Abstract
Twin studies show the established relation between bulimic symptoms and problematic alcohol involvement in adult females is partly due to shared familial factors, specifically shared genetic effects. However, it is unclear if similar shared etiological factors exist during adolescence or in males. We examined the familial overlap (i.e., genetic and common environmental correlations) between bulimic symptoms and various levels of alcohol involvement in 16–17-year-old female and male same-sex twin pairs using sex-specific biometrical twin modeling. Bulimic symptoms were assessed with the Eating Disorder Inventory-2. Alcohol involvement included alcohol use in the last month, having ever been intoxicated, and alcohol intoxication frequency. Results revealed three distinct patterns. First, in general, phenotypic correlations indicated statistically similar associations between bulimic symptoms and alcohol involvement in girls and boys. Second, common environmental overlap was significant for the bivariate associations including having ever been intoxicated. Third, moderate genetic correlations were observed between all bulimic symptoms and alcohol involvement in girls and moderate common environmental correlations were observed in boys for the more risky/deviant levels of involvement. Similar to adults, there is familial overlap between bulimic symptoms and alcohol involvement in adolescent girls and boys. These results could inform symptom- and sex- specific, developmentally-targeted prevention and intervention programs for the comorbidity between bulimic symptoms and alcohol involvement.
Keywords: bulimia nervosa, bulimic symptoms, eating disorder, disordered eating, alcohol use, comorbidity, twin study
There is an established association between bulimia nervosa (BN) and alcohol involvement in adult females (Gadalla & Piran, 2007; Harrop & Marlatt, 2010; Root et al., 2010). For example, one large epidemiological examination of this comorbidity observed the prevalence of alcohol abuse or dependence at 22% in BN (Root, et al., 2010) and a meta-analysis showed a significant association between an alcohol use disorder and binge eating and purging (Gadalla & Piran, 2007). Women with BN also report more alcohol-related negative consequences compared with women without BN (Dunn, Larimer, & Neighbors, 2002). Despite the established association between BN and related symptomatology and problematic alcohol involvement, fundamental questions remain as to whether these findings in adult females can be translated to adolescents and males.
Although the association between BN and alcohol involvement has been less widely explored during adolescence, findings parallel those observed in adults. For example, adolescents with BN have a 3-fold increased risk for an alcohol use disorder compared with peers without BN (Swanson, Crow, Le Grange, Swendsen, & Merikangas, 2011). Specific bulimic symptoms are also associated with alcohol involvement during adolescence. In late adolescent girls, a significant positive correlation was observed between alcohol use and misuse and compensatory behaviors (von Ranson, Iacono, & McGue, 2002). The reverse was also true such that girls who reported lifetime alcohol intoxication had significantly higher compensatory behavior and binge eating scores (von Ranson, et al., 2002). Binge drinking in adolescence also shows significant associations with bulimic symptoms including purging behaviors and diet pill use (Eichen, Conner, Daly, & Fauber, 2012; Field et al., 2014; Stickley et al., 2015). In adolescent boys specifically, those who reported engaging in purging behaviors were approximately four times more likely to have binge drank in the past 30 days than their male peers who did not endorse purging (Stickley, et al., 2015).
A prospective association between adolescent BN and related disorders and alcohol involvement also exists. At age 14–15 girls with BN engaged in heavier alcohol use at age 20 compared with girls without BN (Patton, Coffey, & Sawyer, 2003) and girls with purging disorder are twice as likely to later engage in binge drinking (Field et al., 2012). Further, women with BN at age 24 were disproportionately more likely to report at age 16 being severely intoxicated when they last drank alcohol compared with those without BN; along with more frequent concurrent alcohol intoxication and alcohol-related problems (Mustelin et al., 2016). Thus, the presence of bulimic-related symptoms or alcohol involvement during adolescence may increase the risk for the later development of the other behavior.
Despite the significant and prospective associations between bulimic symptoms and alcohol involvement the mechanism underlying this comorbidity is largely unknown, but both behaviors may share risk factors such as emotional dysregulation, impulsivity, or additional psychopathological traits (Fischer, Settles, Collins, Gunn, & Smith, 2012; Grilo, Sinha, & O’Malley, 2002; Slane, Klump, McGue, & Iacono, 2014a). An acquired preparedness model has also been proposed to account for this comorbidity. It posits that individual dispositions (e.g., impulsivity) increase vulnerability for both bulimic symptoms and alcohol involvement via an interaction between this disposition and learning events, which in turn lead to the development of reinforcement expectancies from the bulimic symptom, alcohol use, or both (Fischer, et al., 2012; Schaumberg & Earleywine, 2013). We have additionally hypothesized that both have a common familial predisposition (Munn-Chernoff & Baker, 2016). This common familial predisposition may, in part, explain the presence of shared risk factors or an underlying disposition.
Twin studies are a particularly useful technique to obtain an indication of the mechanism underlying the association between two traits as twin studies can decompose the amount and source of shared etiological risk between two traits—which non-twin association studies cannot do. In regard to shared familial risk, twin studies identify two sources: shared genetic or common environmental (i.e., environmental effects that create similarity in family members) risk. Indeed, there is accumulating evidence from twin studies showing there is a shared familial predisposition, namely shared genetic overlap, between BN and alcohol involvement at both the diagnostic and symptom level in adult females (Munn-Chernoff & Baker, 2016). Specifically, in one twin study examining the shared etiological risk between BN and a multitude of substance use disorders, genetic factors were the only shared familial factors identified between BN and an alcohol use disorder (Baker, Mitchell, Neale, & Kendler, 2010). Of all of the substance use disorders examined, BN and an alcohol use disorder also showed the strongest genetic overlap.
To date, only one twin study has examined the familial covariance between bulimic symptoms and alcohol involvement in males (Munn-Chernoff et al., 2013). Confirming findings with women only, genetic factors were the only shared familial factors identified. Specifically, this study observed 7% overlap in the genetic liability for binge eating and alcohol dependence in both adult males and females (Munn-Chernoff, et al., 2013). Importantly however, the aforementioned studies have mostly focused on BN and related symptomatology and problematic alcohol involvement in adult females. It is unknown whether similar familial/genetic associations are observed across differing levels of alcohol involvement (e.g., non-problematic use) in both females and males. Such findings could aid in advancing symptom- and sex- specific etiologic models of comorbidity.
Finally, despite the growing body of work examining shared etiologic factors between bulimic symptoms and alcohol involvement, studies addressing whether shared etiological factors exist in younger cohorts of girls and boys are scant. This is an important area of inquiry given the significant and prospective associations observed in this age group. Moreover, twin studies examining the genetic and environmental influences on eating disorder symptoms and alcohol involvement independently in adolescent populations suggest developmental differences exist for the familial effects. Indeed, common environmental effects are more important in the risk for these behaviors during adolescence, with little or no influence from genetic factors, whereas the reverse is true during adulthood—genetic effects are most important in influencing risk with little or no influence from the common environment (Baker, Maes, Larsson, Lichtenstein, & Kendler, 2011; Fairweather-Schmidt & Wade, 2015; Geels et al., 2012; Klump, Burt, McGue, & Iacono, 2007). Similar developmental differences may exist in the overlap between bulimic symptoms and alcohol involvement such that common environmental factors play a more important role in the association between these behaviors compared with genetic factors during adolescence. Such findings could inform developmentally-targeted prevention and intervention programs.
Thus, the purpose of this investigation is to fill in the gaps in the literature in regard to the etiological overlap between bulimic symptoms and alcohol involvement by examining whether shared familial factors (e.g., genetic or common environment) exist in adolescent girls and boys. We focus on bulimic symptoms as this has been the focus of the adult literature. Alcohol involvement includes three classes of involvement: alcohol use in the last month, alcohol intoxication, and frequency of alcohol intoxication. We focus on these levels of involvement in order to capture the varying degrees of alcohol involvement that could occur during adolescence as well as use that could be considered less risky/deviant (use in the last month) versus more risky/deviant (intoxication frequency) for an adolescent population (Johnston, O’Malley, Bachman, & Schulenberg, 2013).
