Abstract
Background
In sub-Saharan Africa, malaria is frequently overdiagnosed as the cause of an undifferentiated febrile illness, whereas arboviral illnesses are presumed to be underdiagnosed.
Methods
Sera from 385 febrile Kenyan children, who presented to 1 of 4 clinical sites, were tested using microscopy and real-time molecular assays for dengue virus (DENV), chikungunya virus (CHIKV), malaria, and Leptospira.
Results
Malaria was the primary clinical diagnosis for 254 patients, and an arboviral infection (DENV or CHIKV) was the primary diagnosis for 93 patients. In total, 158 patients (41.0%) had malaria and 32 patients (8.3%) had CHIKV infections. Compared with real-time polymerase chain reaction, microscopy demonstrated a percent positive agreement of 49.7%. The percentage of malaria cases detected by microscopy varied significantly between clinical sites. Arboviral infections were the clinical diagnosis for patients on the Indian Ocean coast (91 of 238, 38.2%) significantly more often than patients in the Lake Victoria region (2 of 145, 1.4%; P < .001). However, detection of CHIKV infections was significantly higher in the Lake Victoria region (19 of 145 [13.1%] vs 13 of 239 [5.4%]; P = .012).
Conclusions
The clinical diagnosis of patients with an acute febrile illness, even when aided by microscopy, remains inaccurate in malaria-endemic areas, contributing to inappropriate management decisions.
Keywords: chikungunya, Kenya, malaria, molecular diagnosis, serum
An acute, undifferentiated febrile illness is a common clinical syndrome throughout the world and presents significant diagnostic challenges due to the myriad possible causes and the potential for patients to develop severe disease depending on the etiology [1–3]. In the tropics, accurate laboratory diagnostics are frequently unavailable, even for etiologies that may result in severe disease, such as malaria, salmonellosis, or dengue virus (DENV). In sub-Saharan Africa, malaria remains atop the differential diagnosis for patients with an undifferentiated febrile illness, because it is both potentially life-threatening and treatable with oral medication [2, 4]. However, malaria is often overdiagnosed clinically, including frequent diagnoses in patients from regions with low transmission [1, 5–7]. This was demonstrated in a study from Tanzania where 60.7% of patients who were admitted with a febrile illness had suspected malaria, but only 1.6% had confirmed disease [1].
Malaria transmission in Kenya ranges from areas of high transmission near Lake Victoria and Uganda to highland areas without documented transmission. Malaria is frequently the clinical diagnosis for Kenyan children who present with an acute, undifferentiated fever, and microscopy on thick and thin blood smears remains in wide use for laboratory confirmation, although this method has well documented limitations [8, 9]. Molecular testing has proven to be more sensitive than microscopy for the detection of Plasmodium species [8, 10, 11], although most studies that report a significant increase in Plasmodium detection have used whole-blood samples and focused on surveillance testing in asymptomatic individuals [11–14]. Our group, among others, has reported on the use of serum for malaria detection by real-time PCR (rtPCR) [10, 15, 16]. In a study of 317 symptomatic patients from Nigeria, malaria rtPCR using serum or plasma proved to be significantly more sensitive than microscopy for the detection of Plasmodium falciparum, without a significant decrease in specificity [10]. It is notable that this method, which is the same assay used in the current study, demonstrated similar sensitivity to the BinaxNOW Malaria rapid diagnostic test (Alere) with a trend toward improved specificity [10].
In contrast to malaria, arboviral infections are thought to be underdiagnosed in Africa. In a study from Tanzania, 7.9% of patients admitted with a febrile illness had chikungunya virus (CHIKV) infections confirmed by reverse transcription (RT)-PCR, although none had chikungunya listed as an admitting diagnosis [1]. A separate study of febrile Tanzanian children found that 38.2% had DENV and 4.7% had presumptive CHIKV infection, but dengue, chikungunya, and malaria could not be clinically distinguished [17]. Seroprevalence studies in Kenya have reported anti-CHIKV antibodies in 20%–42% of participants [18, 19]. In 2004, coastal Kenya was affected by a large CHIKV outbreak that subsequently spread to countries across the Indian Ocean, Asia, and the South Pacific [20]. However, few reports have characterized acute arboviral infections in Africa outside of an epidemic setting.
The objectives of the current study were to (1) determine the incidence of DENV, CHIKV, Plasmodium, and Leptospira infections among Kenyan children with an acute undifferentiated febrile illness using molecular diagnostics and (2) use molecular test results to evaluate the sensitivity of microscopy for the diagnosis of malaria, the accuracy of clinical diagnoses for malaria and chikungunya, and the characteristics of patients diagnosed with malaria and/or chikungunya in this pediatric population.
METHODS
Ethics Statement
Study protocol was reviewed and approved by the Stanford University Institutional Review Board (no. 31488) and the Kenya Medical Research Institute Scientific and Ethical Review Committee (SSC 2611).
