Abstract
Triple-negative breast cancer (TNBC) is an aggressive breast cancer subtype for which there are no approved targeted therapies. Preclinical and early clinical trials indicate that up to 50% of TNBC express androgen receptor (AR) and are potentially responsive to anti-androgens. However, the function of AR in TNBC and the mechanisms by which AR-targeted therapy reduces tumor burden are largely unknown. We hypothesized that AR maintains a cancer stem cell (CSC)-like tumor initiating population and serves as an anti-apoptotic factor that facilitates anchorage independence and metastasis. Anchorage-independent growth was assessed in TNBC cells cultured in forced suspension and apoptosis was measured with cleaved-caspase 3 antibody. CSC-like populations were assessed in vitro using ultra low attachment plates, CD44/CD24 staining, the ALDEFLUOR assay, and single cell mammosphere formation efficiency (MFE) assays. Tumor-initiating capacity was assessed in vivo using a limiting dilution assay. Lastly, the ability of a combination of enzalutamide (Enza) and paclitaxel to inhibit TNBC tumor growth was assessed in vivo. AR increased in TNBC cells in forced suspension culture compared to attached conditions. Cells that expressed AR resisted detachment-induced apoptosis. The CSC-like population increased in suspension culture and decreased following AR inhibition. Pre-treatment with Enza decreased the tumor-initiating capacity of TNBC cells. Enza decreased tumor volume and viability when administered simultaneously or subsequent to chemotherapy, but simultaneous treatment more effectively suppressed tumor recurrence. AR-targeted therapies may enhance the efficacy of chemotherapy even in TNBC with low AR expression by targeting a CSC-like cell population with anchorage independent, invasive potential.
Keywords: Androgen receptor, triple-negative breast cancer, cancer stem-like cells, chemotherapy
INTRODUCTION
Triple-negative breast cancer (TNBC) is an aggressive breast cancer (BC) subtype for which no effective targeted therapies are currently available. Women with TNBC have a peak risk of recurrence between 1–3 years, an increased likelihood of distal recurrence, and a higher mortality rate within the first five years compared to other BC subtypes [1]. While most metastatic TNBC initially respond to cytotoxic chemotherapy, the majority of patients experience recurrence following treatment [2]. Studies suggest that chemotherapy-resistant cancer cells with stem-like properties may repopulate the tumor [3–6]. Thus, therapies that target the cancer stem cell (CSC)-like population in combination with chemotherapy may prevent tumor recurrence.
Androgen receptor (AR) protein is expressed to some degree in at least half of all TNBC [7–15], and results of clinical trials targeting AR are promising [14, 16]. However, little is known about the function of AR in TNBC and the mechanisms by which AR antagonists improve TNBC outcomes. In ER+ breast cancer, progesterone and progesterone receptors support the expansion of breast cancer tumor-initiating cells [17], and estrogen and progesterone expand the normal mouse mammary stem cell population, even though it is thought that the stem cells themselves lack estrogen and progesterone receptors (ER, PR) [18, 19]. PR and AR recognize similar consensus DNA response elements [20] and regulate many of the same genes [21]. In TNBC, AR binds to chromatin at sites more similar to ER in ER+ BC than AR in prostate cancer [22]. Recent studies also suggest that AR may regulate genes associated with CSCs in prostate cancer [23, 24]. Finally, we previously reported that the most striking effect of the AR antagonist enzalutamide (Enza) on TNBC was a dramatic decrease in anchorage-independent growth of multiple TNBC cell lines [7].
Based on these data, we hypothesized that in TNBC, in the absence of ER and PR, AR supports a CSC-like population. Identification of AR as a target that maintains a tumor-initiating population may alter the clinical treatment strategy. AR antagonists may, in fact, not only target AR+ tumor cells, but also a chemotherapy-resistant CSC population. Our research demonstrates that AR is increased in TNBC under anchorage-independent conditions, and AR inhibition decreases CSC-like populations in vitro andtumor initiation in vivo. Lastly, Enza enhances the efficacy of chemotherapy in a preclinical model and reduces recurrence when given simultaneously with chemotherapy.
MATERIALS AND METHODS
Cell Culture
All cell lines were authenticated in 2014 by Short Tandem Repeat DNA Profiling (Promega; Fitchburg, WI) at the University of Colorado Cancer Center (UCCC) Tissue Culture Core, and testing for mycoplasma was performed every three months. Only cells under five passages were used in this study. SUM159PT cells were purchased from the UCCC Tissue Culture Core in 2013 and were grown in Ham’s F-12 with 5% fetal bovine serum (FBS), penicillin/streptomycin (P/S), hydrocortisone, insulin, HEPES and L-glutamine supplementation. BT549 cells, purchased from the ATCC in 2008, were grown in RPMI Medium 1640 with 10% FBS, P/S and insulin. MDA-MB-453 (MDA453) cells, purchased from ATCC in 2012, were grown in DMEM Medium with 10% FBS and P/S.
SUM159PT-TGL cells were generated by stable retroviral transduction with a SFG-NES-TGL vector, encoding a triple fusion of thymidine kinase, green fluorescent protein and luciferase and sorted for green fluorescent protein. SUM159PT and MDA453 AR knockdown cells were generated by lentiviral transduction of shRNAs targeting AR (pMISSION VSV-G, Sigma Aldrich; St Louis, MO), including AR shRNA 3715 (shAR15) and AR shRNA 3717 (shAR17). Lentiviral transduction of pMISSION shRNA NEG (shNEG) was used as a non-targeting control. Plasmids were purchased from the University of Colorado Functional Genomics Core Facility.
Cellular Assays and Reagents
Cells were treated with 20μM Enza (Medivation; San Francisco, CA) and 10 nM dihydrotestosterone (DHT, Sigma Aldrich). 20 μM Enza approximates the IC50 of the cell lines studied and is a clinically achievable concentration. Circulating plasma Cmax values for Enza and its active metabolite (N-desmethyl enzalutamide) are 16.6μg/ml (23% CV) and 12.7μg/ml (30% CV) respectively (Enza package insert Exposure Rationale), which is equivalent to approximately 60μM total active drug in plasma at steady state.
Forced Suspension Culture
Poly-2-hydroxyethyl methacrylate (poly-HEMA, Sigma) was reconstituted in 95% ethanol to 12 mg/ml. Culture plates were coated with poly-HEMA and incubated overnight to allow ethanol evaporation. Plates were washed with phosphate-buffered saline (PBS) prior to use.
Gene Expression Array Analysis
BT549 cells were grown in either attached or forced suspension condition in quadruplicate for 24 hours. RNA was harvested using the Trizol method and hybridized onto Affymetrix Human Gene 1.0ST arrays at the University of Colorado Denver Genomics and Microarray Core, following the manufacturer’s instructions. The array data is available in the Gene Expression Omnibus (GEO) database as GSE95472.
Microarray analysis was performed using Partek Genomics Suite (Partek, Inc.) and MetaCore Pathway Analysis software (Thomson Reuters). One-way ANOVA analysis was performed to determine differentially expressed genes between the two treatment groups. Significantly differentially expressed genes were imported to MetaCore for pathway analysis, including identification of altered canonical pathways. Determination of potentially AR-regulated genes was performed by gene searches on the Androgen Responsive Gene Database (http://argdb.fudan.edu.cn/geneshow_basic.php).