We address two specific questions: (a) what are the observed phenotypic associations between bulimic symptoms and alcohol involvement and are these associations similar in adolescent girls and boys; and (b) which familial factor (i.e., genetic and/or common environment) primarily accounts for the association between bulimic symptoms and alcohol involvement in girls and boys? Due to the power limitations of twin models to assess sex differences (Prescott & Gottesman, 1993; Verhulst, 2016), we were unable to directly test whether statistically significant differences exist in the familial overlap between girls and boys. However, we are able to assess which familial factor is important within each sex.
Method
Participants
The present sample, the Swedish Twin study of Child and Adolescent Development (TCHAD), began with all twin pairs born in Sweden between May 1985 and December 1986 (Lichtenstein, Tuvblad, Larsson, & Carlström, 2007). Same-sex monozygotic (MZ) and dizygotic (DZ) twin pairs were recruited through the Swedish Medical Birth Registry and identified twins and their parents were mailed study questionnaires (Lichtenstein & Svartengren, 1997). Participants have completed four assessment Waves. Information from Wave 3 was included in the present study, when the twins were 16–17-years-old, as this is when bulimic symptom information was assessed. The response rate at Wave 3 for all twins contacted was 82% (Lichtenstein, et al., 2007). The total sample included 422 and 453 same-sex male and female MZ twins and 306 and 339 same-sex male and female DZ twins, respectively. Zygosity of twins was determined based on computer algorithms of questionnaire responses created from analyses of twin pairs with known zygosity (Lichtenstein, et al., 2007). The Ethics Committee of Karolinska Institutet, Stockholm, Sweden, approved questionnaires used in TCHAD and the University of North Carolina Institutional Review Board approved this project.
Measures
Bulimic Symptoms
Bulimic symptoms were examined with the Eating Disorder Inventory-II (EDI; Garner, 1991); a self-report questionnaire designed to measure behaviors and attitudes relevant to eating disorders. Specifically, the Bulimia (BU) subscale of the EDI was used. The BU subscale assesses the tendency toward episodes of binge eating that may be followed with the impulse to induce vomiting. The Swedish version of the EDI has been translated and validated on a female population (Nevonen, Clinton, & Norring, 2006; Norring & Sohlberg, 1988). Although the EDI was created for use with female populations, it functions similarly in males. The EDI differentiates between males with eating disorders and controls (Olivardia, Pope, Mangweth, & Hudson, 1995) and produces the same factor structure and similar factor loadings and intercorrelations in males and females (Baker et al., 2009; Spillane, Boerner, Anderson, & Smith, 2004). Cronbach’s alpha coefficients for this sample were .53 for girls, .60 for boys, and .64 for the total sample.
The EDI was scored as indicated by the EDI manual (Garner, 1991). Missing data were handled as follows: if the participant responded to more than 75% of items but less than 100% of items, missing item values were mean imputed. If there were less than 75% completed items available, the subscale score was considered missing (Baker, et al., 2009). After initial scoring the BU subscale had to be re-classified due to the categorical nature of the alcohol involvement data. BU was dichotomized into participants that received a subscale score of zero versus those who did not.
Alcohol Involvement
Self-reported alcohol use in the last month, having ever been intoxicated, and frequency of alcohol intoxication were assessed by questionnaire. For use in the last month participants were asked, if in the last month, they had been drinking beer, wine, or liquor and response options included: (0) No; (1) Yes, once; and (2); Yes, several times. Having ever been intoxicated was dichotomously coded, indicating whether or not the participant had ever been intoxicated. Three frequency of alcohol intoxication categories were created based on the question “how often do you get drunk when you drink alcohol?”. Categories included: (0) never been intoxicated; (1) get intoxicated when drink alcohol sometimes (i.e., sometimes, only at parties); or (3) get intoxicated often (i.e., every time drinking, always). For alcohol intoxication and frequency of alcohol intoxication, participants who denied any lifetime alcohol use were coded as missing. This was done because each variable is conditional on having initiated use and including non-users in twin models can bias the genetic and environmental estimates. Similar approaches have been used widely in the substance use literature (e.g., Dick, Meyers, Rose, Kaprio, & Kendler, 2011; Edwards, Maes, Pedersen, & Kendler, 2011; Palmer et al., 2013).
Statistical Analyses
Phenotypic analyses
Before completing twin modeling, sex-specific phenotypic correlations were examined between bulimic symptoms and alcohol involvement. Those associations that showed a sex-specific correlation of .20 or above (which corresponds to at least a small-to-moderate effect size (Cohen, 1992)) were followed-up in twin correlations and models. If the phenotypic correlation was less than .20, we did not complete twin modeling. Although it is possible shared etiological overlap exists between two variables with a small phenotypic association, if there is no phenotypic association between two traits, parsing this negligible association into genetic and environmental components is limitedly informative. This also limits the number of statistical tests being completed to only those with an empirical rationale. Similar techniques have been used previously (e.g., Koren et al., 2014).
Twin correlations
Within-trait cross-twin correlations were calculated for bulimic symptoms and alcohol involvement variables by zygosity to obtain an initial indication on the genetic and environmental estimates for each variable independently. Next, cross-twin cross-trait correlations were calculated by zygosity, based on the initial sex-specific phenotypic correlations described above, to obtain a potential snapshot of whether genetic or environmental factors are important for the observed phenotypic association.
For each, additive genetic factors (A: effects represent the cumulative impact of many genes) are suggested if the MZ twin correlation is approximately twice that of the DZ twin correlation. Common environment (C: environmental factors which make twins/families similar) is suggested if the MZ and DZ correlations are similar. Individual-specific environmental factors (E: serve to make twins dissimilar and includes measurement error) are indicated if the MZ correlation is less than 1.0 (for the within-trait cross-twin correlations) or less than the observed phenotypic correlation (for the cross-twin cross-trait correlations).
Twin analyses
A Cholesky decomposition fitted using Mx (Neale, 1991) was used to decompose the association between bulimic symptoms and alcohol involvement into genetic and environmental components (Figure 1). The final sample included 219 and 229 male and female MZ twins and 157 and 175 male and female DZ twins, respectively. Analyses were conducted using a categorical, raw data approach, which allows information from both complete (n = 734) and incomplete (n = 46) pairs to be included.
Figure 1.
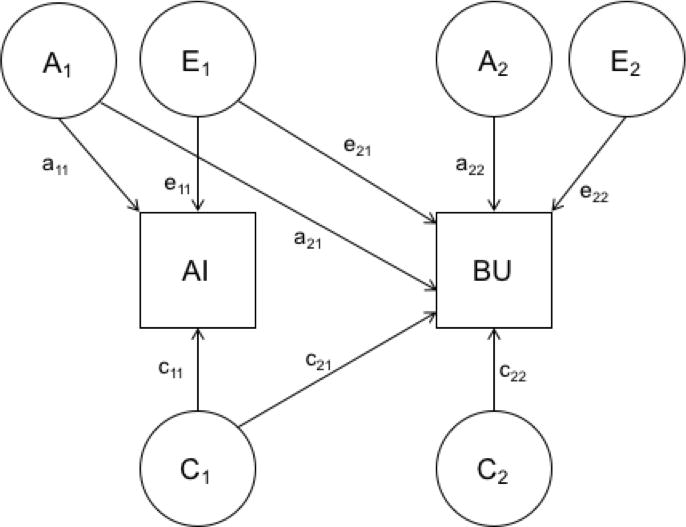
Cholesky decomposition of genetic and environmental covariance between alcohol involvement (AI) and bulimic (BU) symptoms.
The Cholesky decomposition estimates the proportion of variance for bulimic symptoms and alcohol involvement accounted for by additive genetic (a2), common environmental (c2), and individual-specific environmental (e2) factors. Along with the estimates for the proportion of variance attributable to genetic and environmental factors for bulimic symptoms and alcohol involvement independently, the Cholesky decomposition also provides estimates of the genetic (ra), common environment (rc), and individual-specific environment (re) correlations between the variables of interest. These correlations provide readily interpretable estimates of the proportion of overlapping genetic and environmental factors between two traits. For example, if the genetic correlation is estimated at 1.00, this indicates that bulimic symptoms and the alcohol variable of interest share all of their genetic factors; if this correlation is zero, then there are no shared genetic factors between the two phenotypes. Given that genetic and environmental correlations are free from measurement error, it is possible to obtain observed correlations of +/− 1.00.