Clinical Samples
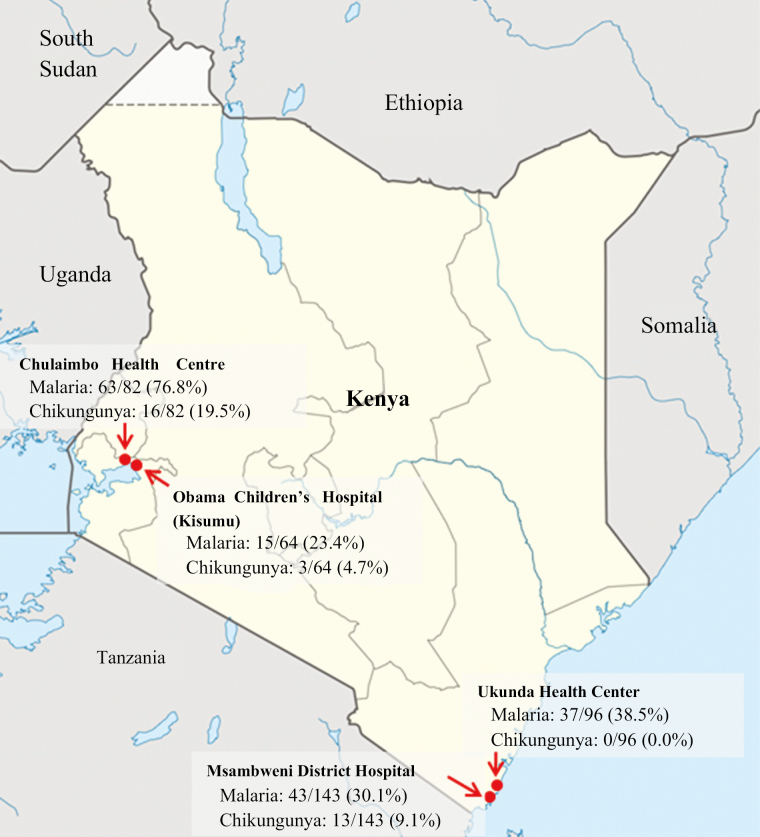
Acute-phase serum samples from 385 patients enrolled in an ongoing, acute febrile illness surveillance study in coastal and western Kenya were tested for this study. In brief, children less than 18 years of age who presented with an acute febrile illness (≤5 days duration) and no localizing signs or symptoms were enrolled at 4 study sites in Kenya: Chulaimbo Health Centre, Obama Children’s Hospital in Kisumu, Msambweni District Hospital, and Ukunda Health Center (Figure 1). Written informed consent was provided by parents or guardians for all participants in the study, and children 7 years and older provided assent. Patients were evaluated by study physicians, and management decisions, including anti-infective therapy and disposition, were made at the discretion of the care providers in accordance with local practice.
Figure 1.
Map of Kenya showing the 4 study sites and incidence of Plasmodium and chikungunya virus detection. Study sites were located on the Indian Ocean coast (Ukunda [urban] and Msambweni [rural]) and in the Lake Victoria region (Chulaimbo [rural] and Kisumu [town]).
For testing in the current study, patients who presented within the first 3 days of fever onset were selected from the overall study cohort. With a target sample size of 400 patients, a small number of patients who presented at later time points were included. Microscopy results were available at the time of the study visit: after physicians recorded a primary clinical diagnosis but before decisions regarding antimalarial treatment. Standardized study questionnaires, containing information on epidemiologic and clinical variables, were completed during the acute visit.
Thick and thin blood smears were performed at presentation. Thick smears were used for malaria detection. Microscopy was considered negative for malaria if no parasites were observed after review of 200 high-powered fields of a thick smear. Thin smears were used for confirmation and speciation. Rapid diagnostic tests are not routinely used at these locations.
Molecular Testing
Acute-phase serum samples were shipped to Stanford University on dry ice and stored at −80°C until nucleic acid extraction was performed. Total nucleic acids were extracted from 200 µL of serum using an easyMAG instrument (bioMerieux, Durham, NC) and a 60-µL elution volume. All samples were tested using internally controlled, real-time, multiplex nucleic acid amplification tests for DENV, CHIKV, Leptospira, Plasmodium species, and P falciparum, as previously described [3, 21]. Plasmodium species in these multiplexes were detected using a pan-Plasmodium rtPCR targeting the 18S gene [3]. The pan-Plasmodium assay was performed as a component of 2 multiplex tests: (1) the DLM assay for DENV, Leptospira, and malaria detection; and (2) an assay for the detection of DENV, CHIKV, Leptospira, and malaria [3, 21]. The analytical performance of the pan-Plasmodium assay was similar in both assays (Supplementary Figure 1). Samples that tested positive in the pan-Plasmodium assay but negative for P falciparum were tested using species-specific rtPCRs, as described previously [22]. For the purposes of this study, malaria was diagnosed by the detection of Plasmodium deoxyribonucleic acid in serum using the pan-Plasmodium and/or P falciparum rtPCRs. Microscopy results were interpreted in relation to rtPCR results.
Statistics
Sixty-one variables from the standardized study questionnaire (Supplementary Table 1) were included in analyses to identify associations between epidemiologic and clinical factors and certain diagnostic test results. Age-specific cutoff values were used to identify patients with tachycardia, tachypnea, and hypotension (systolic blood pressure) [23]. Categorical variables were compared using Fisher’s exact test for variables with 2 discrete outcomes or χ2 tests for variables with 3 or more outcomes. Continuous variables were compared using t tests. A kappa statistic was calculated to compare results of blood smear and molecular testing. GraphPad software (GraphPad, San Diego, CA) was used to calculate Fisher’s exact tests, χ2 tests, t tests, and kappa statistics. For the comparison of pan-Plasmodium cycle threshold (Ct) values and parasite quantitation by microscopy, Pearson’s correlation coefficient was calculated at socscistatistics.com.
