Abstract
Background
Up to 60% of individuals with moderate to severe chronic hemiparetic stroke experience excessive involuntary wrist/finger flexion that constrains functional hand movements including hand opening. It’s not known how stroke-induced brain injury impacts volitional hand opening and grasping forces as a result of the expression of abnormal coupling between shoulder abduction and wrist/finger flexion or the flexion synergy.
Objective
The goal of this study is to understand how shoulder abduction loading affects volitional hand opening and grasping forces in individuals with moderate to severe chronic hemiparetic stroke.
Methods
36 individuals (stroke: 26, control: 10) were recruited for this study. Each participant was instructed to perform maximal hand opening and grasping forces while the arm was either fully supported or lifted with a weight equal to 25% or 50% of the participant’s maximum shoulder abduction torque. Hand pentagon area, defined as the area formed by the tips of thumb and fingers, was calculated during hand opening. Forces were recorded during grasping.
Results
In individuals with moderate stroke, increasing shoulder abduction loading reduced the ability to maximally open the hand. In individuals with severe stroke, who were not able to open the hand, grasping forces were generated and increased with shoulder abduction loading. Stroke individuals also showed a reduced ability to control volitional grasping forces due to the enhanced expression of flexion synergy.
Conclusions
Shoulder abduction loading reduced the ability to volitionally open the hand and control grasp forces after stroke. Neural mechanisms and clinical implications of these findings are discussed.
Introduction
A majority of individuals with stroke report impaired upper extremity function, particularly of the hand, as a major problem.1 In addition to wrist and finger weakness being greater for extensors compared to flexors2–3 and a significant loss of finger individuation,4–6 lifting of the paretic arm results in abnormal involuntary coupling with wrist and finger flexion. This abnormal coupling is proportional to the amount of lifting or shoulder abduction7 and is commonly referred to as the “flexion synergy”.8 A review of past studies shows that the paretic hand post stroke has been mostly studied in isolation, i.e. with the arm fully supported against gravity,2–5 thus neglecting the deleterious effects of flexion synergy on hand function. Even though more recently the effect of the flexion synergy was considered during lifting and reaching,7 it was studied with the paretic hand relaxed. Failure to study hand dysfunction within the context of proximal joint demands has impeded the progression of the field in its attempts to discern the mechanisms responsible for hand dysfunction and subsequently develop more effective targeted rehabilitation interventions.
The goal of this study is therefore to determine, for the first time, the effect of the expression of the flexion synergy on volitional hand function, examined by measuring the effect of various levels of shoulder abduction loading on hand opening and grasping. This should advance our understanding on how the paretic hand function is impacted by proximal joint demands post stroke. Our findings are that individuals with moderate to severe chronic hemiparetic stroke showed a reduced ability to volitionally open the paretic hand with increased shoulder abduction loading, quantified by a decreased hand pentagon area. The ability to control volitional grasping forces was also reduced with increased shoulder abduction loading. Note that neither of these effects were found in control participants. The underlying neural mechanisms and clinical implications of these findings are discussed. Parts of this work has been previously published in a conference proceeding.9
Methods
Participants
A total of 36 individuals (Stroke: 26, Control: 10) participated in this study. Participant demographics are listed in Table 1. Control participants were age-matched to the stroke participants and reported no history of cerebral vascular accidents. Stroke participants were selected from the Clinical Neuroscience Research Registry that is housed in the Rehabilitation Institute of Chicago, as well as from individuals residing in the Chicago area who wished to participate in the study. Qualified stroke participants met the following inclusion criteria: 1) Sustained a unilateral lesion at least one year prior to participation in this project; 2) Paresis confined to one side; 3) Absence of a brainstem and/or cerebellar lesion; 4) Absence of severe concurrent medical problems (e.g. cardiorespiratory impairment, changes in management of hypertension); 5) Absence of any acute or chronic painful condition in the upper extremities or spine; 6) Capacity to provide informed consent; 7) Ability to elevate their limb against gravity up to horizontal and to generate some active elbow extension; and 8) Fugl-Meyer Assessment (FMA)10 within the range of 10~40 out of a possible 66 and 2~5 out of a possible 7 in Chedoke-McMaster Stroke Hand Assessment (CMSAh).11 Stroke individuals were assigned to one of two groups: individuals with severe impairment (N=13, FMA=10~25/CMSAh=2~3) and individuals with moderate impairment (N=13, FMA=26~40/CMSAh=4~5). All participants gave informed consent for participation in this study, which was approved by the Institutional Review Board of Northwestern University in accordance with the ethical standards stipulated by the 1964 Declaration of Helsinki for research involving human subjects.
Table 1.
Participant demographics
| Stroke | Control | |
|---|---|---|
| Age (Yrs.) | 59±9 (40–71) | 55±12 (42–83) |
| Gender (M/F) | 19/7 | 6/4 |
| Time since stroke (Yrs) | 11±7 (1–28) | |
| Sides of tested UE* (L/R) | 16/10 | 0/10 |
| UE FMA | 26±10 (12–39) | |
| CMSAh | 3±1 (2–5) |
Abbreviations: Yrs = years; M = male; F = female; L = left; R = right; UE = upper extremity; FMA = Fugl-Meyer Assessment; CMSAh = Chedoke-McMaster Stroke Assessment (hand); Values are listed as Mean±SD (range).
In this experiment, the stroke subjects were tested at the paretic UE while the control subjects were tested at the dominant UE.