Within the Cholesky decomposition, alcohol involvement was included in the model first and bulimic symptoms second as our aim was to determine the proportion of variance in bulimic symptoms that are shared with alcohol involvement. Thus, the variance of bulimic symptoms is parsed into components attributable to the genetic and environmental effects on alcohol involvement (a21, c21, e21 from Figure 1) and bulimic symptom-specific genetic and environmental effects (a22, c22, e22 from Figure 1). In other words, the amount of genetic and environmental variance on bulimic symptoms accounted for by alcohol involvement can be assessed—providing estimates of the amount of genetic (a22 / a21 + a22 from Figure 1), common environment (c22 / c21 + c22 from Figure 1), and individual-specific environment (e22 / e21 + e22 from Figure 1) variance shared with alcohol involvement and the amount of variance unique to bulimic symptoms.
The fit of the full ACE model, which estimates all genetic and environmental paths from Figure 1 for each phenotype and their covariance, was initially compared to two nested submodels: the AE model, which estimates only genetic and individual-specific environmental variance and covariance and the CE model, which estimates only common and individual-specific environmental variance and covariance.1 The full model and nested submodels were compared using the negative log-likelihood of the models and Akaike’s Information Criterion (AIC; 2*log−likelihood+2*k where k is the number of estimated parameters) (Akaike, 1987). The difference in twice the negative log-likelihood of the models, is, given certain regularity conditions, distributed as a chi-square. A significant (p < .05) change in chi-square indicates a significantly worse model fit; thus, the model is rejected. Lower AIC values indicate a better balance between parsimony and goodness-of-fit and thus models with lower AIC values are preferred and retained as the best-fitting model. After comparing the fit of the full model to the nested submodels, an initial best-fit model was chosen based on chi-square change and AIC.
Once an initial best-fit model was determined from the models described above, we compared additional nested submodels to this initial best-fit model to assess the importance of any familial (e.g., genetic or common environmental) covariance observed between bulimic symptoms and alcohol involvement. Specifically, these follow-up analyses compared model fit to the initially identified best-fit model in the first round of model testing (described above) to additional nested submodels, which respectively dropped the genetic or common environmental covariance path (a21 or c21 from Figure 1; which assesses whether the observed genetic or common environmental covariance/correlation is significant) or the genetic or common environmental path specific to bulimic symptoms (a22 or c22 from Figure 1; which assesses whether all of the genetic or common environmental effects for bulimic symptoms are shared with alcohol involvement) from the model. We chose a priori to only test those submodels that directly examine our aim to assess the importance of familial covariance (i.e., genetic or common environmental covariance) between bulimic symptoms and alcohol involvement to limit the number of submodels tested and thus, statistical analyses completed.
All twin models were completed separately for girls and boys, based on the sex-specific phenotypic correlations. Due to power constraints, we were unable to directly test for sex differences in the familial overlap between girls and boys (i.e., fitting models which constrain female and male estimates and correlations to equality). Simulations show that exceptionally large sample sizes are needed to detect sex differences in twin models (Prescott & Gottesman, 1993; Verhulst, 2016) and our own analyses confirm a lack of power. Thus, we complete sex-specific bivariate models that show which familial factors are important for covariance for each sex; however, we cannot directly test the statistical significance of any observed difference.
Results
Descriptives
The mean score for BU was similar in girls (M = .51; SD = 1.3) and boys (M = .45; SD = 1.2), and approximately one quarter of the girls (24.0%; n = 212) and boys (23.0%; n = 272) scored above a zero. As can be seen in Table 1, the frequencies of alcohol involvement behaviors were also roughly similar in girls and boys. In general, half the sample reported alcohol use in the last month and a majority of the participants reported having ever been intoxicated. In regard to frequency of alcohol intoxication, 55%–61% reported no intoxication when drinking alcohol, 16%–18% reported alcohol intoxication sometimes, and 23%–27% reported alcohol intoxication nearly every time alcohol is consumed.
Table 1.
Frequency of Alcohol Involvement by Sex (Boys Shown in Parentheses)
| Alcohol Involvement Frequency | |||
|---|---|---|---|
| No | Once/Sometimes | Often/Yes | |
| Alcohol Use in Last Montha | 44.2%; n = 371 (50.0%; n = 348) |
27.1%; n = 228 (25.5%; n = 181) |
29.0%; n= 241 (25.5%; n = 181) |
| Ever Intoxicated | 23.0%; n = 139 (30.0%; n = 147) |
– | 77.5%; n = 475 (71.0%; n = 357) |
| Intoxication Frequency | 55.1%; n = 474 (61.0%; n = 453) |
18.2%; n = 157 (16.2%; n = 121) |
27.0%; n = 230 (23.1%; n = 172) |
Note.
= Variable coded as no, once, and several times. % = percentage of sample. n = number of individuals in sample.
Percentages may not equal 100% due to rounding.
Phenotypic Associations
For a majority of the phenotypic polychoric correlations, the observed correlations were similar in girls and boys (Table 2). In fact, a Fisher r-to-z transformation indicated that the observed correlations between BU and alcohol use in the last month and frequency of alcohol intoxication were not significantly different between girls and boys. In contrast, the phenotypic correlation for BU-having ever been intoxicated was significantly greater for boys (r = .40) compared with girls (r = .21). All phenotypic associations between BU and the three alcohol involvement variables met the .20 threshold for follow-up twin modeling.
Table 2.
Phenotypic Correlations (95% confidence Interval) Between Bulimic Symptoms and Alcohol Involvement by Sex
| Girls | Boys | |||||
|---|---|---|---|---|---|---|
| Alcohol Use in Last Month | Ever Intoxicated | Intoxication Frequency | Alcohol Use in Last Month | Ever Intoxicated | Intoxication Frequency | |
| Bulimic Symptoms | .30 (.20; .40) |
.21 (.10; .36) |
0.23 (.13; .34) |
.22 (.10; .33) |
.40* (.24; .54) |
.22 (.11; .34) |
Note.
Fisher r-to-z transformation indicating a significant difference in correlation estimates between girls and boys (p < .01).
Twin Correlations
Twin correlations are provided in Table 3. In general, MZ correlations were nearly twice as high compared with DZ correlations for all phenotypes in both sexes indicating the presence of genetic effects. Moreover, for both sexes, the MZ correlations for the alcohol involvement variables were substantial indicating that genetic factors may be more important than individual-specific effects. In general, the cross-twin cross-trait correlations suggest that genetic effects are important for the association between BU and alcohol involvement in girls and common environment may be important for these associations in boys.
Table 3.
Twin Correlations (95% Confidence Interval) for Bulimic Symptoms and Alcohol Involvement
| MZF | DZF | MZM | DZM | |
|---|---|---|---|---|
|
Within-trait, Cross-twin Correlations |
||||
| BU | .50 (.29; .70) |
.36 (.11; .62) |
.45 (.23; .67) |
.26 (−.03; .55) |
| Drank Alcohol in Last Month | .75 (.65; .84) |
.56 (.40; .71) |
.80 (.72; .89) |
.57 (.40; .73) |
| Ever Intoxicated | .94 (.87; 1.00) |
.46 (.10; .85) |
.84 (.70; .98) |
.70 (.60; 1.00) |
| Intoxication Frequency | .82 (.74; .90) |
.65 (.51; .80) |
.90 (.85; .96) |
.73 (.60; .85) |
|
Cross-twin, Cross-trait Correlations |
||||
| Drank Alcohol in Last Month | .32 (.20; .46) |
.20 (.03; .36) |
.15 (−.03; .32) |
.09 (−.08; .27) |
| Ever Intoxicated | .24 (.04; .44) |
−.02 (−.28; .24) |
.12 (−.11; .35) |
.26 (.01; .51) |
| Intoxication Frequency | .32 (.18; .46) |
.05 (−.13; .22) |
−.01 (−.18; .17) |
.20 (.02; .38) |
Note. MZF=monozygotic female twins; DZF=dizygotic female twins; MZM=monozygotic male twins; DZM=dizygotic male twins. BU = bulimic symptoms.