Multivariable analyses were performed to determine associations between epidemiologic and clinical variables and the following: (1) Plasmodium detection in the pan-Plasmodium assay and (2) positive blood smear results among patients who tested positive in the pan-Plasmodium assay. R software was used to produce generalized linear models including all combinations of variables evaluated in univariate analysis and identify the best-fit model. Model fit was assessed using Akaike information criterion [24].
RESULTS
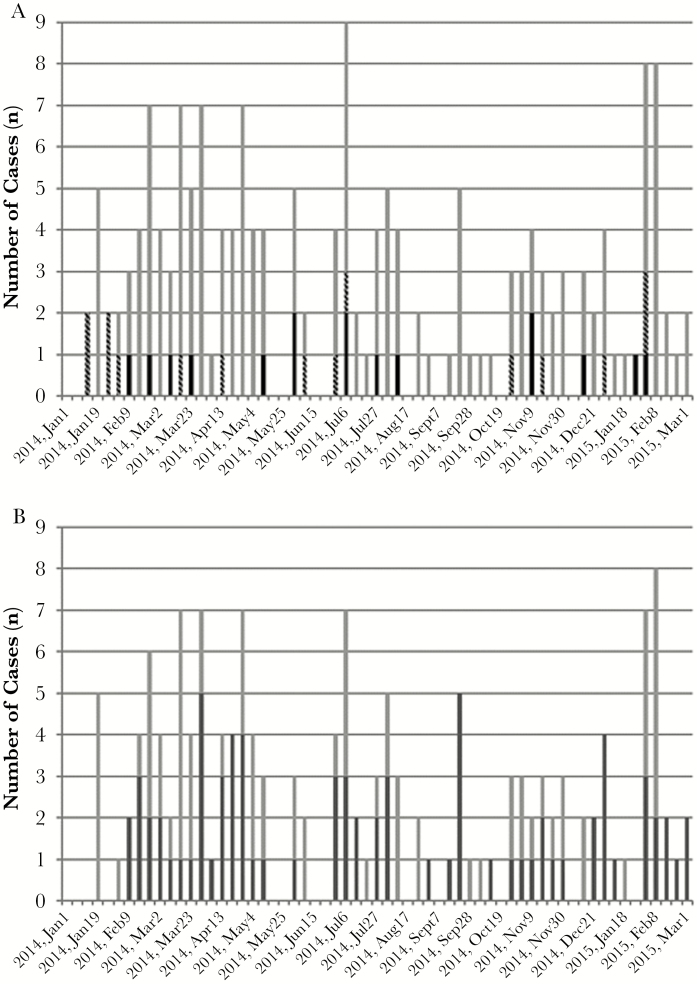
Samples were tested from 385 patients who presented between January 16, 2014 and July 3, 2015. Characteristics of the study population are shown in Table 1, and the distribution of patients by study site is shown in Table 2. Date of birth was recorded for 379 patients (98.4%), and study questionnaires were otherwise complete for 383 patients (99.5%). In total, 158 patients (41.0%) had malaria detected by rtPCR, 32 patients (8.3%) had CHIKV detected, and 1 patient was positive for Leptospira (0.3%). No DENV infections were confirmed in this population. Fifteen patients had coinfections with P falciparum and CHIKV. Infections were detected throughout the study period (Figure 2A), with a peak during the early rainy season in 2014 (late February–May). A spike in infections occurred in July 2014, which coincided with a late rainy season that year and increased testing at the Msambweni and Ukunda sites.
Table 1.
History, Clinical Presentation, and Management of Febrile Kenyan Children, Including Patients With Plasmodium and/or CHIKV Detected by Molecular Methods
| Patient Dataa | All Patients n (%) | Pan-Plasmodium Positive, n (%)b | CHIKV Positive n (%)b | Negative n (%) |
|---|---|---|---|---|
| Number, n | 383 | 157 | 32 | 209 |
| Gender, female | 193 (50.4) | 87 (55.4) | 15 (50.0) | 100 (47.8) |
| Age, years, mean (SD) | 5.05 (3.46) | 5.68 (3.42)c | 4.55 (3.27) | 4.7 (3.49) |
| Days postsymptom onset, mean (SD) | 2.46 (0.86) | 2.50 (1.02) | 2.28 (0.68) | 2.44 (0.75) |
| Bed nets, always used | 308 (80.4) | 115 (73.2)c | 27 (84.4) | 177 (84.7) |
| History of malaria | 295 (77.0) | 132 (84.1)c | 29 (90.6)c | 148 (70.8) |
| Screened windows | 136 (35.5) | 35 (22.3)c | 7 (21.9)c | 95 (45.4) |
| Signs and Symptoms | ||||
| Febrile (temperature ≥38°C) | 383 (100) | 157 (100) | 32 (100) | 209 (100) |
| Headache | 166 (43.3) | 82 (52.2)c | 12 (37.5) | 79 (37.8) |
| Joint pain | 91 (23.8) | 36 (22.9) | 7 (21.9) | 52 (24.9) |
| Tachycardia | 44 (11.6) | 24 (15.3) | 7 (21.9) | 18 (8.8) |
| Tachypnea | 82 (21.8) | 26 (16.7)d | 5 (16.3) | 54 (26.5) |
| Hypotensive | 26 (6.9) | 5 (3.2)d | 3 (9.4) | 19 (9.3) |
| Hepatosplenomegaly | 26 (6.8) | 9 (5.7) | 2 (6.2) | 16 (7.7) |
| Abnormal joint exam | 80 (20. 9) | 24 (15.3)d | 4 (12.5) | 54 (25.8) |
| Rash or petechiae | 26 (6.8) | 5 (3.2)c | 2 (6.2) | 22 (10.5) |
| Microscopy, positive, n/N (%) | 79 of 365 (21.6) | 71 of 143 (49.7)c | 8 of 27 (29.6)c | 6 of 205 (2.9) |
| Primary Diagnosis and Treatment | ||||
| Malaria | 254 (66.3) | 125 (79.6)c | 24 (75.0) | 119 (56.9) |
| Arboviral illnesse | 93 (24.3) | 21 (13.4)c | 8 (25.0) | 64 (30.6) |
| Antimalarials | 129 (33.7) | 100 (63.7)c | 20 (62.5)c | 22 (10.5) |
| Antibiotics | 277 (73.3) | 99 (63.1)c | 19 (59.4)c | 170 (81.3) |
Abbreviations: CHIKV, chikungunya virus; DENV, dengue virus; SD, standard deviation.
