Introduction
As people are living longer, the disease burden is shifting towards diseases of old age, including an increasing prevalence of dementia, most commonly due to Alzheimer’s disease (AD). The risk of developing dementia doubles every 5 years after the age of 65 years.1 There are currently 5.1 million Americans aged over 65 years living with dementia, and this number is expected to reach 13.8 million by the year 2050.2
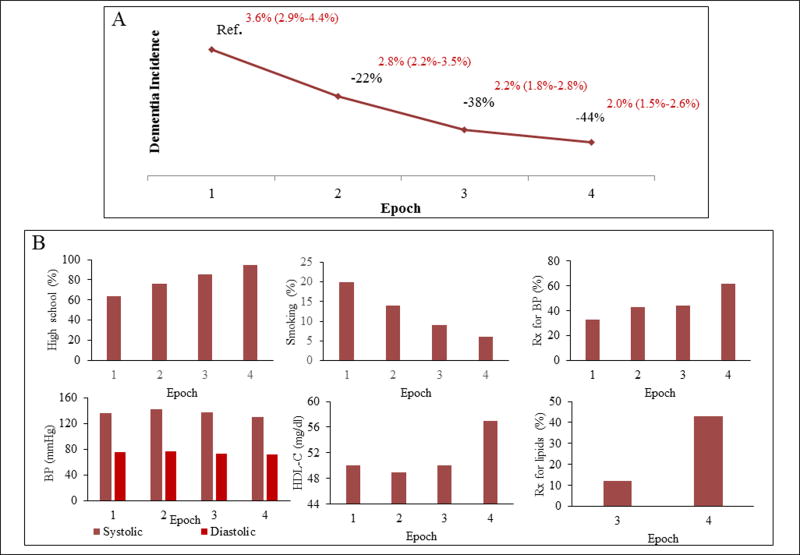
Despite an increasing prevalence of dementia, the Framingham Heart Study (FHS) recently showed that the age-specific incidence of dementia has steadily declined over the previous three decades (Figure 1).3 In other words, whereas the overall number of people affected by dementia will continue to increase due to population aging, an individual’s risk of developing dementia by a specific age has decreased by as much as 20% each decade over the past 30 years. Other studies have also pointed towards a declining incidence of dementia in the USA and Europe.4–6 The Health and Retirement Study also reported a decline in dementia prevalence in the USA from the year 2000 to 2012.7 Collectively, these studies provide hope that some cases of dementia can be prevented or delayed.
Figure 1.
Trends in (A) dementia incidence and (B) education and vascular risk factors over four non-overlapping epochs in the Framingham Heart Study. The baseline examination period was from 1977–1983 for the first epoch, 1986–1991 for the second epoch, 1992–1998 for the third epoch, and 2004–2008 for the fourth epoch. Data are from Satizabal et al.3 BP = blood pressure, HDL-C = high density liporptein cholesterol, high school = completion of high school education, Rx = treatment.
The factors underlying changing temporal trends in dementia are not entirely clear. It is important to understand what underlies the reduction in dementia incidence so that we can promote those factors most likely to reduce dementia incidence even further. This is crucial because, to date, we have no drugs that can permanently halt or reverse clinical dementia. Uncovering modifiable risk factors that can be targeted for the primary prevention of dementia may represent the most feasible approach to managing the growing dementia epidemic.
This review examines whether better cardiovascular health, including better management of stroke risk factors, could be responsible for the observed declining incidence of dementia. We draw on evidence from the FHS as well as other community-based prospective cohort studies.
Vascular contributions to dementia
The brain pulses with each heartbeat. Each thought requires the complex coordination of neuronal firing coupled with local increases in blood flow - The brain does not store energy and is dependent on constant blood flow to meet metabolic demands and to remove metabolic waste such as amyloid beta (Aβ) oligomers. Sudden gross disruption to brain blood flow has obvious and deleterious consequences for the brain, as in the case of stroke and anoxic brain injury. However, vascular brain disease can accumulate silently, and this is far more common than overt stroke. This has been confirmed by the widespread use of structural brain imaging, which has identified markers of small vessel disease, such as lacunar infarcts, white matter hyperintensities, and more recently enlarged perivascular spaces, microinfarcts and cerebral microbleeds in the brains of many elderly adults who present without overt neurological symptoms or signs of a stroke. However, the silent (covert) accumulation of cerebrovascular disease may not be benign. Covert cerebrovascular disease appears to increase the risk of dementia,8 and autopsy studies identify the co-occurrence of vascular and AD-type pathology.9
Historically, dementia due to cerebrovascular disease and AD were considered mutually exclusive and diagnostic criteria (DSM-IV-TR) forbade a diagnosis of probable AD when cognitive deficits were likely due to cerebrovascular disease. Diagnostic criteria have since changed on the weight of evidence linking cerebrovascular disease and AD. In 2002, an influential review by de la Torre challenged current dogma by highlighting the links between vascular risk factors and AD.10 More recently, the American Heart Association (AHA) and American Stroke Association (ASA) released a scientific statement on vascular cognitive impairment (VCI) outlining the importance of vascular contributions to cognitive impairment and dementia.11 Acknowledgment that vascular risk factors contribute to the etiology of dementia provokes a tantalizing question; can improvement in cardiovascular health reduce the risk of developing dementia? In the following section, we examine evidence linking specific vascular risk factors to dementia occurrence. We discuss temporal trends in such risk factors to scrutinize whether improvements in vascular health can explain the declining incidence of dementia.
