] frequency counts) lack the necessary variance and power for twin modeling in a community sample. Fortunately, our prior work in a sample that partially overlaps with the current sample strongly supported the criterion validity of the MEBS Binge Eating scale for assessing OBEs. Specifically, twins who endorsed OBEs on a structured interview scored significantly higher on the MEBS Binge Eating scale than twins who did not report OBEs, twins who reported objective overeating without loss of control, and twins who reported loss of control without objective overeating (Racine et al., 2013). Thus, the MEBS Binge Eating subscale not only distinguishes the presence versus absence of binge eating, but also differentiates among individuals who have one versus both of the core behavioral components of binge eating.
(Youth) Eating Disorder Examination-Questionnaire ((Y)EDE-Q; Fairburn & Beglin, 1994; Goldschmidt, Doyle, & Wilfley, 2007)
The EDE-Q and YEDE-Q are self-report questionnaires that were adapted from the Eating Disorder Examination and Child Eating Disorder Examination (EDE), respectively. The 5-item Restraint subscale of the EDE-Q/YEDE-Q was used to assess dietary restraint, defined as trying to limit food intake, exclude certain foods, and obey rigid dieting rules (e.g., “Have you tried to avoid (not to eat) any foods which you like in order to influence (change) your shape or weight?”). Restraint items are rated on a 7-point scale from 0 (no days) to 6 (every day) based on the past 28 days. The EDE-Q Restraint scale demonstrates acceptable psychometric properties in clinical and non-clinical samples (Berg, Peterson, Frazier, & Crow, 2012). Internal consistency for the Restraint subscale was excellent in our sample (α = .81). Correlations between the EDE-Q and EDE Restraint scales suggest good convergent validity across questionnaire and interview methods (r = .71; Mond, Hay, Rodgers, Owen, & Beumont, 2004). Finally, criterion validity of the EDE-Q Restraint subscale is established, as women with EDs score significantly higher than healthy controls (Berg et al., 2012).
The Sociocultural Attitudes toward Appearance Questionnaire-3 (SATAQ-3; Thompson, van den Berg, Roehrig, Guarda, & Heinberg, 2004)
The SATAQ-3 was used to assess appearance pressures and thin-ideal internalization. The 7-item Appearance Pressures subscale assesses the extent to which participants endorse feeling pressure from media sources to engage in behavior that may move them toward appearance ideals (e.g., “I’ve felt pressure from TV and magazines to be thin”). The 9-item General Internalization subscale captures the extent to which participants want to look like individuals from various media sources (e.g., “I would like my body to look like the models who appear in magazines”). The construct assessed by the General Internalization subscale is most often referred to as “thin-ideal internalization” (e.g., Fitzsimmons-Craft et al., 2012; Myers & Crowther, 2007; Suisman et al., 2012), but also has been referred to as “media internalization” in some studies (e.g., Rodgers, McLean, & Paxton, 2015). SATAQ items are rated on a 5-point scale from 1 (definitely disagree) to 5 (definitely agree). Both subscales demonstrated excellent internal consistency in the current sample (pressures: α = .91; internalization: α = .91) and in prior samples (a’s >.90; Thompson et al., 2004). Finally, individuals with EDs obtain higher scores on these scales compared to controls (Thompson et al., 2004).
Covariates
Given correlations between our predictor variables and both age and body mass index (BMI; see Table 1), age and BMI were included as covariates in phenotypic and etiologic analyses. BMI was calculated ([weight in kg])/[height in m]2) from assessments of height and weight made using a wall-mounted ruler or tape measure and digital scale, respectively.
Table 1.
Descriptive Statistics and Pearson Correlations
| Variable | Binge eating | Negative urgency | Appearance pressures | Thin-ideal internalization | Body dissatisfaction | Dietary restraint | Age | Body mass index |
|---|---|---|---|---|---|---|---|---|
| Descriptive Statistics: | ||||||||
| M (SD) | 1.06 (1.42) | 2.08 (0.61) | 2.34 (1.02) | 2.52 (0.95) | 1.46 (1.85) | 0.57 (0.94) | 16.87 (2.59) | 23.32 (5.41) |
| Range | 0–7.00 | 1.00–3.75 | 1.00–5.00 | 1.00–5.00 | 0–6.00 | 0–5.40 | 11–25 | 15.41–54.60 |
| Correlations: | ||||||||
| Binge eating | -- | -- | -- | -- | -- | -- | -- | -- |
| Negative urgency | .47*** | -- | -- | -- | -- | -- | -- | -- |
| Appearance pressures | .32*** | .28*** | -- | -- | -- | -- | -- | |
| Thin-ideal internalization | .34*** | .34*** | .79*** | -- | -- | -- | -- | -- |
| Body dissatisfaction | .38*** | .26*** | .43*** | .39*** | -- | -- | -- | |
| Dietary restraint | .36*** | .25*** | .46*** | .46*** | .53*** | -- | -- | -- |
| Age | .02 | −.05 | .23*** | .14*** | .06 | −.01 | -- | -- |
| Body mass index | .15*** | .08** | .22*** | .12*** | .54*** | .27*** | .17*** | -- |
p < .01;
p < .001
Statistical Analyses
Prior to analyses, dietary restraint and binge eating were log transformed (log10X + 1) to correct for kurtosis and positive skew.
Phenotypic associations
Pearson correlations were used to initially examine variable relationships. Hierarchical linear models (HLMs) were then fit to investigate the moderating effects of the four ED-specific risk factors on the association between negative urgency and binge eating. HLMs allowed us to control for the non-independent twin data (i.e., by nesting a level 1 variable [twin] within a level 2 unit [family]). Since HLM does not provide standardized regression coefficients, all variables were first standardized to interpret unstandardized estimates as something close to standardized effects. Separate HLMs were run for each ED-specific moderator. Significant interactions were plotted and probed using the online tool specific to HLM described in Preacher, Curran, & Bauer (2006). We examined the significance of simple slopes (i.e., the relationship between the independent variable and dependent variable at chosen levels of the moderator) and computed the regions of significance (i.e., moderator values at which the relationship between the independent variable and dependent variable is significant).
Etiologic associations
First, a bivariate Cholesky decomposition model was used to examine genetic and environmental associations between negative urgency and binge eating. This allowed us to quantify the degree of genetic/environmental overlap between these constructs in the full sample (i.e., independent of level of ED risk factor) and replicate the large genetic and moderate non-shared environmental correlations between negative urgency and binge eating previously reported in a sub-set of the current sample (63% of the HBMC sample; 45% of the total sample; Racine et al., 2013). The bivariate model decomposes the variance within and covariance between two phenotypes into additive genetic (A; genetic influences that add across genes), shared environmental (C; non-genetic factors that make co-twins similar to one another), and non-shared environmental (E; non-genetic factors that make co-twins different from one another, including measurement error) influences. Genetic and environmental correlations are obtained by standardizing genetic and environmental covariance matrices; these correlations index the extent to which the genetic/environmental influences on negative urgency and binge eating are the same.
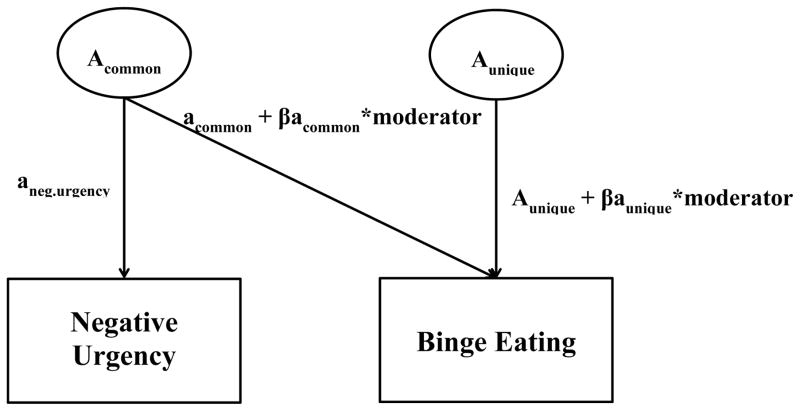
Next, we used an extension of the bivariate model (i.e., gene-environment interaction [GxE] in presence of gene-environment correlation [rGE] model; Purcell, 2002) to investigate the influence of ED-specific moderators on genetic and environmental associations between negative urgency and binge eating. As shown in Figure 1, the bivariate model provides three path estimates for genetic influences: 1) genetic effects on negative urgency; 2) genetic effects on binge eating that are attributable to negative urgency (“common”); and 3) genetic effects unique to binge eating (“unique”). In the GxE in the presence of rGE extension of the bivariate model, the addition of two beta terms allows one to examine: 1) whether a measured variable influences the genetic covariance between negative urgency and binge eating; and 2) whether a measured variable influences the genetic influences unique to binge eating, after controlling for the covariation between negative urgency and binge eating. Shared and non-shared environmental influences are estimated in this model in the same way as genetic influences, but are not included in Figure 1.