aComplete information was available for 383 of 385 patients (99.5%).
bFifteen patients had Plasmodium falciparum-CHIKV coinfections detected.
c P ≤ .01 compared with negative patients.
d P ≤ .05 compared with negative patients.
ePrimary diagnosis of CHIKV or DENV.
Table 2.
Factors Associated With Plasmodium Detection Using the Pan-Plasmodium Assay
| Patient Data | All Patients n (%) | Plasmodium Positive n (%) | Negative n (%)a | Univariate | Multivariable Analysisc | ||
|---|---|---|---|---|---|---|---|
| P Valueb | Odds (Ln) | Standard Error | P Value | ||||
| Number, nd | 383 | 157 | 209 | ||||
| Gender, female | 193 (50.4) | 87 (55.4) | 100 (47.8) | — | |||
| Age, years, mean (SD) | 5.05 (3.46) | 5.68 (3.42) | 4.7 (3.49) | .008 | |||
| Days postsymptom onset, mean (SD) | 2.46 (0.86) | 2.50 (1.02) | 2.44 (0.75) | — | |||
| Study Site | <.001 | ||||||
| Chulaimbo Health Centre | 82 (21.3) | 63 (39.9) | 14 (6.7) | 1.87e | 0.31 | <.001 | |
| Obama Children’s Hospital | 64 (16.6) | 15 (9.5) | 47 (22.4) | ||||
| Msambweni District Hospital | 143 (37.1) | 43 (27.2) | 90 (42.7) | ||||
| Ukunda Health Center | 96 (24.9) | 37 (23.4) | 59 (28.1) | ||||
| Bed nets, always used | 308 (80.4) | 115 (73.3) | 177 (84.7) | .008 | −0.88 | 0.28 | .002 |
| Signs and Symptoms | |||||||
| Tachycardia | 44 (11.6) | 24 (15.3) | 18 (8.8) | .069 | 0.68 | 0.37 | .064 |
| Tachypnea | 82 (21.8) | 26 (16.7) | 54 (26.5) | .030 | −0.64 | 0.30 | .032 |
Abbreviations: CHIKV, chikungunya virus; SD, standard deviation.
aSamples tested negative for Plasmodium and CHIKV by molecular methods.
b P values for univariate analysis comparing results for malaria positive and negative patients.
cResults displayed for variables that remained in the best-fit model.
dComplete information available for 157 patients positive for Plasmodium and 209 negative patients.
eCoded in multivariable analysis as “Chulaimbo Health Centre” versus “Other”.
Figure 2.
(A) Distribution of Plasmodium and chikungunya virus (CHIKV) infections by epidemiologic week, displayed as the date of the Sunday at the start of the week. Chikungunya virus infections are shown in black, Plasmodium infections are shown in gray, and CHIKV-P falciparum coinfections are shown in gray-black hatched bars. (B) Distribution of Plasmodium cases that were detected by real-time polymerase chain reaction (rtPCR) and microscopy (dark gray bars) and those detected only by rtPCR (negative by microscopy; light gray bars). Data are shown for the period January 2014–March 2015; only 3 cases were tested from April to July 2015.
Malaria Cases
Malaria was the primary clinical diagnosis for 254 patients, 125 (49.2%) of whom tested positive by rtPCR. Malaria incidence was significantly higher at Chulaimbo Health Centre (63 of 82, 76.8%) than at any other study site (23.4%–38.5%; P < .001 for all comparisons; Figure 1). Plasmodium falciparum was detected in 150 of 158 malaria cases (94.9%) overall. Species were confirmed for 5 of the remaining 8 cases using species-specific rtPCR: Plasmodium malariae, 2; Plasmodium ovale, 2; Plasmodium vivax, 1. Six patients reported taking antimalarial medication at the time of the acute visit: 3 were positive for malaria by rtPCR; 2 were positive for CHIKV; and 1 tested negative.
Demographic data, patient history, and clinical presentation for malaria cases and negative patients are shown in Table 1. In univariate analysis, malaria cases differed significantly from negative patients on several factors (Table 1; Supplementary Table 2). Also of note, malaria cases were tachypneic and/or hypotensive significantly less often than negative cases (Table 1). Multivariable analysis was performed to identify factors independently associated with malaria detection (Table 2). The most important factor identified in the best-fit model was study site. Relative to patients at the other 3 sites, the odds of a patient at Chulaimbo Health Centre having malaria were 6.5. Reporting “always” for bed net use was more frequent among negative patients and remained significant in the final model. In terms of clinical findings, tachypnea remained significantly associated with negative patients. Tachycardia, which was not significant in univariate analysis, remained associated with malaria cases in the final model but did not reach statistical significance (P = .064).