Vascular risk factors: Associations with dementia and trends over time
Stroke and dementia
A series of strokes leading to dementia was once referred to as multi-infarct dementia, characterized by a stepwise decline in cognitive function.12 Nowadays, multi-infarct dementia is considered as just one cause of vascular dementia (VaD) and stroke is a recognized risk factor for AD as well as VaD. A systematic review and meta-analysis of 14,730 adults, including 862 with a history of stroke and 13,868 controls, demonstrated that a history of stroke increased the risk of AD dementia by 59%.13 The presence of vascular risk factors attenuated, but failed to explain completely, the association between stroke and AD. Stroke can cause a cascade of inflammatory processes and alter the permeability of the blood-brain barrier, exposing the brain to toxins originating in the systemic circulation.13 A clinical stroke may also only reflect the ‘tip of the iceberg’ in the sense that stroke patients may harbor widespread small vessel disease, which may compromise cerebral hemodynamics beyond the site of the clinical lesion. In the presence of AD pathology, a stroke may also serve as a catalyst for the onset of clinically symptomatic dementia. In summary, a history of stroke is a risk factor for dementia.
Trends in stroke risk
Cohort studies have reported a declining stroke incidence in both the US and Europe. The FHS reported a decrease in the age-adjusted incidence of stroke between 1950 and 2004.14 The Rotterdam study reported a reduced risk of stroke between 1990 and 2008 in men only15 whereas the Atherosclerosis Risk in the Communities study reported a reduced risk of stroke incidence between 1987 and 2011, but only in those aged 65 years or older.16 Thus, the incidence of stroke appears to be declining, with some differences reported by age and sex.
Hypertension and dementia
Hypertension is a major risk factor for stroke and cerebrovascular disease, which in turn increases the risk of dementia. In addition to increasing the risk of hemorrhage, sustained exposure to high-pressure flow can alter the brains auto-regulatory thresholds and cause remodeling of small cerebral vessels leading to ischemic injury.17 Associations between hypertension and cognitive impairment are modified by the age at which blood pressure (BP) is measured, with midlife hypertension being associated with later-life cognitive decline across numerous studies.18 Hypertension also appears to increase the risk of incident VaD,19 yet its relationship with incident AD remains unclear.20 The evidence is also lacking as to whether treating hypertension can lower the risk of developing incident dementia,21 although the Systolic-Hypertension in Europe study reported that BP lowering therapy reduced the risk of dementia by 55%.22 Results from the systolic BP intervention trial: memory and cognition in decreased hypertension (SPRINT-MIND), which are anticipated in 2017, may add clarity on this issue. A recent AHA position statement concluded that although substantial evidence links hypertension to the development of cognitive impairment, it remains unclear whether BP lowering treatment prevents or reverses cognitive decline.23
Trends in hypertension and its treatment
In the FHS, BP levels have fallen since the 1970s.24 In the National Health and Nutrition Examination Survey (NHANES) study, the prevalence of hypertension fell between 1960 to 1991, before increasing between 1999 to 2000.25 In Europe, the Rotterdam study reported an increase in the rates of hypertension between 1990 and 2000. Treatment for hypertension did not increase over time in the Rotterdam study, except in the oldest males.15 In contrast, changes in the treatment of BP appear to have been more aggressive in the US, with both the FHS and Cardiovascular Health Study reporting increases over time in the number of participants treated for high BP.24, 26 Thus, treatment of hypertension has increased in the US as has BP control in hypertension.27
Smoking and dementia
Smoking is a major risk factor for cardiovascular disease, including stroke,28 as well as all-cause dementia, AD and cognitive decline.29 Meta-analysis suggests that current smokers, relative to never smokers and former smokers, have a 79% and 70% increased risk of AD, respectively.29 Mechanisms linking smoking to dementia may include concomitant vascular disease, chronic low-grade inflammation, and oxidative stress.29
Trends in smoking
Census data suggests that rates of smoking have steadily decreased in the US for more than half a century.30 On a global scale, the estimated prevalence of daily smoking has decreased since 1980, although the total number of smokers has increased owing to population growth and improved survival among smokers.31 For persons aged 50 years in the FHS, the prevalence of cigarette smoking has decreased from 44% to 19% among those without diabetes and from 58% to 17% among those with diabetes.24 In Europe, analysis of nine countries suggests greater reductions in the rates of smoking between 1985 and 2000 in persons who were highly educated.32 Thus, overall, there have been large reductions in the global rates of smoking.
Lipids and dementia
Cholesterol regulates the production and clearance of brain Aβ.33 The APOE gene is the strongest genetic risk factor for sporadic AD and is involved in cholesterol transport. In the FHS, suboptimal high-density lipoprotein cholesterol levels (≤40 mg/dL) and a high TC/HDL-C ratio (≥5) are associated with incident stroke, whereas low-density lipoprotein cholesterol levels are not.34 However, in the FHS, we did not find an association between plasma total cholesterol and the risk of AD dementia.35 In contrast, a 2008 review of 18 prospective studies suggested that high total cholesterol in midlife but not later life may be associated with an increased risk of all-cause dementia and AD.36 The reviewed studies did not report any significant associations between total cholesterol and the risk of VaD. Despite some observational studies suggesting that statin use may protect against the risk of dementia,37 a 2016 Cochrane review of randomized, controlled trials concluded that the use of statins in later-life to individuals at risk of vascular disease did not prevent dementia.38 In summary, high midlife total cholesterol may be associated with an increased risk of dementia although current evidence does not suggest that cholesterol-lowering therapy reduces the risk of dementia.
