Abstract
A major barrier to successful use of allogeneic hematopoietic cell transplantation is acute graft-versus-host disease (aGVHD), a devastating condition that arises when donor T cells attack host tissues. With current technologies, aGVHD diagnosis is typically made after end-organ injury and often requires invasive tests and tissue biopsies. This impacts patient prognosis as treatments are dramatically less effective at late disease stages. Here we show that a novel positron emission tomography (PET) radiotracer, 2′-deoxy-2′-[18F]fluoro-9-β-D-arabinofuranosylguanine ([18F]F-AraG), targeted towards two salvage kinase pathways preferentially accumulates in activated primary T cells. [18F]F-AraG PET imaging of a murine aGVHD model enabled visualization of secondary lymphoid organs harboring activated donor T cells prior to clinical symptoms. Tracer biodistribution in healthy humans showed favorable kinetics. This new PET strategy has great potential for early aGVHD diagnosis, enabling timely treatments and improved patient outcomes. [18F]F-AraG may be useful for imaging activated T cells in various biomedical applications.
Keywords: acute graft-versus-host disease (aGVHD), T cells, positron emission tomography (PET), 9-(β-D-Arabinofuranosyl) guanine (AraG), mouse model
Introduction
Allogeneic hematopoietic cell transplantation (HCT) is increasingly being used as a potentially curative treatment for hematological malignancies and other life-threatening diseases (1). In malignancy treatment, HCT can destroy residual tumor cells that persist after other treatments, an effect called the graft-versus-tumor (GVT) reaction. The two most important causes of non-relapse mortality are infections and acute graft-versus-host disease (aGVHD) (2). While infections can be successfully treated, at the time of diagnosis aGVHD patients are less responsive to current treatments. Thus, the challenge for widespread adoption of HCT is either avoidance or better management of aGVHD, but arguably the diagnosis and treatment of this devastating disease has only marginally improved in the last 20 years.
aGVHD is an immunological disorder where donor CD4+ and CD8+ T cells migrate to secondary lymphoid organs and are activated by innate immune cells that become reactive by the conditioning therapy for HCT (1). These alloreactive donor T cells then undergo massive proliferation and home to various organs (gastrointestinal tract, liver and skin) and attack host cells via direct cytolysis or cytokine production causing a wide range of symptoms (3). Incidence rates are ∼20-50% for patients receiving human leukocyte antigen (HLA)-matched related donor transplants and incidence and severity of aGVHD increases according to HLA disparity (4). Overall grade is known to have a major effect on patient outcome, with grade I or II cases associated with little morbidity and mortality, and grade III-IV cases associated with high mortality (5). Since long term survival of grade III-IV aGVHD is a dismal 10-20% (6), it is important to detect patients who will progress to high-grade aGVHD earlier in their course. Dramatic differences in response rate with disease stage have been reported including 63% to 95% for grade II, 17% to 30% for grade III, and 0% to 6% for grade IV patients (7). Unfortunately, early aGVHD diagnosis is difficult due to its non-specific symptoms and numerous differential diagnoses (8), and typically a diagnosis occurs when overt symptoms such as skin, GI, or liver problems become apparent (9). Invasive endoscopic guided biopsy (often from liver or GI tract) are required to confirm an aGVHD(4), but bleeding tendency and critical illness associated with HCT make it near impossible to perform these procedures. Blood biomarkers are under active investigation but to date no biomarker has been validated for early aGVHD diagnosis (10,11). These critical issues dramatically delay the onset and effectiveness of potentially life-saving pre-emptive or early treatment interventions. Thus, non-invasive methods that can diagnose aGVHD at earlier stages are urgently needed but entirely lacking. An imaging method that can quantitatively track alloreactive donor T cell dynamics and stratify the risk of aGVHD prior to overt symptoms could provide an ideal solution.
Positron emission tomography (PET) has several key strengths including high sensitivity (10-9 to 10-12 M), quantitative capability, and limitless depth of penetration (12). To date, few PET radiotracers have been developed for imaging the immune system and even fewer for specific imaging of distinct immune cell types such as activated T cells (13-15). Our group recently developed the radiotracer 2′-deoxy-2′-[18F]fluoro-9-β-D-arabinofuranosyl guanine ([18F]F-AraG) (16) (Supplementary Fig. 1a). [18F]F-AraG is an analog of arabinosyl guanine (AraG), a compound identified to have specific cytotoxicity towards T-lymphocytes and T-lymphoblastoid cells versus other immune cell types (17-19). Nelarabine, the water-soluble AraG prodrug, is indicated for the treatment of patients with refractory/relapsed T-cell acute lymphoblastic leukemia (T-ALL) and T-cell lymphoblastic lymphoma (T-LBL) (20). AraG enters cells via one of two nucleoside transporters followed by the initial and rate-limiting phosphorylation of AraG to AraG-monophosphate (AraGMP) by either cytosolic deoxycytidine kinase (dCK) or mitochondrial deoxyguanosine kinase (dGK) (Supplementary Fig. 1b) (20,21). Continued phosphorylation to AraG-triphosphate (AraGTP) can inhibit DNA synthesis and induce cytotoxic effects specifically in T lymphocytes and T-lymphoblastoid cells (18,20). Thus [18F]F-AraG PET imaging may provide a novel method for imaging of activated T cell dynamics in living subjects without toxicity since the tracer is given in very low (ng-μg) mass levels. This technology could allow clinicians to utilize existing or novel interventions in a timely fashion and to more effectively monitor the outcome of such treatments. The ultimate utility of such efforts would be to prevent progression to higher-grade disease and improve patient outcome.
Materials and Methods
Cell Lines
Jurkat (ATCC, Manassas, VA), Ramos (ATCC, Manassas, VA), and Daudi (ATCC, Manassas, VA) cells were cultured in RPMI-1640 containing 10% Fetal Bovine Serum (FBS) and 1× Antibiotic-Antimycotic (Anti-Anti) solution. For Ramos cells, the FBS was heat inactivated at 56°C for 30 minutes. HL-60 (ATCC, Manassas, VA) cells were cultured in Iscove's Modified Dulbecco's Medium (IMDM) containing 20% FBS and 1× Anti-Anti. CCRF-CEM (ATCC, Manassas, VA), Ara-C8D (dCK- or CCRF-CEM-dCK-, a kind gift from Dr. Beverly Mitchell, Stanford University), Ara-C8D dCK+ (dCK+) and Ara-C8D dGK+ (dGK+; the latter two kind gifts from Dr. Varsha Gandhi, MD Anderson) were grown in low glucose DMEM with 10% FBS. HeLa, MDA-MB-231 (ATCC, Manassas, VA), PyMT (a kind gift from Dr. Heike Daldrup-Link, Stanford University) cells were maintained in DMEM containing 10% FBS and 1× Anti-Anti. MeWo (ATCC, Manassas, VA) cells were maintained in MEM containing 10% FBS and 1× Anti-Anti. CHO-K1 cells (ATCC, Manassas, VA) were maintained in F-12K medium containing 10% FBS and 1× Anti-Anti. All cells routinely tested negative for mycoplasma using the MycoAlert mycoplasma detection kit (Lonza, Walkersville, MD; last testing: December 2014). All cell lines were used for experiments within a month of thawing, acquired from the various sources (primarily ATCC) between 2011 and 2014, and cell line authentication was not performed. All cells were maintained at 37 °C in a humidified atmosphere containing 5 % CO2.