Real-time quantitative polymerase chain reaction
RNA was isolated by Trizol (Invitrogen; Grand Island, NY) and cDNA was synthesized from 1 μg total RNA, using M-Mulv reverse transcriptase enzyme (Promega). SYBR green quantitative gene expression analysis was performed using the following primers: AR forward, 5′-CTCACCAAGCTCCTGGACTC-3′ and AR reverse, 5′-CAGGCAGAAGACATCTGAAAG-3′; GR forward, 5′-ACTGCCCCAAGTGAAAACAGA-3′ and GR reverse, 5′-ATGAACAGAAATGGCAGACATTT-3′; β–ACTIN forward, 5′-CTGTCCACCTTCCAGCAGATG-3′ and β–ACTIN reverse, 5′-CGCAACTAAGTCATAGTCCGC-3′; and RPL13A forward, 5′-CCTGGAGGAGAAGAGGAAAGAGA-3′ and RPL13A reverse, 5′-TTGAGGACCTCTGTGTATTTGTCAA-3′. Relative gene expression was calculated using the comparative cycle threshold method and values were normalized to β-ACTIN or RPL13A.
Immunoblotting
Whole cell protein extracts (50 μg) were denatured, separated on SDS-PAGE gels and transferred to polyvinylidene fluoride membranes. After blocking in 3% bovine serum albumin in Tris-buffered saline–Tween, membranes were probed overnight at 4°C. Primary antibodies include: AR (PG-21, 1:500 dilution; EMD Millipore, Darmstadt, Germany), GR (1:1000 dilution; Cell Signaling), and α-Tubulin (clone B-5-1-2, 1:30,000 dilution; Sigma Aldrich). Following secondary antibody incubation, results were detected using Western Lightning Chemiluminescence Reagent Plus (Perkin Elmer).
Immunocytochemistry and Immunofluorescence
Cells were plated in poly-HEMA coated or uncoated plates for 24–96 hours, fixed in 10% neutral buffered formalin, pelleted in histogel (ThermoFisher), and paraffin embedded by the UC Denver Tissue Biobanking and Processing Core. Slides were deparaffinized in a series of xylenes and ethanols, and antigens were heat retrieved in either 10 mM citrate buffer pH 6.0 or 10 mM Tris/1 mM EDTA pH 9.0. Antibodies used were AR clone 441 (Dakocytomation; Carpinteria, CA) or AR clone SP107 (Cell Marque; Rocklin, CA). The Vectastain Elite ABC kit (Vector Laboratories Inc., Burlingame, CA) or Envision horseradish peroxidase (Dakocytomation) were used for detection, and Tris-buffered saline with 0.05% Tween 20 was used for all washes. Representative images were taken using a BX40 microscope (Olympus, Center Valley, PA) with a SPOT Insight Mosaic 4.2 camera and software (Diagnostic Instruments, Inc., Sterling Heights, MI).
For Immunofluorescence, antibodies to cleaved-caspase 3 (Cell Signaling Technology, Danvers, MA) and AR (clone SP107) were used in duplex with TSA Plus Cyanine 3.5 and Fluorescein kits respectively per manufacturer’s instructions (Perkin Elmer, Waltham, MA). Images were taken using a BX40 microscope, DP73 camera, and cellSens standard software (Olympus). In addition, slides were scanned using a Vectra 3 quantitative pathology imaging system and inForm 2.2 software was used to obtain a percent positive score for cells in n=3 fields after both cell segmentation and phenotype analysis (Perkin Elmer).
CSC-like Population Analysis
For the mammosphere formation assays single cells were plated in 96-well ultra-low attachment plates in 200μL per well of Mammocult media (Stem Cell Technologies; Vancouver, BC, Canada). After incubating for one week, the number of mammospheres present was determined by microscopy. Mammosphere criteria included at least five cells and a length of at least 50μm. The mammosphere formation efficiency (MFE) was calculated as the number of mammospheres formed divided by the number of single cells plated. CD44/CD24 (BD Biosciences; San Jose, CA) staining was conducted per the manufacturer’s protocol by flow cytometry analysis. The ALDEFLUOR™ assay (Stem Cell Technologies) was performed per the manufacturer’s protocol. ALDEFLUOR -positive and -negative cell populations were sorted with the assistance of the University of Colorado Cancer Center Flow Cytometry Shared Resource on the MoFlo XDP100 cell sorter (Beckman Coulter Life Sciences; Indianapolis, IN).
Tumor Studies
Xenograft experiments were approved by the University of Colorado Institutional Animal Care and Use Committee (IACUC protocol 83614(01)1E) and conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. For the limiting dilution study, a range of 102–106 SUM159PT-TGL cells were mixed with Matrigel (BD Biosciences; Franklin Lakes, New Jersey) and bilaterally injected into the mammary fat pads of five female, athymic nu/nu mice (Taconic; Germantown, NY). Cells were treated before injection with 20μM Enza or vehicle control (1% DMSO) for 72 hours. Tumor burden was assessed by luciferase activity and by caliper measurements (tumor volume was calculated as volume = (length × width2)/2). Mice were euthanized by carbon dioxide asphyxiation followed by cervical dislocation, and the tumors were harvested.
For the Enza and paclitaxel (Pac) study, 106 SUM159PT-TGL cells were mixed with Matrigel (BD Biosciences) and bilaterally injected into the mammary fat pads of female, athymic nu/nu mice (Taconic). Tumor burden was assessed by luciferase activity and by caliper measurements. Once tumors were established, mice were matched into groups based on the total tumor burden as assessed by luciferase activity and by caliper measurements. Mice were administered Enza in their chow (approximate 50 mg/kg daily dose). Enza was mixed with ground mouse chow (Research Diets Inc.; New Brunswick, NJ) at 0.43 mg/g chow. The feed was irradiated and stored at 4°C before use. Mice in the control group received the same ground mouse chow but without Enza. Mice were given free access to either Enza formulated chow or control chow throughout the study period. Mice were given Pac (LTK Labs; St Paul, MN) i.p. at a dose of 10 mg/kg/d for five consecutive days. Stock Pac was prepared in 50% ethanol and 50% Kolliphor EL (Sigma), then diluted in PBS to a concentration of 1 mg/ml. Mice were euthanized by carbon dioxide asphyxiation followed by cervical dislocation and tumors were harvested.
Statistical Significance
Statistical significance was evaluated using a two-tailed student’s t-test or ANOVA with GraphPad Prism software. Binomial probability was used to calculate significance of MFE assays. Extreme limiting dilution analysis (ELDA) was used to calculate stem cell frequency and significance of tumor initiation frequency of the limiting dilution assay. A p-value of less than 0.05 was considered statistically significant.