Microscopy Positive Versus Microscopy Negative Malaria
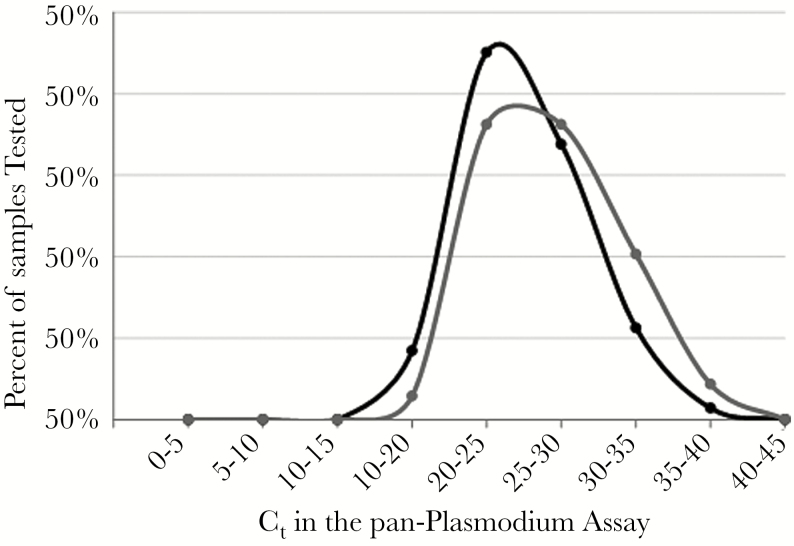
Microscopy results were available for 365 of 385 patients (94.8%). Microscopy and rtPCR demonstrated moderate agreement (kappa 0.50; 95% confidence interval [CI], 0.41–0.59) (Supplementary Table 3). Although microscopy had a 96.4% negative agreement with rtPCR, the percent positive agreement was only 49.7%. The proportion of malaria cases detected only by rtPCR did not vary over the study period (Figure 2B). Although the mean pan-Plasmodium Ct value was lower for samples with positive microscopy (25.04, standard deviation [SD] = 3.93) compared with those with negative microscopy (26.72, SD = 4.36; P = .017), the distributions of Ct values demonstrated marked overlap (Figure 3).
Figure 3.
Distribution of threshold cycle (Ct) values in the pan-Plasmodium assay for samples with positive microscopy (●, black line) or negative microscopy (●, gray line).
Among malaria cases diagnosed by rtPCR, patients who had positive and negative microscopy were similar with regards to demographic information, history, and clinical presentation (Table 3; Supplementary Table 4). Although more malaria cases were detected at each site using rtPCR, a significantly higher proportion of PCR-positive malaria cases were missed by microscopy at Ukunda Health Center (32 of 37, 86.5%) compared with any of the remaining sites (Msambweni, 22 of 43 [51.2%]; Obama, 6 of 9 [40.0%]; and Chulaimbo, 12 of 48 [25%]; P ≤ .001 for all comparisons). In multivariable analysis of malaria cases detected by rtPCR with positive or negative microscopy results, the odds of having a microscopy positive malaria case at Ukunda Health Center were 0.09 compared with the other 3 sites combined (Table 3). Average parasite burden, quantified using pan-Plasmodium Ct values, was significantly lower at Msambweni District Hospital (mean Ct 28.72, SD = 6.02) compared with other sites (P < .05 for each comparison). Ukunda Health Center had, on average, the highest parasite burden (mean Ct 24.83, SD = 4.63), although this did not differ significantly from Chulaimbo Health Centre (26.46, SD = 4.52) or Obama Children’s Hospital (25.04, SD = 3.07).
Table 3.
Factors Associated With Blood Smear Results for Patients Who Tested Positive in the Pan-Plasmodium Assay
| Patient Data | Pan-Plasmodium Assay Positive | Univariate | Multivariable Analysisa | |||
|---|---|---|---|---|---|---|
| Blood Smear Positive n (%) | Blood Smear Negative n (%) | P Value | Odds (Ln) | Standard Error | P Value | |
| Number, nb | 71 | 72 | ||||
| Gender, female | 36 (50.70) | 40 (55.56) | — | |||
| Age, years, mean (SD) | 5.75 (3.40) | 5.59 (3.34) | — | |||
| Days postsymptom onset, mean (SD) | 2.52 (1.29) | 2.56 (0.69) | — | |||
| Study Site | <.001 | |||||
| Chulaimbo Health Center | 36 (50.7) | 12 (16.7) | ||||
| Obama Children’s Hospital | 9 (12.7) | 6 (8.3) | ||||
| Msambweni District Hospital | 21 (29.6) | 22 (30.6) | ||||
| Ukunda Health Center | 5 (7.0) | 32 (44.4) | −2.43c | 0.60 | <.001 | |
| History of malaria | 67 (94.4) | 51 (70.8) | <.001 | 1.49 | 0.64 | .020 |
| Signs and Symptoms | ||||||
| Tachycardia | 15 (21.1) | 4 (5.6) | .007 | 0.49 | 0.62 | .431 |
| Headache | 39 (54.9) | 36 (50.0) | — | 1.34 | 0.47 | .004 |
| Abdominal pain | 15 (21.1) | 28 (38.9) | .028 | −0.83 | 0.50 | .099 |
Abbreviations: DNA, deoxyribonucleic acid; rtPCR, real-time polymerase chain reaction; SD, standard deviation.
aResults displayed for variables that remained in the best-fit model.
bMicroscopy results available for 143 patients with Plasmodium DNA detected by rtPCR.
cCoded for multivariable analysis as “Ukunda Health Center” versus “Other”.