Tracers
AraG was purchased from R.I. Chemicals, Inc. (Orange County, CA). Tritiated AraG ([3H]AraG) and tritiated 1-(2-deoxy-2-fluoro-arabinofuranosyl)-cytosine ([3H]F-AraC) were purchased from Moravek Biochemicals (Brea, CA). [18F]F-AraG was synthesized as previously described (16). [18F]F-AraG for human imaging was prepared according to the approved IND Chemistry Manufacturing and Control (CMC) procedures using the Neptis® perform PET synthesizer, a modification of the published procedure (16). The synthesis details are provided in supplemental information. Prior to injection in human subjects the prepared [18F]F-AraG passed all quality specifications.
Primary T Cell Isolation and Activation
Mouse primary T cells were isolated via FAC sorting from spleens of BALB/c mice (Charles River) using an APC-conjugated Anti-Mouse CD90.2 (Thy-1.2) antibody (eBioscience, Inc. San Diego, CA). Purified T cells were maintained for 2 days in RPMI-1640 containing 10% FBS and 1% penicillin/streptomycin at an initial density of 1×106 cells/ml. For activation, cells were incubated with mouse T-Activator CD3/CD28 Dynabeads® per the manufacturer's instructions (bead:cell ratio of 1:10; Life Technologies, Grand Island, NY). Tracer uptake experiments were performed 48 hours after activation.
Human PBMCs were isolated from whole blood (obtained at the Stanford Blood Center) using Ficoll-Paque Plus following the manufacturer's instructions (GE Healthcare). T cells were isolated via magnetic-activated cell sorting (MACS) using the Naïve Pan T Cell Isolation Kit per the manufacturer's instructions (Miltenyi Biotec, Auburn, CA). To assess successful purification (>90% T cells), both PBMCs and sorted cells were immunostained with a human CD3-FITC antibody (Miltenyi Biotec, Auburn, CA) and the percentage of CD3-positive cells was determined under a fluorescent microscope (EVOS FL cell imaging system, Life Technologies, Grand Island, NY). T cells were activated with T cell activation/expansion kit as per the manufacturer's instructions (Miltenyi Biotec, Auburn, CA). Tracer uptake experiments were performed 48 hours after activation.
Tracer Uptake and Efflux Assays for Immune Cells
5×105 (primary mouse T cells), 2×106 (human primary T cells), or 1×106 (cell lines) cells in 1 ml of Hanks balanced salt solution (HBSS) were incubated with tracer (([18F]F-AraG (5 μCi), [3H]AraG (2 μCi), or [3H]F-AraC (1 μCi)) for 1 hour to measure uptake. For competition assays, cells were incubated with or without 100 μM AraG during tracer incubation (R.I. Chemical, Inc., Orange, CA). To assess tracer efflux (percentage retained), cells were centrifuged at 1000 × g for 5 minutes after 1 hour tracer incubation, supernatant was aspirated, and cells were incubated in 1 ml of HBSS for an additional hour. To measure [18F]F-AraG radioactivity within cells we used a method previously described (22). Briefly, 400 μl of cell suspension was transferred onto 200 μl of an 88:12 silicon oil (density = 1.05) and mineral oil (density = 0.877) mixture and centrifuged at 10,000 rpm for 3 minutes. This enables efficient separation of radioactivity in media and cell pellets. Tubes were frozen in liquid nitrogen, pellets were transferred to scintillation tubes, and radioactivity was measured using an automated γ counter (Cobra II; Packard). For [3H]AraG or [3H]F-AraC measurement, 20 μl of 2N KOH was layered under the oil mixture (23). 40 μl of 1N HCl and 10 ml of scintillation liquid was added sequentially to cell pellets, and radioactivity was measured using an LS 6500 Multi-Purpose scintillation counter (Beckman Coulter). Using the remaining cell fraction, total cell number was determined using an automated cell counter (Nexcelom Bioscience, Lawrence, MA). Data was expressed as percentage uptake per 105 cells.
aGVHD Mouse Model
All procedures performed on animals were approved by Stanford University's Institutional Animal Care and Use Committee and were within the guidelines of humane care of laboratory animals (Protocol #27293). Both control (n=10) and aGVHD mice (n=11) were generated as previously described (11,24,25). Briefly, recipient 8-16 week old female BALB/c mice (Balb/cJ; 18-22 g; Jackson Laboratories) were irradiated with 800 cGy in two split doses on day 0 with an electron linear accelerator. T cell-depleted bone marrow (TCD-BM) cells were obtained from wild-type 8-16 week old female C57Bl/6 donor mice (control mice; C57Bl/6J; 18-22 g; Jackson Laboratories). Donor T cells were obtained from the spleens of firefly luciferase (Luc+) 8-16 week old female C57Bl/6-L2G85 (18-22 g) donor mice (11,26). T cell depletion and donor T cell selection were performed using CD4 and CD8 magnetic beads (Miltenyi Biotec). All mice received intravenous injection of 5×106 TCD-BM cells to initiate hematopoietic reconstitution. For aGVHD mice, they also received 1×106 Luc+ T cells co-injected with the TCD-BM cells. For visualization of donor derived T cells in immunofluorescence experiments, 8-16 week old female Luc+gfp+ C57Bl/6 mice (18-22 g) were used as the T cell donor (27).
Bioluminescence Imaging (BLI), Positron Emission Tomography (PET) and Tracer Biodistribution Studies
For BLI, mice were anesthetized and imaging was performed using an IVIS Spectrum cooled charge-coupled device imaging system (Perkin Elmer, Waltham, MA) 5 minutes after an intraperitoneal injection of the substrate D-luciferin (3 mg). Images were analyzed with Living Image Software 4.1 (Perkin Elmer, Waltham, MA).
For PET/CT imaging, mice were anesthetized and injected with ∼200 μCi of [18F]F-AraG via the lateral tail-vein. Sixty minutes after injection, static PET images (10-minute acquisition time) were collected using a microPET/CT hybrid Inveon scanner (Siemens). A CT image was acquired just before each PET scan to provide an anatomic reference frame for the respective PET data. All PET images were reconstructed with a 3-dimensional ordered-subsets expectation maximization algorithm and co-registered with CT images using the Inveon Research Workplace (IRW) image analysis software (version 4.0; Siemens). To quantify tracer uptake in PET images, 3-dimensional regions of interest were drawn over the CLN, a 50% threshold was applied to each selected region, and partial volume corrected uptake values were expressed as percentage injected dose per gram of tissue (%ID/g).