Trends in total cholesterol and high-density lipoprotein cholesterol
In the FHS, we have observed a decrease in total cholesterol levels and increases in high-density lipoprotein levels over time.3, 24, 39 The NHANES study reported decreases in total cholesterol from 1960 to 200240 whereas neither the NHANES nor Rotterdam studies found changes in high-density lipoprotein levels from 1960–2002 and 1990–2000, respectively.
Atrial Fibrillation (AF) and dementia
Two independent meta-analyses published between 2011 and 2012 demonstrate that AF is associated with an increased risk of dementia.41, 42 Estimates of the increased risk attributable to AF ranged from 42–100% across the two studies. One of these meta-analyses suggested that the association with dementia was observed primarily in post-stroke patients.42 Mechanisms linking AF to dementia, other than a history of overt stroke, may stem from ischemic brain injury caused by reduced cardiac output, covert strokes, and microthromboemboli.41
Trends in Atrial Fibrillation
The FHS reported an increase in the prevalence and incidence of AF between 1958 to 2007, perhaps as a consequence of enhanced survival.43 The Rotterdam study reported no consistent change in AF incidence between 1990 and 2000.15
Education and dementia
The declining incidence of dementia in the FHS was only observed in persons with a high school diploma.3 Numerous studies have suggested a protective effect of education against dementia and clinical AD.44, 45 The protective effect of education does not appear to be caused entirely by better vascular health or healthier lifestyles.44, 46 With higher educational attainment may come greater cognitive reserve, protecting against or delaying the clinical manifestations of dementia.46
Trends in education
Educational attainment has been increasing steadily in the US. Only 25% of adults older than 24 years had completed a high school diploma or higher in the year 1940.47 This percentage rose to 50% in 1967, 75% in 1986 and 88% in 2015. Similarly, the percentage of persons holding a bachelor’s degree grew from 5% in 1940 to 33% in 2015.47
Diabetes, obesity, and dementia
Our own research had found that, although diabetes was not associated with an increase in the risk of AD overall, patients with diabetes who were without other major risk factors for AD were almost 3 times as likely to develop AD dementia.48 A large meta-analysis of 14 studies reported that persons with type 2 diabetes were at a 60% increased risk of developing dementia.49 Diabetes may increase dementia risk by causing cerebrovascular disease rather than AD pathology; a study of approximately 2400 autopsies demonstrated that diabetes was associated with brain infarction but not AD-type pathology.50
Some contradictory findings have been reported on obesity and the risk of dementia. The association appears to be age-dependent and non-linear. One meta-analysis of prospective cohort studies found a U-shaped association between body mass index and dementia risk, with obesity predicting an 80% increase in the risk of incident AD dementia.51 With respect to the influence of age, midlife obesity has been associated with an increased risk of later-life dementia,51, 52 whereas later life obesity has been associated with a reduced risk of dementia.52 The relationship between midlife obesity and dementia may be underpinned by higher vascular risk, and the influence of adipokines such as leptin.53 Later life obesity may indicate a reduced risk of AD given that weight loss may signify poor health, frailty and the beginning of a neurodegenerative process. Thus, midlife obesity and diabetes have been associated with increased dementia risk whereas a decline in body mass index may precede incident dementia.
Diabetes and obesity: bucking the trend
Unlike many of the aforementioned vascular risk factors, rates of obesity and diabetes are increasing in the FHS and the US.3, 54, 55 It is unclear how rising rates of diabetes and obesity will influence future trends in dementia. Jones and Green warn against complacency, noting that, as the burden of dementia is malleable, it can easily relapse.56
Other vascular risk factors
There are many other vascular risk factors, not discussed here, that are associated with dementia (i.e. homocysteine, diet, lifestyle). For example, the FHS demonstrated that elevated homocysteine was associated with an increased risk of AD dementia57 and that fortification of enriched grain products with folic acid in the 1990s was linked with increases in folate status and reductions in homocysteine concentrations.58
Do temporal trends in vascular risk factors contribute to the declining incidence of dementia?
We have observed tremendous improvements in BP and other stroke risk factors over the same period in which we have observed a declining incidence of dementia. Studies have used Population Attributable Risk (PAR) estimates in an attempt to quantify the contribution of vascular risk factors to the global burden of AD. Results suggest that approximately half of AD cases might be attributable to a set of seven modifiable risk factors: smoking, physical inactivity, midlife hypertension, diabetes, low educational attainment, and depression.59 However, after accounting for non-independence between risk factors, the number of AD cases potentially attributable to modifiable risk factors reduced to one-third.59 Vascular risk factors often co-exist, and it is unclear whether the presence of any one risk factor is sufficient to cause dementia on its own. Thus, it cannot be assumed that reducing a single vascular risk factor will meaningfully lower the global burden of dementia.