Figure 1. GxE in Presence of rGE Model.
Acommon = genetic factor common to negative urgency and binge eating; Aunique = genetic factor unique to binge eating; aneg.urgency = total genetic effects on negative urgency; acommon = genetic effects on binge eating that are attributable to negative urgency; βacommon = moderation coefficient for common genetic path; aunique = genetic effects unique to binge eating; βaunique = moderation coefficient for unique genetic path.
Typically, the GxE in the presence of rGE model has been used to examine whether a measured variable moderates genetic influences on a single outcome variable (GxE) after accounting for shared genetic variance between the measured variable and the outcome variable (rGE, see Racine, Burt, Iacono, McGue, & Klump, 2011 for an example). In this case, researchers are interested in the significance of the unique genetic moderation coefficient (βaunique; see Figure 1) for the single outcome variable. In contrast, the βacommon coefficient is of particular interest in the current study, as this coefficient indicates whether the genetic covariance between negative urgency and binge eating significantly varies based on an individual’s level of the ED-specific moderator. This approach is consistent with prior work that has utilized the GxE in the presence of rGE model to examine moderation of etiologic covariance between two phenotypes (Slane, Klump, McGue, & Iacono, 2014; Wang, Deater-Deckard, Petrill, & Thompson, 2012).
Similar to phenotypic analyses, we investigated each ED moderator variable in a separate GxE in the presence of rGE model. Scores on the ED-specific moderators were categorized into four groups to ensure an adequate sample size at each level of the moderator. Appearance pressures and thin-ideal internalization scores were grouped into quartiles. Body dissatisfaction and dietary restraint scores could not be grouped into quartiles given that approximately half of the participants had a score of 0 on these measures; thus, categories were formed to ensure that a minimum of 100 participants fell into each group.3
Biometric models were fit using full-information raw data techniques in Mx (Neale, 1995). Raw data techniques treat missing data as missing-at-random and allow for the retention of twin pairs in which one twin in a pair is missing data on a main variable. Full ACE models were fit and compared to AE nested sub-models. Given extant research suggesting little-to-no shared environmental influences on impulsivity (Bezdjian, Baker, & Tuvblad, 2011) and binge eating (Bulik et al. 1998), we expected AE models to provide a better fit to the data than ACE models. Additional submodels (e.g., those in which moderation coefficients were constrained to zero) were not fit in order to minimize model comparisons and ensure that confidence intervals were not artificially narrowed (Sullivan & Eaves, 2002). Instead, the significance of moderator estimates was determined by examining whether confidence intervals overlapped with 0. Model fit was examined by taking the difference in minus twice the log likelihood between the full ACE and the nested AE models. This comparison results in a χ2 difference test, with the degrees of freedom representing the difference between the full and nested models. A non-significant chi square test suggests that the more parsimonious model (i.e., the model with fewer estimated parameters and more degrees of freedom) is preferred. Akaike’s information criterion (AIC) was also used as an index of model fit, with lower AICs indicating better model fit.
Results
Phenotypic Associations
Descriptive statistics and Pearson correlations are presented in Table 1. Inspection of means, standard deviations, and ranges suggested sufficient variability for most constructs. Descriptive statistics for MEBS subscales were similar to those reported in previous twin studies (Klump et al. 2002; Racine et al. 2011). Further, 4.8% and 11.2% of our sample scored above the clinical cut-offs for MEBS Binge Eating and Body Dissatisfaction, respectively (cutoffs = 4; von Ranson et al. 2005). SATAQ-3 means were on par with those from previous studies of adolescent females (e.g., Wilksch, Tiggemann, & Wade, 2006), but the mean and standard deviation for the EDE-Q Restraint subscale was lower than norms for adolescent and adult females (Carter, Stewart, & Fairburn, 2001; Mond, Hay, Rodgers, & Owen, 2006). As expected, all variables of interest were significantly correlated with one another (rs = .25–.79; see Table 1). BMI was correlated with all variables, and age was correlated with appearance pressures and thin-ideal internalization, supporting the inclusion of these covariates.
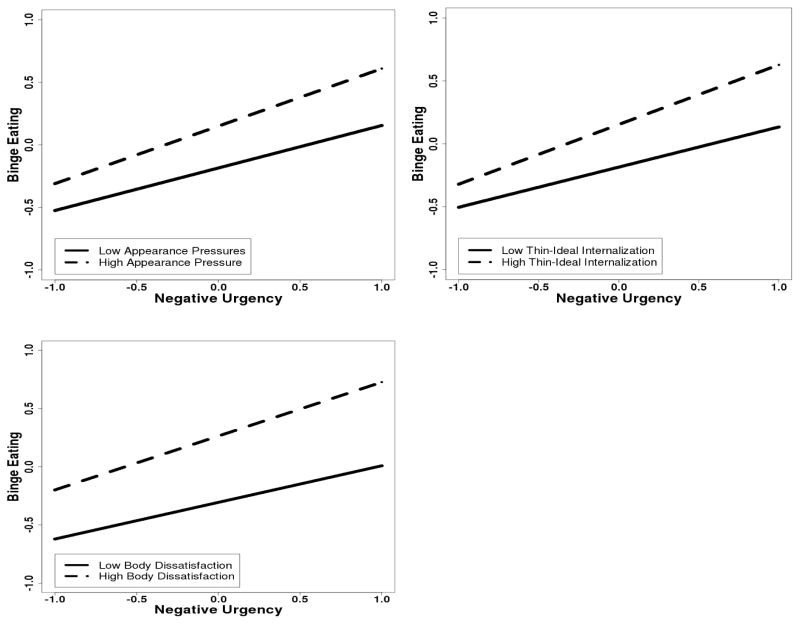
Results from the HLMs examining ED-specific variables as moderators of phenotypic negative urgency-binge eating associations are presented in Table 2. Negative urgency, appearance pressures, thin-ideal internalization, body dissatisfaction, and dietary restraint were significantly associated with binge eating, after controlling for age and BMI. Moreover, appearance pressures, thin-ideal internalization, and body dissatisfaction significantly moderated the relationship between negative urgency and binge eating, but dietary restraint did not (see Table 2). Plotting the significant interactions revealed that, as expected, the relationship between negative urgency and binge eating was stronger at higher levels of appearance pressures, thin-ideal internalization, and body dissatisfaction (see Figure 2). Interestingly, however, probing the interactions using simple slopes and regions of significance analyses indicated that negative urgency was significantly related to binge eating across all possible values of each moderator, highlighting the strength of the main effect of negative urgency on binge eating.
Table 2.