Experimental setup
The experiment was carried out using an arm coordination training 3-D system (ACT3D, Figure 1A), which consists of a modified HapticMaster robot (Moog-FCR B.V., the Netherlands) and a Biodex chair and T-base support system (Biodex Medical Systems, Shirley, NY). The ACT3D allows for free movement in three dimensions and was used to modulate forces applied to the arm while lifting the arm thus changing shoulder abduction (SABD) loading levels.12–14 Prior to the experiment, each participant’s maximum SABD torque was measured using a manual dynamometer (Lafayette Instrument Company, Lafayette, IN, USA) placed just proximal to the axis of rotation of the elbow, close to the lateral epicondyle of the humerus, in a limb configuration of 85° shoulder abduction, 45° shoulder flexion, 90° elbow flexion. This limb configuration depicted the arm posture at the start of the reach where the participant lifted the upper arm to the side of the body to the level almost parallel to the horizontal plane through the shoulder and then positioned with the tip of the fingers aligned with the mid-sagittal plan in front of the body. Once the maximum SABD torque was obtained, each participant was seated in a Biodex chair (Biodex Medical Systems, Shirley, NY) with the trunk strapped to the back of the chair to prevent unwanted movement of the upper body. The to-be-tested forearm was placed in a forearm orthosis and the fingers/palm placed over a cylinder. The cylinder was rigidly attached to the orthosis and instrumented with a pressure sensor mat (Pressure Profile System Inc., Los Angeles, CA, USA, Figure 1C). The orthosis/cylinder setup was also rigidly coupled to the end effector of the ACT3D. The sensor mat allowed real-time measurement of the pressure generated under each finger and thumb during the hand grasping task (Figure 1D). The grasping pressure measurements were converted to provide a distribution of forces generated by the thumb and fingers. Hand kinematic data was collected using two Optotrak camera systems (Optotrak 3020 and Optotrak Certus, Northern Digital Inc., Waterloo, ON, Canada). Five infrared, light-emitting diode markers were placed on the tip of the thumb and each finger. Nine additional markers were embedded in a Styrofoam ball to form a rigid body which was placed on the back of the hand (Figure 1C). The rigid body provided a dynamic reference for the markers on the thumb and fingers, so that upper limb movement did not affect the kinematic measurements of the thumb and fingers.
Figure 1.
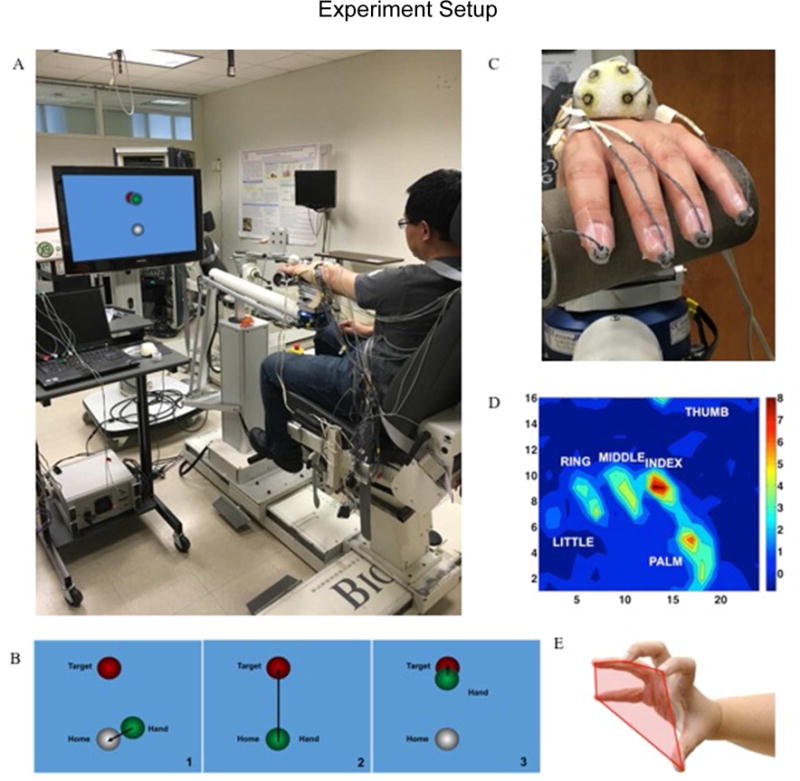
A) ACT3D system; B) visual feedback during the task; C) a close-up of the hand attachment which shows the rigid body, the TactArray sensory mat, and all the markers attached to the fingertips; D) an example of force (unit: N) distribution measured by the TactArray sensory mat; E) demonstration of the hand pentagon area.
Protocol
At the beginning of the task, participants were first required to find the home position and then to reach out to a distant target (Figure 1B). The reaching target was located one arm length away in front of the participant on the horizontal plane at the level of 90° shoulder abduction. Once the participant successfully acquired the target, he/she was instructed to either lift up (a SABD task) or not (table task). Each participant was given 2 seconds to lift the arm off the table (SABD task), or stay relaxed (table task). After 2 seconds, while maintaining the arm in a lifted (SABD task) or supported position (table task), the participant was instructed to perform one of the two hand tasks, either hand opening or grasping with a maximal effort. Participants were required to continue to maximally open the hand or grasp for a period of 5 seconds, followed by relaxation of the hand. SABD tasks included two levels of loading: 25% or 50% of maximum SABD torque, and the table condition in which the participant’s arm was fully supported. ACD3D was programmed to generate forces in the vertical direction resulting in a percentage of the participant’s maximum shoulder abduction once the arm is lifted off the haptic table (i.e. approximately an increase of 5° to 10° shoulder abduction). Ten to twelve repetitions of each hand task (N=2) at each level of SABD (N=3) were performed in a randomized order.
Data collection and analysis
A Hand pentagon area (HPA), shown to be an effective measure in evaluating hand opening ability,15 was used as the primary measure to quantify hand opening. HPA was defined as the area of the pentagon formed by the tip of the thumb and fingers (Figure 1E). All participants were asked to rest their hand on the cylinder prior to the trial and the resting HPA formed by the initial hand posture on the cylinder served as baseline. The pentagon area was baseline corrected to zero while the hand was relaxed on the cylinder and then normalized to the maximal HPA to facilitate between-subject comparisons during hand opening. To calculate the area, HPA was broken down into three triangular areas, each of which was formed by the thumb and two fingertips, as shown in the equation,
where S∆ denotes the triangular area, T, I, M, R, L are abbreviations for thumb, index, middle, ring and little fingers. Normalized HPA was calculated by dividing HPA by the maximal HPA measured when the hand was placed on a flat surface with maximal finger abduction. Peak HPA value was first identified during the hand opening period, and then an averaged HPA over a 100ms time window, centered at the peak value, was calculated as the HPA for one trial during a certain abduction condition. Marker location was collected at a sampling rate of 30 Hz. One participant in the severe group and three participants in the moderate group were excluded from data analysis due to difficulty in tracking the thumb position.