Insight from the Framingham Heart Study
The declining incidence of dementia in the FHS coincides with a decline in stroke incidence and vascular risk factors, and better treatment of those vascular risk factors (Figure 1). However, in our recent analysis, trends in vascular risk factors did not convincingly account for the temporal trends in dementia (Table 1).3 In our study, first, we included adjustments for baseline levels of individual vascular risk factors, an aggregate measure of vascular risk in the Framingham Stroke Risk Profile and disease events such as stroke. Second, we included adjustments for midlife (age 50 years) vascular risk factors. We did this because data comparing the 2nd and 3rd generation of FHS participants who were studied at average ages of 62 and 42 respectively suggest that the impact of vascular risk factors on an individual may be greater earlier in life.60, 61 Thus, when comparing the brain age of a hypertensive person with a normotensive person using an aggregate imaging measure, a difference of 2 years was noted at age 62, versus 7 years at age 42.
Table 1.
Adjusted temporal trends in dementia incidence
| Dementia cases (N) |
Observation periods (N) |
5-year HR (95% CI) | Trend | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Adjustment for | Epoch 1 | Epoch 2 | Epoch 3 | Epoch 4 | HR (95% CI) | P | ||
| Age and sex | 371 | 9015 | Ref | 0.78 (0.59–1.04) | 0.62 (0.47–0.83) | 0.56 (0.41–0.77) | 0.80 (0.72–0.90) | <0.001 |
| Baseline systolic BP | 371 | 9009 | Ref | 0.80 (0.60–1.06) | 0.62 (0.47–0.83) | 0.55 (0.41–0.75) | 0.80 (0.72–0.89) | <0.001 |
| Midlife Systolic BP | 284 | 7418 | Ref | 0.76 (0.56–1.01) | 0.61 (0.46–0.81) | 0.54 (0.40–0.75) | 0.79 (0.71–0.89) | <0.001 |
| Baseline Type-2 diabetes | 308 | 8466 | Ref | 0.70 (0.52–0.95) | 0.62 (0.46–0.83) | 0.47 (0.33–0.66) | 0.76 (0.67–0.86) | <0.001 |
| Midlife Type-2 diabetes | 284 | 7418 | Ref | 0.76 (0.55–1.06) | 0.55 (0.39–0.76) | 0.50 (0.35–0.71) | 0.76 (0.67–0.86) | <0.001 |
| Preexisting and incident Stroke | 371 | 9015 | Ref | 0.78 (0.59–1.04) | 0.62 (0.47–0.82) | 0.58 (0.42–0.78) | 0.81 (0.72–0.90) | <0.001 |
BP = blood pressure; Ref = reference group.
All models are adjusted for age and sex. The baseline examination period was 1977–1983 for epoch 1, 1986–1991 for epoch 2, 1992–1998 for epoch 3, and 2004–2008 for epoch 4. Data are from Satizabal et al.3
However, such adjustments also did not substantially explain the observed trends in dementia incidence. Third, we examined temporal trends in the effects of stroke, cardiovascular disease and vascular risk factors on dementia incidence. We observed that there was a decrease in the risk of dementia associated with having a stroke. Thirty years ago, the 5-year relative risk of developing dementia was 9 times higher in a person who suffered a stroke compared to persons free of stroke. In the past decade it was only 40% greater. This improvement in risk associated with a stroke is likely due to detection of milder strokes using MRI diffusion weighted imaging, more effective early treatment and later rehabilitation as well as better prevention of recurrent strokes. The risk of dementia associated with heart failure and atrial fibrillation was also less in the third epoch relative to the first.3 However, the lower risk associated with clinical stroke and other clinical cardiovascular events only partly explained the favorable trends in dementia risk in the FHS sample. In summary, improvements in vascular health did not completely account for the declining incidence of dementia in our analyses.
Challenges in understanding the role of vascular risk factors in dementia risk
Should we conclude that the declining temporal trends in dementia are not due to improvements in vascular health? We urge caution in jumping to this conclusion because there are many sources of potential impact that need to be considered (Table 2). Our research has linked numerous vascular risk factors to the risk of developing dementia later in life, including AD, and the complexities of these associations should not be underestimated. The relationship between any one vascular risk factor and dementia is dynamic and can change over the lifespan. Individuals appear to move through different time windows with aging, in each of which, different risk factors assume greater importance. Risk factors may even have different effects depending on age, as in the case of hypertension18 and obesity.52 In a recent issue of Stroke, we demonstrated that adhering to the AHA’s Ideal-Cardiovascular Health guidelines was associated with a reduced risk of incident VaD, but neither all-cause dementia nor AD dementia.64 When we examined Ideal-Cardiovascular Health just 6 years earlier in life, higher scores predicted a reduced risk of incident all-cause dementia and clinical AD. These and other examples demonstrate that noise, complexities in the data or failure to capture cumulated exposure may prevent us from understanding the true role of vascular risk factors in the declining incidence of dementia.
Table 2.