Ninety minutes after tracer administration mice were euthanized, various tissues (blood, heart, small intestine, kidney, liver, lung, muscle, spleen, mesenteric lymph nodes (MLN), and cervical lymph nodes (CLN)) were collected, weighed and radioactivity was measured using an automated γ counter (Cobra II; Packard). Radioactivity was decay-corrected to the time of radiotracer injection using diluted aliquots of the initial administered dose as standards. Data is expressed as percentage injected dose per gram of tissue (%ID/g) values.
Imaging Studies in Humans
Six healthy volunteers (3 women, 3 men; 24-60 years old) were enrolled to study the whole-body kinetics of [18F]F-AraG in humans (IND #123591) at the University of California, San Francisco, under protocols approved by the Institutional Review Board and the Radiation Safety Committee. The following subjects were excluded from participating in this study: pregnant or breastfeeding women, individuals with any type of chronic illness or symptoms of disease, individuals with known or suspected substance abuse, individuals unable or unwilling to comply with the study procedures and individuals with contraindications to MRI. Written informed consents were obtained from the volunteers prior to the initiation of each imaging study. Details of image acquisition and analysis are provided in supplemental information.
Results
AraG selective cytotoxicity is related to the preferential ability of T lymphocytes or T cell derived cancer cells versus other immune cell types to retain AraGTP (19,28). To evaluate the potential utility of [18F]F-AraG, we first assessed uptake and retention across a number of immune cell lines that are all derived from patients with hematological malignancies. Compared to T cell and B lymphoblast cell lines, significantly increased and decreased tracer uptake at 1 hour was seen in myeloid and B lymphoblast cells, respectively (p<0.05; Supplementary Fig. 2a). Importantly, both T cell lines significantly and efficiently (∼100%) retained the tracer (expressed as percentage remaining in cells compared to tracer uptake at 1 hour) compared to all other immune cell lineages (p<0.001; Supplementary Fig. 2b). We also assessed the uptake and retention of a tritiated version of another nucleoside analog radiotracer, 1-(2′-deoxy-2′-[18F]fluoro-D-arabinofuranosyl) cytosine ([18F]F-AraC, also called [18F]FAC). [18F]F-AraC, a substrate for deoxycytidine kinase (dCK), shows higher uptake in proliferating versus resting T cells, and may be useful for visualizing regions of immune activation in vivo with PET (13). We found significantly higher levels of [3H]F-AraC uptake in T lymphocyte cells versus all other immune cell lines, and significantly higher uptake in T lymphoblast line compared to B cell lines (p<0.01; Supplementary Fig. 2c). We also found less [18F]F-AraC retention (<100%) in T cell lines versus [18F]F-AraG, equivalent [18F]F-AraC retention between T lymphoblast, T lymphocyte and myeloid cell lines, significantly decreased retention in B cells versus T lymphoblasts, and significantly decreased retention in B versus T lymphocytes (p<0.05; Supplementary Fig. 2d). Co-incubation with molar excess of non-radiolabeled AraG (100 μM) significantly impaired [18F]F-AraG uptake across all cell types (p<0.05; Supplementary Fig. 3). Significantly higher uptake was also seen across several solid tumor cell lines with [3H]F-AraC versus [18F]F-AraG (p<0.01; Supplementary Fig. 4a), as well as significantly higher retention of [3H]F-AraC in both cervical cancer and melanoma cell lines (p<0.01; Supplementary Fig. 4b). Thus [18F]F-AraG accumulates in cell lines in a manner that is similar to AraG but distinguishable from the AraC-based tracer.
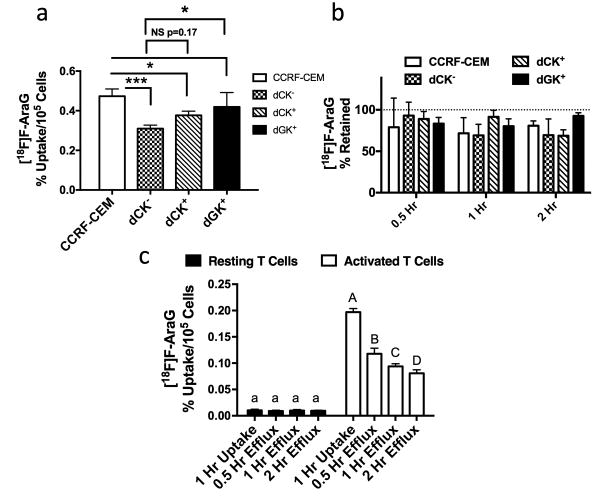
We next evaluated whether [18F]F-AraG accumulates in cells via dCK and/or dGK activity using established cell lines (21). Loss of dCK in mutant CCRF-CEM T lymphoblast cells (dCK-), as confirmed with Western blot analysis (Supplementary Fig. 5), significantly impaired [18F]F-AraG uptake (p<0.001; Fig. 1a). Overexpression of dCK (dCK+) in dCK- cells showed a trend toward increased tracer uptake in dCK+ compared to dCK- cells (p=0.17), whereas dGK overexpression (dGK+) in dCK- cells significantly (p<0.05) increased tracer uptake in dGK+ compared to dCK- cells (Fig. 1a). Uptake levels in dGK+ cells were not significantly different to levels in wild-type CCRF-CEM cells. No effects on tracer retention were seen across any of these cell lines (Fig. 1b). dGK utilization of [18F]F-AraG was further confirmed via significantly higher uptake and retention in CHO-K1 cells overexpressing dGK compared to empty vector transfected cells (p<0.05; Supplementary Fig. 6a/b). Similar studies with [3H]F-AraC showed this tracer accumulated via dCK, but not dGK, and lack of dCK activity significantly reduced retention of the tracer (p<0.05; Supplementary Fig. 7a/b). dCK overexpression had no significant effects on uptake or retention of [18F]F-AraG in this cell type. Our evidence supports that in contrast to [18F]F-AraC that accumulates via dCK alone, but in line with AraG metabolism (21), [18F]F-AraG accumulates in cells via both dCK and dGK activity. These findings support recently published observations in a study on a novel PET tracer metabolized by dCK, which also noted a role of dGK in the metabolism of [18F]F-AraG (29); however, we extend upon these previous findings to highlight the additional role of dCK activity in the metabolism of [18F]F-AraG.
Figure 1. [18F]F-AraG Accumulates in Cells via dCK and dGK Activity and at Increased Levels in Activated Versus Resting Primary Human T Cells.
a) Uptake and b) retention of [18F]F-AraG across wild-type CCRF-CEM T lymphoblasts, mutant CCRF-CEM dCK- cells (dCK-), and dCK- cells overexpressing either dCK (dCK+) or dGK (dGK+) (n=4 per cell type per time point). Significantly less uptake was seen due to the loss of dCK in wild-type cells (CCRF-CEM vs. dCK-), (p<0.001). There was a trend towards higher uptake in dCK+ versus dCK- cells, whereas dGK+ cells had significantly higher uptake compared to dCK- cells and equivalent uptake compared to wild-type cells. No differences in retention were seen across cell types. c) Activated primary human T cells had significantly higher [18F]F-AraG uptake compared to resting T cells at all time points examined (***p<0.001; n=4 per cell state per time point). Data in all graphs are expressed as mean ± SD.