The synergy-induced grasping forces and the total grasping forces were calculated as the sum of the forces generated by the thumb and fingers averaged over the lifting phase without a grasp and with a grasp in a hand grasp task, respectively (Figure 4). The lifting phase without a grasp referred to the duration in which each participant only needed to lift the arm while generating a certain shoulder abduction load without any voluntary grasping (5.5s–8s in Figure 4). The lifting phase with a grasp referred to the duration in which the participant was asked to maximally grasp the cylinder (8s–13s in Figure 4) while lifting the arm with a specific shoulder abduction load. To quantify the grasping forces, the peak value during the hand grasping period was first identified, and then an averaged grasping force over a 100ms time window, centered over the peak value, was calculated as the grasping force per trial for a certain shoulder abduction load level. Volitional component of the grasping forces was defined as the difference between the total grasping forces and the synergy-induced grasping forces. On average, a total of 10 to 12 trials at each condition were included to quantify the total and synergy-induced grasping forces. Palm pressure areas were not considered due to the inconsistent measurements across participants. Maximal grasping forces were calculated as the average of the largest three total grasping forces across all trials and was used for normalization. Finger/thumb pressure was sampled at 100 Hz. One participant in the severe group and two participants in the moderate group were excluded due to difficulty in measuring the thumb forces on the sensory mat.
Figure 4.
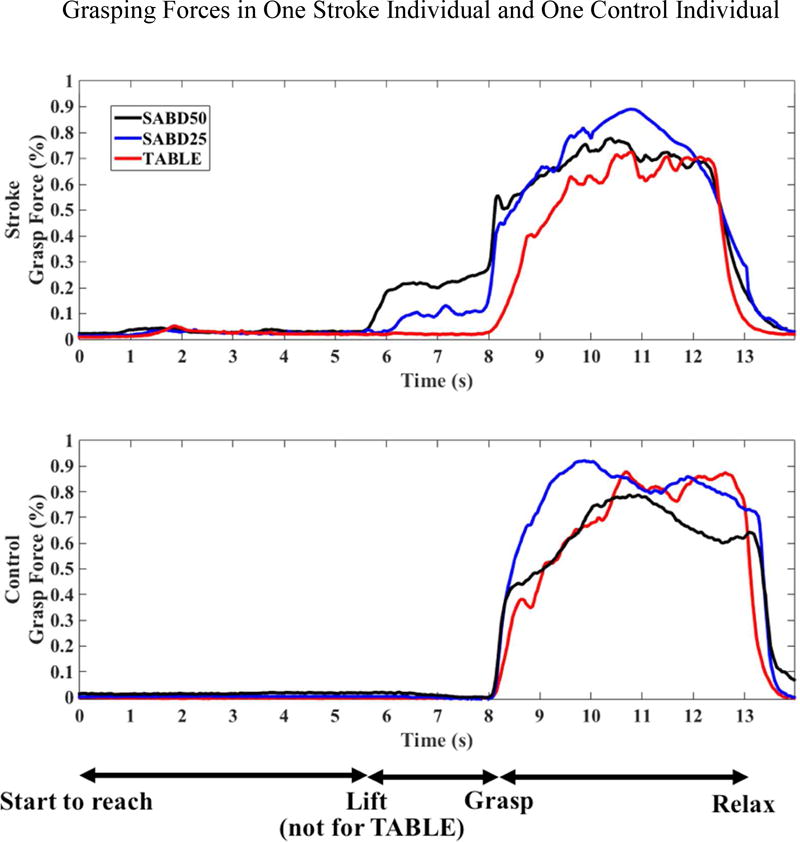
Grasping force changes in (A) one individual with moderate stroke and (B) one control individual. Participants were instructed to start to reach at time 0 second. For SABD loading, at time 5.5 second, subjects were instructed to lift the arm and at time 8 second, subjects were instructed to grasp the cylinder maximally and hold it for five seconds. For the table condition, subjects did not lift the arm. Normalized maximum grasp forces for the individual with stroke: 258.6 N, and for the control individual: 465.3 N.
Statistics
A mixed two-way analysis of variance (ANOVA) with repeated measures was conducted to determine whether loading (Table, SABD25, SABD50), group (severe, moderate, control) and/or their interaction explains the measured changes in HPA and total and synergy-induced grasping forces. Tests for homogeneity of variances and sphericity were run to avoid violating these assumptions. All the data used in this study are normally distributed (Shapiro-wilk test) and thus satisfied the assumptions of an ANOVA. Post hoc comparisons with the Bonferroni adjustment were adopted to compare within-subject differences. Unless specified otherwise, results are presented as mean ± standard error. Statistical significance was set at p<0.05. The statistical analysis was performed using the IBM SPSS version 22 software.
Results
Hand pentagon area during opening
Figure 2 depicts the changes of HPA in one individual with moderate stroke and one control individual between the Table/SABD25/SABD50 conditions when each participant tried to open the hand with a maximal effort. The HPA curves highlight a different trend between these two participants. While the control individual was able to open the hand with a consistent HPA at all levels of SABD condition, the stroke individual’s hand opening was affected by an increasing SABD loading.
Figure 2.