RESULTS
Androgen receptor transcript, protein, and transcriptional activity are upregulated in forced suspension culture compared to adherent conditions
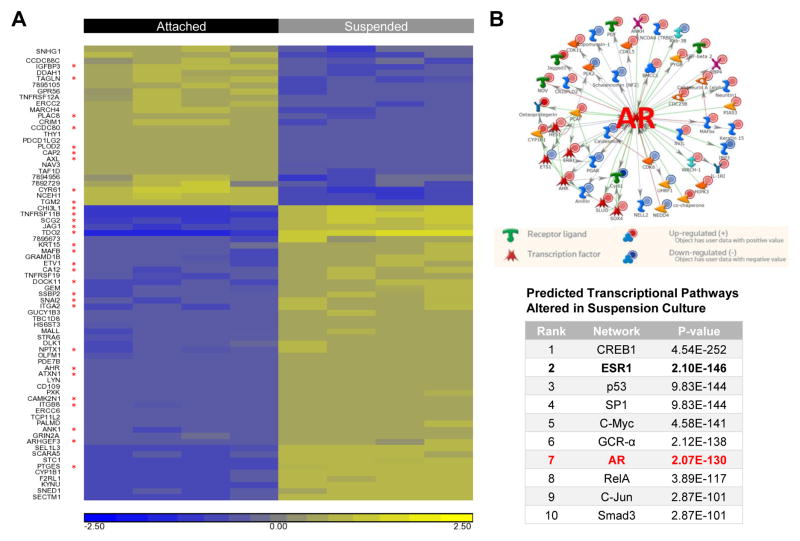
To determine what genes may have a role in the ability of cells to resist anoikis and survive in suspension (mimicking survival in-transit during metastasis), gene expression microarray analysis was conducted on BT549 cells following growth in attached or forced suspension culture for 24 hours (GEO record GSE95472). Data analysis was performed to identify genes significantly altered by more than 2.0 fold between attached and suspended conditions by one-way ANOVA analysis (p < 0.05). Among the 73 genes differentially expressed >2.0-fold, 30 were potentially AR-regulated, as determined by cross-referencing with the Androgen Responsive Gene Database (http://argdb.fudan.edu.cn/geneshow_basic.php) (Fig. 1A). Metacore pathway analysis revealed that AR was one of the top predicted upstream regulators of genes altered in BT549 cells cultured in suspension (Fig. 1B).
Figure 1.
AR-regulated changes in gene expression in TNBC cells cultured in forced suspension. A, Heatmap of genes differentially regulated in BT549 cells grown in attached vs. suspended culture conditions for 24 hours. Biological quadruplicate samples of each treatment group are shown. Genes with > or < 2.0 fold-change difference between attached and suspended conditions (p < 0.05) are shown (yellow indicating increased gene expression and blue indicating decreased gene expression). Red stars indicate potentially AR-regulated genes in the Androgen Responsive Gene Database (http://argdb.fudan.edu.cn/index_info.php). B, MetaCore analysis of BT549 microarray data. Top, pathway analysis illustrating annotated connections between AR and genes altered in suspension culture. Bottom, summary of top transcriptional pathways predicted to be altered in suspension compared to attached culture conditions.
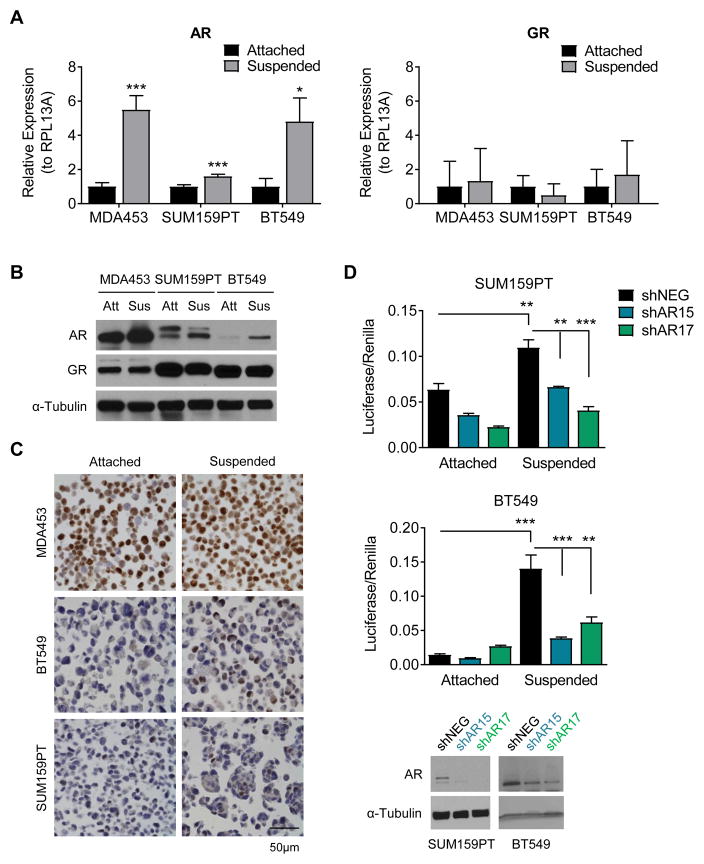
Upon further investigation we found that AR transcript was significantly increased after 24 hours in forced suspension compared to the attached condition in three TNBC cell lines (MDA453, SUM159PT, and BT549) (Fig. 2A). Since many TNBC cell lines also express the glucocorticoid receptor (GR), we examined GR transcript levels and found that they were unchanged between cells in suspension and attached culture (Fig. 2A). Increased AR, but not GR protein was detected in cells in forced suspension culture by western blot after 48 hours (Fig. 2B). The increase in AR protein was confirmed by IHC after 48 hours in attached versus forced suspension culture followed by paraffin embedding (Fig. 2C). Although the western and IHC both demonstrated much lower baseline levels of AR in the BT549 and SUM159PT cells as well as fewer AR+ cells compared to MDA453, IHC revealed that AR clearly increases in a small percentage of cells in the BT549 and SUM159PT. Interestingly, the latter two cell lines aggregated in suspension culture and there was at least one AR+ cell per clump. Indeed, with increased time in suspension culture, the BT549 cells showed enrichment for both the percent AR+ cells and AR staining intensity compared to attached culture conditions (Supplemental Fig 1). AR transcriptional activity on a consensus AR response element (ARE) linked to luciferase was significantly increased in suspended compared to attached conditions in SUM159PT and BT549 cells. This effect was attenuated in cells transduced with shRNAs targeting AR (Fig. 2D).
Figure 2.
AR expression increases in forced suspension culture. A, Quantitative real-time PCR (qRT-PCR) for androgen receptor (AR) and glucocorticoid receptor (GR) gene expression in attached versus forced suspension culture conditions at 24 hours. B, Western blot for AR and GR in attached versus forced suspension culture conditions at 24 hours. α-Tubulin was used as a loading control. C, Immunohistochemistry for AR in attached versus forced suspension culture conditions at 48 hours. Representative images, magnification 400X. D, AR luciferase reporter assay in attached compared to suspended conditions in cells transduced with a non-targeting control (shNEG) or shRNAs targeting AR (shAR15, shAR17). Western blot for AR. α-Tubulin was used as a loading control. Mean ± SD; * p < 0.05, ** p < 0.01, *** p < 0.001.