Hierarchical Linear Models Examining Interactive Effects of Negative Urgency and Eating Disorder-Specific Risk Factors in the Prediction of Binge Eating.
| b (S.E.) | t (df) | p | |
|---|---|---|---|
| Appearance pressures | |||
| Negative urgency | .40 (.03) | 12.99 (858.78) | <.001 |
| Appearance pressures | .17 (.03) | 5.16 (817.53) | <.001 |
| Negative urgency x appearance pressures | .06 (.03) | 2.15 (861.66) | .03 |
| Age | −.01 (.04) | −0.34 (473.10) | .73 |
| BMI | .09 (.03) | 2.87 (626.58) | .004 |
| Thin-ideal internalization | |||
| Negative urgency | .40 (.03) | 12.69 (853.80) | <.001 |
| Thin-ideal internalization | .17 (.03) | 5.29 (810.67) | <.001 |
| Negative urgency x thin-ideal internalization | .08 (.03) | 2.88 (854.58) | .004 |
| Age | .01 (.03) | 0.39 (456.51) | .70 |
| BMI | .12 (.03) | 3.79 (613.19) | <.001 |
| Body dissatisfaction | |||
| Negative urgency | .39 (.03) | 13.70 (939.92) | <.001 |
| Body dissatisfaction | .28 (.03) | 8.25 (929.49) | <.001 |
| Negative urgency x body dissatisfaction | .07 (.03) | 2.71 (940.80) | .007 |
| Age | .03 (.03) | 1.01 (497.07) | .31 |
| BMI | −.05 (.03) | −1.68 (787.53) | .09 |
| Dietary restraint | |||
| Negative urgency | .39 (.03) | 13.91 (934.20) | <.001 |
| Dietary restraint | .23 (.03) | 7.59 (929.61) | <.001 |
| Negative urgency x dietary restraint | .04 (.03) | 1.39 (926.47) | .17 |
| Age | .03 (.03) | 0.95 (490.92) | .34 |
| BMI | .04 (.03) | 1.26 (730.72) | .21 |
Standardized beta estimates are presented in the table, as all variables were standardized prior to conducting the hierarchical linear models. BMI = body mass index
Figure 2.
Interactions between Negative Urgency and Eating Disorder-Specific Risk Factors in the Prediction of Binge Eating
Etiologic Associations
Bivariate model
Replicating previous findings in a sub-set of this sample (Racine et al., 2013), bivariate Cholesky decomposition models revealed significant genetic and non-shared environmental influences on negative urgency and binge eating, as well as significant genetic and non-shared environmental associations between negative urgency and binge eating. The AE model was preferred to the ACE model, given the non-significant chi-square difference test (Δχ2 (Δdf) < 0.001 (3); p > .99) and a lower AIC value (AE: 1290.67 vs ACE: 1296.67). Shared environmental effects were estimated at 0 for both negative urgency and binge eating and, as such, genetic and non-shared environmental estimates were identical in the AE and ACE models. The heritability of negative urgency was estimated at 39% (confidence intervals (CIs): .29, .48), with the remaining variance due to the non-shared environment (61%, CIs: .52, .71). Genetic and non-shared environmental variance estimates for binge eating were similar to those reported in previous research (A: 39%, CIs: .29, .48; E: 61%, CIs: .52, .71). With regards to the etiologic nature of the negative urgency-binge eating association, the genetic correlation was large and significant (ra = .72, CIs: .57, .86) with a smaller, yet significant, non-shared environmental correlation (re = .31, CIs: .21, .40). These genetic and non-shared environmental correlation estimates are very similar to those reported in our previous study (ra = .77; re = .29; Racine et al., 2013). Thus, across the sample, negative urgency and binge eating have very similar genetic influences and exhibit some overlap in their non-shared environmental contributions.
Moderator models
Given bivariate model results, we first confirmed that AE moderator models provided a superior fit to the data than ACE moderator models. We found this to be true for all three ED-specific moderators: appearance pressures: Δχ2 (Δdf) = 0.72 (5); p = .98; AICs: AE = 1104.04; ACE = 1113.32; thin-ideal internalization: Δχ2 (Δdf) < 0.001 (5); p > .99; AICs: AE = 1086.91; ACE = 1096.91; body dissatisfaction: Δχ2 (Δdf) = 0.13 (5); p = .99; AICs: AE = 1214.31; ACE = 1224.17. 4
Path and moderator estimates for the best-fitting AE moderator models are presented in Table 3.5 Path estimates index genetic and environmental contributions to the variance within, and covariance between, negative urgency and binge eating at the lowest level of the moderator (i.e., individuals in the lowest quartile on appearance pressures and thin-ideal internalization; individuals with a score of 0 on body dissatisfaction). Moderator estimates are added to path estimates to calculate the genetic and environmental variance and covariance at each level of the moderator. For example, the genetic covariance between negative urgency and binge eating is calculated using the following equation: covariancea: [Ac + βacommon*moderator value (i.e., 0, 1, 2, 3)]2. Following previous recommendations (Purcell, 2002), unstandardized path and moderator estimates are presented. However, we standardized these estimates to calculate the genetic and environmental correlations between negative urgency and binge eating at each level of the moderator (see Table 4). Similar to simple slopes analyses in traditional moderation models, these correlations index the impact of the moderating variables on genetic and environmental associations between negative urgency and binge eating.
Table 3.
Unstandardized Parameter Estimates from Best-Fitting AE Moderation Models
| Model | Path Estimates | Moderator Estimates | ||
|---|---|---|---|---|
|
| ||||
| Genetic | Non-shared environment | Genetic | Non-shared environment | |
| Appearance Pressures | ||||
| Negative urgency-Total | .63 (.53, .72) | .76 (.70, .83) | -- | -- |
| Binge eating-Common | .31 (.13, .49) | .17 (.03, .30) | .10 (−.01, .20) | .04 (−.04, .12) |
| Binge eating-Unique | .51 (.33, .65) | .57 (.47, .68) | −.05 (−.18, .06) | .10 (.03, .16) |
| Thin-Ideal Internalization | ||||
| Negative urgency-Total | .63 (.53, .72) | .76 (.70, .83) | -- | -- |
| Binge eating-Common | .29 (.13, .45) | .20 (.08, .33) | .10 (.01, .18) | .03 (−.04, .10) |
| Binge eating-Unique | .40 (.19, .57) | .64 (.55, .75) | .02 (−.08, .13) | .05 (−.01, .10) |
| Body Dissatisfaction | ||||
| Negative urgency-Total | .63 (.54, .72) | .76 (.70, .83) | -- | -- |
| Binge eating-Common | .33 (.20, .46) | .18 (.08, .29) | .12 (.01, .21) | .04 (−.03, .12) |
| Binge eating-Unique | .30 (.12, .43) | .66 (.59, .73) | .10 (−.02, .21) | .06 (.01, .13) |
95% confidence intervals for parameter estimates are presented in parentheses. Confidence intervals that do not overlap with 0 indicate statistical significance at p < .05. Total = total genetic/environmental variance for negative urgency. Common = genetic/environmental variance for binge eating that is common to negative urgency. Unique = genetic/environmental variance that is unique to binge eating.
Table 4.
Calculated Genetic (A) and Non-shared Environmental (E) Correlations between Negative Urgency and Binge Eating at Varying Levels of the Eating Disorder-Specific Moderator
|
Correlations
|
||||||
|---|---|---|---|---|---|---|
| Appearance pressures | Thin-ideal internalization | Body dissatisfaction | ||||
|
| ||||||
| A | E | A | E | A | E | |
| Level of Moderator | ||||||
| 0 | .51 | .24 | .52 | .28 | .61 | .25 |
| 1 | .66 | .28 | .65 | .30 | .72 | .30 |
| 2 | .79 | .31 | .76 | .33 | .81 | .34 |
| 3 | .91 | .34 | .86 | .35 | .88 | .38 |
Results from the moderator models suggested that genetic covariance between negative urgency and binge eating was enhanced as appearance pressures, thin-ideal internalization, and body dissatisfaction increased. The moderation coefficients for the genetic influences on binge eating that are shared with negative urgency (“common”) were positive and statistically significant (i.e., confidence intervals did not overlap with 0) in the thin-ideal internalization and body dissatisfaction models, and this moderation coefficient approached significance in the appearance pressures model (see Table 3). The genetic correlations clearly demonstrate an increase in the genetic association between negative urgency and binge eating at higher levels of the moderator (see Table 4). At the lowest level of appearance pressures, thin-ideal internalization, and body dissatisfaction, genetic correlations between negative urgency and binge eating were .51, .52, and .61, respectively, whereas these correlations were .91, .86, and .88 at the highest level of these risk factors. In contrast, the “unique” genetic moderation coefficients were uniformly non-significant (see Table 3), suggesting that disorder-specific risk factors did not impact the magnitude of the genetic influences unique to binge eating (i.e., after accounting for genetic influences shared with negative urgency).