As our goal is to image activated primary T lymphocytes, not T cell lines, uptake and retention of various tracers ([18F]F-AraG, [3H]AraG, and [3H]AraC) in primary murine and human resting and activated T cells was also evaluated. Activated murine T cells (2 days after activation) appeared morphologically distinct (elongated versus round) compared to resting cells (Supplementary Fig. 8a) and accumulated significantly higher (∼7.8-fold) [18F]F-AraG (p<0.001; Supplementary Fig. 8b). A 2-day activation protocol of sorted (>90% CD3+ T cells) human peripheral blood mononuclear cells (PBMCs) resulted in similar morphological changes as seen in murine T cells (Supplementary Fig. 9). Significantly higher uptake in activated versus resting cells was seen with both [3H]AraG and [3H]F-AraC, but retention in both cell states was significantly higher with [3H]AraG (p<0.05; Supplementary Fig. 10). Finally, we found significantly higher uptake (19-fold) of [18F]F-AraG in activated versus resting human T cells (p<0.001; Fig. 1c). Although [18F]F-AraG significantly effluxed out of activated but not resting cells (p<0.05), absolute levels in activated versus resting cells at all time points evaluated were significantly higher (p<0.01). Overall these results suggest the use of [18F]F-AraG may allow selective PET imaging of activated versus resting T cells.
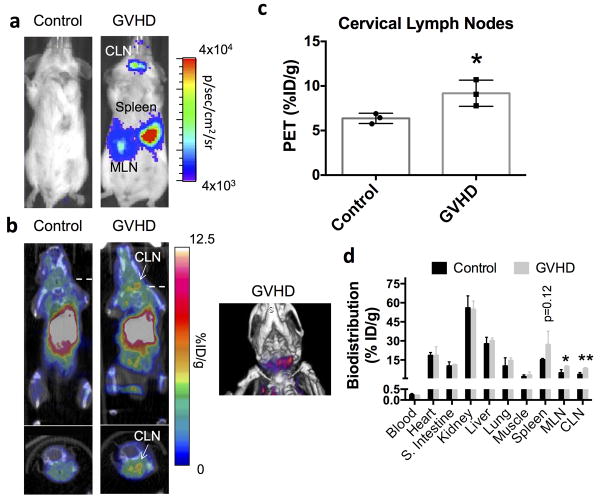
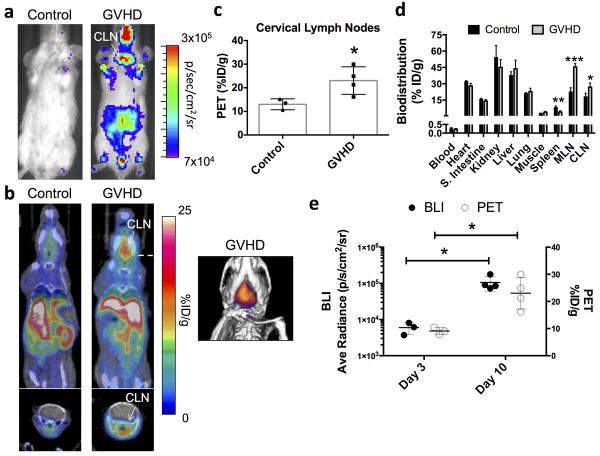
The ability to visualize sites of activated T cell accumulation in aGVHD with [18F]F-AraG PET imaging was assessed in a well-established MHC mismatch aGVHD mouse model (11,24-26). In this model, donor T cells constitutively express the bioluminescence reporter gene firefly luciferase (Luc) enabling in vivo bioluminescence imaging (BLI) to monitor sites of donor T cell accumulation and proliferation over time. We performed in vivo BLI, [18F]F-AraG PET/CT imaging, tracer biodistribution studies, and ex vivo immunofluorescence studies at both day 3 and day 10 after model initiation in both control (irradiated and bone marrow transplanted mice without donor T cells) and aGVHD (irradiated and bone marrow transplanted mice with Luc+ donor T cells) mice. Consistent with previous results (11,24,25) and based on a standard scoring system (30), both day 3 and day 10 mice in this model are considered within the T cell activation phase of the disease. Day 3 aGVHD mice lack noticeable symptoms and are at a time point when T cell activation is being initiated, whereas day 10 mice display clinical symptoms and are considered at the peak of T cell activation. As previously demonstrated (11,24,25), in vivo BLI of aGVHD mice revealed that donor T cells at day 3 had accumulated preferentially in lymphoid organs such as the spleen, mesenteric lymph nodes (MLN) and cervical lymph nodes (CLN) (Fig. 2a). On separate cohorts of mice receiving GFP+ donor T cells we qualitatively confirmed progressively higher donor T cell accumulation within the spleen and CLN from day 3 to day 10 (Supplementary Fig. 11). We also demonstrate that the percentage of donor T cells that are proliferating (Ki67+) – a marker of activation - is higher at later (day 9) versus earlier time points (day 3) (Supplementary Fig. 12). Importantly, [18F]F-AraG PET/CT images taken 1 hour after tracer (∼200 μCi) administration showed qualitatively higher uptake in the CLN in aGVHD versus control mice (Fig. 2b). Quantitative image analysis corroborated our findings revealing significantly higher (∼1.4-fold) [18F]F-AraG uptake in the CLNs of aGVHD mice (p<0.05; Fig. 2c). At 1 hour, tracer uptake within abdominal organs such as the liver and kidneys obscured the ability to visualize in vivo differences in tracer accumulation within the MLN and spleen (Fig. 2b). However, tracer biodistribution studies assessed at 90 minutes after intravenous tracer injection revealed significantly higher [18F]F-AraG accumulation in both the CLN (p<0.05; ∼2.3-fold) and the MLN (p<0.05; ∼2.0-fold), and a trend towards higher uptake in the spleen (p=0.12; Fig. 2d). No significant differences were noted across all other organs between control and aGVHD mice. Thus, at an early stage of disease development prior to clinical symptoms in this model, [18F]F-AraG accumulated in lymphoid organs (MLN, CLN, and spleen) harboring activated T cells and [18F]F-AraG PET imaging enabled visualization of early T cell accumulation within the CLN. At day 10, when donor T cells are highly proliferative (Supplementary Fig. 12), BLI revealed significantly higher total body signal compared to day 3 (p<0.01; Supplementary Fig. 13), and qualitatively higher signal in the CLN region (Fig. 3a vs. Fig. 2a, and Supplementary Fig. 13; note differences in image scale). [18F]F-AraG PET/CT images showed an even more apparent increase in tracer accumulation in the CLN of aGVHD compared to control mice (Fig. 3b), which was quantitatively confirmed via PET image analysis (Fig. 3c). Tracer biodistribution analysis revealed significantly lower tracer uptake in the spleen at this time point (p<0.05), but higher uptake in both the CLN and the MLN (p<0.05 and p<0.01, respectively; Fig. 3d). Amongst aGVHD mice, we also showed significantly higher BLI and PET signal within the CLNs at day 10 versus day 3 (p<0.05; Fig. 3e), indicating promise for monitoring disease burden via this surrogate imaging biomarker.