AR+ cells are resistant to apoptosis
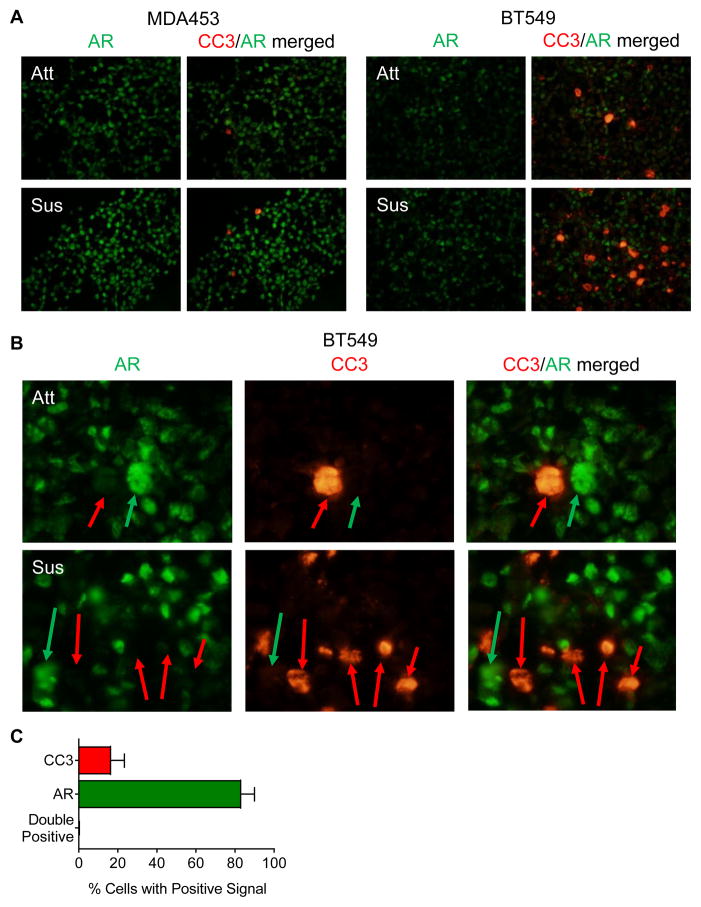
We previously reported that Enza caused a greater than 80% reduction in TNBC cell growth on soft agar [7]. Since AR expression and activity were increased in forced suspension culture, we hypothesized that AR might protect against anoikis (detachment-induced cell death). Dual immunofluorescent staining of AR and the active apoptotic marker cleaved-caspase 3 (CC3) demonstrated increased staining in MDA453 and BT549 cells following 48 hours in forced suspension culture (Fig. 3A). The MDA453 cells, that are highly AR+, showed less CC3 staining than the BT549. Importantly, these two markers were found to be mutually exclusive (Fig. 3B), with less than 1% of cells co-staining for both AR and CC3 (Fig. 3C). In other words, AR+ cells did not undergo apoptosis in suspension culture.
Figure 3.
AR and cleaved-caspase 3 (CC3) increase in TNBC cells cultured in forced suspension and do not co-localize. A–B, TNBC cells were grown in attached and suspended conditions for 48 hours and stained for AR (green) and CC3 (red) using tyramide signal amplification. Representative images; A, magnification 400X, B, magnification 1000X to emphasize CC3 (red arrows) and AR (green arrows) localization. C, image analysis of BT549 suspended cells using inForm 2.2. Percentage of cells positive for Cy3.5 (CC3), FITC (AR), or double positive (CC3+AR) were calculated from the total number of positive cells for n = 3 random 200X fields; mean ± SEM.
A population of CSC-like cells increases in forced suspension conditions
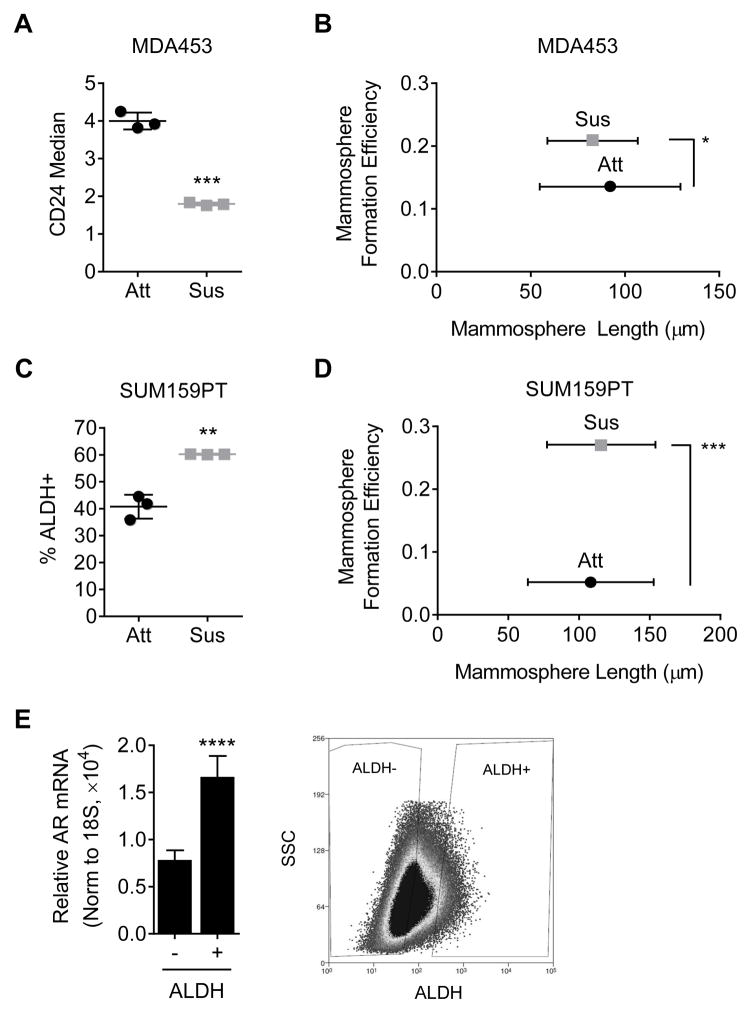
Anchorage independence is associated with stem cells and we therefore expected the CSC-like population to be increased in forced suspension culture conditions. CD44+/CD24− staining was used to identify CSC-like MDA453 cells. Although there were no changes in CD44 staining (data not shown), CD24 staining decreased by 2-fold following suspension culture, suggesting a less luminal phenotype (Fig. 4A, p < 0.001). A 2-fold increase in mammosphere formation efficiency (MFE) was also observed in MDA453 cells placed in suspension culture for 5 days prior to plating the mammosphere assay (Fig. 4B, p < 0.05). These findings were confirmed in a second cell line, SUM159PT, where a 3-day forced suspension culture of SUM159PT cells increased the population of aldehyde dehydrogenase-positive (ALDH+) cells to 60%, compared to 40% in attached culture (Fig. 4C, p < 0.01). Likewise, in a MFE assay, SUM159PT cells suspended 3 days prior to initiation of the assay exhibited a 5-fold increase in MFE (Fig. 4D, p < 0.001). An examination of AR gene expression in the ALDH+ versus ALDH− populations of SUM159PT cells cultured in forced suspension, showed that ALDH+ SUM159PT cells expressed significantly higher AR mRNA levels than the ALDH− population, suggesting that the CSC-like SUM159PT cell population is AR+ (Fig. 4E, p < 0.0001).
Figure 4.