Chikungunya Infections
An arboviral infection was the primary clinical diagnosis for 93 patients, including 70 patients with suspected CHIKV infections and 23 patients with suspected DENV infections. In total, 32 patients had confirmed CHIKV infections, including 17 CHIKV monoinfections and 15 P falciparum-CHIKV coinfections (Supplementary Table 5). Only 8 of 93 patients (8.6%) with a suspected arbovirus infection had a CHIKV infection confirmed by real-time RT-PCR, whereas 21 of 93 patients (22.6%) had malaria. When grouped by region, patients in the Lake Victoria region were significantly less likely to receive a primary clinical diagnosis of an arboviral infection (2 of 145, 1.4%) than patients on the Indian Ocean coast (91 of 238, 38.2%; P < .001; Table 4). However, the incidence of CHIKV infection was significantly higher in the Lake Victoria region (19 of 145, 13.1%) compared with the coast (13 of 238, 5.5%; P = .012).
Table 4.
Primary Diagnosis of an Arboviral Infection (DENV or CHIKV) and Confirmed CHIKV Infections by Region
| Region | Primary Diagnosis of Arbovirus Infection n/N (%)a | Confirmed CHIKV Infections n/N (%) |
|---|---|---|
| Totalb | 93/383 (24.3) | 32/383 (8.4) |
| Lake Victoria regionc | 2/145 (1.4) | 19/145 (13.1) |
| Indian Ocean coastd | 91/238 (38.2) | 13/238 (5.5) |
| P value | <.001 | .012 |
Abbreviations: CHIKV, chikungunya virus; DENV, dengue virus.
aDENV was the primary diagnosis for 1 patient in the Lake Victoria region and 22 patients on the Indian Ocean coast.
bPrimary diagnoses were available for 383 patients.
cCombined data from Chulaimbo Health Centre and Obama Children’s Hospital.
dCombined data from Msambweni District Hospital and Ukunda Health Center.
Few significant differences in patient history or clinical presentation were detected between monoinfected and coinfected patients (Supplementary Table 5). Chikungunya virus-monoinfected patients tended to be younger (mean age in years 3.54, SD = 2.39) than coinfected patients (mean 5.68, SD = 3.82; P = .064). They were also less likely to have a dirt floor (10 of 17, 58.8%) than coinfected patients (14 of 15, 93.3%; P = .041). In the history and clinical exam, loss of appetite, nausea, or vomiting were reported significantly less often for patients with CHIKV monoinfections (5 of 17, 29.4%) compared with other patients (236 of 366, 64.5%; P = .008). Most notably, joint pain or an abnormal joint exam was infrequently documented in CHIKV-infected patients (Table 1), and this did not differ between monoinfected and coinfected patients.
Disposition
Antimalarial treatment decisions demonstrated only moderate agreement with molecular test results: 29 patients treated with antimalarial medication had negative rtPCR results, and 57 patients with malaria were discharged without treatment. Therefore, prescribed treatment was not consistent with the diagnosis by rtPCR in 86 of 383 cases (22.5%).
The majority of patients in this study were discharged home (373 of 383, 97.4%) either with follow-up (192 of 383, 50.1%) or without (181 of 383, 47.3%) (Supplementary Table 2). Ten patients (2.6%) were referred to the hospital or a higher level of care. Patient disposition differed significantly between regions: in the Lake Victoria region, 135 of 145 patients (93.1%) were discharged with follow-up; on the Indian Ocean coast, 57 of 239 patients (23.8%) were discharged with follow-up (P < .001).
DISCUSSION
In the current study, molecular diagnostic assays were implemented to determine the etiology of acute febrile illness among Kenyan children. Clinical diagnosis proved unreliable in this pediatric population, because both malaria and chikungunya were overdiagnosed: 125 of 254 patients (49.2%) with suspected malaria and 8 of 93 patients (8.6%) with a suspected arboviral illness had confirmation of their primary diagnosis. Although the overdiagnosis of malaria has been described by others, this was confirmed in the current study despite use of a more sensitive diagnostic test [1, 5, 25]. In addition, antimalarial treatment decisions demonstrated only moderate agreement with molecular test results, indicating that malaria diagnosis based on clinical findings plus microscopy is inaccurate, rather than simply nonspecific.
The overdiagnosis of CHIKV infections in this population was unexpected and did not correlate with regional incidence rates. Seroprevalence studies have reported similar rates of anti-CHIKV antibody detection in both regions evaluated in this study [18, 19], but clinical suspicion for CHIKV infection was significantly lower in the Lake Victoria region, where the incidence of infection was higher, than on the Indian Ocean coast. Causes of this discordance are likely multiple, but they may be related to the large CHIKV outbreak in 2004 [20], which affected coastal communities and may continue to impact clinical decision making. Nonspecific clinical findings also complicated the diagnosis of chikungunya in this population. It is notable that patients with CHIKV did not have high rates of joint paint or abnormal findings on joint exam. This is consistent with findings from a study of febrile children in Tanzania, where only 5.9% of probable CHIKV cases had joint pain [17].