Twin Analyses
Model fitting results are shown in Table 4 and parameter estimates and genetic and environmental correlations from the full and final best-fit models are shown in Table 5.
Table 4.
Twin Model Fitting Results for Bivariate Associations Between Bulimic Symptoms and Alcohol Involvement in Girls and Boys
| Model | −2lnL | Df | χ2 diff (p) | AIC |
|---|---|---|---|---|
| Girls | ||||
| Alcohol Use in Last Month | ||||
| ACE | 2363.50 | 1563 | – | −764.00 |
| AE | 2366.00 | 1566 | 3.40 (.34) | −766.20 |
| CE | 2370.30 | 1566 | 8.00 (.05) | −762.00 |
| AE-no genetic covariance | 2380.21 | 1567 | 14.40 (<.001) | −754.00 |
| AE-no unique genetic effects on BU | 2379.00 | 1567 | 13.04 (<.001) | −755.12 |
| Ever Intoxicated | ||||
| ACE | 1585.30 | 1449 | – | −1313.00 |
| AE | 1594.00 | 1452 | 9.00 (.03) | −1310.00 |
| CE | 1600.00 | 1452 | 15.00 (<.01) | −1304.20 |
| ACE-no genetic covariance | 1589.00 | 1450 | 3.45 (.06) | −1311.30 |
| ACE-no common environment covariance | 1586.00 | 1450 | .30 (.60) | −1314.50 |
| ACE-no unique genetic effects on BU | 1585.40 | 1450 | .12 (.73) | −1314.60 |
| ACE-no unique common environment on BU | 1585.36 | 1450 | .10 (.75) | −1315.00 |
| Frequency of Intoxication | ||||
| ACE | 2238.84 | 1577 | – | −915.16 |
| AE | 2245.30 | 1580 | 6.40 (.10) | −915.00 |
| CE | 2252.81 | 1580 | 14.00 (<.01) | −907.20 |
| AE-no genetic covariance | 2258.50 | 1580 | 13.22 (<.01) | −903.52 |
| AE-no unique genetic effects on BU | 2260.00 | 1580 | 15.00 (<.01) | −902.13 |
| Boys | ||||
| Alcohol Use in Last Month | ||||
| ACE | 2068.00 | 1424 | – | −780.20 |
| AE | 2071.20 | 1427 | 3.37 (.34) | −783.00 |
| CE | 2081.00 | 1427 | 13.00 (<.01) | −773.40 |
| AE-no genetic covariance | 2080.00 | 1428 | 7.50 (<.01) | −777.30 |
| AE-no unique genetic effects on BU | 2085.33 | 1428 | 14.12 (<.001) | −771.00 |
| Ever Intoxicated | ||||
| ACE | 1430.00 | 1297 | – | −1165.00 |
| AE | 1436.00 | 1300 | 6.20 (.11) | −1164.41 |
| CE | 1434.04 | 1300 | 5.00 (.20) | −1166.00 |
| CE-no common environment covariance | 1438.00 | 1301 | 3.50 (.06) | −1164.50 |
| CE-no unique common environment on BU | 1446.00 | 1301 | 11.50 (<.01) | −1160.00 |
| Frequency of Intoxication | ||||
| ACE | 1918.60 | 1447 | – | −975.42 |
| AE | 1926.30 | 1450 | 7.70 (.05) | −973.74 |
| CE | 1930.00 | 1450 | 11.42 (.01) | −970.10 |
| ACE-no genetic covariance | 1918.66 | 1448 | 1.00 (.78) | −977.34 |
| ACE-no common environment covariance | 1920.00 | 1448 | 0.66 (.42) | −976.75 |
| ACE-no unique genetic effects on BU | 1920.00 | 1448 | 1.00 (.34) | −976.51 |
| ACE-no unique common environment on BU | 1918.60 | 1448 | 0.01 (.96) | −977.42 |
Note. Final best-fit model shown in bold. −2lnL = difference in twice the negative log-likelihood of the models. Df = degrees of freedom. χ2 diff (p) = chi-square difference between full model and submodel and the associated p-value. AIC = Akaike Information Criterion. BU = bulimic symptoms.
Table 5.
Parameter Estimates and Observed Correlations (95% Confidence Interval) from Full and Final Best-Fit Twin Models for Bulimic Symptoms and Alcohol Involvement in Girls and Boys
| Alcohol Involvement | Bulimic Symptoms | Correlations | |||||||
|---|---|---|---|---|---|---|---|---|---|
| a2 | c2 | e2 | a2 | c2 | e2 | ra | rc | re | |
| Girls | |||||||||
| Alcohol Use in Last Month | |||||||||
| ACE | 45 (12; 80) |
30 (0; 58) |
26 (18; 36) |
30 (0; 63) |
16 (0; 52) |
54 (36; 76) |
.27 (−1; 1) |
.70 (−1; 1) |
.12 (−.17; .40) |
| AE |
76 (70; 83) |
– |
24 (17; 33) |
47 (27; 65) |
– |
53 (35; 73) |
.42 (.20;.65) |
– |
.10 (−.17; .38) |
| Ever Intoxicated | |||||||||
| ACE | 50 (23; 83) |
46 (14; 71) |
4 (1; 11) |
37 (27; 65) |
11 (0; 50) |
52 (34; 72) |
.80 (−.06; 1) |
−.40 (−1; 1) |
.14 (−.54; .77) |
| ACE-no unique common environment on BU |
51 (23; 84) |
45 (13; 70) |
4 (1; 11) |
46 (20; 66) |
3 (0; 25) |
51 (34; 71) |
.76 (−.04; 1) |
−1.00a (−1; −1) |
.13 (−.54; .42) |
| Intoxication Frequency | |||||||||
| ACE | 47 (20; 80) |
35 (4; 61) |
18 (12; 27) |
32 (10; 63) |
14 (0; 46) |
54 (36; 70) |
.98 (.11; 1) |
−.53 (−1; 1) |
−.14 (−.42; .20) |
| AE |
84 (76; 90) |
– |
16 (11; 24) |
49 (29; 65) |
– |
51 (35; 71) |
.38 (.20;.60) |
– | −.10 (−.40; .23) |
| Boys | |||||||||
| Alcohol Use in Last Month | |||||||||
| ACE | 52 (21; 85) |
30 (0; 57) |
18 (12; 28) |
41 (0; 65) |
5 (0; 50) |
54 (35; 77) |
.58 (−1; 1) |
−.59 (−1; 1) |
−.01 (−.31; .30) |
| AE |
83 (75; 89) |
– |
17 (11; 26) |
47 (25; 65) |
– |
53 (35; 75) |
.31 (.10;.53) |
– |
.02 (−.28; .32) |
| Ever Intoxicated | |||||||||
| ACE |
37 (0; 38) |
50 (11; 80) |
13 (5; 29) |
42 (0; 64) |
3 (0; 50) |
55 (36; 80) |
.20 (−1; 1) |
.86 (−1; 1) |
.44 (−.10; 80) |
| CE | – |
76 (.63; .85) |
24 (15; 37) |
– |
36 (17; 52) |
64 (48; 83) |
– |
.28 (.01;.55) |
.37 (.07; .63) |
| Intoxication Frequency | |||||||||
| ACE | 30 (12; 61) |
57 (27; 80) |
13 (11; 18) |
40 (0; 63) |
5 (0; 50) |
56 (37; 80) |
−.15 (−1; 1) |
.82 (−1; 1) |
.42 (.04; .70) |
| ACE-no unique common environment on BU |
30 (5; 61) |
57 (30; 78) |
13 (8; 20) |
41 (10; 63) |
3 (0; 30) |
56 (37; 79) |
−.15 (−1; .85) |
1.00a (1; 1) |
.42 (.04; .61) |
Note. Final best-fitting model shown in bold. a2 = heritability. c2 = common environmental parameter estimate. e2 = individual-specific environment parameter estimate. ra = genetic correlation. rc = common environmental correlation. re = individual-specific environmental correlation. BU = bulimic symptoms.