The opposite pattern is true when considering how ED-specific risk factors impact non-shared environmental contributions to binge eating. Moderation coefficients for the non-shared environmental influences on binge eating that are shared with negative urgency (“common”) were uniformly small and non-significant, whereas examination of “unique” moderation coefficients indicated that non-shared environmental influences on binge eating appeared to be greater in individuals who reported higher levels of appearance pressures, thin-ideal internalization, and body dissatisfaction (see Table 3). Taken together, behavioral genetic modelling suggests that ED risk factors moderate the phenotypic relationship between negative urgency and binge eating by increasing the degree to which these two constructs share genetic influences.
Confirmatory bivariate models
Although the GxE in the presence of rGE model has been widely used, there also have been published concerns that the model can yield false positive GxE results and biased parameter estimates (Rathouz, Van Hulle, Rodgers, Waldman, & Lahey, 2008; van Hulle, Lahey, & Rathouz, 2013). The extent to which these concerns apply to our use of the model (i.e., to examine whether etiologic influences shared by two variables are moderated by an independent moderator variable) is unclear. Nonetheless, we confirmed our genetic moderation findings by conducting bivariate models at high and low levels of the moderator variables, defined by both a median split and by extreme groups (i.e., values of 0 and 4 from moderator models).6 The pattern of results in these confirmatory models was identical to that observed in the moderator models. For appearance pressures and body dissatisfaction, estimated genetic correlations between negative urgency and binge eating were significantly larger for individuals high and low on the respective moderator in both sets of analyses (see Table 5). For thin-ideal internalization, the genetic correlations in the median split analysis did not significantly differ (although were in the right direction), but the extreme group analysis revealed significant differences between high and low moderator groups.7 Taken together with our moderation model results, appearance pressures, thin-ideal internalization, and body dissatisfaction appear to significantly moderate genetic influences common to negative urgency and binge eating.
Table 5.
Estimated Genetic Correlations between Negative Urgency and Binge Eating from Confirmatory Bivariate Models Conducted at High and Low Levels of the Eating-Disorder Specific Moderator
|
Correlations
|
||||||
|---|---|---|---|---|---|---|
| Appearance pressures | Thin-ideal internalization | Body dissatisfaction | ||||
|
| ||||||
| Low | High | Low | High | Low | High | |
| Moderator Grouping | ||||||
| Median split | .59 (.30, .88) | .84 (.52, 1.0)† | .79 (.39, 1.0) | .82 (.51, 1.0) | .64 (.33, .91) | .88 (.61, 1.0)† |
| Extreme groups | .76 (.13, 1.0) | .94 (.48, 1.0)† | .62 (−1.0, 1.0) | .79 (.06., 1.0)† | .64 (.33, .91) | 1.0 (.39, 1.0)† |
Correlations in the low and high groups are significantly different from one another at p < .05 based on a Fisher r-to-z transformation test
Discussion
The aim of the current study was to examine whether well-established risk factors for EDs (i.e., appearance pressures, thin-ideal internalization, body dissatisfaction, dietary restraint) moderate associations between negative urgency and binge eating. Using a large sample of twins, this study was the first to investigate moderation effects at both the phenotypic and etiologic (i.e., genetic and environmental) levels of analysis. Appearance pressures, thin-ideal internalization, and body dissatisfaction strengthened phenotypic and genetic associations between negative urgency and binge eating, such that a greater proportion of genetic influences were shared between negative urgency and binge eating at higher levels of these disorder-specific risk factors. Each risk factor had a similar moderating impact on phenotypic and etiologic negative urgency-binge eating associations. In contrast, dietary restraint did not emerge as a moderator of the phenotypic or etiologic relationship between negative urgency and binge eating.
Our results suggest that sociocultural pressures for thinness, and the cognitive consequences that can arise from these pressures (i.e., thin-ideal internalization and body dissatisfaction), are associated with a higher risk for binge eating in individuals with negative urgency. For women with this combination of risk factors, binge eating may be an impulsive behavior that functions to regulate negative emotions, despite the ineffectiveness of binge eating for moving toward cultural ideals of thinness. Notably, however, negative urgency was associated with binge eating even at low levels of these ED-specific risk factors. This finding highlights the strength of the main effect of negative urgency on binge eating and is consistent with a recent study that found that high negative urgency also was associated with elevated loss of control eating frequencies in individuals with low dietary restraint (Emery et al., 2013). Consistent with transdiagnostic models of negative urgency (Cyders, Conskunpinar, VanderVeen, 2016), one possibility is that women with high negative urgency, but low disorder-specific risk, engage in several different maladaptive behaviors in an attempt to regulate emotions, including binge eating. In contrast, disorder-specific variables may interact with negative urgency to place individuals at risk for using a specific maladaptive behavior to cope with negative emotions. This interpretation is consistent with the findings of Racine & Martin (2016), as high negative urgency was associated with dysregulated eating, depressive symptoms, and problematic alcohol use, but interactions among negative urgency and ED risk factors only predicted dysregulated eating. Thus, the increased risk for binge eating that is associated with high negative urgency may be further potentiated in the presence of ED-risk factors. Future research using prospective, longitudinal designs is necessary to investigate how negative urgency and disorder-specific risk factors intersect to predict binge eating as well as other forms of psychopathology.
Our behavior genetic modeling results significantly extend phenotypic findings by suggesting that ED-specific risk factors moderate genetic, rather than environmental, covariance between negative urgency and binge eating. Specifically, at high levels of ED risk factors, individual differences in binge eating were more likely to be accounted for by genetic influences shared with negative urgency, as opposed to genetic influences independent of negative urgency. Consistent with the biopsychosocial model of risk proposed by Culbert et al. (2015), genetic influences on transdiagnostic, trait-based risk factors like negative urgency may be “shaped” by ED-specific risk factors to lead to the development of binge eating and eating disorders. That is, individuals who are genetically “wired” for engaging in emotion-based rash action may be more likely to manifest this genetic predisposition as binge eating in the presence of ED-risk factors, which would serve to strengthen genetic associations between negative urgency and binge eating. Notably, negative urgency and binge eating also shared a relatively large proportion of their genetic risk factors at low levels of ED-specific risk variables, but the presence of ED-risk factors appears to further enhance risk for binge eating via genetically-based mechanisms.
Prospective behavioral genetic research is necessary to examine whether genetic influences on earlier negative urgency predict later binge eating, and whether there are critical developmental windows (e.g., puberty) during which these genetic processes are shaped by ED-specific risk factors. Further, future twin studies should consider whether individual differences in binge eating are more likely to result from genetically-mediated mechanisms that do not overlap with negative urgency (e.g., obesity, ovarian hormones, appetite-regulating hormones; Bulik et al., 2003; Culbert, Racine, & Klump, 2016; Klump et al., 2016) in individuals with low versus high ED-specific risk.