A cancer stem cell-like population is increased in suspension. A, CD24 staining of MDA-MB-453 (MDA453) cells following 5 days in suspension culture. B, mammosphere formation efficiency (MFE) assay of MDA453 cells placed in attached or suspended conditions for 5 days prior to plating. C, ALDEFLUOR (ALDH+) assay of SUM159PT cells placed in suspended conditions for 3 days. DEAB was used as a control to set the gate. D, MFE assay of SUM159PT cells placed in attached or suspended conditions for 3 days prior to plating. E, SUM159PT cells cultured for 3 days in suspension were sorted for ALDH and AR gene expression was measured using quantitative real-time PCR (qRT-PCR). Shown is a representative image of sorted ALDH populations. Mean ± SD; * p < 0.05, ** p < 0.01, *** p < 0.001.
Androgen receptor inhibition decreases the CSC-like population in vitro
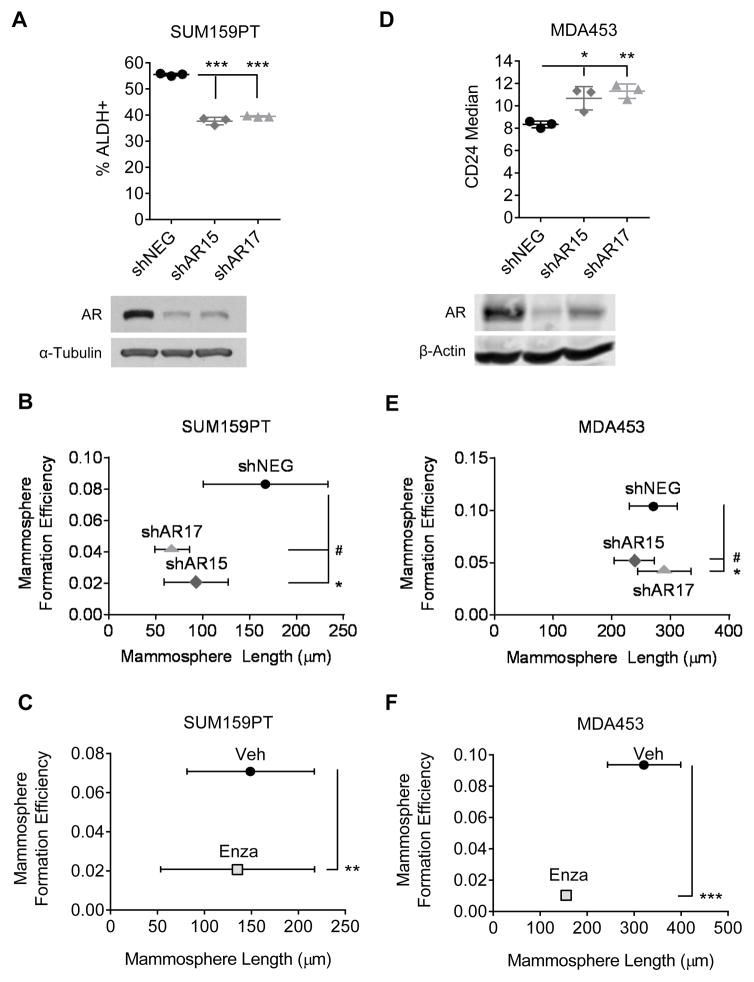
Since we observed that the increase in AR expression and activity coincided with an enrichment of CSC-like cells in suspension culture, we next tested whether inhibiting AR could decrease the CSC-like population. In SUM159PT cells, AR knockdown decreased the percentage of ALDH+ cells from 55% to approximately 40% (Fig. 5A, p < 0.001). A 4-fold decrease in MFE was also observed (Fig. 5B, p < 0.05) following AR knockdown. Finally, treatment with the AR antagonist Enza decreased MFE by nearly 2-fold (Fig. 5C, p < 0.01). These results were recapitulated in the MDA453 cell line. AR knockdown led to a decrease in the CD44+/CD24− cell population, as shown by an increase in the levels of CD24 following knockdown, indicative of a more luminal phenotype (Fig. 5D, p < 0.05). Similarly, AR knockdown decreased MFE in MDA453 by 2-fold (Fig. 5E, p < 0.05). Treatment with Enza decreased MFE in MDA453 (Fig. 5F, p < 0.001), and although a trend toward decreased mammosphere length was observed following AR inhibition, these changes did not reach statistical significance.
Figure 5.
AR inhibition decreases a cancer stem cell-like population. A, ALDEFLUOR assay of SUM159PT cells transduced with a non-targeting control (shNEG) or shRNAs targeting AR (shAR15 and shAR17). Western blot for AR. α-Tubulin is shown as a loading control. B, mammosphere formation efficiency (MFE) assays of SUM159PT cells transduced with shNEG, shAR15, or shAR17 or C, treated with enzalutamide (Enza). D, CD24 staining of MDA-MB-453 (MDA453) cells transduced with shNEG, shAR15, or shAR17. Western blot for AR. β-Actin is shown as a loading control. E, MFE assays of MDA453 cells transduced with shNEG, shAR15, or shAR17 or F, treated with Enza. Mean ± SD; * p < 0.05, # p = 0.06, ** p < 0.01, *** p < 0.001.
Pre-treatment with enzalutamide decreases tumor initiation frequency in vivo
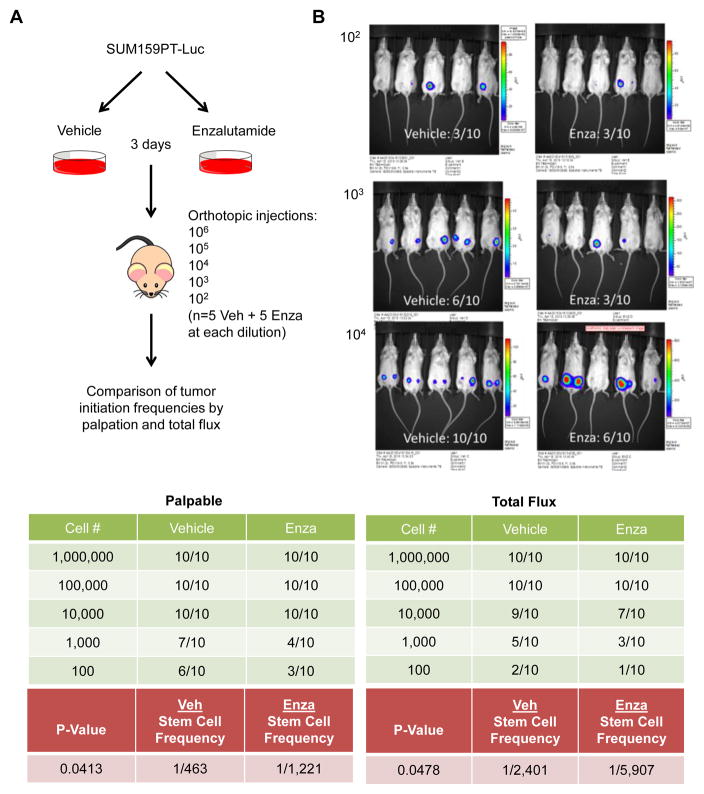
To evaluate the effect of AR inhibition on the CSC property of tumor initiation, an in vivo limiting dilution assay was performed. Luciferase-tagged SUM159PT cells were pretreated for 3 days with either vehicle or Enza in vitro. Mice received bilateral orthotopic injections in the 4th mammary gland of these cells ranging from 102–106 cells and tumor formation was measured by palpation and luciferase activity (Fig. 6A–B, Supplemental Fig. 2). By both analyses, pretreatment with Enza significantly decreased the tumor initiation frequency by approximately 2.5-fold (Fig. 6B bottom, p < 0.05), suggesting that inhibition of AR decreases the tumor forming ability of TNBC cells.