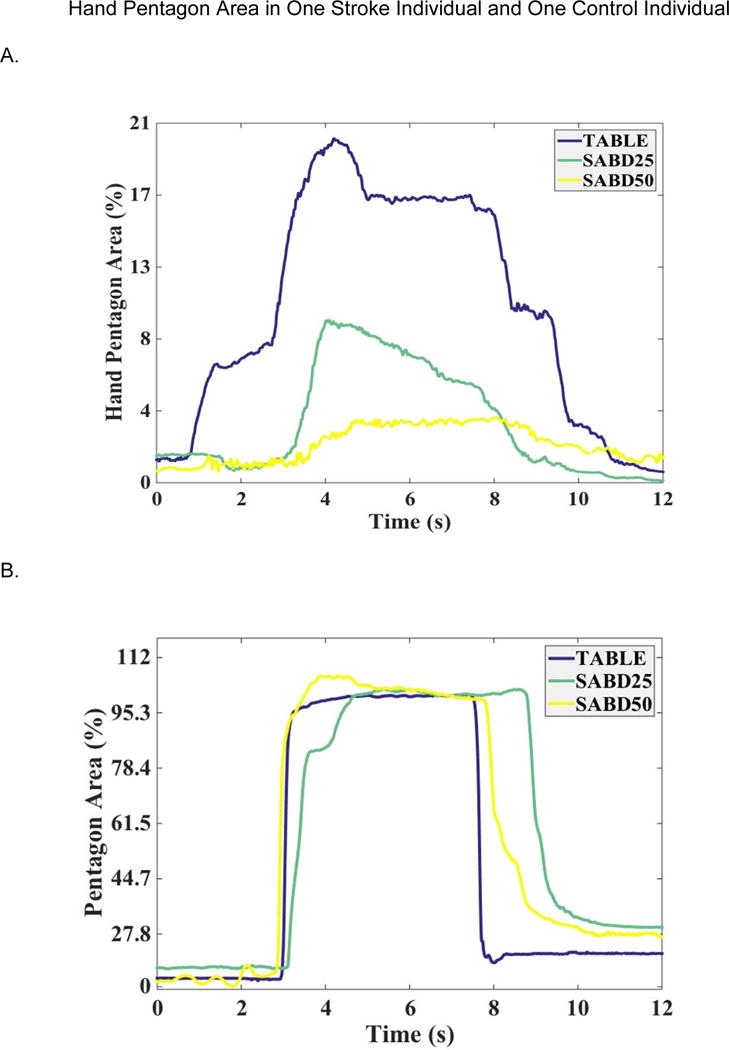
Pentagon area changes in (A) one individual with stroke and (B) one control individual. For the table condition, participants were instructed to open the hand at time 0 second. For SABD loading, at time 0 second, subjects were instructed to lift the arm and at time 2s, subjects were instructed to open the hand maximally and hold it for five seconds. Normalized maximum HPA for the individual with stroke: 119.93 cm2, and for the control individual: 118.63 cm2.
The two-way ANOVA with repeated measures for the maximal HPA found a significant effect of group (p<0.000) and an interaction effect of loading*group (p<0.000). Post hoc analysis with Bonferroni adjustment showed that the control group had the largest HPA, and the moderate group showed significantly greater HPA than the severe group. It was also found that maximal HPA decreased significantly with increased SABD loading in individuals with moderate stroke but not in the severe group nor in the control group (Figure 3A). HPA was not observed in the severe group, but instead these participants generated grasping forces when they were trying to open the hand (Figure 3B). Implementation of the two-way ANOVA for grasping forces during hand opening resulted in a significant effect of loading (p<0.000), group (p<0.000) and an interaction effect of loading*group (p<0.000). Post Hoc with Bonferroni adjustment demonstrated that the severe group generated significantly greater amount of grasping forces during hand opening than the moderate and able-bodied group (p<0.000). The severe group also generated significantly greater grasping forces at 50% of SABD loading than at 25% of SABD loading (p<0.000) and during the table condition (p<0.000). There was no significant difference in grasping forces between SABD loading conditions during hand opening for the moderate group. Grasping forces during hand opening were not observed in the control group.
Figure 3.
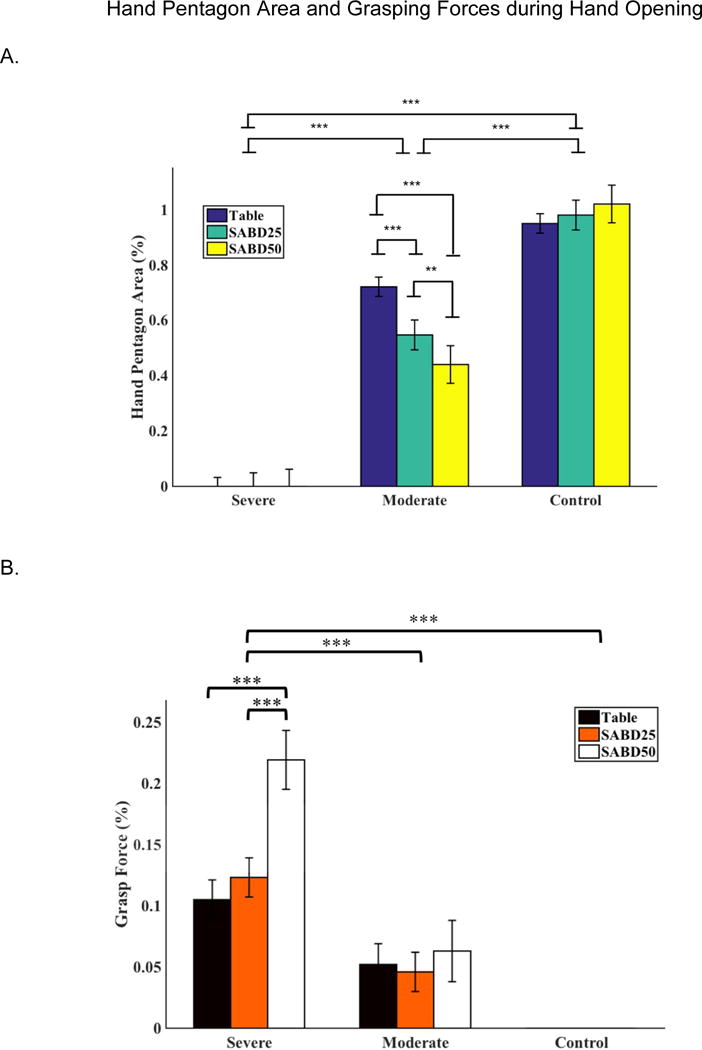
A). Pentagon area for severe (N=12), moderate (N=10), and control (N=10) groups. B). Grasping forces during hand opening for severe (N=12), moderate (N=11), control groups (N=10). *: p<0.05, **: p<0.01; ***: p<0.001.
