Abstract
An increased risk of arteriosclerosis has been noted in cancer survivors. Currently, there are only a few reports available that consider the risk of arteriosclerosis in patients treated with chemotherapy. Patients with an advanced stage B-cell malignant lymphoma are typically treated with a combination therapy of rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP). Complications such as diabetes mellitus (DM), hyperlipidemia (HL), and osteoporosis due to prednisolone and cardiotoxicity due to anthracyclines are well known. However, there are no studies that have investigated the link between R-CHOP therapy and arteriosclerosis. We discussed a patient with follicular lymphoma who was evaluated using cardio-ankle vascular index (CAVI) as an arterial stiffness parameter during R-CHOP therapy in this report. She achived complete remission after the eighth course therapy without complications such as hypertension (HT), HL, DM, and infection. This patient showed elevated CAVI with new plaque formation in the carotid arteries after the end of chemotherapy. These data indicate that R-CHOP therapy may progress the arteriosclerosis.
Keywords: Cardio-ankle vascular index, Arteriosclerosis, Malignant lymphoma, R-CHOP
Introduction
The incidence of tumor-bearing patients has also increased from the prolongation of the average life expectancy, and likewise, the incidence of malignant lymphomas is also increasing. Recently, new molecular targeted drugs have greatly improved the prognosis of cancer patients including malignant lymphoma. With these new drug developments, the importance of systemic management including complications with chemotherapy has also increased.
Follicular lymphoma is a low-grade B-cell malignancy, and patients at an advanced stage are usually treated with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP) therapy [1, 2]. Diabetes mellitus (DM) [3], hyperlipidemia (HL) [4] and osteoporosis [5] are well-known complications of R-CHOP treatment with large amount of steroids; moreover, cardiotoxicity by anthracyclines are also well known [6].
An arterial stiffness parameter, the cardio-ankle vascular index (CAVI), was developed as a marker related to arteriosclerosis including that of the aorta, femoral and tibial artery [7-10]. The principle of CAVI is based on the stiffness parameter β theory proposed by Hayashi et al [11], and it is based on the variance of the arterial pressure that is required to change the vascular diameter. So, the CAVI theoretically does not depend on blood pressure changes during the measurements [7, 8].
Up to now, there were no reports about evaluating the relationships between arteriosclerosis and R-CHOP therapy in the patients with malignant lymphoma. This is the first report evaluating CAVI during R-CHOP therapy in a patient with follicular lymphoma.
Case Report
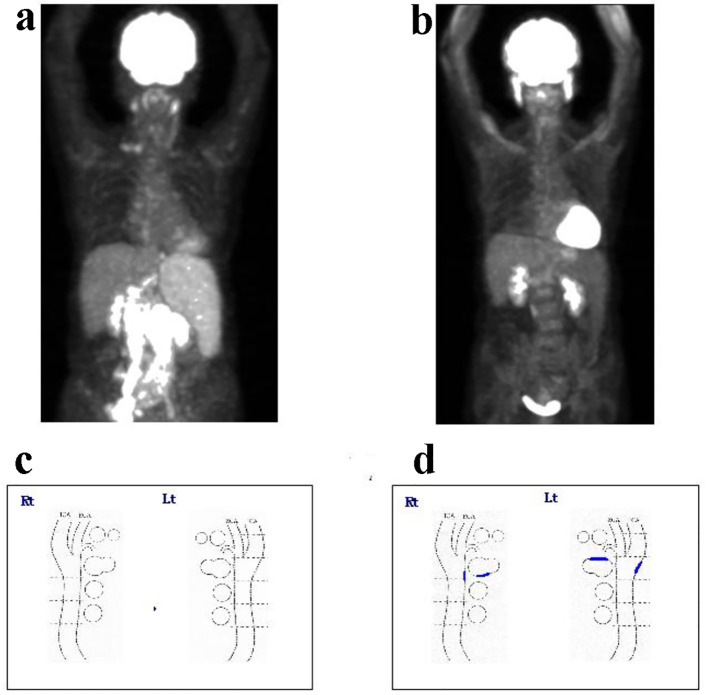
A 68-year-old Japanese female with bilateral edema was admitted to the hospital in April 2015. Laboratory tests showed pancytopenia elevated levels of lactate dehydrogenase and interleukin-2 receptor and hypogammaglobulinemia as follows: white blood cell count 1,810/µL, hemoglobin 10.4 g/dL, platelet count 6.8 × 109/L, lactate dehydrogenase 265 IU/L, interleukin-2 receptor 3,710 U/mL, IgG 373 mg/dL, IgA 48 mg/dL and IgM 23 mg/dL. Computed tomography showed a large bulky mass around the abdominal aorta. Lymph node swelling of bilateral cervical, supraclavicular fossa, and inguinal regions, and splenomegaly were also observed. Invasion of malignant lymphoma was observed in the bone marrow (BM), and the percentage of BM lymphoma cells was 15.2%. Fluorodeoxyglucose-positron emission tomography (FDG-PET) also showed accumulation at the same site (Fig. 1a). After the resection of inguinal lymph node swelling, the patient was diagnosed with follicular lymphoma grade 1, clinical stage IVA. Following admission, the patient was treated with R-CHOP therapy. Until November 2016, eight courses of therapy were completed until November 2016 without complications such as hypertension (HT), HL, DM, and infection. FDG-PET examination after the last chemotherapy cycle revealed complete remission (Fig. 1b).