= the confidence interval for the correlation in this bivariate association in the best-fit model is observed at +/−1.00; +/−1.00 because there are no environmental effects specific to bulimic symptoms that are not shared with alcohol involvement.
Alcohol Use in the Last Month
Comparing the fit of the ACE model to the initial nested submodels indicated that the AE model was the initial best-fitting model for BU-alcohol use in the last month for both girls and boys. Most other submodels could be rejected as the change in chi-square was significant and the AE model had the lowest AIC value. Thus, in the follow-up submodels testing the importance of familial covariance for girls and boys, we made comparisons to the AE model. Because we were able to drop common environment from the model, only two follow-up submodels were examined: an AE model without genetic covariance; and an AE model without unique genetic effects for BU. Both of these models had a significant change in chi-square for both girls and boys and thus could be rejected as a significantly worse fit. Thus, the initial AE model was the final, best-fitting model for BU-alcohol use in the last month for girls and boys.
The results of these final, best-fit models indicated that genetic factors accounted for some of the covariance between bulimic symptoms and alcohol use in the last month. Specifically, for girls, the model showed an estimated ra = .42 (95% CI: .20; .65) and re = .10 (95% CI: −.17; .38) indicating that alcohol use in the last month accounted for 17% of the total heritability of BU and 2% of the total individual-specific environmental effects. For boys, the model estimated ra = .31 (95% CI: .10; .53) and re = .02 (95% CI: −.28; .32), with alcohol use in the last month only accounting for 7% of the total heritability of BU and less than 1% of the total individual-specific environmental effects.
Ever been Intoxicated
For the association between BU-ever been intoxicated in girls the ACE model was the initial best-fitting model. The AE and CE models could be rejected as the change in chi-square was significant and the ACE model had the lowest AIC value. Thus, in the follow-up submodels, we made the following comparisons to the ACE model: an ACE model without genetic covariance; an ACE model without common environmental covariance; an ACE model without unique genetic effects for BU; and an ACE model without unique common environmental effects for BU. According to the fit statistics, the ACE model without unique common environmental effects for BU was the final best-fitting model such that this model had the lowest AIC value and the chi-square change for this model was also the least. This model estimated ra = .76 (95% CI: −.04; 1.00); rc = −1.00 (95% CI: −1.00; −1.00) and re = .13 (95% CI: −.54; .42).
In girls, all of the common environmental effects for BU were shared with alcohol intoxication. However, because this correlation was negative this suggests that the common environmental effects that influence alcohol intoxication decrease the risk for BU. Alcohol intoxication also accounted for less than 1% of the total individual-specific environmental effects for BU yet for 57% of the total heritability. Although the confidence interval for the genetic correlation does include zero, because dropping this correlation from the model resulted in a worse fit, this covariance is likely important. However, this does suggest that the observed correlation in the model and the percentage of covariance accounted for by alcohol intoxication may be imprecise and could vary from the estimate reported here.
For boys, the CE model was the initial best-fitting model as this model had the lowest AIC value. Thus, two follow-up submodels were compared with the fit of the CE model: a model without common environmental covariance; and a model without unique common environmental effects for BU. However, the initial CE model remained the best-fitting model. This CE model estimated rc = .28 (95% CI: .01; .55) and re = .37 (95% CI: .07; .63), with alcohol intoxication accounting for 9% and 14% of the total common environmental effects and individual-specific environmental effects, respectively, for BU.
Alcohol Intoxication Frequency
The AE model was the initial and final best-fitting model for the association between BU-alcohol intoxication frequency in girls. This model estimated ra = .38 (95% CI: .20; .60) and re = −.10 (95% CI: −.40; .23). Thus, alcohol intoxication accounted for 14% of the total heritability and less than 1% of the total individual-specific environmental effects for BU in girls.
For boys, the full ACE model was the initial best-fitting model and an ACE model without unique common environmental effects for BU was the final best-fitting model. This indicates that all of the common environmental effects for BU were attributable to alcohol intoxication frequency in boys. In regard to familial overlap, common environmental factors were more important for the covariance between BU-alcohol intoxication frequency compared with genetic effects such that ra = −.15 (95% CI: −1.00; .85), rc = 1.00 (95% CI: 1.00; 1.00), and re = .42 (95% CI: .04; .61). Specifically, alcohol intoxication frequency accounted for 100% of the common environmental effects for BU whereas only 2% of the total heritability for BU was accounted for by alcohol intoxication frequency. However, the genetic correlation confidence interval did include zero, so these estimates may be imprecise. Finally, 17% of the total individual-specific environmental effects for BU were accounted for by alcohol intoxication frequency.
Discussion
We examined the phenotypic and familial (i.e., genetic or common environment) association between bulimic symptoms and alcohol involvement in adolescent girls and boys, a population that has been largely overlooked in this literature. To date, no study has examined the familial covariance between these phenotypes during adolescence using a twin study design, despite the fact that developmental differences exist in the etiology of each independently and a prospective relationship exists between the two. In general, our results corroborate the adult female literature, which indicates a moderate phenotypic and genetic association between bulimic symptomatology and problematic alcohol involvement (Holderness, Brooks-Gunn, & Warren, 1994; Munn-Chernoff & Baker, 2016). However, there do appear to be some sex- and symptom- specific phenotypic and familial associations during adolescence depending on which level of alcohol involvement is examined.
Indeed, although a majority of the observed phenotypic correlations between bulimic symptoms and alcohol involvement were similar in girls and boys, the correlation between BU-having ever been intoxicated was substantial and significantly greater in boys compared with girls. In contrast, bulimic symptoms showed moderately similar correlations with all levels of alcohol involvement in girls. This suggests that the association between bulimic symptoms and alcohol involvement may be more stable and generalized across differing levels of alcohol involvement in girls. For boys however, this association appears to be strongest and most pronounced with the initiation of more risky/deviant levels of alcohol involvement. This aligns with a previous study examining the association between multiple eating disorder symptoms and binge drinking in adolescent girls and boys (Stickley, et al., 2015). Binge drinking was significantly associated with a majority of the eating disorder symptoms assessed in girls. However, for boys, a significant association was only observed between binge drinking and purging behaviors and feeling overweight.
Confirming findings in the adult female literature (Baker, et al., 2010), we also observed moderate genetic associations between bulimic symptoms and alcohol involvement in adolescent girls. Indeed, in adult females moderate overlap in genetic factors between BN and an alcohol use disorder (Baker, et al., 2010) as well as between alcohol dependence and problematic alcohol use and binge eating and compensatory behaviors (Munn-Chernoff, et al., 2013; Munn-Chernoff et al., 2015; Slane, Burt, & Klump, 2012) has been observed. Taken together, this suggests that genetic factors not only play a role in the co-aggregation of BN and alcohol use disorder diagnoses in adults, similar heritable traits play a role in the co-aggregation at the symptom-level in both adults and adolescents. Specifically, alcohol involvement accounted for up to 57% of the heritability of bulimic symptoms for adolescent girls.
In contrast to the adult female literature, we did observe a symptom specific pattern of familial overlap for BU-having ever been intoxicated such that genetic and common environmental overlap were both important. Although the common environmental effects were small, all of these effects for bulimic symptoms in girls were entirely accounted for by having ever been intoxicated. Interestingly, not only was significant overlap in common environmental factors observed for BU-having ever been intoxicated, this correlation was negative indicating an inverse association. This is a different pattern of familial overlap than has been observed previously and it is currently unclear what common environmental factors can increase the risk for the initiation of alcohol intoxication while decreasing the risk for BU. In comparison to the other levels of alcohol involvement, shared familial overlap (both genetic and common environment) was also greatest between bulimic symptoms and having ever been intoxicated. This provides further support for the hypothesis that the familial/genetic association between eating disorders and alcohol use disorders may be more pronounced and salient with specific symptoms (Munn-Chernoff & Baker, 2016).