Note that the moderate group showed both a hand pentagon area (Figure 3A) and grasping forces generated during hand opening (Figure 3B). This occurred because moderately impaired individuals could not extend all five digits as controls during hand opening. In these participants, there were some digits that could be more extended than others. The number of extending digits varied among participants. As a result, the moderate group generated a HPA, due to partial hand opening, and grasping forces simultaneously. It’s also worth noting that there were error bars for the severely impaired group (Figure 3A). This is because some severely impaired individuals, when asked to maximally open the hand, generated greater finger flexion which resulted in a smaller HPA than the resting HPA. After baseline correction, this smaller HPA was converted into a negative value and therefore created the error bars in Figure 3A.
Grasping force generation
Figure 4 depicts the changes of grasping forces in one individual with moderate stroke and one control individual between the Table/SABD25/SABD50 conditions when each participant tried to grasp with a maximal effort. The most evident difference between the stroke and control figures is that during the lifting phase (5.5s–8s), the stroke individual generated an increased level of synergy-induced grasping forces with SABD loading, in contrast to the control individual. However, the total grasping forces generated during the grasping phase (8–15s) were largely indistinguishable between SABD loading conditions and between groups.
Figure 5 illustrates the group results for grasping forces generated during SABD loading without grasp (in blue, i.e. synergy-induced grasping forces) and with grasp (in blue + yellow, i.e. total grasping forces). The two-way ANOVA with repeated measures found a significant effect of loading (p<0.000), group (p<0.000) and an interaction effect of loading*groups (p=0.000) for synergy-induced grasping forces, but showed no effects of loading (p=0.15), groups (p=0.36) nor loading*groups (p=0.85) for total grasping forces. Post hoc tests with Bonferroni adjustment indicated that both severe and moderate groups showed significantly greater synergy-induced grasping forces than the control group. Synergy-induced grasping forces were also significantly greater in the severe group than the moderate group. Additionally, synergy-induced grasping forces significantly increased with SABD loading in the two stroke groups but not in control group (Figure 5). In contrast, the total grasping forces were not significantly different between groups and remained consistent between SABD loading conditions.
Figure 5.
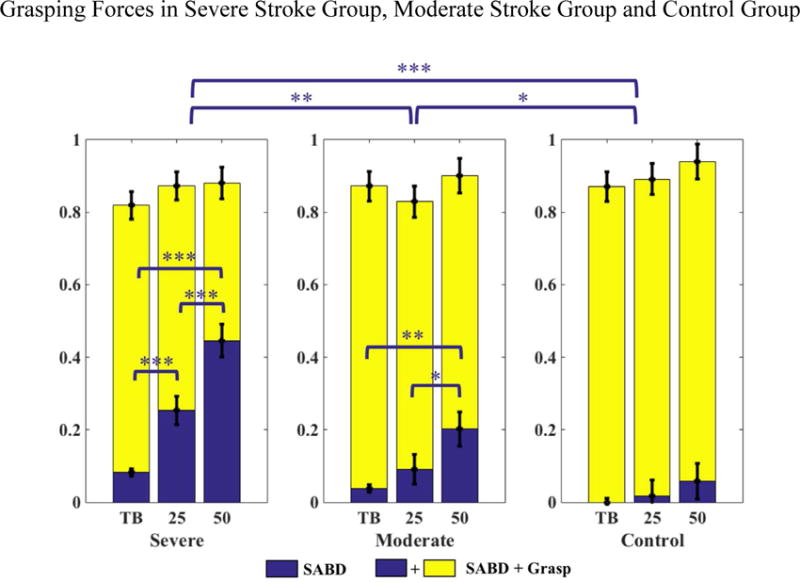
Grasping forces in individuals with severe (N=12) and moderate (N=11) chronic hemiparetic stroke, and control individuals (N=10). Grasping forces generated due to SABD loading (blue) and SABD loading + Grasp (blue + yellow) are shown in each group. The blue bridges and asterisks indicated the within-group and between-group significance in synergy-induced grasping force under the SABD loading conditions. TB: fully supported by the haptic table; 25: 25% of maximal SABD torque; 50: 50% of maximal SABD torque. *: p<0.05, **: p<0.01; ***: p<0.001.
Discussion
The main finding of this study is that following a stroke, increased SABD loading results in greater synergy-induced wrist/finger flexion, which progressively limits the ability to volitionally open the paretic hand or control volitional grasping forces. Individuals with more severe impairment are no longer able to open their hands and actually generated net grasping forces when asked to open the hand.