Figure 1.
The findings of FDG-PET at (a) pretreatment and (b) after eight cycles of R-CHOP. The findings of plaque formation in the carotid artery by ultrasonography at (c) pretreatment and (d) after eight cycles of R-CHOP. After eight cycles of treatment, the patient achieved complete remission. FDG-PET: fluorodeoxyglucose-positron emission tomography; R-CHOP: rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone.
The change in CAVI prior to treatment and immediately before the next chemotherapy cycle was also evaluated. As shown in Figure 2, before the first treatment, the CAVI was 8.8 on the right and 8.9 on the left. After completing eight chemotherapy cycles, CAVI showed a marked elevation at 10.2 and 10.7, respectively. Approximately 3 months after the last chemotherapy cycle, CAVI decreased to 8.9 and 9.1, respectively.
Figure 2.
Clinical course of the patient with blood pressure and CAVI. CAVI was elevated as R-CHOP progressed. CAVI: cardio-ankle vascular index; R-CHOP: rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone.
In addition, intima-media thickness (IMT) and plaque formation in the internal carotid artery were measured during the treatment. After the eight R-CHOP cycles, new plaque formation was observed (Fig. 1c, d) in the bilateral carotid artery without any increase in IMT.
Discussion
CAVI was developed as a marker related to arteriosclerosis, including that of the aorta, femoral, and tibial artery without dependence of blood pressure [7-9]. CAVI is based on the variance of the arterial pressure that is required to change the vascular diameter, and is extremely useful in clinical practices offering a simple and non-invasive method of measurement. As demonstrated by past clinical studies, CAVI was high in arteriosclerotic diseases such as coronary artery disease and cerebral infarction [8, 12], and was also in majority of people with various coronary risk factors [13]. The efficient management of these risk factors reduced CAVI [14].
Pulse wave velocity (PWV) is frequently applied in clinical practice as method for the measurement of arterial stiffness [15]. However, PWV is dependent on blood pressure at the time of measurement. Therefore, PWV is not an appropriate parameter for the evaluation of arterial stiffness.
In patients treated with chemotherapy, veins used as blood access, which are directly exposed to the therapeutic agent, show sclerotic changes. In our patient, CAVI as an evaluation of the arteriosclerosis increased as R-CHOP progressed, and then decreased after chemotherapy termination. However, the CAVI was higher than that prior to treatment.
Previous reports have demonstrated increased carotid IMT in patients with breast cancer treated with anthracycline-based chemotherapy [16]. Sekijima et al (2011) reported that platinum-based chemotherapy was associated with an increased brachial-ankle PWV in patients with gynecological malignancies. Furthermore, it was reported that cisplatin-induced vascular endothelial damage was identified using human umbilical vein endothelial cells in vitro [17]. Moreover, patients with testicular cancer who received chemotherapy showed progression of arteriosclerosis with increasing plasma levels of von Willebrand factor, plasminogen activator inhibitor and tissue-type plasminogen as endothelial markers, and also fibrinogen, C-reactive protein as inflammatory markers [18, 19]. These reports suggested that chemotherapy is a risk factor for the development of arteriosclerosis.
The mechanism of arteriosclerosis progression induced by chemotherapy, the reduction of nitric oxide from endothelial cells was reported apart from complications of metabolic diseases. Nitric oxide inhibits local inflammation, coagulation, and vascular smooth muscle cell proliferation [20, 21]. Hence, the loss of nitric oxide impairs these protective effects and contributes to the development of arteriosclerosis [17].