Studies that compare the sensitivity of microscopy to molecular testing in symptomatic patients frequently document only a small number of discrepant samples [26–30], and few studies have documented a significant increase in case detection using molecular methods [10, 31, 32]. This suggests that molecular testing may only result in a small increase in malaria diagnoses and precludes an evaluation of malaria cases diagnosed only by molecular methods. In the current study of symptomatic children, the use of rtPCR significantly increased malaria diagnosis: 143 cases were detected by rtPCR, 71 of which were positive by microscopy. More importantly, the clinical presentation of malaria cases diagnosed only by rtPCR was similar to that of cases diagnosed by microscopy, consistent with the idea that these are symptomatic malaria cases.
Individuals with a positive molecular test and negative microscopy are typically described as having “submicroscopic parasitemia” [11]. In a systematic review of submicroscopic (asymptomatic) P falciparum parasitemia, the percentage of infections detected by microscopy was higher in areas of high transmission [11]. In our study, the percentage of rtPCR-positive malaria cases detected by microscopy varied significantly by study site, with Ukunda Health Center having the lowest percentage of malaria detected by microscopy but the second highest incidence of malaria based on rtPCR. Differences in the sensitivity of microscopy could not be explained by parasite burden quantified using rtPCR. These findings highlight the variability of diagnostic test performance observed between sites within a specific region and the importance of ongoing proficiency testing. Moreover, our findings indicate that malaria cases diagnosed only by rtPCR do not simply result from very low levels of parasite burden, as the term submicroscopic parasitemia connotes.
Limitations to the current study include the use of only acute-phase serum samples for testing. Although this specimen type is used less often than whole blood for the molecular detection of malaria, we have previously documented the clinical sensitivity and specificity of malaria detection in serum or plasma [3, 10]. It is also possible that children presented with symptomatic CHIKV and/or DENV infections but were no longer viremic. Such cases may have been detected by serologic testing of acute and convalescent samples. Given that the mean day postsymptom onset was 2.46, we expect that most patients would be viremic at presentation. However, Ct values in CHIKV infections detected in this study were later than expected, given the day postsymptom onset of sample collection [33]. Because only data from the acute patient visit was available for this study, we are unable to provide follow-up information on the 57 patients who were diagnosed with malaria but did not receive antimalarials. Given the natural history of P falciparum infections [34], prolonged follow-up will be necessary to document differences in patient outcomes [31].
CONCLUSIONS
In conclusion, we present findings from the implementation of molecular diagnostics to study the etiology of acute febrile illness in Kenyan children. These data demonstrate that the clinical diagnosis of malaria and chikungunya, even when aided by microscopy, is inaccurate and varies by care site in endemic areas. This contributes to inappropriate management decisions, including the use of antimalarials and antibiotics and disposition of patients who may be at risk for severe disease.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Acknowledgments
We thank the staff of the Stanford Clinical Virology Laboratory for assistance in coordinating nucleic acid extractions and real-time reverse-transcription polymerase chain reaction performance. We also thank the study participants, their families, and the field teams in Kenya for contributions to this research.
Financial support. This research was funded by National Institutes of Health grants K08AI110528 (to J. W.) and R01AI102918 (to A. D. L.).
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Crump JA, Morrissey AB, Nicholson WL, et al. Etiology of severe non-malaria febrile illness in Northern Tanzania: a prospective cohort study. PLoS Negl Trop Dis 2013; 7:e2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. D’Acremont V, Kilowoko M, Kyungu E, et al. Beyond malaria–causes of fever in outpatient Tanzanian children. N Engl J Med 2014; 370:809–17. [DOI] [PubMed] [Google Scholar]
- 3. Waggoner JJ, Abeynayake J, Balassiano I, et al. Multiplex nucleic acid amplification test for diagnosis of dengue fever, malaria, and leptospirosis. J Clin Microbiol 2014; 52:2011–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization. World Malaria Report. Geneva: World Health Organization; 2012. [Google Scholar]
- 5. A-Elgayoum SM, El-Rayah EA, Giha HA. In areas of low transmission, is the presumptive treatment of febrile but bloodsmear-negative patients for malaria validated by the results of PCR-based testing? Ann Trop Med Parasitol 2010; 104:573–81. [DOI] [PubMed] [Google Scholar]
- 6. Faucher JF, Aubouy A, Béhéton T, et al. What would PCR assessment change in the management of fevers in a malaria endemic area? A school-based study in Benin in children with and without fever. Malar J 2010; 9:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oladosu OO, Oyibo W. Overdiagnosis and overtreatment of malaria in children that presented with fever in Lagos, Nigeria. ISRN Infect Dis 2013 Available at: 10.5402/2013/914675. Accessed 23 May 2015. [DOI] [Google Scholar]