Challenges and controversies in understanding the role of vascular risk factors in dementia risk
|
Table 2 illustrates some of the challenges in understanding the role of vascular risk factors in the development of incident AD dementia. One important issue is that examining a vascular risk factor at a single time point does not provide information on cumulative exposure. This is a significant limitation due to the long preclinical phase and insidious nature of AD. Cumulated exposure to vascular risk factors could be investigated by using surrogate markers of exposure burden and indicators of preclinical disease.65 As an example, left ventricular hypertrophy, carotid intima-media thickness, aortic atherosclerosis, white matter hyperintensity burden or retinal pathology could be used as indicators of prolonged BP elevation over many years as compared to a BP measurement taken at a single time point. Another issue is the role of genetic variants in determining susceptibility to vascular risk and lifestyle changes.62, 66, 67
We must respond to the evidence and additionally consider non-vascular factors
Whereas the appropriate prevention, treatment, and management of vascular disease are undeniably important for the prevention of stroke and vascular cognitive impairment, we must consider the role of non-vascular factors in the declining incidence of dementia. We may need to go one step further, temporarily hang our white coats, and consider that the explanation for the declining incidence of dementia need not be medical. Changes in education, culture, occupational demands, nutrition, social support and environmental factors are all possible contributing factors remaining to be confirmed or refuted. The answer may also lie in changes experienced in very early life or even in utero.
Conclusions
How we explain the declining incidence of dementia will substantially impact prevention guidelines, therapeutic strategies, research investment and ultimately our ability to further reduce dementia risk in our aging population.56 There is substantial evidence linking vascular risk factors to the risk of incident dementia, including clinical AD. However, the favorable trends in vascular risk factors do not entirely explain the declining incidence of dementia. Despite PAR estimates suggesting that vascular risk factors contribute to a significant portion of AD burden, the degree to which reducing individual vascular risk factors will mitigate the risk of dementia remains unclear, hard to quantify and likely dependent on numerous interacting factors. Careful observational studies in this area will need to extend across multiple, large cohorts and the entire life course from early to late adult life. They should combine large meta-analyses to identify societal and public health interventions most effective in reducing population burden of risk with a search for targeted approaches that may be necessary to go further beyond these ‘low-hanging fruit.' Targeted prevention approaches will likely require that we examine the precise characteristics of the vascular or lifestyle risk factor that is most strongly associated with risk and protection, understand the underlying biological pathways and identify the persons, defined by genetics, concomitant disease or other factors, most likely to benefit from such interventions.
The final proof will rest with methodologically sound intervention studies examining how the treatment of vascular risk factors affects the risk of dementia and surrogate endpoints such as cognition and brain atrophy. However, such intervention studies completed to date have mostly yielded discouraging results.21, 38 We must listen to the evidence and consider the complimentary role of non-vascular factors in the declining incidence of dementia. The favorable trends raise hope that morbidity due to dementia can be delayed and diminished and urge renewed investment in understanding, sustaining and accelerating this trend.
Acknowledgments
Funding Sources: Dr. Pase is funded by an Australian National Health and Medical Research Council Early Career Fellowship (APP1089698). The Framingham Heart Study is supported by the National Heart, Lung, and Blood Institute (contract no. N01-HC-25195 and no. HHSN268201500001I) and by grants from the National Institute on Aging (R01 AG054076, R01 AG049607, R01 AG033193, U01 AG049505, U01 AG052409) and the National Institute of Neurological Disorders and Stroke (NS017950 and UH2 NS100605).
Footnotes
Disclosures: The authors report no potential conflicts of interest and no disclosures.
References