Given that ED risk factors impacted negative urgency-binge eating associations through genetic, rather than environmental mechanisms, it is important to consider candidate genetic/biological mechanisms that may underlie both negative urgency and binge eating and that may be strengthened in the presence of ED-specific risk factors. Although mechanistic research on negative urgency is in its infancy, individuals with high negative urgency are thought to be biologically at risk for behaviors that involve approach tendencies towards rewarding stimuli as well as poor behavioral regulation of the pursuit of long-term goals. Specifically, negative urgency has been theorized to result from a combination of high dopamine and low serotonin levels (Cyders & Smith, 2008). Dopamine mediates approach-related behavior, whereas serotonin is involved in affect-guided planning. Given that serotonin modulates dopamine neurotransmission in some brain areas, low serotonin levels have been hypothesized to leave the effects of dopamine on reward drive and impulsivity uninhibited (Cyders & Smith, 2008). Further, neuroimaging studies reveal associations between self-reported negative urgency and activation in brain regions that underlie both reward valuation and cognitive control (Cyders et al. 2014; Weiland et al., 2014; Wilbertz et al., 2014). Importantly, these same neuromodulators and neurocognitive processes have been implicated in the etiology of binge eating and EDs (Culbert et al., 2015; Schag, Schonleber, Teufel, Zipfel, & Giel, 2013). Thus, negative urgency and binge eating may be genetically related because they are both mediated by a strong “bottom-up” approach system and a weak “top-down” control system. This genetically-mediated association is likely present regardless of disorder-specific moderators, as both our previous report and the current study found that genetic influences on negative urgency and binge eating significantly overlapped, independent of ED-specific moderators. However, ED-specific risk factors may strengthen genetic associations because they may serve to increase approach tendencies, and decrease cognitive control abilities, specifically in relation to food.
In contrast to genetic influences, ED-specific risk factors did not moderate non-shared environmental associations between negative urgency and binge eating but instead impacted the magnitude of non-shared environmental influences unique to binge eating. The fact that ED-specific risk factors impacted negative urgency-binge eating associations through genetic, rather than environmental, mechanisms suggests that environmental experiences that are shared with negative urgency are less important for binge eating than shared neurobiological/neurocognitive mechanisms at high levels of ED risk factors. In contrast, environmental experiences that impact binge eating, independent of negative urgency, appear to contribute more to binge eating among females with high ED-specific risk factors. For example, parental comments about weight or weight-focused peer groups (Marcos, Sebastian, Aubalat, Ausina, & Treasure, 2013) are environmental factors that are unlikely to be associated with negative urgency and that may predict binge eating in girls who feel pressure to look like women they see on the media and who feel negatively about their body.
Contrary to two previous studies in female college students (Emery et al., 2013; Racine & Martin, 2016), we found that dietary restraint exerted a significant main effect, but did not significantly interact with negative urgency to predict binge eating. Although these previous studies also used the EDE-Q Restraint Scale, the fact that our participants spanned pre-adolescence to early adulthood and had lower rates of dietary restraint could account for discrepant findings. Further, binge eating is often accompanied by compensatory behaviors in college students (Kelly-Weeder, 2011), and negative urgency-dietary restraint interactions may be most likely to predict binge eating in individuals with intentions of compensating after binge eating episodes. It also is possible that momentary levels of dietary restraint or restriction may be more relevant for binge eating in individuals with negative urgency than number of days of restrained eating over the past month. Indeed, Pearson and colleagues (2015) recent model posits that dieting behavior may represent a state-based risk factor that precipitates binge eating in individuals high on trait negative urgency. Future ecological momentary assessment research should examine the interactive roles of momentary restriction and negative affect for binge eating in individuals with high and low negative urgency.
Despite the significance of our study for advancing integrative models of binge eating and ED development, we must note several limitations. First, our results are based on cross-sectional data, as all measures were completed at one time point. Although negative urgency and ED risk factors have predicted increases in binge eating in previous longitudinal research (Pearson et al., 2012; Stice et al., 2002), it is unclear whether interactions among these variables prospectively predict binge eating symptoms. Further, although the proposed explanations for our findings focus on negative urgency as a genetically-mediated risk factor for binge eating, it also is important to recognize that binge eating may further reinforce the tendency to act impulsively in response to negative emotions. Specifically, individuals who binge eat may have fewer opportunities to learn effective emotion regulation strategies, and reciprocal reinforcement processes may be especially strong in individuals with ED-specific risk factors because of the increased rates of binge eating associated with these variables.
Second, we used a self-report questionnaire to assess binge eating tendencies in a sample of twins unselected for binge eating or EDs. These study features were necessary to permit sufficient variability to examine moderating effects and power to conduct twin moderation analyses. Although self-report questionnaires have been criticized for overestimating the presence and frequency of binge eating compared to clinical interviews (Fairburn & Beglin, 1994), interview-based measures often underestimate the heritability of psychopathology (Burt, 2009). Thus, there are advantages and disadvantages to both questionnaire and interview assessments when conducting twin studies. Further, our measure of binge eating has been validated against a structured interview and has been shown to distinguish among multiple forms of dysregulated eating (i.e., binge eating, objective overeating, loss of control eating; Racine et al., 2013). Nonetheless, we acknowledge that our binge eating measure assesses both binge eating thoughts and urges, in addition to actions, which is a limitation of our study. It is important to investigate whether interactions among negative urgency and ED-specific risk factors predict the onset and maintenance of clinically significant binge eating and EDs in future longitudinal research.
Third, as noted above, the bivariate moderation model used in the present study has been found to produce false positive GxE results, with alternative non-linear models potentially explaining significant moderation of covariance pathways (Rathouz et al. 2008; van Hulle et al., 2013; Zheng et al., 2015). By running bivariate models at high and low levels of the moderators, we confirmed that ED-risk factors enhance genetic sharing between negative urgency and binge eating. We also did not detect significant non-linear main effects of our ED-specific moderators on binge eating, lending further confidence to our moderator model results. Finally, it is important to note that the detection of etiologic moderation can be influenced by the scaling and distribution of the outcome variables (Purcell, 2002). We used log-transformed binge eating scores, as log transformations have been shown to significantly reduce the false positive rate for GxE (Murray, Molenaar, Johnson, & Krueger, 2016). Nonetheless, the above issues warrant some caution, and our findings need to be replicated in independent samples.
Future directions include investigating genetically-mediated mechanisms that may account for increased phenotypic and genetic associations between negative urgency and binge eating in the presence of ED risk factors. As mentioned above, increased reward sensitivity and decreased cognitive control in response to negative affect are thought to underlie self-reported negative urgency and binge eating (Cyders & Smith, 2008; Schag et al., 2013). The possibility that these neurocognitive processes explain the genetic moderation effects observed in our study should be examined in future research that incorporates measures spanning multiple units of analysis (e.g., circuits, physiology, behavior, self-report). In addition, other important disorder-specific risk factors for binge eating, such as the expectation that eating will alleviate negative affect (Pearson et al., 2012), should be investigated as moderators of genetic and environmental negative urgency-binge eating associations in future work. Although eating expectancies may operate similarly to the ED-specific risk factors included in the current study, the importance of early parental modeling experiences for the development of eating expectancies (e.g., Annus, Smith, Fischer, Hendricks, & Williams, 2007) suggests that this ED-specific risk variable could play a more prominent role in increasing non-shared environmental associations between negative urgency and binge eating.
Finally, if longitudinal studies find that negative urgency-ED risk factor interactions prospectively predict binge eating onset, novel prevention programs that address both sets of risk factors should be considered, particularly for females who are at high genetic risk for negative urgency and binge eating. For example, the Body Project (Stice, Becker, & Yokum, 2013), a well-established program designed to modify ED-risk factors, could be enhanced via assessments of negative urgency and the addition of mindfulness and emotion regulation strategies for individuals with this combination of transdiagnostic and disorder-specific risk factors. Further, interventions that target both emotion-related variables and traditional ED-specific risk factors (e.g., Integrative Cognitive-Affective Treatment; Wonderlich et al., 2014) may be most helpful for individuals who are at genetic risk for negative urgency and binge eating.
Supplementary Material
General Scientific Summary.
This study found that females who reported the tendency to act impulsively in response to negative emotions (i.e., negative urgency) and who endorsed greater eating disorder-specific risk had the highest levels of binge eating. Eating disorder risk factors were found to increase risk for binge eating among those with negative urgency via genetic mechanisms.
Acknowledgments
This research was supported by grants from the National Institute of Mental Health (R01 MH092377 and R01 MH082054) to Drs. Klump, Keel, Burt, Neale, and Boker.
Footnotes
Ten percent of the sample did not complete the Sociocultural Attitudes toward Appearance Questionnaire-3 and were thus missing data for models examining thin-ideal internalization and appearance pressures as moderators.