The patient discussed in this report did not show complications such as DM, HT, and HL during chemotherapy. However, an elevation of CAVI coupled with plaque formation was observed as chemotherapy progressed. The patient did not show an increase in IMT thickness. Naqvi et al reported that carotid plaque is a more powerful parameter to predict the risk of cardiovascular disease as a result of progress of arteriosclerosis compared with carotid IMT [22].
Recently, CAVI was also examined in our few other patients with malignant lymphoma, revealing an elevation tendency with the progression of treatment (data not shown). However, it is difficult to identify the therapeutic agent responsible for this elevation. Long-term assessment in the more patients with malignant lymphoma is necessary to evaluate the relationship between chemotherapy and arteriosclerosis. These studies should include the evaluation of the carotid IMT, plaque formation, and the marker of vascular endothelial damage.
This report discusses the first case with malignant lymphoma showed the elevated CAVI coupled with plaque formation after eight R-CHOP cycles. We thus suggest that R-CHOP therapy is a risk factor for the progression of arteriosclerosis in the patients with malignant lymphoma.
Conflicts of Interest
All authors declare no conflicts of interest.
References
- 1.Prica A, Baldassarre F, Hicks LK, Imrie K, Kouroukis T, Cheung M. Rituximab in Lymphoma and Chronic Lymphocytic Leukaemia: A Practice Guideline. Clin Oncol (R Coll Radiol) 2017;29(1):e13–e28. doi: 10.1016/j.clon.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Izutsu K. Treatment of follicular lymphoma. J Clin Exp Hematop. 2014;54(1):31–37. doi: 10.3960/jslrt.54.31. [DOI] [PubMed] [Google Scholar]
- 3.Lee SY, Kurita N, Yokoyama Y, Seki M, Hasegawa Y, Okoshi Y, Chiba S. Glucocorticoid-induced diabetes mellitus in patients with lymphoma treated with CHOP chemotherapy. Support Care Cancer. 2014;22(5):1385–1390. doi: 10.1007/s00520-013-2097-8. [DOI] [PubMed] [Google Scholar]
- 4.Mai K, Reinecke F, Andres J, Bobbert T, Kraatz J, Wudy SA, Hartmann MF. et al. Effects of hyperlipidaemia on glucocorticoid metabolism: results of a randomized controlled trial in healthy young women. Clin Endocrinol (Oxf) 2011;74(5):551–557. doi: 10.1111/j.1365-2265.2011.03972.x. [DOI] [PubMed] [Google Scholar]
- 5.Lukert BP, Raisz LG. Glucocorticoid-induced osteoporosis: pathogenesis and management. Ann Intern Med. 1990;112(5):352–364. doi: 10.7326/0003-4819-112-5-352. [DOI] [PubMed] [Google Scholar]
- 6.Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F, Civelli M. et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131(22):1981–1988. doi: 10.1161/CIRCULATIONAHA.114.013777. [DOI] [PubMed] [Google Scholar]
- 7.Shirai K, Utino J, Otsuka K, Takata M. A novel blood pressure-independent arterial wall stiffness parameter; cardio-ankle vascular index (CAVI) J Atheroscler Thromb. 2006;13(2):101–107. doi: 10.5551/jat.13.101. [DOI] [PubMed] [Google Scholar]
- 8.Shirai K, Hiruta N, Song M, Kurosu T, Suzuki J, Tomaru T, Miyashita Y. et al. Cardio-ankle vascular index (CAVI) as a novel indicator of arterial stiffness: theory, evidence and perspectives. J Atheroscler Thromb. 2011;18(11):924–938. doi: 10.5551/jat.7716. [DOI] [PubMed] [Google Scholar]
- 9.van Popele NM, Mattace-Raso FU, Vliegenthart R, Grobbee DE, Asmar R, van der Kuip DA, Hofman A. et al. Aortic stiffness is associated with atherosclerosis of the coronary arteries in older adults: the Rotterdam Study. J Hypertens. 2006;24(12):2371–2376. doi: 10.1097/01.hjh.0000251896.62873.c4. [DOI] [PubMed] [Google Scholar]