- 1.Corrada MM, Brookmeyer R, Paganini-Hill A, Berlau D, Kawas CH. Dementia incidence continues to increase with age in the oldest old the 90+ study. Ann of Neurol. 2010;67:114–121. doi: 10.1002/ana.21915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alzheimer’s Association. 2015 alzheimer's disease facts and figures. Alzheimer Dement. 2015;11:332–384. doi: 10.1016/j.jalz.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Satizabal CL, Beiser AS, Chouraki V, Chêne G, Dufouil C, Seshadri S. Incidence of dementia over three decades in the framingham heart study. New Engl J Med. 2016;374:523–532. doi: 10.1056/NEJMoa1504327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rocca WA, Petersen RC, Knopman DS, Hebert LE, Evans DA, Hall KS, et al. Trends in the incidence and prevalence of alzheimer's disease, dementia, and cognitive impairment in the united states. Alzheimer Dement. 2011;7:80–93. doi: 10.1016/j.jalz.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matthews FE, Arthur A, Barnes LE, Bond J, Jagger C, Robinson L, et al. A two-decade comparison of prevalence of dementia in individuals aged 65 years and older from three geographical areas of england: Results of the cognitive function and ageing study i and ii. Lancet. 2013;382:1405–1412. doi: 10.1016/S0140-6736(13)61570-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qiu C, von Strauss E, Backman L, Winblad B, Fratiglioni L. Twenty-year changes in dementia occurrence suggest decreasing incidence in central stockholm, sweden. Neurology. 2013;80:1888–1894. doi: 10.1212/WNL.0b013e318292a2f9. [DOI] [PubMed] [Google Scholar]
- 7.Langa KM, Larson EB, Crimmins EM, Faul JD, Levine DA, Kabeto MU, et al. A comparison of the prevalence of dementia in the united states in 2000 and 2012. JAMA Intern Med. 2017;177:51–58. doi: 10.1001/jamainternmed.2016.6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Debette S, Beiser A, DeCarli C, Au R, Himali JJ, Kelly-Hayes M, et al. Association of mri markers of vascular brain injury with incident stroke, mild cognitive impairment, dementia, and mortality: The framingham offspring study. Stroke. 2010;41:600–606. doi: 10.1161/STROKEAHA.109.570044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.White L, Small BJ, Petrovitch H, Ross GW, Masaki K, Abbott RD, et al. Recent clinical-pathologic research on the causes of dementia in late life: Update from the honolulu-asia aging study. J Geriatr Psychiatry and Neurol. 2005;18:224–227. doi: 10.1177/0891988705281872. [DOI] [PubMed] [Google Scholar]
- 10.de la Torre JC. Alzheimer disease as a vascular disorder: Nosological evidence. Stroke. 2002;33:1152–1162. doi: 10.1161/01.str.0000014421.15948.67. [DOI] [PubMed] [Google Scholar]
- 11.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, et al. Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hachinski VC, Lassen NA, Marshall J. Multi-infarct dementia. A cause of mental deterioration in the elderly. Lancet. 1974;304:207–209. doi: 10.1016/s0140-6736(74)91496-2. [DOI] [PubMed] [Google Scholar]
- 13.Zhou J, Yu JT, Wang HF, Meng XF, Tan CC, Wang J, et al. Association between stroke and alzheimer's disease: Systematic review and meta-analysis. J Alz Dis. 2015;43:479–489. doi: 10.3233/JAD-140666. [DOI] [PubMed] [Google Scholar]
- 14.Carandang RA, Wolf PA. Trends in stroke over the past 50 years: The framingham study. Cardiol Rev. 2007;24:21–23. [Google Scholar]
- 15.Wieberdink RG, Ikram MA, Hofman A, Koudstaal PJ, Breteler MMB. Trends in stroke incidence rates and stroke risk factors in rotterdam, the netherlands from 1990 to 2008. Eur J Epidemiol. 2012;27:287–295. doi: 10.1007/s10654-012-9673-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koton S, Schneider ALC, Rosamond WD, Shahar E, Sang Y, Gottesman RF, et al. Stroke incidence and mortality trends in us communities, 1987 to 2011. JAMA. 2014;312:259–268. doi: 10.1001/jama.2014.7692. [DOI] [PubMed] [Google Scholar]
- 17.Scuteri A, Nilsson PM, Tzourio C, Redon J, Laurent S. Microvascular brain damage with aging and hypertension: Pathophysiological consideration and clinical implications. J Hypertens. 2011;29:1469–1477. doi: 10.1097/HJH.0b013e328347cc17. [DOI] [PubMed] [Google Scholar]
- 18.Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005;4:487–499. doi: 10.1016/S1474-4422(05)70141-1. [DOI] [PubMed] [Google Scholar]
- 19.Sharp SI, Aarsland D, Day S, Sønnesyn H, Ballard C. Hypertension is a potential risk factor for vascular dementia: Systematic review. Int J Geriatr Psychiatry. 2011;26:661–669. doi: 10.1002/gps.2572. [DOI] [PubMed] [Google Scholar]
- 20.Power MC, Weuve J, Gagne JJ, McQueen MB, Viswanathan A, Blackera D. The association between blood pressure and incident alzheimer disease: A systematic review and meta-analysis. Epidemiology. 2011;22:646–659. doi: 10.1097/EDE.0b013e31822708b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGuinness B, Todd S, Passmore P, Bullock R. Blood pressure lowering in patients without prior cerebrovascular disease for prevention of cognitive impairment and dementia. Cochrane Database Syst Rev. 2009;4:CD004034. doi: 10.1002/14651858.CD004034.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forette F, Seux ML, Staessen JA, Thijs L, Babarskiene MR, Babeanu S, et al. The prevention of dementia with antihypertensive treatment: New evidence from the systolic hypertension in europe (syst-eur) study. Arch Intern Med. 2002;162:2046–2052. doi: 10.1001/archinte.162.18.2046. [DOI] [PubMed] [Google Scholar]
- 23.Iadecola C, Yaffe K, Biller J, Bratzke LC, Faraci FM, Gorelick PB, et al. Impact of hypertension on cognitive function: A scientific statement from the american heart association. Hypertension. 2016;68:e67–e94. doi: 10.1161/HYP.0000000000000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Preis SR, Pencina MJ, Hwang SJ, D'Agostino RB, Savage PJ, Levy D, et al. Trends in cardiovascular disease risk factors in individuals with and without diabetes mellitus in the framingham heart study. Circulation. 2009;120:212–220. doi: 10.1161/CIRCULATIONAHA.108.846519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the united states, 1988–2000. JAMA. 2003;290:199–206. doi: 10.1001/jama.290.2.199. [DOI] [PubMed] [Google Scholar]