In regards to the familial overlap between bulimic symptoms and alcohol involvement in boys, alcohol involvement only accounted for a small portion of the genetic effects for bulimic symptoms ranging from 0% to 7%. In contrast, common environment played a more important and significant role in covariance for the risky/deviant levels of alcohol involvement. Specifically, for having ever been intoxicated and intoxication frequency, common environment was the familial factor contributing most to the covariance, accounting for 14% and 100% of the common environmental effects for bulimic symptoms, respectively. This suggests differential patterns of familial co-aggregation for bulimic symptoms and varying levels of alcohol involvement for adolescent boys. Specifically, common environmental covariance appears important for the initiation and engagement in more risky/deviant levels of alcohol involvement whereas genetic factors play a small but significant role in covariance for less risky/deviant levels of use.
Finally, previous research has suggested a developmental trajectory for the genetic and common environmental factors for eating disorder symptoms in girls and alcohol involvement in girls and boys, whereby common environment is important at younger ages and decreases in importance with age, beginning around adolescence (Baker, Maes, Larsson, Lichtenstein, & Kendler, 2011; Fairweather-Schmidt & Wade, 2015; Geels et al., 2012; Klump, Burt, McGue, & Iacono, 2007). Although the age of our sample is around the time genetic and common environmental factors tend to alter in importance, which is likely reflected by the univariate estimates, our findings indicate a potential developmental trajectory for covariance. This is specifically true for having ever been intoxicated in girls and more risky/deviant levels of alcohol involvement in boys such that common environmental effects may be important for covariance during adolescence, which has not been observed in adults. However, because our study was not longitudinal, we are unable to directly examine a developmental trajectory for covariance.
It is unknown what genetic and common environmental factors may account for the familial covariance between bulimic symptoms and alcohol involvement during adolescence. However, previous studies examining risk factors and correlates of eating disorders (and related traits) and alcohol use disorders (and related traits) independently provide interesting candidates. In regard to the overlap in genetic effects observed for a majority of the bivariate associations, these effects may be mediated through a third variable such as a personality characteristic or additional psychopathology. In other words, the observed shared genetic factors may be attributable to the genetic factors responsible for a third variable, not assessed in this study.
For example, the negative urgency component of impulsivity has shown strong associations with both bulimic-related symptoms (Fischer, Smith, & Cyders, 2008; Racine et al., 2013) and alcohol use (Coskunpinar, Dir, & Cyders, 2013; Stautz & Cooper, 2013). Adolescents who are more likely to engage in rash behaviors under distress may be more likely to engage in bulimic-related behaviors and/or alcohol use. To date, only one study has explored this possibility and no effect of a dysregulated personality cluster (characterized by behavioral disinhibition and emotional dysregulation) on the shared genetic effects between BN symptoms and alcohol use disorder symptoms were observed (Slane, et al., 2014a). However, an effect could be found for other personality characteristics, such as negative urgency, especially given that a substantial genetic correlation has been observed between negative urgency and binge eating (Racine, et al., 2013). These shared genetic factors may also serve as the start of the cascade of the acquired preparedness model—genes may predispose an individual to a certain trait such as negative urgency which, directly or indirectly, lead to risky behaviors such as disordered eating and alcohol use.
In regard to common environmental factors that may account for the familial overlap between bulimic symptoms and having ever been intoxicated and intoxication frequency in boys, childhood experiences may play a significant role. For example, parental depression is significantly associated with the development of an eating disorder (Bould et al., 2015; Jacobi, Hayward, de Zwaan, Kraemer, & Agras, 2004) and also increases risk for a substance use disorder in adolescents (Lieb, Isensee, Hofler, Pfister, & Wittchen, 2002; Tully, Iacono, & McGue, 2008). Parental substance use problems are also a well-established risk factor for offspring alcohol involvement (Cranford, Zucker, Jester, Puttler, & Fitzgerald, 2010; Hawkins, Catalano, & Miller, 1992) and increase risk for an eating disorder (Jacobi, et al., 2004). Indeed, children who report parental misuse of alcohol have higher rates of binge eating, use of compensatory behaviors, weight dissatisfaction, higher frequencies of dieting as well as demonstrate increased rates of eating pathology in general (Chandy, Harris, Blum, & Resnick, 1994). Thus, parental mental illness, such as depression or substance abuse, may increase offspring risk for bulimic symptoms, alcohol problems, or both.
Parental monitoring and parental attitudes toward substance use may also play a role. Parental supervision and monitoring are protective against adolescent substance use (Hawkins, et al., 1992; Thompson, Roemer, & Leadbeater, 2015) whereas parental attitudes toward substance use account for a proportion of the common environmental variance for male offspring alcohol use (Baker, Maes, & Kendler, 2012). Adolescent boys who receive less parental supervision and/or whom have parents with more relaxed attitudes about substance use may be more likely to initiate and engage in more risky/deviant levels of alcohol involvement (i.e., intoxication) as well as to engage in other risky behaviors, such as bulimic symptoms, increasing the risk for both.
Although we cannot say for certain whether the differences observed in common environmental covariance between girls and boys are statistically significant, the fact that the genetic influences on certain aspects of alcohol involvement may emerge in girls earlier than boys could play a role in these differences (Meyers et al., 2014; Rhee et al., 2003). In other words, if common environmental effects are not important for alcohol involvement (and/or bulimic symptoms) independently for girls, they cannot influence covariance. Further, parental mental illness, low parental monitoring, and/or parental attitudes towards substance use taken together with the fact adolescent boys tend to exhibit more engagement in risky behaviors compared with adolescent girls (Ellis et al., 2012) may, in part, explain why common environmental covariance was observed for boys but not girls. Adolescent boys could be more apt to use limited parental monitoring/engagement as a window to engage in risky behaviors. Importantly however, these environments themselves can be partially influenced by genetic effects and thus, could also reflect a genotype-environment correlation (i.e., tendency for individuals to experience environments that are correlated with their genotype) (Kendler & Baker, 2007).
The results of this study must be considered within the context of its limitations. First, our sample size is modest—although not insignificant for a twin study—impacting the associated statistical power. As previously mentioned, extremely large sample sizes are needed to statistically detect sex differences in twin models (Prescott & Gottesman, 1993; Verhulst, 2016). Although our findings suggest there may be potential differential patterns of familial co-aggregation for bulimic symptoms and alcohol involvement between girls and boys, we did not have the power to test whether these patterns are statistically different so direct comparisons between female and male results should not be made.
Second, the behaviors were relatively low base-rate traits. Combined with the associated sample size, this may have influenced the precision of the estimates and percentage of overlapping covariance obtained, the wide confidence intervals, and the ability to detect both significant genetic and common environmental effects in the final, best-fit model. For example, for BU-having ever been intoxicated in boys we were able to entirely drop A from the model yet for the other bivariate associations genetic effects were important. This is likely because of the substantial common environmental effects present for having ever been intoxicated—providing the power necessary in this model to detect common environment (Visscher, Gordon, & Neale, 2008). However, our sample size may not have been large enough to significantly detect both A and C in the final model (Neale, Eaves, & Kendler, 1994). Often with twin models the power to detect common environment is limited and thus C is routinely dropped from models in favor of an AE model (Neale, et al., 1994; Visscher, et al., 2008). Because having ever been intoxicated was substantially influenced by common environment—corroborating previous studies (Edwards, Larsson, Lichtenstein, & Kendler, 2011; Viken, Kaprio, Koskenvuo, & Rose, 1999)—our modeling showed the opposite pattern. Nonetheless, here our goal is to identify shared familial risk between bulimic symptoms and alcohol involvement, not obtain univariate estimates of the genetic and environmental effects for bulimic symptoms and alcohol involvement, which has been explored numerous times. Therefore, the covariance patterns observed in the models are expected to be accurate.