Figure 6.
Tumor initiation frequency of SUM159PT cells pre-treated with enzalutamide. A, schematic of experimental design. B, Tumor initiation frequency. Top, luciferase activity, measured as total flux, in mice injected with 102–104 cells pre-treated for 3 days with vehicle control (Veh) or enzalutamide (Enza). Bottom, summary of the number of mice per group with tumors as determined by palpation (left) and luciferase activity/total flux (right).
Combined enzalutamide plus paclitaxel is more effective than paclitaxel alone
CSCs are resistant to chemotherapy and may repopulate the tumor following treatment [3–6]. Given that AR inhibition decreases the CSC-like population in vitro and in vivo (Fig. 5–6), AR inhibition combined with chemotherapy may prove to be more effective than chemotherapy alone. In vitro, both Enza and Pac inhibit SUM159PT cell growth more than 50% (p < 0.001). However, the combination of drugs inhibited cell growth by 75% (Supplemental Fig. 3A, p < 0.01).
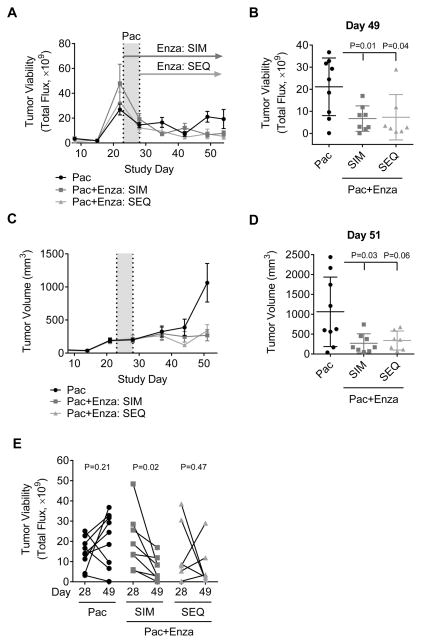
To determine in vivo whether the combination of AR inhibition and chemotherapy is more effective than chemotherapy alone, Enza was administered to mice either simultaneously (SIM) or subsequent (SEQ) to paclitaxel (Pac) and these mice were compared to mice administered Pac alone (see Supplemental Fig. 3B for experimental design and Supplemental Fig. 3C for matched group randomization). Tumor viability analysis demonstrated that the addition of Enza (whether SIM or SEQ) significantly decreased tumor viability compared to Pac alone (Fig. 7A and B). Tumor volume was also significantly smaller in both groups that received Pac plus Enza compared to Pac alone (p < 0.05) (Fig. 7C and D). Only mice treated SIM with Enza and Pac showed a statistically significant reduction in tumor viability (Fig. 7E), indicating that only this group did not exhibit recurrance. These findings suggest that giving Enza in combination with chemotherapy may effectively reduce recurrance following cessasion of chemotherapy treatment.
Figure 7.
Enzalutamide and paclitaxel combination therapy is more effective than paclitaxel alone. SUM159PT-TGL cells were bilaterally injected into the 4th mammary fat pads of female, athymic nu/nu mice. Once tumors were established, mice were randomized and matched into three groups. Mice were administered 10 mg/kg/d paclitaxel (Pac) for five days. Group 1 started enzalutamide (Enza) treated measure simultaneously with Pac (SIM), while Group 2 started Enza subsequent to Pac (SEQ). A, tumor cell luciferase activity as measured by total flux over time. B, comparison of total flux on study day 49. C, tumor volume over time. D, comparison of tumor volume on study day 51. E, total flux per mouse between the final day of Pac treatment (Day 28) and Day 49. Mean ± SD.
DISCUSSION
Our data demonstrate the novel finding that AR mRNA, protein, and transcriptional activity are upregulated in TNBC cell lines under anchorage-independent conditions. We previously published that the anti-androgen Enza dramatically reduced growth on soft agar, a classic measure of anchorage-independence, in multiple TNBC cell lines [7]. The data presented here indicate that AR is anti-apoptotic and its increase in forced suspention culture protects against cell death under anchorage-independent conditions. This data is consistent with previous studies demonstrating that AR+ tumors retain AR expression in metastatic disease [25, 26]. Interestingly, Lawson et al. recently reported single cell sequencing of circulating tumor cells from breast cancer patient-derived xenografts (PDX) and found AR among the transcripts upregulated in circulating tumor cells (CTCs) and low-burden metastases compared to the primary tumor (21.5 fold) [27]. Increased AR transcript in CTCs, along with our data on AR protein action in forced suspension, and clinical data showing retention of AR in metastatic disease [25, 26], suggest that AR may support survival in transit during metastasis.
The present data also demonstrate, both in vitro and in vivo, that AR inhibition decreases a CSC-like population. A recent manuscript by Feng et al. reporting an increase in cancer cell stemness and invasive potential following increased AR expression and activation in ER+ MCF7 breast cancer cells [28]. Furthermore, AR is highly retained in lung metastases in the MMTV-PyMT mammary carcinoma model that arise from tumors that are intially ER+/PR+/AR+ [29]. Our finding that AR inhibition affects a CSC-like population challenges the theory that AR targeted therapies will be most effective in tumors with high AR [30–32], because even if AR is only expressed in a small number of cells it can still influnce whether latent CSC-like cells can persist after therapy and cause recurrant disease. Indeed, preliminary data from the Phase II trial of Enza in TNBC demonstrated that the degree of AR immunostaining itself was not the best predictor of who will receive the most benefit from AR targeted therapy, but rather a signature of genes indicative of AR activity [16]. If AR targeted therapies decrease a CSC-like population capable of repopulating a tumor to form local or distant recurrence, then even patients with relatively low AR expression may benefit from anti-androgens, since these cells represent a slow growing CSC-like population that can survive chemotherapy. This finding is not unprecidented since breast cancer patients with as low as 1% ER positivity in their primary tumors can benefit from ER targeted therapies, although a definative mechanism for this phenomenon has not been elucidated.