- 8. Gonçalves L, Subtil A, de Oliveira MR, et al. Bayesian latent class models in malaria diagnosis. PLoS One 2012; 7:e40633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ohrt C, Purnomo, Sutamihardja MA, et al. Impact of microscopy error on estimates of protective efficacy in malaria-prevention trials. J Infect Dis 2002; 186:540–6. [DOI] [PubMed] [Google Scholar]
- 10. Waggoner JJ, Okangba C, Mohamed-Hadley A, et al. Molecular testing for Plasmodium falciparum by use of serum or plasma and comparison with microscopy and rapid diagnostic testing in febrile Nigerian patients. J Clin Microbiol 2015; 53:3596–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Okell LC, Ghani AC, Lyons E, Drakeley CJ. Submicroscopic infection in Plasmodium falciparum-endemic populations: a systematic review and meta-analysis. J Infect Dis 2009; 200:1509–17. [DOI] [PubMed] [Google Scholar]
- 12. Ndao M, Bandyayera E, Kokoskin E, et al. Comparison of blood smear, antigen detection, and nested-PCR methods for screening refugees from regions where malaria is endemic after a malaria outbreak in Quebec, Canada. J Clin Microbiol 2004; 42:2694–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rantala AM, Taylor SM, Trottman PA, et al. Comparison of real-time PCR and microscopy for malaria parasite detection in Malawian pregnant women. Malar J 2010; 9:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vallejo AF, Martínez NL, González IJ, et al. Evaluation of the loop mediated isothermal DNA amplification (LAMP) kit for malaria diagnosis in P. vivax endemic settings of Colombia. PLoS Negl Trop Dis 2015; 9:e3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gal S, Fidler C, Turner S, et al. Detection of Plasmodium falciparum DNA in plasma. Ann N Y Acad Sci 2001; 945:234–8. [DOI] [PubMed] [Google Scholar]
- 16. Lamikanra AA, Dobaño C, Jiménez A, et al. A direct comparison of real time PCR on plasma and blood to detect Plasmodium falciparum infection in children. Malar J 2012; 11:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chipwaza B, Mugasa JP, Selemani M, et al. Dengue and chikungunya fever among viral diseases in outpatient febrile children in Kilosa district hospital, Tanzania. PLoS Negl Trop Dis 2014; 8:e3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. LaBeaud AD, Banda T, Brichard J, et al. High rates of o’nyong nyong and chikungunya virus transmission in coastal Kenya. PLoS Negl Trop Dis 2015; 9:e0003436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sutherland LJ, Cash AA, Huang YJ, et al. Serologic evidence of arboviral infections among humans in Kenya. Am J Trop Med Hyg 2011; 85:158–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Powers AM. Chikungunya. Clin Lab Med 2010; 30:209–19. [DOI] [PubMed] [Google Scholar]
- 21. Waggoner JJ, Ballesteros G, Gresh L, et al. Clinical evaluation of a single-reaction real-time RT-PCR for pan-dengue and chikungunya virus detection. J Clin Virol 2016; 78:57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lefterova MI, Budvytiene I, Sandlund J, et al. Simple real-time PCR and amplicon sequencing method for identification of Plasmodium species in human whole blood. J Clin Microbiol 2015; 53:2251–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. New York State Department of Health. Pediatric Assessment Reference Card Available at: http://www.health.ny.gov/professionals/. Accessed 1 November 2016.
- 24. Burnham KP, Anderson DR. Multimodal inference: understanding AIC and BIC in model selection. Sociol Methods Res 2004; 33:261–304. [Google Scholar]
- 25. Ghai RR, Thurber MI, El Bakry A, et al. Multi-method assessment of patients with febrile illness reveals over-diagnosis of malaria in rural Uganda. Malar J 2016; 15:460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Boonma P, Christensen PR, Suwanarusk R, et al. Comparison of three molecular methods for the detection and speciation of Plasmodium vivax and Plasmodium falciparum. Malar J 2007; 6:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mohon AN, Elahi R, Khan WA, et al. A new visually improved and sensitive loop mediated isothermal amplification (LAMP) for diagnosis of symptomatic falciparum malaria. Acta Trop 2014; 134:52–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Paglia MG, Vairo F, Bevilacqua N, et al. Molecular diagnosis and species identification of imported malaria in returning travellers in Italy. Diagn Microbiol Infect Dis 2012; 72:175–80. [DOI] [PubMed] [Google Scholar]
- 29. Rakotonirina H, Barnadas C, Raherijafy R, et al. Accuracy and reliability of malaria diagnostic techniques for guiding febrile outpatient treatment in malaria-endemic countries. Am J Trop Med Hyg 2008; 78:217–21. [PubMed] [Google Scholar]
- 30. Vo TK, Bigot P, Gazin P, et al. Evaluation of a real-time PCR assay for malaria diagnosis in patients from Vietnam and in returned travellers. Trans R Soc Trop Med Hyg 2007; 101:422–8. [DOI] [PubMed] [Google Scholar]
- 31. Menge DM, Ernst KC, Vulule JM, et al. Microscopy underestimates the frequency of Plasmodium falciparum infection in symptomatic individuals in a low transmission highland area. Am J Trop Med Hyg 2008; 79:173–7. [PMC free article] [PubMed] [Google Scholar]
- 32. Andrade BB, Reis-Filho A, Barros AM, et al. Towards a precise test for malaria diagnosis in the Brazilian Amazon: comparison among field microscopy, a rapid diagnostic test, nested PCR, and a computational expert system based on artificial neural networks. Malar J 2010; 9:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Waggoner JJ, Gresh L, Vargas MJ, et al. Viremia and clinical presentation in Nicaraguan patients infected with zika virus, chikungunya virus, and dengue virus. Clin Infect Dis 2016; 63:1584–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ashley EA, White NJ. The duration of Plasmodium falciparum infections. Malar J 2014; 13:500. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.