The Minnesota Eating Behavior Survey (MEBS; previously known as the Minnesota Eating Disorder Inventory (M-EDI)) was adapted and reproduced by special permission of Psychological Assessment Resources, Inc., 16204 North Florida Avenue, Lutz, Florida 33549, from the Eating Disorder Inventory (collectively, EDI and EDI-2) by Garner, Olmstead, & Polivy (1983) Copyright 1983 by Psychological Assessment Resources, Inc. Further reproduction of the MEBS is prohibited without prior permission from Psychological Assessment Resources, Inc.
Model fitting was examined using additional data preparations (e.g., dichotomous scores, tertiles), and the same pattern of results was obtained in all cases (data not shown).
We do not present twin moderation models for dietary restraint since restraint did not significantly moderate the phenotypic negative urgency-binge eating association. As expected, twin moderation models revealed that dietary restraint did not significantly moderate genetic and/or environmental covariance between negative urgency and binge eating (data not shown).
Path and moderator estimates from full ACE moderator models are available as Supplemental Material.
The primary disadvantage to this approach is that it requires co-twins to have the same score on the level of the moderator variable. As such, these models excluded 42–45% and 61–81% of our sample in median split and extreme group analyses, respectively.
As recommended by Van Hulle et al. (2013) and Zheng, Van Hulle & Rathouz (2015), we also confirmed that our significant moderation effects were not instead explained by non-linear main effects of the moderator on the dependent variable. For each ED-specific moderator, non-linear main effects were non-significant, with the GxE in presence of rGE model providing a better fit to the data than the non-linear main effects model (data not shown).
Portions of this manuscript were presented at the International Conference on Eating Disorders, Boston, Massachusetts, April 23–25, 2015.
References
- Anestis MD, Selby EA, Joiner TE. The role of urgency in maladaptive behaviors. Behaviour Research and Therapy. 2007;45:3018–3029. doi: 10.1016/j.brat.2007.08.012. http://doi.org/10.1016/j.brat.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Annus AM, Smith GT, Fischer S, Hendricks M, Williams SF. Associations among family-of-origin food-related experiences, expectancies, and disordered eating. International Journal of Eating Disorders. 2007;40:179–186. doi: 10.1002/eat.20346. http://doi.org/10.1002/eat.20346. [DOI] [PubMed] [Google Scholar]
- Arens AM, Gaher RM, Simons JS. Child maltreatment and deliberate self-harm among college students: Testing mediation and moderation models for impulsivity. American Journal of Orthopsychiatry. 2012;82:328–337. doi: 10.1111/j.1939-0025.2012.01165.x. http://doi.org/10.1111/j.1939-0025.2012.01165.x. [DOI] [PubMed] [Google Scholar]
- Berg KC, Peterson CB, Frazier P, Crow SJ. Psychometric evaluation of the eating disorder examination and eating disorder examination-questionnaire: A systematic review of the literature. International Journal of Eating Disorders. 2012;45:428–438. doi: 10.1002/eat.20931. http://doi.org/10.1002/eat.20931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezdjian S, Baker LA, Tuvblad C. Genetic and environmental influences on impulsivity: A meta-analysis of twin, family and adoption studies. Clinical Psychology Review. 2011;31:1209–1223. doi: 10.1016/j.cpr.2011.07.005. http://doi.org/10.1016/j.cpr.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulik CM, Sullivan PF, Kendler KS. Heritability of binge-eating and broadly defined bulimia nervosa. Biological Psychiatry. 1998;44:1210–1218. doi: 10.1016/s0006-3223(98)00280-7. [DOI] [PubMed] [Google Scholar]
- Bulik CM, Sullivan PF, Kendler KS. Genetic and environmental contributions to obesity and binge eating. International Journal of Eating Disorders. 2003;33:293–298. doi: 10.1002/eat.10140. http://doi.org/10.1002/eat.10140. [DOI] [PubMed] [Google Scholar]
- Burt SA. Rethinking environmental contributions to child and adolescent psychopathology: A meta-analysis of shared environmental influences. Psychological Bulletin. 2009;135:608–637. doi: 10.1037/a0015702. http://doi.org/10.1037/a0015702. [DOI] [PubMed] [Google Scholar]
- Burt SA, Klump KL. The Michigan State University Twin Registry (MSUTR): An update. Twin Research and Human Genetics. 2013;16:344–350. doi: 10.1017/thg.2012.87. http://doi.org/10.1017/thg.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter JC, Stewart DA, Fairburn CG. Eating Disorder Examination Questionnaire: Norms for young adolescent girls. Behaviour Research and Therapy. 2001;39:625–632. doi: 10.1016/s0005-7967(00)00033-4. [DOI] [PubMed] [Google Scholar]
- Culbert KM, Racine SE, Klump KL. Research Review: What we have learned about the causes of eating disorders - a synthesis of sociocultural, psychological, and biological research. Journal of Child Psychology and Psychiatry. 2015;56:1141–1164. doi: 10.1111/jcpp.12441. http://doi.org/10.1111/jcpp.12441. [DOI] [PubMed] [Google Scholar]
- Culbert KM, Racine SE, Klump KL. Hormonal factors and disturbances in eating disorders. Current Psychiatry Reports. 2016;18:1–16. doi: 10.1007/s11920-016-0701-6. http://doi.org/10.1007/s11920-016-0701-6. [DOI] [PubMed] [Google Scholar]
- Cyders MA, Coskunpinar A, VanderVeen JD. Urgency: A transdiagnostic endophenotype for maladaptive risk taking. In: Zeigler-Hill V, Marcus DK, editors. The dark side of personality: Science and practice in social, personality, and clinical psychology. Washington, DC: American Psychological Association; 2016. pp. 157–188. [Google Scholar]
- Cyders MA, Dzemidzic M, Eiler WJ, Coskunpinar A, Karyadi K, Kareken DA. Negative urgency and ventromedial prefrontal cortex responses to alcohol cues: fMRI evidence of emotion-based impulsivity. Alcoholism: Clinical and Experimental Research. 2014;38:409–417. doi: 10.1111/acer.12266. http://doi.org/10.1111/acer.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyders MA, Smith GT. Emotion-based dispositions to rash action: Positive and negative urgency. Psychological Bulletin. 2008;134:807–828. doi: 10.1037/a0013341. http://doi.org/10.1037/a0013341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery RL, King KM, Fischer SF, Davis KR. The moderating role of negative urgency on the prospective association between dietary restraint and binge eating. Appetite. 2013;71:113–119. doi: 10.1016/j.appet.2013.08.001. http://doi.org/10.1016/j.appet.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Fairburn CG, Beglin SJ. Assessment of eating disorders: Interview or self-report questionnaire? International Journal of Eating Disorders. 1994;16:363–370. http://doi.org/10.1002/1098-108X(199412)16:4<363::AID-EAT2260160405>3.0.CO;2-#. [PubMed] [Google Scholar]
- Fischer S, Peterson CM, McCarthy D. A prospective test of the influence of negative urgency and expectancies on binge eating and purging. Psychology of Addictive Behaviors. 2013;27:294–300. doi: 10.1037/a0029323. http://doi.org/10.1037/a0029323. [DOI] [PubMed] [Google Scholar]