- 26.Psaty BM, Manolio TA, Smith NL, Heckbert SR, Gottdiener JS, Burke GL, et al. Time trends in high blood pressure control and the use of antihypertensive medications in older adults: The cardiovascular health study. Arch Int Med. 2002;162:2325–2332. doi: 10.1001/archinte.162.20.2325. [DOI] [PubMed] [Google Scholar]
- 27.Egan BM, Zhao Y, Axon R. Us trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA. 2010;303:2043–2050. doi: 10.1001/jama.2010.650. [DOI] [PubMed] [Google Scholar]
- 28.Wolf PA, D'Agostino RB, Kannel WB, Bonita R, Belanger AJ. Cigarette smoking as a risk factor for stroke. The framingham study. JAMA. 1988;259:1025–1029. [PubMed] [Google Scholar]
- 29.Anstey KJ, Von Sanden C, Salim A, O'Kearney R. Smoking as a risk factor for dementia and cognitive decline: A meta-analysis of prospective studies. Am J Epidemiol. 2007;166:367–378. doi: 10.1093/aje/kwm116. [DOI] [PubMed] [Google Scholar]
- 30.Center for Disease Control and Prevention. Trends in current cigarette smoking among high school students and adults, united states, 1965–2014. Center for Disease Control and Prevention. [Acessed 12/31/2016]; https://www.cdc.gov/tobacco/data_statistics/tables/trends/cig_smoking/
- 31.Ng M, Freeman MK, Fleming TD, Robinson M, Dwyer-Lindgren L, Thomson B, et al. Smoking prevalence and cigarette consumption in 187 countries, 1980–2012. JAMA. 2014;311:183–192. doi: 10.1001/jama.2013.284692. [DOI] [PubMed] [Google Scholar]
- 32.Giskes K, Kunst AE, Benach J, Borrell C, Costa G, Dahl E, et al. Trends in smoking behaviour between 1985 and 2000 in nine european countries by education. J Epidemiol Community health. 2005;59:395–401. doi: 10.1136/jech.2004.025684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Puglielli L, Tanzi RE, Kovacs DM. Alzheimer's disease: The cholesterol connection. Nature Neurosci. 2003;6:345–351. doi: 10.1038/nn0403-345. [DOI] [PubMed] [Google Scholar]
- 34.Pikula A, Beiser AS, Wang J, Himali JJ, Kelly-Hayes M, Kase CS, et al. Lipid and lipoprotein measurements and the risk of ischemic vascular events: Framingham study. Neurology. 2015;84:472–479. doi: 10.1212/WNL.0000000000001202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tan ZS, Seshadri S, Beiser A, Wilson PW, Kiel DP, Tocco M, et al. Plasma total cholesterol level as a risk factor for alzheimer disease: The framingham study. Arch Intern Med. 2003;163:1053–1057. doi: 10.1001/archinte.163.9.1053. [DOI] [PubMed] [Google Scholar]
- 36.Anstey KJ, Lipnicki DM, Low LF. Cholesterol as a risk factor for dementia and cognitive decline: A systematic review of prospective studies with meta-analysis. Am J Geriatr Psychiatry. 2008;16:343–354. doi: 10.1097/JGP.0b013e31816b72d4. [DOI] [PubMed] [Google Scholar]
- 37.Jick H, Zornberg GL, Jick SS, Seshadri S, Drachman DA. Statins and the risk of dementia. Lancet. 2000;356:1627–1631. doi: 10.1016/s0140-6736(00)03155-x. [DOI] [PubMed] [Google Scholar]
- 38.McGuinness B, Craig D, Bullock R, Passmore P. Statins for the prevention of dementia. Cochrane Database of Syst Rev. 2016;1:CD003160. doi: 10.1002/14651858.CD003160.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ingelsson E, Massaro JM, Sutherland P, Jacques PF, Levy D, Dagostino RB, et al. Contemporary trends in dyslipidemia in the framingham heart study. Arch Int Med. 2009;169:279–286. doi: 10.1001/archinternmed.2008.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carroll MD, Lacher DA, Sorlie PD, Cleeman JI, Gordon DJ, Wolz M, et al. Trends in serum lipids and lipoproteins of adults, 1960–2002. JAMA. 2005;294:1773–1781. doi: 10.1001/jama.294.14.1773. [DOI] [PubMed] [Google Scholar]
- 41.Santangeli P, Di Biase L, Bai R, Mohanty S, Pump A, Cereceda Brantes M, et al. Atrial fibrillation and the risk of incident dementia: A meta-analysis. Heart Rhythm. 2012;9:1761–1768. doi: 10.1016/j.hrthm.2012.07.026. [DOI] [PubMed] [Google Scholar]
- 42.Kwok CS, Loke YK, Hale R, Potter JF, Myint PK. Atrial fibrillation and incidence of dementia: A systematic review and meta-analysis. Neurology. 2011;76:914–922. doi: 10.1212/WNL.0b013e31820f2e38. [DOI] [PubMed] [Google Scholar]
- 43.Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, McManus DD, et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the framingham heart study: A cohort study. Lancet. 2015;386:154–162. doi: 10.1016/S0140-6736(14)61774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ott A, Breteler MMB, Van Harskamp F, Claus JJ, Van der Cammen TJM, Grobbee DE, et al. Prevalence of alzheimer's disease and vascular dementia: Association with education. The rotterdam study. Brit Med J. 1995;310:970–973. doi: 10.1136/bmj.310.6985.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R. Influence of education and occupation on the incidence of alzheimer's disease. JAMA. 1994;271:1004–1010. [PubMed] [Google Scholar]
- 46.Ngandu T, von Strauss E, Helkala EL, Winblad B, Nissinen A, Tuomilehto J, et al. Education and dementia: What lies behind the association? Neurology. 2007;69:1442–1450. doi: 10.1212/01.wnl.0000277456.29440.16. [DOI] [PubMed] [Google Scholar]
- 47.Ryan C, Bauman K. Educational attainment in the united states: 2015. United States Census. [Accessed 31/12/2016]; http://www.census.gov/content/dam/Census/library/publications/2016/demo/p20-578.pdf.