Third, we were somewhat limited by the behaviors queried and assessment measures used in TCHAD and thus we were not able to make comparisons across more varied or “traditional” levels of alcohol involvement (e.g., age at first use, frequency of use). However, it is not possible to capture information on all types of behaviors in large scale twin studies, which often query about a wide variety of topics, in order to decrease participant burden. Additionally, because our alcohol involvement data were categorical, we had to categorize the BU subscale. However, mean scores for BU were on par with other community samples (Nevonen, et al., 2006; Slane, Klump, McGue, & Iacono, 2014b; Stice, Burton, & Shaw, 2004), and therefore the distribution of our categories is expected to be similar to the distribution of previous studies (e.g., the percentage of participants scoring a zero). Similarly, alcohol involvement rates were in-line with rates of involvement for 16-year-old Swedish adolescents around the time of Wave 3 TCHAD data collection such that 46% of Swedish girls and 52% of Swedish boys reported alcohol use in the last month and 62% of girls and boys reported lifetime alcohol intoxication (ESPAD, 2003). However, rates were less than the lifetime prevalence of alcohol use and intoxication observed for 10th graders in the United States (Johnston, O’Malley, & Bachman, 2002) near the TCHAD data collection timeframe—which may impact the generalizability of our results. Finally, because we do not have longitudinal information on both alcohol involvement and bulimic symptoms, we were only able to examine a single snapshot of development. We were not able to directly assess whether the familial covariance between bulimic symptoms and alcohol involvement changes across differing developmental periods/age groups.
Our results suggest a common familial diathesis between bulimic symptoms and alcohol involvement during adolescence. It appears that genetic factors play an important role in this familial diathesis for girls across all of these associations and common environmental factors play an important role for boys in more risky/deviant levels of use. However, future research is needed to determine whether these sex differences in covariance are statistically different from one another. Given the prospective association between bulimic symptoms and alcohol involvement, these findings could inform symptom- and sex- specific, developmentally-targeted prevention and intervention programs for the comorbidity between BN and bulimic symptoms and alcohol involvement. Future studies should additionally focus on identifying the mechanisms underlying familial relatedness, as well as possible differences in covariance across varying age groups including younger and older individuals.
General Scientific Summary.
This study shows that family factors play a role in why bulimic symptoms and alcohol involvement are associated in adolescent girls and boys. Genetic factors appear to play an important role in this association for girls. Family environmental factors influence the association between bulimic symptoms and alcohol intoxication in adolescent boys.
Acknowledgments
Dr. Baker was supported by grant NIH K01MH106675.
Footnotes
An E model, which estimates only individual-specific environmental variance and covariance, was not fit as, based on numerous published studies, there is substantial empirical evidence that this is a poor fitting model for bulimic symptoms, alcohol involvement, and their covariance. There is also substantial evidence for the importance of genetic and/or common environmental effects for the variance and/or covariance of bulimic symptoms, alcohol involvement, and their covariance.
Contributor Information
Jessica H. Baker, Department of Psychiatry, University of North Carolina
Melissa A. Munn-Chernoff, Department of Psychiatry, University of North Carolina
Paul Lichtenstein, Department of Medical Epidemiology and Biostatistics, Karolinska Institutet.
Henrik Larsson, Department of Medical Epidemiology and Biostatistics, Karolinska Institutet and Department of Medical Science Örebro University.
Hermine Maes, Department of Genetics, Virginia Commonwealth University.
Kenneth S. Kendler, Department of Psychiatry, Virginia Commonwealth University
References
- Akaike H. Factor analysis and aic. Psychometrika. 1987;52:317–332. [Google Scholar]
- Baker JH, Maes HH, Kendler KS. Shared environmental contributions to substance use. Behavior Genetics. 2012;42:345–353. doi: 10.1007/s10519-011-9516-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JH, Maes HH, Larsson H, Lichtenstein P, Kendler KS. Sex differences and developmental stability in genetic and environmental influences on psychoactive substance consumption from early adolescence to young adulthood. Psychological Medicine. 2011;41:1907–1916. doi: 10.1017/S003329171000259X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JH, Maes HH, Lissner L, Aggen SH, Lichtenstein P, Kendler KS. Genetic risk factors for disordered eating in adolescent males and females. Journal of Abnormal Psychology. 2009;118:576–586. doi: 10.1037/a0016314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JH, Mitchell KS, Neale MC, Kendler KS. Eating disorder symptomatology and substance use disorders: prevalence and shared risk in a population based twin sample. International Journal of Eating Disorders. 2010;43:648–658. doi: 10.1002/eat.20856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bould H, Koupil I, Dalman C, DeStavola B, Lewis G, Magnusson C. Parental mental illness and eating disorders in offspring. International Journal of Eating Disorder. 2015;48:383–391. doi: 10.1002/eat.22325. [DOI] [PubMed] [Google Scholar]
- Chandy J, Harris L, Blum R, Resnick M. Disordered eating among adolescents whose parents misues alcohol: Protective and risk factors. International Journal of Addictions. 1994;29:505–516. doi: 10.3109/10826089409047396. [DOI] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychological Bulletin. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Coskunpinar A, Dir AL, Cyders MA. Multidimensionality in impulsivity and alcohol use: a meta-analysis using the UPPS model of impulsivity. Alcoholism: Clinincal and Experimental Research. 2013;37:1441–1450. doi: 10.1111/acer.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cranford JA, Zucker RA, Jester JM, Puttler LI, Fitzgerald HE. Parental alcohol involvement and adolescent alcohol expectancies predict alcohol involvement in male adolescents. Psychology of Addictive Behavior. 2010;24:386–396. doi: 10.1037/a0019801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Meyers JL, Rose RJ, Kaprio J, Kendler KS. Measures of current alcohol consumption and problems: two independent twin studies suggest a complex genetic architecture. Alcoholism: Clinincal and Experimental Research. 2011;35:2152–2161. doi: 10.1111/j.1530-0277.2011.01564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn EC, Larimer ME, Neighbors C. Alcohol and drug-related negative consequences in college students with bulimia nervosa and binge eating disorder. International Journal of Eating Disorders. 2002;32:171–178. doi: 10.1002/eat.10075. [DOI] [PubMed] [Google Scholar]
- Edwards AC, Larsson H, Lichtenstein P, Kendler KS. Early environmental influences contribute to covariation between internalizing symptoms and alcohol intoxication frequency across adolescence. Addictive Behaviors. 2011;36:175–182. doi: 10.1016/j.addbeh.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards AC, Maes HH, Pedersen NL, Kendler KS. A population-based twin study of the genetic and environmental relationship of major depression, regular tobacco use and nicotine dependence. Psychological Medicine. 2011;41:395–405. doi: 10.1017/S0033291710000589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichen DM, Conner BT, Daly BP, Fauber RL. Weight perception, substance use, and disordered eating behaviors: comparing normal weight and overweight high-school students. Journal of Youth and Adolescence. 2012;41:1–13. doi: 10.1007/s10964-010-9612-8. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Del Giudice M, Dishion TJ, Figueredo AJ, Gray P, Griskevicius V, Wilson DS. The evolutionary basis of risky adolescent behavior: implications for science, policy, and practice. Developmental Psychology. 2012;48:598–623. doi: 10.1037/a0026220. [DOI] [PubMed] [Google Scholar]
- European School Survey Project on Alcohol and Other Drugs (ESPAD) The 2003 ESPAD Report. 2003 Retrieved Februrary 2 2017, 2017, from http://www.dldocs.stir.ac.uk/documents/espad2003.pdf.