Figure 2. Bioluminescence and [18F]F-AraG PET Imaging of Donor T Cell Dynamics During the Initiation of T Cell Activation in a Mouse Model of acute GVHD.
a) Bioluminescence imaging of control and GVHD mice 3 days after HCT revealed homing of luciferase-positive donor T cells to secondary lymphoid organs such as the spleen, cervical lymph nodes (CLN), and mesenteric lymph nodes (MLN) in GVHD mice. b) Representative [18F]F-AraG PET/CT images (10 minute static scan; 60-70 minutes post-tracer administration; ∼200 μCi) at this time point showed visibly higher tracer uptake in the CLN of GVHD mice versus control mice (n=3 per group). c) Quantitative region of interest PET image analysis of the CLN corroborated our qualitative assessments, demonstrating significantly (*p<0.05) higher tracer uptake in the CLN of GVHD mice versus control mice (%ID/g; percentage injected dose per gram of tissue). d) Biodistribution studies (90 minutes after tracer administration) also supported our imaging findings showing significantly (*p<0.05) higher tracer uptake in CLNs in GVHD versus control mice. Significantly (*p<0.05) higher tracer uptake was also apparent in the T-cells harboring MLN and a trend (p=0.12) towards higher uptake was seen in the spleen. Data in all graphs are expressed as mean ± SD.
Figure 3. Bioluminescence and [18F]F-AraG PET Imaging of Donor T Cell Dynamics During the Peak of T Cell Activation in a Mouse Model of acute GVHD.
a) Bioluminescence imaging of control and GVHD mice 10 days after HCT revealed more widespread distribution of luciferase-positive donor T cells in GVHD mice but still localized accumulation within the cervical lymph nodes (CLN). Note the order of magnitude difference in scale between the images shown here and those in Fig. 2a (Day 3 after cell transplantation). b) Representative [18F]F-AraG PET/CT images (10 minute static scans, 60-70 post-tracer administration; ∼200 μCi) at this time point showed visibly higher tracer uptake in the CLN of GVHD (n=4) versus control mice (n=3). c) Quantitative region of interest PET image analysis of the CLN corroborated our qualitative assessments, demonstrating significantly higher tracer uptake in the CLN of GVHD versus control mice (*p<0.05) (%ID/g; percentage injected dose per gram of tissue). d) Biodistribution studies (90 minutes after tracer administration) also supported our imaging findings showing significantly higher tracer uptake in CLNs in GVHD versus control mice. Significantly higher tracer uptake was also apparent in the MLN (*p<0.05). At this time point, significantly lower tracer uptake was noted within the spleen (*p<0.05). e) Comparison of Day 3 and Day 10 bioluminescence and PET image analysis of CLNs shows both significantly higher BLI and PET signals on Day 10 compared to Day 3. Data in all graphs are expressed as mean ± SD.
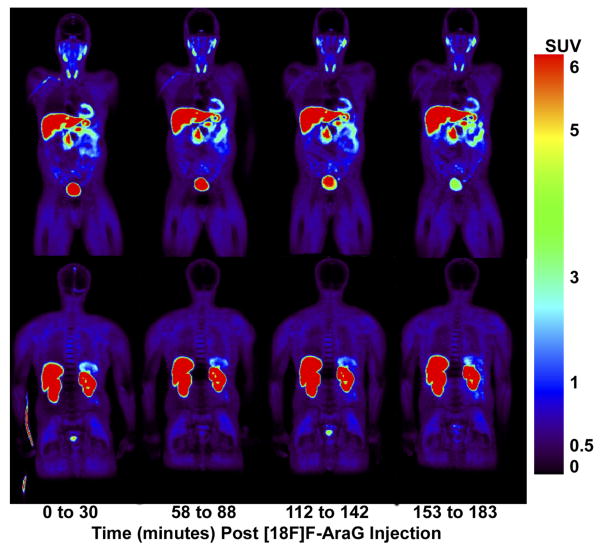
To further explore the suitability of [18F]F-AraG for human studies, [18F]F-AraG PET/CT scans were performed on six healthy human volunteers (Fig. 4). [18F]F-AraG exhibited hepatobiliary and renal clearance with highest uptake in associated organs with SUV mean values (normalized to body weight) of 13.47 ±1.46, 20.72±5.26 and 17.27±4.19 in liver, right and left kidneys respectively at 47-77 minutes post tracer injection (Supplementary Table 1). High uptake was also observed in the myocardium as seen in mice, while relatively low background was observed in the thorax and lower abdomen. No significant adverse events or blood lab changes were observed due to tracer administration in any of the volunteers other than slightly increased urine esterase levels. Radiation dosimetry results are shown in Supplementary Table 2.
Figure 4. Whole body PET images of [18F]F-AraG in a healthy human volunteer.
Frontal (top panel) and dorsal (bottom panel) coronal PET images taken immediately after intra-venous injection of the tracer and at serial time points in one of the 6 humans imaged. Highest activity is observed in the liver, kidneys and bladder, associated with [18F]F-AraG clearance from the body. Uptake is also observed in the heart and spleen while relatively low background activity is observed in all other tissues. Similar images were obtained from the other 5 subjects.
Discussion
aGVHD is a T cell mediated disease and the major complication of allogeneic HCT. aGVHD diagnosis is predominantly based on clinical symptoms, is often one of exclusion, and is typically confirmed with invasive biopsies that have their own complications. Work on diagnostic blood assays have identified biomarkers, but more work needs to be done to validate these in multicenter prospective studies prior to widespread clinical use (31). Moreover, while noninvasive conventional imaging has played a role in aGVHD management, the structural manifestations that are detectable often only present at the latest of disease stages (32,33). Molecular imaging of aGVHD, which may yield more sensitive detection, has been very limited, mostly focusing on PET imaging using [18F]FDG (34), a non-specific tracer with high uptake in many cell types and conditions. Overall, there is still no established single blood or imaging biomarker that is used for early diagnosis of aGVHD. A translationally-relevant molecular imaging method that can non-invasively visualize activated T cell dynamics within living subjects could enable earlier definitive aGVHD diagnosis and quantitative risk stratification to permit more timely and effective interventional strategies. Here we show that, like the T cell specific cytotoxic drug AraG, the PET tracer [18F]F-AraG is a substrate for cellular dCK and dGK activity, preferentially accumulates in both murine and human activated versus resting T cells, and [18F]F-AraG PET imaging allowed quantitation of progressively increasing alloreactive T cell accumulation in the CLN of aGVHD mice. Thus this technology is an exciting new tool with high translational potential that can enable activated T cell imaging in aGVHD mice and could be the missing link for earlier detection of this devastating disease.