Estrogen and progesterone do expand the stem cell population in the normal mammary gland and breast cancer [18, 19]. In AR+ TNBC, AR binds to many of the same sites on chromatin as ER binds in ER+ BC [22]. In immortal mammary epithelial cells amphiregulin (AREG), transcriptionally regulated by ER, supports the expansion of stem cells [18, 19, 33]. Our previously published data demonstrated that activation of AR in TNBC cell lines increased AREG production in vitro and in vivo and treatment with Enza significantly abbrogated AREG [7]. In the present study, we identified genes altered by 24 hours in forced suspension culture in the TNBC line BT549 (Figure 1A). Interestingly, many of the top genes that increase in suspension culture are known to be AR regulated and encode secreted factors such as JAG1 (a ligand for Notch receptors and target of canonical WNT signaling pathway in progenitor cells), chitinase (CHI3L1 or YKL40, which is well known to be androgen-regulated in prostate cancer), and RANKL/TNFRSF11B or osteoprotegerin ligand. In short, any and all of these AR-regulated secreted proteins could mediate survival of an anchorage independent, stem like population. AR+ cells may support a CSC-like population through such paracrine action or may themselves have stem-like properties as indicated by this study. Although there is a very low percentage of CD44+ cells in the Luminal AR MDA453 line, as reported previously [34], and this does not change with suspension culture (data not shown), CD24 staining decreased by 2-fold following suspension culture, perhaps suggesting a slightly less luminal or more flexible phenotype (Fig. 4A, p < 0.001). The percentage of cells positive for AR at baseline in the MDA453 is high, but does increase in suspension (Figure 2B, C and 3A). AR confers a luminal gene expression pattern by binding to chromatin at sites where ER binds in ER+ lines [22] and indeed we observe that the majority of MDA453 cells do not die in forced suspension (3A), indicating that there can be cells that are luminal, but resistant to anoikis (a trait usually associated with basal/mesenchymal/stem-like cells). Our data demonstrating that when AR is knocked down the CD24+ population increases (Figure 5D) and the mammosphere formation efficiency decreases with the anti-androgen enzalutamide (5F), indicate that AR affects these CSC-like traits. Further studies are needed to elucidate the mechanisms by which AR supports a CSC-like population or whether the AR+ cells themselves are the CSC-like tumor initiating population. However, we find AR transcript to be more abundant in ALDH+ SUM159PT cells. The data show that AR inhibition decreases mammosphere formation efficiency in vitro and tumor initiation in vivo and that a strategy simultaneously targeting the more slowly proliferating AR+ cells with Enza along with the more rapidly proliferating cells with chemotherapy, may be the most effective at preventing recurrence.
Given the success of of Enza (Xtandi) in metastatic TNBC (NCT01889238) [16], the next trials will test efficacy of Enza in combination with standard of care chemotherapy as frontline therapy and in the neoadjuvant setting. The need for improved chemotherapy among AR+ TNBC tumors is highlighted in a study by Masuda et al. demonstrating that AR+ TNBCs (luminal AR subtype) have a poor pathologic complete response compared to other subtypes [35]. This is likely because AR+ tumors proliferate more slowly and are therefore less responsive to chemotherapeutic agents. Enza may increase the efficacy of chemotherapy by decreasing the CSC population that is insensitive to chemotherapy and repopulates the tumor post-chemotherapy to cause relapse [3–6]. The present study indicates that the combination of Enza and chemotherapy is more effective than chemotherapy alone in a preclinical model. Further, although both Enza plus chemotherapy regimens (simultaneous and subsequent) were more effective than chemotherapy alone, simultaneous treatment yielded ignificantly more decreased tumor volume and viability and no tumor regrowth upon cessation of chemotherapy.
In summary, this study provides preclinical evidence supporting chemotherapy combination trials with Enza, and it shows promise that the addition of a targeted therapy may improve outcomes for patients with TNBC. The first clinical trial (NCT01889238), showed an impressive clinical benefit rate when given to women with metastatic disease where the majority of patients had already received chemotherapy [16]. The next trial with Enza in AR+ disease will involve Enza plus chemotherapy compared to chemotherapy alone as adjuvant therapy in metastatic, then non-metastatic disease. The ENDEAR Phase III trial in TNBC is designed to test Enza in combination with paclitaxel in metastatic AR+ TNBC (ENzalutamiDe in Combination With PaclitaxEl Chemotherapy or as Monotherapy Versus Placebo With Paclitaxel in Patients With Advanced, Diagnostic-Positive, Triple-Negative BReast Cancer for tumors that test positive for a proprietary gene expression profile). There are already several other trials now ongoing with Enza and Pac combination therapy, including as neoadjuvant therapy (NCT02684227, NCT02689427, NCT02138383). Indeed, if AR targeted therapies continue to be successful in the clinic, they may become the first successful targeted therapy for TNBC.
Supplementary Material
Acknowledgments
FINANCIAL SUPPORT
DOD BCRP Clinical Translational Award BC120183 W81XWH-13-1-0090 to JKR and AE, R01 CA187733-01A1 to JKR, and NIH NCI NRSA F31 CA192807-02 to VNB
The authors also acknowledge the Genomics and Microarray Core, the Tissue Culture Core, the Flow Cytometry Shared Resource and other shared resources of Colorado’s NIH/NCI Cancer Center Support Grant P30CA046934.
Footnotes
CONFLICTS OF INTEREST
The authors declare no competing interests.
References
- 1.Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–34. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 2.Liedtke C, Mazouni C, Hess KR, Andre F, Tordai A, Mejia JA, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26:1275–81. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 3.Kabos P, Haughian JM, Wang X, Dye WW, Finlayson C, Elias A, Horwitz KB, Sartorius CA. Cytokeratin 5 positive cells represent a steroid receptor negative and therapy resistant subpopulation in luminal breast cancers. Breast Cancer Res Treat. 2011;128:45–55. doi: 10.1007/s10549-010-1078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu MF, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst. 2008;100:672–9. doi: 10.1093/jnci/djn123. [DOI] [PubMed] [Google Scholar]
- 6.Bhola NE, Balko JM, Dugger TC, Kuba MG, Sanchez V, Sanders M, Stanford J, Cook RS, Arteaga CL. TGF-beta inhibition enhances chemotherapy action against triple-negative breast cancer. J Clin Invest. 2013;123:1348–58. doi: 10.1172/JCI65416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barton VN, D’Amato NC, Gordon MA, Lind HT, Spoelstra NS, Babbs BL, et al. Multiple molecular subtypes of triple-negative breast cancer critically rely on androgen receptor and respond to enzalutamide in vivo. Mol Cancer Ther. 2015;14:769–78. doi: 10.1158/1535-7163.MCT-14-0926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Micello D, Marando A, Sahnane N, Riva C, Capella C, Sessa F. Androgen receptor is frequently expressed in HER2-positive, ER/PR-negative breast cancers. Virchows Arch. 2010;457:467–76. doi: 10.1007/s00428-010-0964-y. [DOI] [PubMed] [Google Scholar]