- Fischer S, Settles R, Collins B, Gunn R, Smith GT. The role of negative urgency and expectancies in problem drinking and disordered eating: Testing a model of comorbidity in pathological and at-risk samples. Psychology of Addictive Behaviors. 2012;26:112–123. doi: 10.1037/a0023460. http://doi.org/10.1037/a0023460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzsimmons-Craft EE, Harney MB, Koehler LG, Danzi LE, Riddell MK, Bardone-Cone AM. Explaining the relation between thin ideal internalization and body dissatisfaction among college women: The roles of social comparison and body surveillance. Body Image. 2012;9:43–49. doi: 10.1016/j.bodyim.2011.09.002. http://doi.org/10.1016/j.bodyim.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Goldschmidt AB, Doyle AC, Wilfley DE. Assessment of binge eating in overweight youth using a questionnaire version of the Child Eating Disorder Examination with instructions. International Journal of Eating Disorders. 2007;40:460–467. doi: 10.1002/eat.20387. http://doi.org/10.1002/eat.20387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly-Weeder S. Binge drinking and disordered eating in college students. Journal of the American Academy of Nurse Practitioners. 2011;23:33–41. doi: 10.1111/j.1745-7599.2010.00568.x. http://doi.org/10.1111/j.1745-7599.2010.00568.x. [DOI] [PubMed] [Google Scholar]
- Klump KL, McGue M, Iacono WG. Genetic relationships between personality and eating attitudes and behaviors. Journal of Abnormal Psychology. 2002;111:380–389. doi: 10.1037//0021-843x.111.2.380. http://doi.org/10.1037/0021-843X.111.2.380. [DOI] [PubMed] [Google Scholar]
- Klump KL, O’Connor SM, Hildebrandt BA, Keel PK, Neale M, Sisk CL, … Burt SA. Differential effects of estrogen and progesterone on genetic and environmental risk for emotional eating in women. Clinical Psychological Science. 2016 doi: 10.1177/2167702616641637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Perkins PS, Burt SA, McGue M, Iacono WG. Puberty moderates genetic influences on disordered eating. Psychological Medicine. 2007;37:627–634. doi: 10.1017/S0033291707000189. http://doi.org/10.1017/S0033291707000189. [DOI] [PubMed] [Google Scholar]
- Lykken DT, Bouchard TJ, McGue M, Tellegen A. The Minnesota Twin Family Registry: Some initial findings. Acta Geneticae Medicae Et Gemellologiae. 1990;39:35–70. doi: 10.1017/s0001566000005572. [DOI] [PubMed] [Google Scholar]
- Lynam DR, Smith GT, Whiteside SP, Cyders MA. The UPPS-P: Assessing five personality pathwways to impulsive behavior. West Lafayette, IN: Purdue Universtiy; 2006. [Google Scholar]
- Marcos YQ, Sebastián MJQ, Aubalat LP, Ausina JB, Treasure J. Peer and family influence in eating disorders: A meta-analysis. European Psychiatry. 2013;28:199–206. doi: 10.1016/j.eurpsy.2012.03.005. http://doi.org/10.1016/j.eurpsy.2012.03.005. [DOI] [PubMed] [Google Scholar]
- Mond JM, Hay PJ, Rodgers B, Owen C, Beumont PJV. Validity of the Eating Disorder Examination Questionnaire (EDE-Q) in screening for eating disorders in community samples. Behaviour Research and Therapy. 2004;42:551–567. doi: 10.1016/S0005-7967(03)00161-X. http://doi.org/10.1016/S0005-7967(03)00161-X. [DOI] [PubMed] [Google Scholar]
- Mond JM, Hay PJ, Rodgers B, Owen C. Eating Disorder Examination Questionnaire (EDE-Q): Norms for young adult women. Behaviour Research and Therapy. 2006;44:53–62. doi: 10.1016/j.brat.2004.12.003. http://doi.org/10.1016/j.brat.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Murray AL, Molenaar D, Johnson W, Krueger RF. Dependence of gene-by-environment interactions (GxE) on scaling: comparing the use of sum scores, transformed sum scores and IRT scores for the phenotype in tests of GxE. Behavior Genetics. 2016;46:552–572. doi: 10.1007/s10519-016-9783-5. http://doi.org/10.1007/s10519-016-9783-5. [DOI] [PubMed] [Google Scholar]
- Myers TA, Crowther JH. Sociocultural pressures, thin-ideal internalization, self-objectification, and body dissatisfaction: Could feminist beliefs be a moderating factor? Body Image. 2007;4:296–308. doi: 10.1016/j.bodyim.2007.04.001. http://doi.org/10.1016/j.bodyim.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Neale M. Mx: Statistical Modeling. Richmond, VA: Virginia Commonwealth University; 1995. [Google Scholar]
- Neumark-Sztainer D, Paxton SJ, Hannan PJ, Haines J, Story M. Does body satisfaction matter? Five-year longitudinal associations between body satisfaction and health behaviors in adolescent females and males. Journal of Adolescent Health. 2006;39:244–251. doi: 10.1016/j.jadohealth.2005.12.001. http://doi.org/10.1016/j.jadohealth.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Neumark-Sztainer D, Wall M, Guo J, Story M, Haines J, Eisenberg M. Obesity, disordered eating, and eating disorders in a longitudinal study of adolescents: How do dieters fare 5 years later? Journal of the American Dietetic Association. 2006;106:559–568. doi: 10.1016/j.jada.2006.01.003. http://doi.org/10.1016/j.jada.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Pearson CM, Combs JL, Zapolski TCB, Smith GT. A longitudinal transactional risk model for early eating disorder onset. Journal of Abnormal Psychology. 2012;121:707–718. doi: 10.1037/a0027567. http://doi.org/10.1037/a0027567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson CM, Wonderlich SA, Smith GT. A risk and maintenance model for bulimia nervosa: From impulsive action to compulsive behavior. Psychological Review. 2015;122:516–535. doi: 10.1037/a0039268. http://doi.org/10.1037/a0039268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson CM, Zapolski TCB, Smith GT. A longitudinal test of impulsivity and depression pathways to early binge eating onset. International Journal of Eating Disorders. 2015;48:230–237. doi: 10.1002/eat.22277. http://doi.org/10.1002/eat.22277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polivy J, Herman P. Dieting and binging: A causal analysis. American Psychologist. 1985;40:193–201. doi: 10.1037//0003-066x.40.2.193. http://doi.org/10.1037/0003-066X.40.2.193. [DOI] [PubMed] [Google Scholar]
- Peeters H, Van Gestel S, Vlietinck R, Derom C, Derom R. Validation of a telephone zygosity questionnaire in twins of known zygosity. Behavior Genetics. 1998;28:159–163. doi: 10.1023/a:1021416112215. [DOI] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17:117–133. doi: 10.1007/BF01537962. http://doi.org/10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interactions in multiple linear regression, multilevel modeling, and latent curve analysis. Journal of Educational and Behavioral Statistics. 2006;31:437–448. [Google Scholar]
- Purcell S. Variance components models for gene–environment interaction in twin analysis. Twin Research and Human Genetics. 2002;5:554–571. doi: 10.1375/136905202762342026. http://doi.org/10.1375/twin.5.6.554. [DOI] [PubMed] [Google Scholar]
- Racine SE, Burt SA, Iacono WG, McGue M, Klump KL. Dietary restraint moderates genetic risk for binge eating. Journal of Abnormal Psychology. 2011;120:119–128. doi: 10.1037/a0020895. http://doi.org/10.1037/a0020895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine SE, Burt SA, Keel PK, Sisk CL, Neale MC, Boker S, Klump KL. Examining associations between negative urgency and key components of objective binge episodes. International Journal of Eating Disorders. 2015;48:527–531. doi: 10.1002/eat.22412. http://doi.org/10.1002/eat.22412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine SE, Keel PK, Burt SA, Sisk CL, Neale M, Boker S, Klump KL. Exploring the relationship between negative urgency and dysregulated eating: Etiologic associations and the role of negative affect. Journal of Abnormal Psychology. 2013;122:433–444. doi: 10.1037/a0031250. http://doi.org/10.1037/a0031250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine SE, Martin SJ. Exploring divergent trajectories: Disorder-specific moderators of the association between negative urgency and dysregulated eating. Appetite. 2016;103:45–53. doi: 10.1016/j.appet.2016.03.021. http://dx.doi.org/10.1016/j.appet.2016.03.021. [DOI] [PubMed] [Google Scholar]