Possible neural mechanisms
Volitional hand function, particularly requiring fine and fractionated finger control, relies on the integrity and volume of the corticospinal tract.16–17 Following a stroke, the individual’s ability to volitionally control the hand is often seriously affected due to a loss of corticospinal projections from the ipsilesional hemisphere. The neural mechanisms underlying the expression of flexion synergy are not yet fully known, but it has been suggested that residual undamaged bulbospinal pathways, particularly the reticulospinal tract, may play a role in post-stroke hand function.7,9,18–19 For example in the monkey primate, recovery of upper limb function after damage to the motor system utilizes the reticulospinal tract in order to relay motor commands targeting motoneurons projecting to extrinsic and intrinsic hand muscles, especially of finger flexors.18–19 More specifically, it was found that mono- and disynaptic inputs from reticulospinal fibers to the forearm flexor and intrinsic hand muscles were significantly increased after a lesion of the pyramidal tract, while inputs to the extensors showed no change.19 In humans there is indirect evidence, using transcranial magnetic stimulation (TMS), that stroke-induced losses in corticospinal and corticoreticular pathways (collectively called corticofugal pathways) from the ipsilesional hemisphere may result in strengthening of the connections in the contralesional corticoreticulospinal pathways.20–23 The upregulated use of the contralesional corticoreticulospinal tract may explain relatively greater preservation of wrist and finger flexion than the extension given the connectivity of the contralesional reticulospinal system.24–25 For instance, monkey primate studies using spike-triggered averaging to explore the effect of reticulospinal stimulation on arm and hand muscles, demonstrated that the reticulospinal tract tends to facilitate flexors and suppress extensors ipsilaterally, and facilitate extensors and suppress flexors contralaterally.24–25 Consequently, an increased reliance on the contralesional corticoreticulospinal pathway, as a function of shoulder abduction, may cause abnormal muscle coactivation patterns between shoulder abduction and elbow and wrist/finger flexion due to the extensive branching of this pathway at the level of the spinal cord.19,26–28 Additionally, an upregulated corticoreticulospinal pathway20 is expected to increase its neuromodulatory drive which may further enhance the reticulospinal’s ionotopic effect on wrist and finger flexor motoneurons post stroke.29 Previous studies examined the effect of such upregulated neural drive by increasing shoulder abduction loading on the paretic side and found that hand flexion was involuntarily coupled to the shoulder and elbow after stroke.7
In this study, the reduced amount of HPA in the moderate group and the increased level of grasping forces in the severe group as a function of SABD loading during hand opening can be explained by an increased reliance on the reticulospinal tract. There is also indirect evidence that after a stroke the contralesional corticoreticulospinal tract has been upregulated,20,30 which results in a deviation of kinematics and kinetics of the impaired hand with a flexion bias. Interestingly, the SABD loading did not show an impact on the total grasping forces, while the synergy-induced grasping forces increased. This suggests that more severely impaired individuals have lower capability to control volitional grasp, especially when lifting with a greater SABD loading due to the increased contribution of synergy-induced grasping forces. Compared to the table condition, a greater portion of neural resources may be allocated to driving the shoulder when the participants lift with SABD loading, leaving more limited neural resources to volitionally drive the hand. In contrast, control participants were not affected by the SABD loading and maintained a consistent level of maximal volitional grasping forces.
Impact of changes in muscle properties at the paretic hand
Alterations in muscle properties such as muscle atrophy, muscle stiffness, and contracture have been reported along with neurologic changes following stroke.2,3,31 While the relative contribution of these muscular changes to hand dysfunction within the context of flexion synergy is still not fully understood, past studies have suggested that the impact of muscle properties on the hand impairment is quite small in comparison with the contribution from neural sources. For instance, from Kamper’s work in measuring mechanical contribution to hand impairment, it was shown that muscle contracture in the paretic hand represented at most 20% of the peak isometric extension in the non-paretic hand, suggestive of small effect of the muscle property changes on hand impairment compared to neural-induced motor impairments2. Another study investigating muscle atrophy after stroke reported that muscle size in the paretic hand was reduced with respect to the muscle size in the non-paretic hand, but it further addressed that the relatively small percent atrophy observed in this study could not account for the marked motor impairments in hemiparetic stroke.31
In our study, we strived to reduce the impact of biomechanical factors on the paretic hand by purposefully placing the fingers over the cylinder allowing unconstrained finger extension (Figure 1) rather than using an isometric setup or being inside a cast. The initial flexed hand posture also permitted us to greatly reduce the possible contribution of passive stiffness generated at the wrist and fingers. Additionally, passive stiffness induced pressures were measured by the sensory mat and were baseline corrected to eliminate its impact on the grasping measures. By making these efforts to minimize the potential impact of these muscle property changes, thus allowing us to concentrate on synergy-induced grasping forces, total grasping forces, as well as changes of the hand pentagon area.
Clinical implications for recovery of hand function after stroke
Use of the corticoreticulospinal tract has been suggested as a potential candidate for the recovery of hand function post stroke.18–19 Although outputs from the reticulospinal tract are considered to be too weak to significantly activate motoneurons of hand muscles,19 it is believed that the neuromodulatory component of the reticulospinal system has been upregulated as well following stroke-induced loss of corticoreticular projections.29 After such a change in neuromodulation mediated via reticulospinal tract, the synaptic drive could be enhanced to strengthen the activation of especially finger flexor motoneurons.20,34 However, an upregulated reticulospinal tract may also come at a cost of deleterious effects especially when activating shoulder abductors, resulting in more significant involuntary flexion generated at the wrist and fingers and thus preventing any effective recovery of hand function.7 Given the results from the present study, an upregulated reticulospinal tract reduces the ability to volitionally open the hand and results in the generation grasping forces, especially among individuals with more severe impairment. Thus, the use of the corticoreticulospinal tract for the purpose of hand recovery may not be functionally effective. While the increased SABD loading did not affect the total grasping forces during hand grasping, the reduction in volitional control is related to the increased synergistic drive presumably by progressively relaying more on the corticoreticulospinal tract, which is believed to act as the backup system to the corticospinal pathway.18–19,32
Using inhibitory transcranial cathodal direct current stimulation (tDCS) over the contralesional motor cortex has shown to be effective in enhancing motor skills acquisition of the paretic hand following a mild stroke.33 Other efforts aiming at reducing the reliance on the contralesional corticoreticulospinal tract may also have a positive effect on the recovery of arm function. For instance, improvement in the reaching area was reported following an intervention implementing progressive SABD loading35–36 possibly by using the remaining corticofugal resources from the lesioned hemisphere. Similarly, facilitatory anodal tDCS over the lesioned motor cortex increases reaching distance during 25% of maximum SABD loading following stroke.37 However, it is not yet clear whether such interventions can be extended to the recovery of hand function. To establish an effective treatment for the hand, it is recommended that clinical efforts should target on reducing the impact of neural drive descending via the corticoreticulospinal tract, either through induced inhibition of the reticular formation or through the increased use of remaining corticofual projections from the lesioned hemisphere to reduce the expression of the flexion synergy and increase dexterity of the paretic hand.