Inclusive of aGVHD, selective non-invasive imaging of particular immune cell populations would have broad applicability in both immune-based disease detection and immunotherapy monitoring. Along these lines, the PET tracer [18F]F-AraC was previously developed by screening deoxynucleoside analogs for preferential uptake in activated versus resting T cells (13). However, as with AraC, the selectivity of [18F]F-AraC for accumulation in activated T cells in vivo is less than ideal (13). It is also subject to rapid in vivo deamination via cytidine deaminase activity, and although newer tracers avoiding this issue are being pursued (35,36), the primary applications being pursued for [18F]F-AraC are now tumor imaging. In contrast to AraC, AraG has shown remarkably selective cytotoxic effects towards T-lymphoblastoid cells and T-lymphocytes compared to other immune cell lineages (17-19). Based on this knowledge, we previously described the chemical synthesis of the PET tracer [18F]F-AraG in an attempt to develop a more selective activated T cell molecular imaging probe(16). Here we describe detailed mechanistic studies and in vivo imaging of [18F]F-AraG in a relevant T-cell mediated disease model. This is important as our tracer is a fluorinated version of AraG, and even though studies have shown that F-AraG behaves similarly to AraG (37), this may not have held true at low tracer concentrations. Our results in cultured cells support similar mechanisms of uptake and cell specificity as AraG since our tracer was preferentially retained in T cell lines versus other immune cell lineages (17-19,28), a competition assay with excess AraG inhibited uptake of our tracer across all immune cell lines, and our tracer utilizes both dCK and dGK activity to accumulate into cells (21). dGK utilization is supported by our biodistribution results in mice and humans showing moderately high tracer accumulation in the liver and heart, two organs with high dGK expression (38). Unlike AraC and [18F]F-AraC, which are solely phosphorylated by dCK (13), AraG and [18F]F-AraG are substrates for both dCK and dGK activity, with dCK described as a low affinity, high specific activity enzyme and dGK as a high affinity, low specific activity enzyme. The KM of [3H]AraG for dGK and dCK are 10 and 100 μM, respectively (21), and thus our tracer at low nM doses should preferentially utilize the dGK pathway, if available. However, dCK appears to be playing a significant role in [18F]F-AraG uptake as loss of dCK in the T lymphoblast cell line (CCRF-CEM) resulted in significantly lower intracellular levels (Figure 1). The distinct mechanisms of uptake of [18F]F-AraG compared to [18F]F-AraC (i.e., dGK phosphorylation) is also supported by the differences in the uptake and efflux of both tracers across immune cell lines, solid tumor cell lines, and resting and primary activated T cells. Biodistribution in humans is also different between the two tracers with [18F]F-AraC showing low liver and high splenic uptake (36), whereas [18F]F-AraG showed high liver and moderate splenic uptake. Overall, our data strongly supports the uniqueness of our tracer for imaging cell populations with high dCK and dGK activity including activated T cells.
Based on studies in the same mouse model we used here, aGVHD has two phases of disease progression called the activation phase and effector phase (11,24,25,39). From an early diagnostic perspective, it would be ideal if subjects at risk for high-grade GVHD could be identified during the activation phase to fortify immunosuppressive therapy. Several candidate blood biomarkers have shown promise for early detection of aGVHD including a panel of proteomic blood biomarkers capable of diagnosis approximately 15 days before definitive diagnosis (10). Cellular biomarkers of aGVHD in the blood have also been explored. Bauerlein et al. have shown that, during their migration to effector organs, peripheral blood detection of alloreactive T cells expressing homing receptors (α4β7 integrin and P-selectin ligand) can be used to define a diagnostic window for the improved treatment of aGVHD (11). We believe that [18F]F-AraG PET imaging that can quantify activated T cell accumulation within the CLN during the activation phase may provide a complementary technique to these blood-based diagnostics to provide more definitive early aGVHD diagnosis. Hypothetically, [18F]F-AraG PET may even enable earlier diagnosis than a blood-based strategy by detection of T cells in priming sites prior to migration of cells or shedding of cellular proteins into the peripheral blood - a hypothesis that we are currently testing.
Several other imaging techniques have also shown promise for definitive diagnosis of aGVHD, particularly gastrointestinal manifestations of the disease, including magnetic resonance imaging (MRI), computed tomography (CT), high-resolution transabdominal ultrasound and color Doppler ultrasound (32,33). These techniques primarily focus on detection of edema and thickening of the intestinal wall during advanced stages of aGVHD, and are therefore likely limited for early disease detection. [18F]FDG PET has also shown promise for imaging intestinal inflammation associated with aGVHD in both mouse models and patients with early clinical symptoms, even allowing prediction and monitoring of therapy response in these patients (34). However, the utility of [18F]FDG PET imaging for early detection prior to clinical symptoms is not known and the non-specific nature of [18F]FDG imaging for a variety of activated immune cell types should enable a selective advantage of [18F]F-AraG PET imaging for this purpose.
Despite the high sensitivity of PET imaging for low levels of molecular targets it remains a challenge to detect scarce target cell numbers in small organs or organs near areas of non-specific tracer accumulation (i.e. areas with high background signal). There is inherently a limit to the spatial resolution and cellular sensitivity that is achievable with PET. One limitation of our study was the inability to visualize in vivo differences of tracer uptake in both the MLN and spleen, two organs we know harbor activated donor T cells. While our tracer ex vivo biodistribution results supported higher uptake in the MLN of aGVHD versus control mice at both early- and late-stages of disease we were unable to identify the MLN in our PET (or CT) images. This was mostly due to high tracer uptake in adjacent organs such as the kidneys and liver at 1 hour after tracer administration. While this is problematic in mice due to their small size and closely positioned organs, we believe this will be less of a concern in humans as supported by the low tracer uptake in the GI tract in human PET images of normal volunteers. The spleen showed an interesting pattern of tracer uptake in ex vivo biodistribution analysis with a trend towards higher tracer uptake in GVHD versus control mice at day 3 and significantly lower uptake at day 10. This contrasts with the data supporting more donor T cells in the spleen at day 10 compared to day 3. Although the reason for these differences is being actively investigated, the spleen has a more heterogeneous cellular composition than lymph nodes with T cells constituting a minor portion of this, so changes in overall cell composition or numbers may result in differences in tracer uptake as the disease progresses. We also found significantly decreased splenic weights between aGVHD and control mice at day 10 (0.06±0.01 vs. 0.12±0.01; p<0.01). This suggests that at day 10 the spleen of GVHD mice may have been a site of extensive tissue damage, resulting in a lower absolute number of splenocytes (which may also take up the tracer at low levels), and lower overall tracer accumulation. We do see some tracer uptake in the spleen in human PET images so it will need to be determined whether [18F]F-AraG accumulates in this organ at higher or lower levels in GVHD patients. As with dCK-targeted radiotracers (36), we are also actively pursuing alternative radioactive AraG analogs that may have different pharmacokinetic profiles.