- Rathouz PJ, Van Hulle CA, Rodgers JL, Waldman ID, Lahey BL. Specification, testing, and intepretation of gene-by-measured-environment interaction models in the presence of gene-environment correlation. Behavior Genetics. 2008;38:301–305. doi: 10.1007/s10519-008-9193-4. http://doi.org/10.1007/s10519-008-9193-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers RF, McLean SA, Paxton SJ. Longitudinal relationships among internalization of the media ideal, peer social comparison, and body dissatisfaction: Implications for the tripartite influence model. Developmental Psychology. 2015;51:706–713. doi: 10.1037/dev0000013. http://doi.org/10.1037/dev0000013. [DOI] [PubMed] [Google Scholar]
- Schag K, Schönleber J, Teufel M, Zipfel S, Giel KE. Food-related impulsivity in obesity and Binge Eating Disorder - a systematic review. Obesity Reviews. 2013;14:477–495. doi: 10.1111/obr.12017. http://doi.org/10.1111/obr.12017. [DOI] [PubMed] [Google Scholar]
- Schnitzler CE, von Ranson KM, Wallace LM. Adding thin-ideal internalization and impulsiveness to the cognitive–behavioral model of bulimic symptoms. Eating Behaviors. 2012;13:219–225. doi: 10.1016/j.eatbeh.2012.02.007. http://doi.org/10.1016/j.eatbeh.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Slane JD, Klump KL, McGue M, Iacono G. Genetic and environmental factors underlying comorbid bulimic behaviors and alcohol use disorders: A moderating role for the dysregulated personality cluster? European Eating Disorders Review. 2014;22:159–169. doi: 10.1002/erv.2284. http://doi.org/10.1002/erv.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GT, Fischer S, Cyders MA, Annus AM, Spillane NS, McCarthy DM. On the validity and utility of discriminating among impulsivity-like traits. Assessment. 2007;14:155–170. doi: 10.1177/1073191106295527. http://doi.org/10.1177/1073191106295527. [DOI] [PubMed] [Google Scholar]
- Smith GT, Guller L, Zapolski TCB. A comparison of two models of urgency: Urgency predicts both rash action and depression in youth. Clinical Psychological Science. 2013;1:266–275. doi: 10.1177/2167702612470647. http://doi.org/10.1177/2167702612470647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E. A prospective test of the dual-pathway model of bulimic pathology: Mediating effects of dieting and negative affect. Journal of Abnormal Psychology. 2001;110:124–135. doi: 10.1037//0021-843x.110.1.124. http://doi.org/10.1037/0021-843X.110.1.124. [DOI] [PubMed] [Google Scholar]
- Stice E. Interactive and mediational etiologic models of eating disorder onset: Evidence from prospective studies. Annual Review of Clinical Psychology. 2016;12 doi: 10.1146/annurev-clinpsy-021815-093317. http://doi.org/10.1146/annurev-clinpsy-021815-093317. [DOI] [PubMed] [Google Scholar]
- Stice E, Becker CB, Yokum S. Eating disorder prevention: Current evidence-base and future directions. International Journal of Eating Disorders. 2013;46:478–485. doi: 10.1002/eat.22105. http://doi.org/10.1002/eat.22105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Presnell K, Spangler D. Risk factors for binge eating onset in adolescent girls. Health Psychology. 2002;21:131–138. [PubMed] [Google Scholar]
- Suisman JL, O’Connor SM, Sperry S, Thompson JK, Keel PK, Burt SA, … Klump KL. Genetic and environmental influences on thin-ideal internalization. International Journal of Eating Disorders. 2012;45:942–948. doi: 10.1002/eat.22056. http://doi.org/10.1002/eat.22056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF, Eaves LJ. Evaluation of analyses of univariate discrete twin data. Behavior Genetics. 2002;32:221–227. doi: 10.1023/a:1016025229858. [DOI] [PubMed] [Google Scholar]
- Thompson JK, Heinberg LJ, Altabe M, Tantleff-Dunn S. Exacting beauty: Theory, assessment, and treatment of body image disturbance. Washington, DC: American Psychological Association; 1999. [Google Scholar]
- Thompson JK, van den Berg P, Roehrig M, Guarda AS, Heinberg LJ. The Sociocultural Attitudes towards Appearance Scale-3 (SATAQ-3): Development and validation. International Journal of Eating Disorders. 2004;35:293–304. doi: 10.1002/eat.10257. http://doi.org/10.1002/eat.10257. [DOI] [PubMed] [Google Scholar]
- Van Hulle CA, Lahey BB, Rathouz PJ. Operating characteristics of alternative statistical methods for detecting gene-by-measured environment interaction in the presence of gene-environment correlation in twin and sibling studies. Behavior Genetics. 2013;43:71–84. doi: 10.1007/s10519-012-9568-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Ranson KM, Klump KL, Iacono WG, McGue M. The Minnesota Eating Behavior Survey: A brief measure of disordered eating attitudes and behaviors. Eating Behaviors. 2005;6:373–392. doi: 10.1016/j.eatbeh.2004.12.002. http://doi.org/10.1016/j.eatbeh.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Wang Z, Deater-Deckard K, Petrill SA, Thompson LA. Externalizing problems, attention regulation, and household chaos: A longitudinal behavioral genetic study. Development and Psychopathology. 2012;24:755–769. doi: 10.1017/S0954579412000351. http://doi.org/10.1017/S0954579412000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland BJ, Heitzeg MM, Zald D, Cummiford C, Love T, Zucker RA, Zubieta JK. Relationship between impulsivity, prefrontal anticipatory activation, and striatal dopamine release during rewarded task performance. Psychiatry Research: Neuroimaging. 2014;223:244–252. doi: 10.1016/j.pscychresns.2014.05.015. http://doi.org/10.1016/j.pscychresns.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel KR, Weinstock J, Vander Wal JS, Weaver TL. Examining the role of negative urgency in a predictive model of bulimic symptoms. Eating Behaviors. 2014;15:343–349. doi: 10.1016/j.eatbeh.2014.04.014. http://doi.org/10.1016/j.eatbeh.2014.04.014. [DOI] [PubMed] [Google Scholar]
- Whiteside SP, Lynam DR. The Five Factor Model and impulsivity: Using a structural model of personality to understand impulsivity. Personality and Individual Differences. 2001;30:669–689. http://doi.org/10.1016/S0191-8869(00)00064-7. [Google Scholar]
- Wilbertz T, Deserno L, Horstmann A, Neumann J, Villringer A, Heinze HJ, Schlagenhauf F. Response inhibition and its relation to multidimensional impulsivity. Neuroimage. 2014;103:241–248. doi: 10.1016/j.neuroimage.2014.09.021. [DOI] [PubMed] [Google Scholar]
- Wilksch SM, Tiggemann M, Wade TD. Impact of interactive school-based media literacy lessons for reducing internalization of media ideals in young adolescent girls and boys. International Journal of Eating Disorders. 2006;39:385–393. doi: 10.1002/eat.20237. http://doi.org/10.1002/eat.20237. [DOI] [PubMed] [Google Scholar]
- Wonderlich SA, Peterson CB, Crosby RD, Smith TL, Klein MH, Mitchell JE, Crow SJ. A randomized controlled comparison of integrative cognitive-affective therapy (ICAT) and enhanced cognitive-behavioral therapy (CBT-E) for bulimia nervosa. Psychological Medicine. 2014;44:543–553. doi: 10.1017/S0033291713001098. http://doi.org/10.1017/S0033291713001098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapolski TCB, Stairs AM, Settles RF, Combs JL, Smith GT. The measurement of dispositions to rash action in children. Assessment. 2010;17:116–125. doi: 10.1177/1073191109351372. http://doi.org/10.1177/1073191109351372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Van Hulle CA, Rathouz PJ. Comparing alternative biometric models with and without gene-by-measured environment interaction in behavior genetic designs: Statistical operating characteristics. Behavior Genetics. 2015;45:480–491. doi: 10.1007/s10519-015-9710-1. http://doi.org/10.1007/s10519-015-9710-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Yu C, Zhang W, Bao Z, Jiang Y, Chen Y, Zhen S. Peer victimization, deviant peer affiliation and impulsivity: Predicting adolescent problem behaviors. Child Abuse & Neglect. 2016;58:39–50. doi: 10.1016/j.chiabu.2016.06.008. http://doi.org/10.1016/j.chiabu.2016.06.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.