Extensor weakness undoubtedly plays an important role in the inability to open the hand in the presence of the flexion synergy, particularly in more severely impaired individuals2. As the results shown in Figure 3, the severe group showed no sign of opening not only because of the increased wrist and finger flexion, but possibly also due to the very weak activation of the extensors. Efforts aimed at reducing the impact of the flexion synergy will help to decrease the grasping forces generated during hand opening, but may not be as effective in severely impaired individuals compared to moderately impaired individuals due to the presence of extensor weakness.2
Future studies
Future studies should focus on understanding the structure and function of the corticoreticulospinal tract and its impact on hand disability following stroke. Quantification of the volume and integrity of contralesional corticoreticular projections and ipsilesional corticofugal projections using magnetic resonance imaging may help to elucidate the changes in neural drive and advance our understanding of neural mechanisms underlying the expression of the flexion synergy. Additionally, the progressive increase in SABD loading while reaching and opening the hand to grasp an object should be included in any future intervention, especially in individuals with moderate upper extremity impairment who retain some ability to open the hand. This is likely to reduce the negative effects of the flexion synergy on hand function over time.
Acknowledgments
We would like to thank Dr. Ana Maria Acosta for providing valuable feedback regarding the interpretation of the findings shown in this paper.
Funding
The authors disclosed receipt of the following financial support for the research: The study was supported by NIH R01HD039343.
Footnotes
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Broeks JG, Lankhorst GJ, Rumping K, Prevo AJ. The long-term outcome of arm function after stroke: results of a follow-up study. Disabil Rehabil. 1999;21(8):357–64. doi: 10.1080/096382899297459. [DOI] [PubMed] [Google Scholar]
- 2.Kamper DG, Fischer HC, Cruz EG, Rymer WZ. Weakness is the primary contributor to finger impairment in chronic stroke. Arch Phys Med Rehabil. 2006;87(9):1262–9. doi: 10.1016/j.apmr.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 3.Kamper DG, Cruz EG, Siegel MP. Stereotypical fingertip trajectories during grasp. J Neurophysiol. 2003;90(6):3702–10. doi: 10.1152/jn.00546.2003. [DOI] [PubMed] [Google Scholar]
- 4.Lang CE, Schieber MH. Differential impairment of individuated finger movements in humans after damage to the motor cortex or the corticospinal tract. J Neurophysiol. 2003;90(2):1160–70. doi: 10.1152/jn.00130.2003. [DOI] [PubMed] [Google Scholar]
- 5.Lang CE, Schieber MH. Reduced muscle selectivity during individuated finger movements in humans after damage to the motor cortex or corticospinal tract. J Neurophysiol. 2004;91(4):1722–33. doi: 10.1152/jn.00805.2003. [DOI] [PubMed] [Google Scholar]
- 6.Raghavan P, Petra E, Krakauer JW, Gordon AM. Patterns of impairment in digit independence after subcortical stroke. J Neurophysiol. 2006;95(1):369–78. doi: 10.1152/jn.00873.2005. [DOI] [PubMed] [Google Scholar]
- 7.Miller LC, Dewald JP. Involuntary paretic wrist/finger flexion forces and EMG increase with shoulder abduction load in individuals with chronic stroke. Clin Neurophysiol. 2012;123(6):1216–25. doi: 10.1016/j.clinph.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunnstrom S. Movement therapy in hemiplegia: a neurophysiological approach. New York: Harper and Row; 1970. [Google Scholar]
- 9.Lan Y, Yao J, Dewald J. Increased shoulder abduction loads decreases volitional finger extension in individuals with chronic stroke: Preliminary findings. Conf Proc IEEE Eng Med Biol Soc. 2014;2014:5808–11. doi: 10.1109/EMBC.2014.6944948. [DOI] [PubMed] [Google Scholar]
- 10.Fugl-Meyer AR, Jääskö L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med. 1975;7(1):13–31. [PubMed] [Google Scholar]
- 11.Gowland C, Stratford P, Ward M, Moreland J, Torresin W, Van Hullenaar S, Sanford J, Barreca S, Vanspall B, Plews N. Measuring physical impairment and disability with the Chedoke-McMaster Stroke Assessment. Stroke. 1993;24(1):58–63. doi: 10.1161/01.str.24.1.58. [DOI] [PubMed] [Google Scholar]
- 12.Sukal TM, Ellis MD, Dewald JP. Shoulder abduction-induced reductions in reaching work area following hemiparetic stroke: neuroscientific implications. Exp Brain Res. 2007;183(2):215–23. doi: 10.1007/s00221-007-1029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellis MD, Sukal T, DeMott T, Dewald JP. Augmenting clinical evaluation of hemiparetic arm movement with a laboratory-based quantitative measurement of kinematics as a function of limb loading. Neurorehabil Neural Repair. 2008;22(4):321–9. doi: 10.1177/1545968307313509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellis MD, Lan Y, Yao J, Dewald JP. Robotic quantification of upper extremity loss of independent joint control or flexion synergy in individuals with hemiparetic stroke: a review of paradigms addressing the effects of shoulder abduction loading. J Neuroeng Rehabil. 2016;13(1) doi: 10.1186/s12984-016-0203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Supuk T, Kodek T, Bajd T. Estimation of hand preshaping during human grasping. Med Eng Phys. 2005;27(9):790–7. doi: 10.1016/j.medengphy.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 16.Lawrence DG, Kuypers HG. The functional organization of the motor system in the monkey. I. The effects of bilateral pyramidal lesions. Brain. 1968;91(1):1–14. doi: 10.1093/brain/91.1.1. [DOI] [PubMed] [Google Scholar]
