Abstract
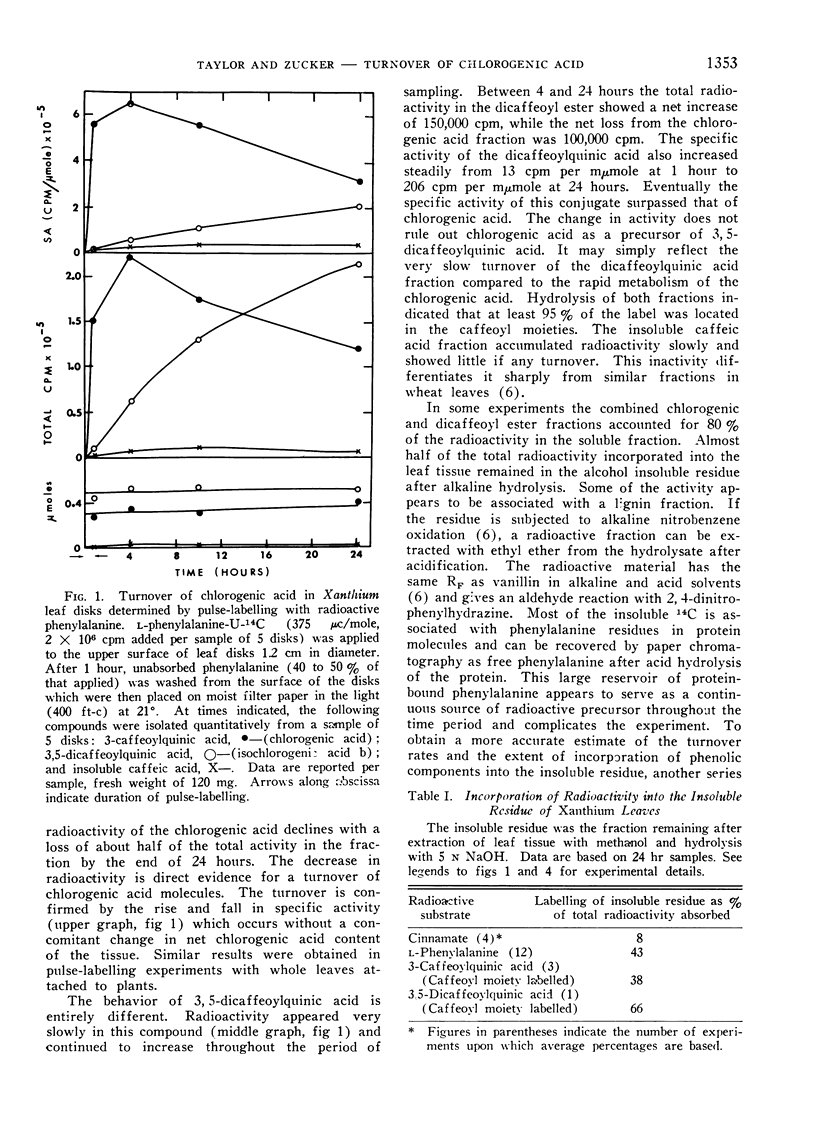
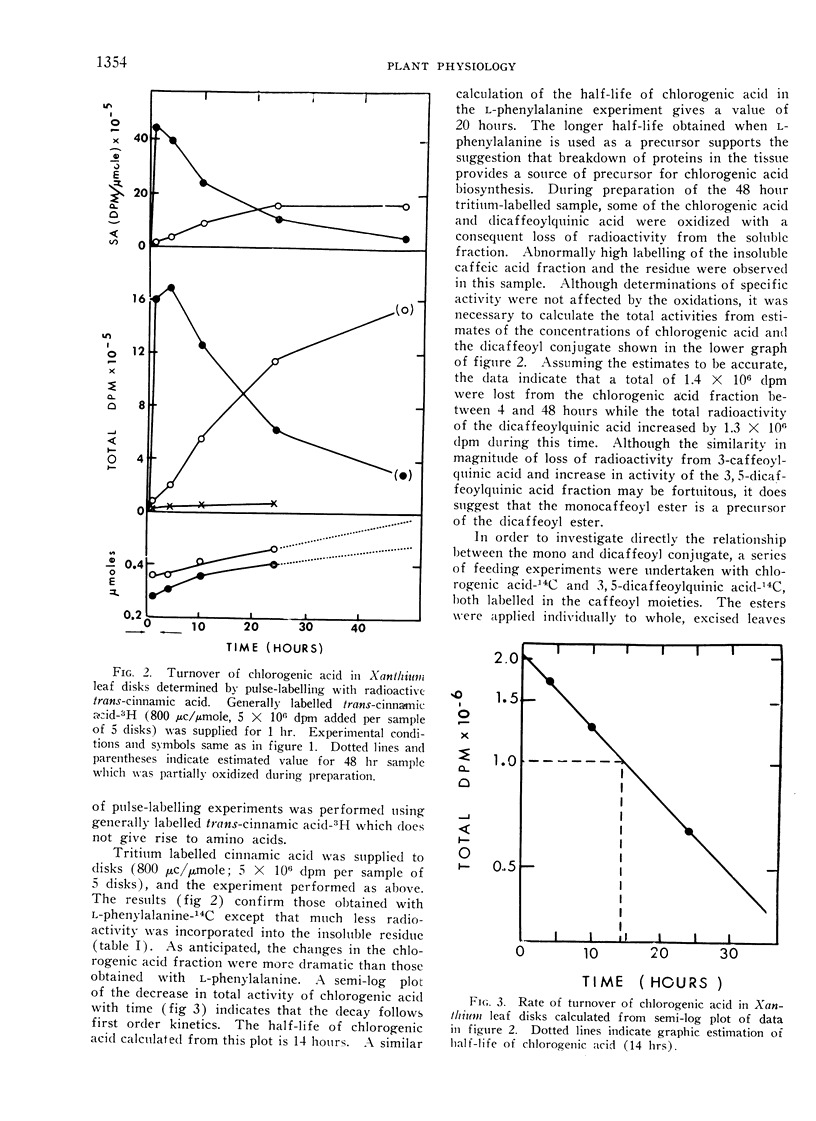
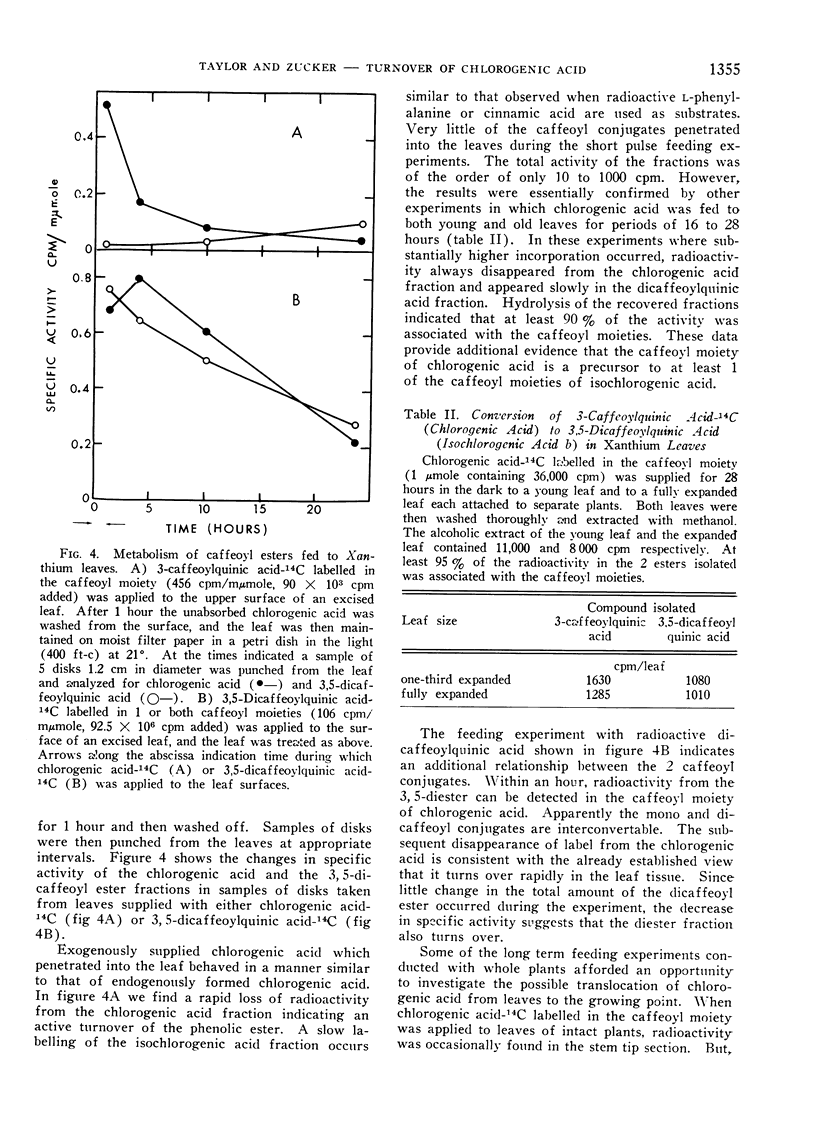
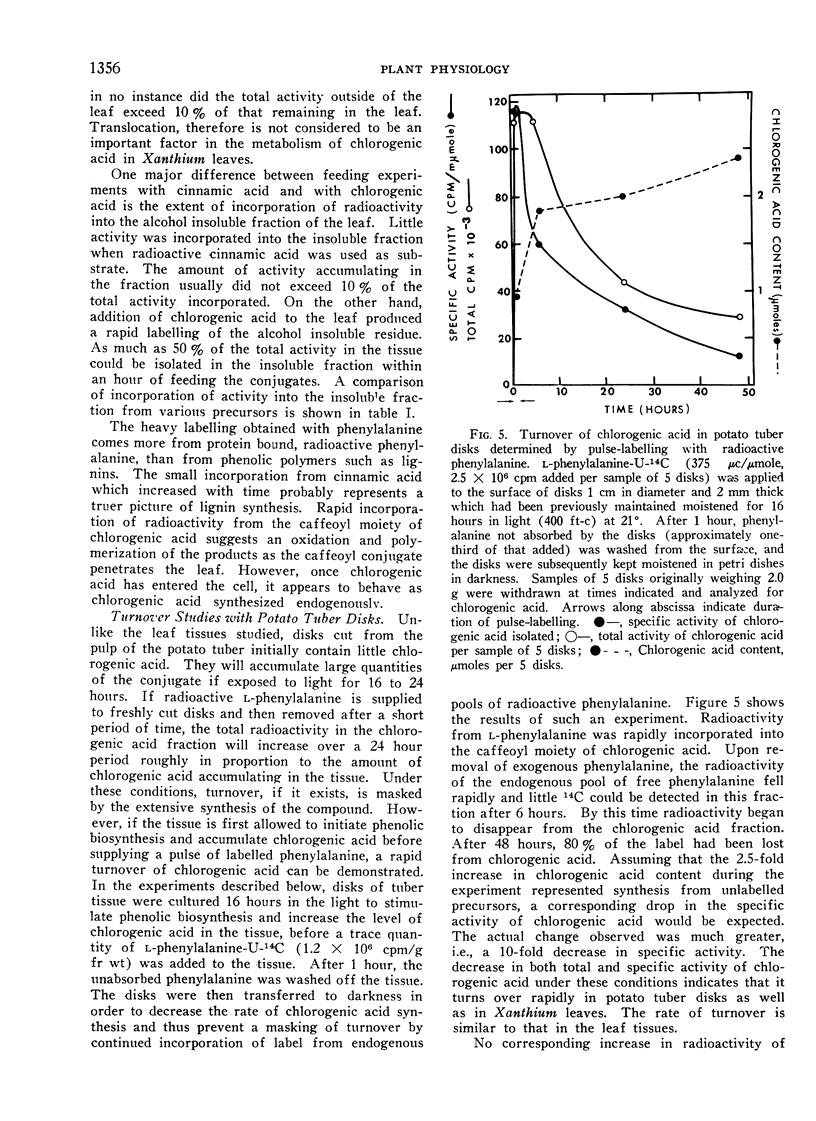
The active turnover of chlorogenic acid (3-caffeoylquinic acid3), a major phenolic component of Xanthium leaves and potato tuber disks, has been demonstrated in these tissues. Pulse-labelling experiments with radioactive l-phenylalanine and trans-cinnamic acid as well as direct feeding experiments with chlorogenic acid-14C labelled in the caffeoyl moiety have been employed in the turnover studies. The rate of turnover is calculated to be on the order of 50 to 100 mμmoles per hour per gram fresh weight of tissue.