- 10.Yamashina A, Tomiyama H, Takeda K, Tsuda H, Arai T, Hirose K, Koji Y. et al. Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertens Res. 2002;25(3):359–364. doi: 10.1291/hypres.25.359. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi K, Handa H, Nagasawa S, Okumura A, Moritake K. Stiffness and elastic behavior of human intracranial and extracranial arteries. J Biomech. 1980;13(2):175–184. doi: 10.1016/0021-9290(80)90191-8. [DOI] [PubMed] [Google Scholar]
- 12.Saiki A, Sato Y, Watanabe R, Watanabe Y, Imamura H, Yamaguchi T, Ban N. et al. The Role of a Novel Arterial Stiffness Parameter, Cardio-Ankle Vascular Index (CAVI), as a Surrogate Marker for Cardiovascular Diseases. J Atheroscler Thromb. 2016;23(2):155–168. doi: 10.5551/jat.32797. [DOI] [PubMed] [Google Scholar]
- 13.Sato Y, Nagayama D, Saiki A, Watanabe R, Watanabe Y, Imamura H, Yamaguchi T. et al. Cardio-Ankle Vascular Index is Independently Associated with Future Cardiovascular Events in Outpatients with Metabolic Disorders. J Atheroscler Thromb. 2016;23(5):596–605. doi: 10.5551/jat.31385. [DOI] [PubMed] [Google Scholar]
- 14.Nagayama D, Saiki A, Endo K, Yamaguchi T, Ban N, Kawana H, Ohira M. et al. Improvement of cardio-ankle vascular index by glimepiride in type 2 diabetic patients. Int J Clin Pract. 2010;64(13):1796–1801. doi: 10.1111/j.1742-1241.2010.02399.x. [DOI] [PubMed] [Google Scholar]
- 15.Torii S, Arima H, Ohkubo T, Fujiyoshi A, Kadota A, Takashima N, Kadowaki S. et al. Association between Pulse Wave Velocity and Coronary Artery Calcification in Japanese men. J Atheroscler Thromb. 2015;22(12):1266–1277. doi: 10.5551/jat.30247. [DOI] [PubMed] [Google Scholar]
- 16.Kalabova H, Melichar B, Ungermann L, Dolezal J, Krcmova L, Kasparova M, Plisek J. et al. Intima-media thickness, myocardial perfusion and laboratory risk factors of atherosclerosis in patients with breast cancer treated with anthracycline-based chemotherapy. Med Oncol. 2011;28(4):1281–1287. doi: 10.1007/s12032-010-9593-1. [DOI] [PubMed] [Google Scholar]
- 17.Sekijima T, Tanabe A, Maruoka R, Fujishiro N, Yu S, Fujiwara S, Yuguchi H. et al. Impact of platinum-based chemotherapy on the progression of atherosclerosis. Climacteric. 2011;14(1):31–40. doi: 10.3109/13697137.2010.522278. [DOI] [PubMed] [Google Scholar]
- 18.Nuver J, Smit AJ, Sleijfer DT, van Gessel AI, van Roon AM, van der Meer J, van den Berg MP. et al. Microalbuminuria, decreased fibrinolysis, and inflammation as early signs of atherosclerosis in long-term survivors of disseminated testicular cancer. Eur J Cancer. 2004;40(5):701–706. doi: 10.1016/j.ejca.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 19.Vaughn DJ, Palmer SC, Carver JR, Jacobs LA, Mohler ER. Cardiovascular risk in long-term survivors of testicular cancer. Cancer. 2008;112(9):1949–1953. doi: 10.1002/cncr.23389. [DOI] [PubMed] [Google Scholar]
- 20.Kinlay S, Fang JC, Hikita H, Ho I, Delagrange DM, Frei B, Suh JH. et al. Plasma alpha-tocopherol and coronary endothelium-dependent vasodilator function. Circulation. 1999;100(3):219–221. doi: 10.1161/01.CIR.100.3.219. [DOI] [PubMed] [Google Scholar]
- 21.Tousoulis D, Davies G, Toutouzas P. Vitamin C increases nitric oxide availability in coronary atherosclerosis. Ann Intern Med. 1999;131(2):156–157. doi: 10.7326/0003-4819-131-2-199907200-00022. [DOI] [PubMed] [Google Scholar]
- 22.Naqvi TZ, Lee MS. Carotid intima-media thickness and plaque in cardiovascular risk assessment. JACC Cardiovasc Imaging. 2014;7(10):1025–1038. doi: 10.1016/j.jcmg.2013.11.014. [DOI] [PubMed] [Google Scholar]