- 17.Lawrence DG, Kuypers HG. The functional organization of the motor system in the monkey. II. The effects of lesions of the descending brain-stem pathways. Brain. 1968;91(1):15–36. doi: 10.1093/brain/91.1.15. [DOI] [PubMed] [Google Scholar]
- 18.Baker SN. The primate reticulospinal tract, hand function and functional recovery. J Physiol. 2011;589(Pt 23):5603–12. doi: 10.1113/jphysiol.2011.215160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baker SN, Zaaimi B, Fisher KM, Edgley SA, Soteropoulos DS. Pathways mediating functional recovery. Prog Brain Res. 2015;218:389–412. doi: 10.1016/bs.pbr.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 20.Schwerin S, Dewald JP, Haztl M, Jovanovich S, Nickeas M, MacKinnon C. Ipsilateral versus contralateral cortical motor projections to a shoulder adductor in chronic hemiparetic stroke: implications for the expression of arm synergies. Exp Brain Res. 2008;185(3):509–19. doi: 10.1007/s00221-007-1169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwerin SC, Yao J, Dewald JP. Using paired pulse TMS to facilitate contralateral and ipsilateral MEPs in upper extremity muscles of chronic hemiparetic stroke patients. J Neurosci Methods. 2011;195(2):151–60. doi: 10.1016/j.jneumeth.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alagona G, Delvaux V, Gérard P, et al. Ipsilateral motor responses to focal transcranial magnetic stimulation in healthy subjects and acute-stroke patients. Stroke. 2001;32(6):1304–9. doi: 10.1161/01.str.32.6.1304. [DOI] [PubMed] [Google Scholar]
- 23.Strens LH, Fogelson N, Shanahan P, Rothwell JC, Brown P. The ipsilateral human motor cortex can functionally compensate for acute contralateral motor cortex dysfunction. Curr Biol. 2003;13(14):1201–5. doi: 10.1016/s0960-9822(03)00453-6. [DOI] [PubMed] [Google Scholar]
- 24.Davidson AG, Buford JA. Bilateral actions of the reticulospinal tract on arm and shoulder muscles in the monkey: stimulus triggered averaging. Exp Brain Res. 2006;173(1):25–39. doi: 10.1007/s00221-006-0374-1. [DOI] [PubMed] [Google Scholar]
- 25.Davidson AG, Schieber MH, Buford JA. Bilateral spike-triggered average effects in arm and shoulder muscles from the monkey pontomedullary reticular formation. J Neurosci. 2007;27(30):8053–8. doi: 10.1523/JNEUROSCI.0040-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peterson BW. The reticulospinal system and its role in the control of movement. In: Bames CD, editor. Brainstem control of spinal cord function. Orlando (FL): Academic Press; 1984. pp. 27–86. [Google Scholar]
- 27.Peterson BW, Pitts NG, Fukushima K. Reticulospinal connections with limb and axial motoneurons. Exp Brain Res. 1979;36:1–20. doi: 10.1007/BF00238464. [DOI] [PubMed] [Google Scholar]
- 28.Illert M, Lundberg A, Padel Y, Tanaka R. Integration in descending motor pathways controlling the forelimb in the cat. 5. Properties of and monosynaptic excitatory convergence on C3–C4 propriospinal neurones. Exp Brain Res. 1978;33(1):101–30. doi: 10.1007/BF00238798. [DOI] [PubMed] [Google Scholar]
- 29.McPherson JG, Ellis MD, Heckman CJ, Dewald JP. Evidence for increased activation of persistent inward currents in individuals with chronic hemiparetic stroke. J Neurophysiol. 2008;100(6):3236–43. doi: 10.1152/jn.90563.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jang SH, Chang CH, Lee J, Kim CS, Seo JP, Yeo SS. Functional role of the corticoreticular pathway in chronic stroke patients. Stroke. 2013;44(4):1099–104. doi: 10.1161/STROKEAHA.111.000269. [DOI] [PubMed] [Google Scholar]
- 31.Triandafilou K, Kamper D. Investigation of hand muscle atrophy in stroke survivors. Clin Biomech. 2012;27(3):268–72. doi: 10.1016/j.clinbiomech.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Honeycutt CF, Kharouta M, Perreault EJ. Evidence for reticulospinal contributions to coordinated finger movements in humans. J Neurophysiol. 2013;110(7):1476–1483. doi: 10.1152/jn.00866.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zimerman M, Heise KF, Hoppe J, Cohen LG, Gerloff C, Hummel FC. Modulation of training by single-session transcranial direct current stimulation to the intact motor cortex enhances motor skill acquisition of the paretic hand. Stroke. 2012;43(8):2185–91. doi: 10.1161/STROKEAHA.111.645382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drew T, Dubuc R, Rossignol S. Discharge patterns of reticulospinal and other reticular neurons in chronic, unrestrained cats walking on a treadmill. J Neurophysiol. 1986;55(2):375–401. doi: 10.1152/jn.1986.55.2.375. [DOI] [PubMed] [Google Scholar]
- 35.Ellis MD, Holubar BG, Acosta AM, Beer RF, Dewald JP. Modifiability of abnormal isometric elbow and shoulder joint torque coupling after stroke. Muscle Nerve. 2005;32(2):170–8. doi: 10.1002/mus.20343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ellis MD, Sukal-Moulton T, Dewald JP. Progressive shoulder abduction loading is a crucial element of arm rehabilitation in chronic stroke. Neurorehabil Neural Repair. 2009;23(8):862–9. doi: 10.1177/1545968309332927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yao J, Drogos J, Veltink F, et al. The effect of transcranial direct current stimulation on the expression of the flexor synergy in the paretic arm in chronic stroke is dependent on shoulder abduction loading. Front Hum Neurosci. 2015;9:262. doi: 10.3389/fnhum.2015.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]