In Xanthium leaves chlorogenic acid is in part converted to an isochlorogenic acid identified by silica gel chromatography as 3,5-dicaffeoylquinic acid. Radioactivity of the caffeoyl moiety of chlorogenic acid is also incorporated into lignin-like insoluble polymers in the leaf. Turnover of chlorogenic acid in tuber tissue is largely accounted for by the incorporation of the caffeoyl moiety into insoluble polymers in the tissue.
The significance of chlorogenic acid turnover is discussed in relation to the perception of the photoperiodic stimulus by leaves and to the possible role of chlorogenic acid in lignin synthesis.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CLICK R. E., HACKETT D. P. THE ROLE OF PROTEIN AND NUCLEIC ACID SYNTHESIS IN THE DEVELOPMENT OF RESPIRATION IN POTATO TUBER SLICES. Proc Natl Acad Sci U S A. 1963 Aug;50:243–250. doi: 10.1073/pnas.50.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANSON K. R., ZUCKER M. The biosynthesis of chlorogenic acid and related conjugates of the hydroxycinnamic acids. Chromatographic separation and characterization. J Biol Chem. 1963 Mar;238:1105–1115. [PubMed] [Google Scholar]
- Hanson K. R. Chlorogenic acid biosynthesis. Chemical synthesis and properties of the mono-O-cinnamoylquinic acids. Biochemistry. 1965 Dec;4(12):2719–2731. doi: 10.1021/bi00888a023. [DOI] [PubMed] [Google Scholar]
- LEVY C. C., ZUCKER M. Cinnamyl and p-coumaryl esters as intermediates in the biosynthesis of chlorogenic acid. J Biol Chem. 1960 Aug;235:2418–2425. [PubMed] [Google Scholar]
- McCALLA D. R., NEISH A. C. Metabolism of phenylpropanoid compounds in Salvia. II. Biosynthesis of phenolic cinnamic acids. Can J Biochem Physiol. 1959 Apr;37(4):537–547. [PubMed] [Google Scholar]
- Stafford H. A. Factors Controlling the Synthesis of Natural and Induced Lignins in Phleum and Elodea. Plant Physiol. 1965 Sep;40(5):844–851. doi: 10.1104/pp.40.5.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. O. Some Effects of Photoperiod on the Biosynthesis of Phenylpropane Derivatives in Xanthium. Plant Physiol. 1965 Mar;40(2):273–280. doi: 10.1104/pp.40.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker M. Induction of Phenylalanine Deaminase by Light and its Relation to Chlorogenic Acid Synthesis in Potato Tuber Tissue. Plant Physiol. 1965 Sep;40(5):779–784. doi: 10.1104/pp.40.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]