In conclusion, we have shown that [18F]F-AraG PET imaging may provide crucial information to aid in the detection of activated T cell dynamics during the activation phase of aGVHD. Future work will focus on monitoring the effects of novel immunosuppressive therapies in this model. Additionally, the preliminary studies in healthy human volunteers have been promising with low background in the thorax and GI tract, making it favorable for thoracic and pelvic imaging. Favorable kinetics of [18F]F-AraG in humans along with the preclinical data support its potential as a promising tracer for early detection of GVHD and warrants its further clinical evaluation in aGVHD patients. Continued development of [18F]F-AraG as a selective T cell imaging agent in HCT patients is ongoing. Considering the paucity of activated T cell PET tracers in the clinic, this work also lays the foundation for future use of [18F]F-AraG PET for visualizing other T cell mediated diseases (e.g., multiple sclerosis, rheumatoid arthritis, Type 1 diabetes mellitus, etc.), evaluating endogenous T cell dynamics during the pathogenesis of other diseases (e.g. cancer or infection), and monitoring the effectiveness of T cell-based immunotherapies.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the support of the Stanford Center for Innovation in In-Vivo Imaging (SCI3) for all preclinical imaging experiments and Dr. Frezghi Habte for help with PET image analysis. We would also like to thank Dr. Beverly Mitchell (Stanford University) for providing the dCK- cell line, Dr. Varsha Gandhi (MD Anderson) for providing the dCK+ and dGK+ cell lines, and Dr. Heike Daldrup-Link (Stanford University) for providing the PyMT cell line. We are also grateful to Xinrui Yan for supporting the preclinical studies, Samuel Quezada (Cellsight Technologies) for supporting clinical studies and Mirwais Wardak for insightful comments on the manuscript. We acknowledge the assistance of Vahid Ravanfar acquiring the patient images on the GE PET/MR.
Financial Support: This work was funded by several grants including NCI ICMIC P50CA114747 (S.S. Gambhir), NCI RO1 R01CA201719 (S.S. Gambhir), Ben & Catherine Ivy Foundation (S.S. Gambhir), and Phase II SBIR Contract HHSN261201300063C (S.S. Yaghoubi).
Abbreviations list
- aGVHD
acute graft-versus-host disease
- PET
positron emission tomography
- [18F]F-AraG
2′-deoxy-2′-[18F]fluoro-9-β-D-arabinofuranosylguanine
- HCT
hematopoietic cell transplantation
- HLA
human leukocyte antigen
- AraG
arabinosyl guanine
- T-ALL
T-cell acute lymphoblastic leukemia
- T-LBL
T-cell lymphoblastic lymphoma
- AraGMP
AraG-monophosphate
- dCK
deoxycytidine kinase
- dGK
deoxyguanosine kinase
- AraGTP
AraG-triphosphate
- Anti-Anti
Antibiotic-Antimycotic
- [3H]F-AraC
tritiated 1-(2-deoxy-2-fluoro-arabinofuranosyl)-cytosine
- CMC
Chemistry Manufacturing and Control
- MACS
magnetic-activated cell sorting
- HBSS
Hanks balanced salt solution
- TCD-BM
T cell-depleted bone marrow
- IRW
Inveon Research Workplace
- Luc
firefly luciferase
- BLI
bioluminescence imaging
- MLN
mesenteric lymph nodes
- CLN
cervical lymph nodes
Footnotes
Conflict of Interest: Dr. Yaghoubi and Dr. Gambhir are founders of CellSight Technologies Inc. that has licensed the rights to [18F]AraG from Stanford University.
References
- 1.McDonald-Hyman C, Turka LA, Blazar BR. Advances and challenges in immunotherapy for solid organ and hematopoietic stem cell transplantation. Sci Transl Med. 2015;7:280rv2. doi: 10.1126/scitranslmed.aaa6853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gratwohl A, Brand R, Frassoni F, Rocha V, Niederwieser D, Reusser P, et al. Cause of death after allogeneic haematopoietic stem cell transplantation (HSCT) in early leukaemias: an EBMT analysis of lethal infectious complications and changes over calendar time. Bone Marrow Transplant. 2005;36:757–69. doi: 10.1038/sj.bmt.1705140. [DOI] [PubMed] [Google Scholar]
- 3.Ferrara JLM, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373:1550–61. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tabbara IA, Zimmerman K, Morgan C, Nahleh Z. Allogeneic hematopoietic stem cell transplantation: complications and results. Archives of Internal Medicine. 2002;162:1558–66. doi: 10.1001/archinte.162.14.1558. [DOI] [PubMed] [Google Scholar]
- 5.Ferrara JL, Deeg HJ. Graft-versus-host disease. The New England Journal of Medicine. 1991;324:667–74. doi: 10.1056/NEJM199103073241005. [DOI] [PubMed] [Google Scholar]
- 6.Jamani K, Russell JA, Daly A, Stewart D, Savoie L, Duggan P, et al. Prognosis of grade 3-4 acute GVHD continues to be dismal. Bone Marrow Transplant. 2013;48:1359–61. doi: 10.1038/bmt.2013.59. [DOI] [PubMed] [Google Scholar]
- 7.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–8. [PubMed] [Google Scholar]
- 8.Malard F, Mohty M. New insight for the diagnosis of gastrointestinal acute graft-versus-host disease. Mediators of Inflammation. 2014;2014:701013. doi: 10.1155/2014/701013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vogelsang GB, Lee L, Bensen-Kennedy DM. Pathogenesis and treatment of graft-versus-host disease after bone marrow transplant. Annual Review of Medicine. 2003;54:29–52. doi: 10.1146/annurev.med.54.101601.152339. [DOI] [PubMed] [Google Scholar]
- 10.De Bock M, Beguin Y, Leprince P, Willems E, Baron F, Deroyer C, et al. Comprehensive plasma profiling for the characterization of graft-versus-host disease biomarkers. Talanta. 2014;125:265–75. doi: 10.1016/j.talanta.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 11.Bäuerlein CA, Riedel SS, Baker J, Brede C, Garrote ALJ, Chopra M, et al. A diagnostic window for the treatment of acute graft-versus-host disease prior to visible clinical symptoms in a murine model. BMC Medicine. 2013;11:134. doi: 10.1186/1741-7015-11-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.James ML, Gambhir SS. A Molecular Imaging Primer: Modalities, Imaging Agents, and Applications. Physiological Reviews. 2012;92:897–965. doi: 10.1152/physrev.00049.2010. [DOI] [PubMed] [Google Scholar]
- 13.Radu CG, Shu CJ, Nair-Gill E, Shelly SM, Barrio JR, Satyamurthy N, et al. Molecular imaging of lymphoid organs and immune activation by positron emission tomography with a new [18F]-labeled 2′-deoxycytidine analog. Nature Medicine. 2008;14:783–8. doi: 10.1038/nm1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Gialleonardo V, Signore A, Glaudemans AWJM, Dierckx RAJO, de Vries EFJ. N-(4-18F-fluorobenzoyl)interleukin-2 for PET of human-activated T lymphocytes. Journal of Nuclear Medicine. 2012;53:679–86. doi: 10.2967/jnumed.111.091306. [DOI] [PubMed] [Google Scholar]