- 9.Collins LC, Cole KS, Marotti JD, Hu R, Schnitt SJ, Tamimi RM. Androgen receptor expression in breast cancer in relation to molecular phenotype: results from the Nurses’ Health Study. Mod Pathol. 2011;24:924–31. doi: 10.1038/modpathol.2011.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mrklic I, Pogorelic Z, Capkun V, Tomic S. Expression of androgen receptors in triple negative breast carcinomas. Acta Histochem. 2013;115:344–8. doi: 10.1016/j.acthis.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Safarpour D, Pakneshan S, Tavassoli FA. Androgen receptor (AR) expression in 400 breast carcinomas: is routine AR assessment justified? Am J Cancer Res. 2014;4:353–68. [PMC free article] [PubMed] [Google Scholar]
- 12.Thike AA, Yong-Zheng Chong L, Cheok PY, Li HH, Wai-Cheong Yip G, Huat Bay B, Tse GM, Iqbal J, Tan PH. Loss of androgen receptor expression predicts early recurrence in triple-negative and basal-like breast cancer. Mod Pathol. 2014;27:352–60. doi: 10.1038/modpathol.2013.145. [DOI] [PubMed] [Google Scholar]
- 13.Qi JP, Yang YL, Zhu H, Wang J, Jia Y, Liu N, et al. Expression of the androgen receptor and its correlation with molecular subtypes in 980 chinese breast cancer patients. Breast Cancer (Auckl) 2012;6:1–8. doi: 10.4137/BCBCR.S8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gucalp A, Tolaney S, Isakoff SJ, Ingle JN, Liu MC, Carey LA, et al. Phase II trial of bicalutamide in patients with androgen receptor-positive, estrogen receptor-negative metastatic Breast Cancer. Clin Cancer Res. 2013;19:5505–12. doi: 10.1158/1078-0432.CCR-12-3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Traina T. Stage I results from MDV3100-11: a 2 stage study of enzalutamide (Enza), an androgen receptor (AR) inhibitor, in advanced AR+ triple-negative breast cancer (TNBC) 2014 [Google Scholar]
- 16.Traina TAMK, Yardley DA, O’Shaughnessy J, Cortes J, Awarda A, Kelly CM, Trudeau ME, Schmid P, Gianni L, Garcia-Estevez L, Nanda R, Ademuyiwa FO, Chan S, Steinberg JL, Blaney ME, Tudor TC, Uppal H, Peterson AC, Hudis CA. Results from a phase 2 study of enzalutamide (ENZA), an androgen receptor (AR) inhibitor, in advanced AR+ triple-negative breast cancer (TNBC) 2015 [Google Scholar]
- 17.Horwitz KB, Dye WW, Harrell JC, Kabos P, Sartorius CA. Rare steroid receptor-negative basal-like tumorigenic cells in luminal subtype human breast cancer xenografts. Proc Natl Acad Sci U S A. 2008;105:5774–9. doi: 10.1073/pnas.0706216105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graham JD, Mote PA, Salagame U, van Dijk JH, Balleine RL, Huschtscha LI, Reddel RR, Clarke CL. DNA replication licensing and progenitor numbers are increased by progesterone in normal human breast. Endocrinology. 2009;150:3318–26. doi: 10.1210/en.2008-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asselin-Labat ML, Vaillant F, Sheridan JM, Pal B, Wu D, Simpson ER, et al. Control of mammary stem cell function by steroid hormone signalling. Nature. 2010;465:798–802. doi: 10.1038/nature09027. [DOI] [PubMed] [Google Scholar]
- 20.Beato M, Herrlich P, Schutz G. Steroid hormone receptors: many actors in search of a plot. Cell. 1995;83:851–7. doi: 10.1016/0092-8674(95)90201-5. [DOI] [PubMed] [Google Scholar]
- 21.Cloke B, Huhtinen K, Fusi L, Kajihara T, Yliheikkila M, Ho KK, et al. The androgen and progesterone receptors regulate distinct gene networks and cellular functions in decidualizing endometrium. Endocrinology. 2008;149:4462–74. doi: 10.1210/en.2008-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robinson JL, Macarthur S, Ross-Innes CS, Tilley WD, Neal DE, Mills IG, Carroll JS. Androgen receptor driven transcription in molecular apocrine breast cancer is mediated by FoxA1. EMBO J. 2011;30:3019–27. doi: 10.1038/emboj.2011.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Q, Li W, Zhang Y, Yuan X, Xu K, Yu J, et al. Androgen receptor regulates a distinct transcription program in androgen-independent prostate cancer. Cell. 2009;138:245–56. doi: 10.1016/j.cell.2009.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu J, Yu J, Mani RS, Cao Q, Brenner CJ, Cao X, et al. An integrated network of androgen receptor, polycomb, and TMPRSS2-ERG gene fusions in prostate cancer progression. Cancer Cell. 2010;17:443–54. doi: 10.1016/j.ccr.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cimino-Mathews A, Hicks JL, Illei PB, Halushka MK, Fetting JH, De Marzo AM, Park BH, Argani P. Androgen receptor expression is usually maintained in initial surgically resected breast cancer metastases but is often lost in end-stage metastases found at autopsy. Hum Pathol. 2012;43:1003–11. doi: 10.1016/j.humpath.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McNamara KM, Moore NL, Hickey TE, Sasano H, Tilley WD. Complexities of androgen receptor signalling in breast cancer. Endocr Relat Cancer. 2014;21:T161–81. doi: 10.1530/ERC-14-0243. [DOI] [PubMed] [Google Scholar]
- 27.Lawson DA, Bhakta NR, Kessenbrock K, Prummel KD, Yu Y, Takai K, et al. Single-cell analysis reveals a stem-cell program in human metastatic breast cancer cells. Nature. 2015;526:131–5. doi: 10.1038/nature15260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feng J, Li L, Zhang N, Liu J, Zhang L, Gao H, et al. Androgen and AR contribute to breast cancer development and metastasis: an insight of mechanisms. Oncogene. 2016 doi: 10.1038/onc.2016.432. [DOI] [PubMed] [Google Scholar]
- 29.Christenson JL, Butterfield KT, Spoelstra NS, Norris JD, Josan JS, Pollock JA, et al. MMTV-PyMT and Derived Met-1 Mouse Mammary Tumor Cells as Models for Studying the Role of the Androgen Receptor in Triple-Negative Breast Cancer Progression. Horm Cancer. 2017;8:69–77. doi: 10.1007/s12672-017-0285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doane AS, Danso M, Lal P, Donaton M, Zhang L, Hudis C, Gerald WL. An estrogen receptor-negative breast cancer subset characterized by a hormonally regulated transcriptional program and response to androgen. Oncogene. 2006;25:3994–4008. doi: 10.1038/sj.onc.1209415. [DOI] [PubMed] [Google Scholar]
- 31.Farmer P, Bonnefoi H, Becette V, Tubiana-Hulin M, Fumoleau P, Larsimont D, et al. Identification of molecular apocrine breast tumours by microarray analysis. Oncogene. 2005;24:4660–71. doi: 10.1038/sj.onc.1208561. [DOI] [PubMed] [Google Scholar]
- 32.Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, Pietenpol JA. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–67. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Booth BW, Boulanger CA, Anderson LH, Jimenez-Rojo L, Brisken C, Smith GH. Amphiregulin mediates self-renewal in an immortal mammary epithelial cell line with stem cell characteristics. Exp Cell Res. 2010;316:422–32. doi: 10.1016/j.yexcr.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marotta LL, Almendro V, Marusyk A, Shipitsin M, Schemme J, Walker SR, et al. The JAK2/STAT3 signaling pathway is required for growth of CD44(+)CD24(−) stem cell-like breast cancer cells in human tumors. J Clin Invest. 2011;121:2723–35. doi: 10.1172/JCI44745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Masuda H, Baggerly KA, Wang Y, Zhang Y, Gonzalez-Angulo AM, Meric-Bernstam F, et al. Differential response to neoadjuvant chemotherapy among 7 triple-negative breast cancer molecular subtypes. Clin Cancer Res. 2013;19:5533–40. doi: 10.1158/1078-0432.CCR-13-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.