- 48.Akomolafe A, Beiser A, Meigs JB, Au R, Green RC, Farrer LA, et al. Diabetes mellitus and risk of developing alzheimer disease: Results from the framingham study. Arch Neurol. 2006;63:1551–1555. doi: 10.1001/archneur.63.11.1551. [DOI] [PubMed] [Google Scholar]
- 49.Chatterjee S, Peters SAE, Woodward M, Mejia Arango S, Batty GD, Beckett N, et al. Type 2 diabetes as a risk factor for dementia in women compared with men: A pooled analysis of 2.3 million people comprising more than 100,000 cases of dementia. Diabetes care. 2016;39:300–307. doi: 10.2337/dc15-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abner EL, Nelson PT, Kryscio RJ, Schmitt FA, Fardo DW, Woltjer RL, et al. Diabetes is associated with cerebrovascular but not alzheimer's disease neuropathology. Alzheimer Dement. 2016;12:882–889. doi: 10.1016/j.jalz.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beydoun MA, Beydoun HA, Wang Y. Obesity and central obesity as risk factors for incident dementia and its subtypes: A systematic review and meta-analysis. Obesity Rev. 2008;9:204–218. doi: 10.1111/j.1467-789X.2008.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fitzpatrick AL, Kuller LH, Lopez OL, Diehr P, O'Meara ES, Longstreth WT, Jr, et al. Midlife and late-life obesity and the risk of dementia: Cardiovascular health study. Arch Neurol. 2009;66:336–342. doi: 10.1001/archneurol.2008.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lieb W, Beiser AS, Vasan RS, Tan ZS, Au R, Harris TB, et al. Association of plasma leptin levels with incident alzheimer’s disease and mri measures of brain aging: The framingham study. JAMA. 2009;302:2565–2572. doi: 10.1001/jama.2009.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the united states, 1988–2012. JAMA. 2015;314:1021–1029. doi: 10.1001/jama.2015.10029. [DOI] [PubMed] [Google Scholar]
- 55.Okosun IS, Chandra KMD, Boev A, Boltri JM, Choi ST, Parish DC, et al. Abdominal adiposity in u.S. Adults: Prevalence and trends, 1960–2000. Prev Med. 2004;39:197–206. doi: 10.1016/j.ypmed.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 56.Jones DS, Greene JA. Is dementia in decline? Historical trends and future trajectories. New Engl J Med. 2016;374:507–509. doi: 10.1056/NEJMp1514434. [DOI] [PubMed] [Google Scholar]
- 57.Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D'Agostino RB, et al. Plasma homocysteine as a risk factor for dementia and alzheimer's disease. New Engl J Med. 2002;346:476–483. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- 58.Jacques PF, Selhub J, Bostom AG, Wilson PWF, Rosenberg IH. The effect of folic acid fortification on plasma folate and total homocysteine concentrations. New Engl J Med. 1999;340:1449–1454. doi: 10.1056/NEJM199905133401901. [DOI] [PubMed] [Google Scholar]
- 59.Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of alzheimer's disease: An analysis of population-based data. Lancet Neurol. 2014;13:788–794. doi: 10.1016/S1474-4422(14)70136-X. [DOI] [PubMed] [Google Scholar]
- 60.Maillard P, Seshadri S, Beiser A, Himali JJ, Au R, Fletcher E, et al. Effects of systolic blood pressure on white-matter integrity in young adults in the framingham heart study: A cross-sectional study. Lancet Neurol. 2012;11:1039–1047. doi: 10.1016/S1474-4422(12)70241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seshadri S, Wolf PA, Beiser A, Elias MF, Au R, Kase CS, et al. Stroke risk profile, brain volume, and cognitive function: The framingham offspring study. Neurology. 2004;63:1591–1599. doi: 10.1212/01.wnl.0000142968.22691.70. [DOI] [PubMed] [Google Scholar]
- 62.Pase MP, Beiser A, Aparicio H, DeCarli C, Vasan RS, Murabito J, et al. Interarm differences in systolic blood pressure and the risk of dementia and subclinical brain injury. Alzheimer Dement. 2016;12:438–445. doi: 10.1016/j.jalz.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hofman A, Ott A, Breteler MMB, Bots ML, Slooter AJC, van Harskamp F, et al. Atherosclerosis, apolipoprotein e, prevalence of dementia and alzheimer's disease in the rotterdam study. Lancet. 1997;349:151–154. doi: 10.1016/S0140-6736(96)09328-2. [DOI] [PubMed] [Google Scholar]
- 64.Pase MP, Beiser A, Enserro D, Xanthakis V, Aparicio H, Satizabal CL, et al. Association of ideal cardiovascular health with vascular brain injury and incident dementia. Stroke. 2016;47:1201–1206. doi: 10.1161/STROKEAHA.115.012608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Seshadri S. Indices of subclinical atherosclerosis: Signposts on the highway to disease. J Am Coll Cardiol Cardiovasc Img. 2014;7:1116–1118. doi: 10.1016/j.jcmg.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Qi Q, Chu AY, Kang JH, Jensen MK, Curhan GC, Pasquale LR, et al. Sugar-sweetened beverages and genetic risk of obesity. New Engl J Med. 2012;367:1387–1396. doi: 10.1056/NEJMoa1203039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Erickson KI, Banducci SE, Weinstein AM, Macdonald AW, 3rd, Ferrell RE, Halder I, et al. The brain-derived neurotrophic factor val66met polymorphism moderates an effect of physical activity on working memory performance. Psychol Sci. 2013;24:1770–1779. doi: 10.1177/0956797613480367. [DOI] [PMC free article] [PubMed] [Google Scholar]