- 15.Tavaré R, McCracken MN, Zettlitz KA, Knowles SM, Salazar FB, Olafsen T, et al. Engineered antibody fragments for immuno-PET imaging of endogenous CD8+ T cells in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:1108–13. doi: 10.1073/pnas.1316922111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Namavari M, Chang YF, Kusler B, Yaghoubi S, Mitchell BS, Gambhir SS. Synthesis of 2′-deoxy-2′-[18F]fluoro-9-β-D-arabinofuranosylguanine: a novel agent for imaging T-cell activation with PET. Molecular Imaging and Biology. 2011;13:812–8. doi: 10.1007/s11307-010-0414-x. [DOI] [PubMed] [Google Scholar]
- 17.Cohen A, Lee JW, Gelfand EW. Selective toxicity of deoxyguanosine and arabinosyl guanine for T-leukemic cells. Blood. 1983;61:660–6. [PubMed] [Google Scholar]
- 18.Shewach DS, Daddona PE, Ashcraft E, Mitchell BS. Metabolism and selective cytotoxicity of 9-beta-D-arabinofuranosylguanine in human lymphoblasts. Cancer Research. 1985;45:1008–14. [PubMed] [Google Scholar]
- 19.Shewach DS, Mitchell BS. Differential metabolism of 9-beta-D-arabinofuranosylguanine in human leukemic cells. Cancer Research. 1989;49:6498–502. [PubMed] [Google Scholar]
- 20.Roecker AM, Stockert A, Kisor DF. Nelarabine in the treatment of refractory T-cell malignancies. Clinical Medicine Insights Oncology. 2010;4:133. doi: 10.4137/CMO.S4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodriguez CO, Mitchell BS, Ayres M, Eriksson S, Gandhi V. Arabinosylguanine is phosphorylated by both cytoplasmic deoxycytidine kinase and mitochondrial deoxyguanosine kinase. Cancer Research. 2002;62:3100–5. [PubMed] [Google Scholar]
- 22.Bauer C, Bauder-Wuest U, Mier W, Haberkorn U, Eisenhut M. 131I-labeled peptides as caspase substrates for apoptosis imaging. J Nucl Med. 2005;46:1066–74. [PubMed] [Google Scholar]
- 23.Nakanishi T, Ross DD, Mitsuoka K. Methods to Evaluate Transporter Activity in Cancer. Totowa, NJ: Humana Press; 2010. pp. 105–20. [DOI] [PubMed] [Google Scholar]
- 24.Beilhack A, Schulz S, Baker J, Beilhack GF, Nishimura R, Baker EM, et al. Prevention of acute graft-versus-host disease by blocking T-cell entry to secondary lymphoid organs. Blood. 2008;111:2919–28. doi: 10.1182/blood-2007-09-112789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beilhack A, Schulz S, Baker J, Beilhack GF, Wieland CB, Herman EI, et al. In vivo analyses of early events in acute graft-versus-host disease reveal sequential infiltration of T-cell subsets. Blood. 2005;106:1113–22. doi: 10.1182/blood-2005-02-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cao YA, Wagers AJ, Beilhack A, Dusich J, Bachmann MH, Negrin RS, et al. Shifting foci of hematopoiesis during reconstitution from single stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:221–6. doi: 10.1073/pnas.2637010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim BS, Nishikii H, Baker J, Pierini A, Schneidawind D, Pan Y, et al. Treatment with agonistic DR3 antibody results in expansion of donor Tregs and reduced graft-versus-host disease. Blood. 2015;126:546–57. doi: 10.1182/blood-2015-04-637587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verhoef V, Fridland A. Metabolic basis of arabinonucleoside selectivity for human leukemic T- and B-lymphoblasts. Cancer Research. 1985;45:3646–50. [PubMed] [Google Scholar]
- 29.Kim W, Le TM, Wei L, Poddar S, Bazzy J, Wang X, et al. [18F]CFA as a clinically translatable probe for PET imaging of deoxycytidine kinase activity. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(15):4027–32. doi: 10.1073/pnas.1524212113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cooke KR, Kobzik L, Martin TR, Brewer J, Delmonte J, Crawford JM, et al. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation: I. The roles of minor H antigens and endotoxin. Blood. 1996;88:3230–9. [PubMed] [Google Scholar]
- 31.Levine JE, Braun TM, Harris AC, Holler E, Taylor A, Miller H, et al. A prognostic score for acute graft-versus-host disease based on biomarkers: a multicentre study. Lancet Haematol. 2015;2:e21–9. doi: 10.1016/S2352-3026(14)00035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mentzel HJ, Kentouche K, Kosmehl H, Gruhn B, Vogt S, Sauerbrey A, et al. US and MRI of gastrointestinal graft-versus-host disease. Pediatric Radiology. 2002;32:195–8. doi: 10.1007/s00247-001-0613-3. [DOI] [PubMed] [Google Scholar]
- 33.Kalantari BN, Mortele KJ, Cantisani V, Ondategui S, Glickman JN, Gogate A, et al. CT features with pathologic correlation of acute gastrointestinal graft-versus-host disease after bone marrow transplantation in adults. AJR American Journal of Roentgenology. 2003;181:1621–5. doi: 10.2214/ajr.181.6.1811621. [DOI] [PubMed] [Google Scholar]
- 34.Stelljes M, Hermann S, Albring J, Köhler G, Löffler M, Franzius C, et al. Clinical molecular imaging in intestinal graft-versus-host disease: mapping of disease activity, prediction, and monitoring of treatment efficiency by positron emission tomography. Blood. 2008;111:2909–18. doi: 10.1182/blood-2007-10-119164. [DOI] [PubMed] [Google Scholar]
- 35.Shu CJ, Campbell DO, Lee JT, Tran AQ, Wengrod JC, Witte ON, et al. Novel PET probes specific for deoxycytidine kinase. Journal of Nuclear Medicine. 2010;51:1092–8. doi: 10.2967/jnumed.109.073361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwarzenberg J, Radu CG, Benz M, Fueger B, Tran AQ, Phelps ME, et al. Human biodistribution and radiation dosimetry of novel PET probes targeting the deoxyribonucleoside salvage pathway. European Journal of Nuclear Medicine and Molecular Imaging. 2011;38:711–21. doi: 10.1007/s00259-010-1666-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Montgomery JA, Shortnacy AT, Carson DA, Secrist JA. 9-(2-Deoxy-2-fluoro-b-D-arabinofuranosyl)guanine: a metabolically stable cytotoxic analogue of 2′-deoxyguanosine. Journal of Medicinal Chemistry. 1986;29:2389–92. doi: 10.1021/jm00161a041. [DOI] [PubMed] [Google Scholar]
- 38.Johansson M, Karlsson A. Cloning and expression of human deoxyguanosine kinase cDNA. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(14):7258–62. doi: 10.1073/pnas.93.14.7258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Leeuwen L, Guiffre A, Atkinson K, Rainer SP, Sewell WA. A two-phase pathogenesis of graft-versus-host disease in mice. Bone Marrow Transplant. 2002;29:151–8. doi: 10.1038/sj.bmt.1703328. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.