- Fairweather-Schmidt AK, Wade TD. Changes in genetic and environmental influences on disordered eating between early and late adolescence: a longitudinal twin study. Psychological Medicine. 2015;45:3249–3258. doi: 10.1017/S0033291715001257. [DOI] [PubMed] [Google Scholar]
- Field AE, Sonneville KR, Crosby RD, Swanson SA, Eddy KT, Camargo CA, Jr, Micali N. Prospective associations of concerns about physique and the development of obesity, binge drinking, and drug use among adolescent boys and young adult men. JAMA Pediatrics. 2014;168:34–39. doi: 10.1001/jamapediatrics.2013.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field AE, Sonneville KR, Micali N, Crosby RD, Swanson SA, Laird NM, Horton NJ. Prospective association of common eating disorders and adverse outcomes. Pediatrics. 2012;130:e289–295. doi: 10.1542/peds.2011-3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer S, Settles R, Collins B, Gunn R, Smith GT. The role of negative urgency and expectancies in problem drinking and disordered eating: testing a model of comorbidity in pathological and at-risk samples. Psychology of Addictive Behaviors. 2012;26:112–123. doi: 10.1037/a0023460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer S, Smith GT, Cyders MA. Another look at impulsivity: a meta-analytic review comparing specific dispositions to rash action in their relationship to bulimic symptoms. Clinical Psychology Review. 2008;28:1413–1425. doi: 10.1016/j.cpr.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadalla T, Piran N. Co-occurrence of eating disorders and alcohol use disorders in women: a meta analysis. Archives of Womens Mental Health. 2007;10:133–140. doi: 10.1007/s00737-007-0184-x. [DOI] [PubMed] [Google Scholar]
- Garner D. Eating Disorders Inventory-2: Professional Manual. Odessa, FL: Psychological Assessment Resources, Inc; 1991. [Google Scholar]
- Geels LM, Bartels M, van Beijsterveldt TC, Willemsen G, van der Aa N, Boomsma DI, Vink JM. Trends in adolescent alcohol use: effects of age, sex and cohort on prevalence and heritability. Addiction. 2012;107:518–527. doi: 10.1111/j.1360-0443.2011.03612.x. [DOI] [PubMed] [Google Scholar]
- Grilo CM, Sinha R, O’Malley SS. Eating disorders and alcohol use disorders. NIAAA Publications. 2002 Retrieved July 18, 2016, from http://pubs.niaaa.nih.gov/publications/arh26-2/151-160.htm.
- Harrop EN, Marlatt GA. The comorbidity of substance use disorders and eating disorders in women: prevalence, etiology, and treatment. Addictive Behaviors. 2010;35:392–398. doi: 10.1016/j.addbeh.2009.12.06. [DOI] [PubMed] [Google Scholar]
- Hawkins JD, Catalano RF, Miller JY. Risk and protective factors for alcohol and other drug problems in adolescence and early adulthood: implications for substance abuse prevention. Psychological Bulletin. 1992;112:64–105. doi: 10.1037/0033-2909.112.1.64. [DOI] [PubMed] [Google Scholar]
- Holderness C, Brooks-Gunn J, Warren M. Co-morbidity of eating disorders and substance abuse. Review of the literature. International Journal of Eating Disorders. 1994;16:1–35. doi: 10.1002/1098-108x(199407)16:1<1::aid-eat2260160102>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Jacobi C, Hayward C, de Zwaan M, Kraemer H, Agras W. Coming to terms with risk factors for eating disorders: application of risk terminology and suggestions for a general taxonomy. Psychological Bulletin. 2004;130:19–65. doi: 10.1037/0033-2909.130.1.19. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG. Monitoring the Future national survey results on adolescent drug use: Overview of key findings, 2001. 2002 Retrieved July 29, 2016, 2016, from http://www.monitoringthefuture.org/pubs/monographs/overview2001.pdf.
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future National Results on Adolescent Drug Use: Overview of Key Findings. 2013 Retrieved July 29, 2016 from www.monitoringthefuture.org/pubs/monographs/mtf-overview2012.pdf.
- Kendler KS, Baker JH. Genetic influences on measures of the environment: a systematic review. Psychological Medicine. 2007;37:615–626. doi: 10.1017/S0033291706009524. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Schmitt E, Aggen SH, Prescott CA. Genetic and environmental influences on alcohol, caffeine, cannabis, and nicotine use from early adolescence to middle adulthood. Archives of General Psychiatry. 2008;65:674–682. doi: 10.1001/archpsyc.65.6.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Burt SA, McGue M, Iacono WG. Changes in genetic and environmental influences on disordered eating across adolescence: a longitudinal twin study. Archives of General Psychiatry. 2007;64:1409–1415. doi: 10.1001/archpsyc.64.12.1409. [DOI] [PubMed] [Google Scholar]
- Koren R, Munn-Chernoff MA, Duncan AE, Bucholz KK, Madden PA, Heath AC, Agrawal A. Is the relationship between binge eating episodes and personality attributable to genetic factors? Twin Research and Human Genetics. 2014;17:65–71. doi: 10.1017/thg.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein P, Svartengren M. Genes, environments, and sex: factors of importance in atopic diseases in 7–9-year-old Swedish twins. Allergy. 1997;52:1079–1086. doi: 10.1111/j.1398-9995.1997.tb00179.x. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P, Tuvblad C, Larsson J, Carlström E. The Swedish Twin Study of CHild and Adolescent Development: The TCHAD-Study. Twin Research and Human Genetics. 2007;10:67–73. doi: 10.1375/twin.10.1.67. [DOI] [PubMed] [Google Scholar]
- Lieb R, Isensee B, Hofler M, Pfister H, Wittchen HU. Parental major depression and the risk of depression and other mental disorders in offspring: a prospective-longitudinal community study. Archives of General Psychiatry. 2002;59:365–374. doi: 10.1001/archpsyc.59.4.365. [DOI] [PubMed] [Google Scholar]
- Meyers JL, Salvatore JE, Vuoksimaa E, Korhonen T, Pulkkinen L, Rose RJ, Dick DM. Genetic influences on alcohol use behaviors have diverging developmental trajectories: a prospective study among male and female twins. Alcoholism: Clinical and Experimental Research. 2014;38:2869–2877. doi: 10.1111/acer.12560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn-Chernoff MA, Baker JH. A primer on the genetics of comorbid eating disorders and substance use disorders. European Eating Disorders Review. 2016;24:91–100. doi: 10.1002/erv.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn-Chernoff MA, Duncan AE, Grant JD, Wade TD, Agrawal A, Bucholz KK, Heath AC. A twin study of alcohol dependence, binge eating, and compensatory behaviors. Journal of Studies on Alcohol and Drugs. 2013;74:664–673. doi: 10.15288/jsad.2013.74.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn-Chernoff MA, Grant JD, Agrawal A, Sartor CE, Werner KB, Bucholz KK, Duncan AE. Genetic overlap between alcohol use disorder and bulimic behaviors in European American and African American women. Drug and Alcohol Dependence. 2015;153:335–340. doi: 10.1016/j.drugalcdep.2015.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustelin L, Latvala A, Raevuori A, Rose RJ, Kaprio J, Keski-Rahkonen A. Risky drinking behaviors among women with eating disorders-A longitudinal community-based study. International Journal of Eating Disorders. 2016;49:563–571. doi: 10.1002/eat.22526. [DOI] [PubMed] [Google Scholar]
- Neale M. Statistical Modelling with Mx. Richmond, VA: Box 980710, Richmond VA 23298: Department of Psychiatry; 1991. [Google Scholar]
- Neale M, Eaves L, Kendler K. The power of the classical twin study to resolve variation in threshold traits. Behavior Genetics. 1994;24:239–258. doi: 10.1007/BF01067191. [DOI] [PubMed] [Google Scholar]
- Nevonen L, Clinton D, Norring C. Validating the EDI-2 in three Swedish female samples: eating disorder patients psychiatric outpatients and normal controls. Nordic Journal of Psychiatry. 2006;60:44–50. doi: 10.1080/08039480500504537. [DOI] [PubMed] [Google Scholar]
- Norring C, Sohlberg S. Eating Disorder Inventory in Sweden: description, cross-cultural comparison, and clinical utility. Acta Psychiatrica Scandinavica. 1988;78:567–575. doi: 10.1111/j.1600-0447.1988.tb06386.x. [DOI] [PubMed] [Google Scholar]
- Olivardia R, Pope H, Mangweth B, Hudson J. Eating disorders in college men. American Journal of Psychiatry. 1995;152:1279–1285. doi: 10.1176/ajp.152.9.1279. [DOI] [PubMed] [Google Scholar]
- Palmer RH, Young SE, Corley RP, Hopfer CJ, Stallings MC, Hewitt JK. Stability and change of genetic and environmental effects on the common liability to alcohol, tobacco, and cannabis DSM-IV dependence symptoms. Behavior Genetics. 2013;43:374–385. doi: 10.1007/s10519-013-9599-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton GC, Coffey C, Sawyer SM. The outcome of adolescent eating disorders: findings from the Victorian Adolescent Health Cohort Study. European Child & Adolescent Psychiatry. 2003;12(Suppl 1):I25–29. doi: 10.1007/s00787-003-1104-x. [DOI] [PubMed] [Google Scholar]
- Prescott CA, Gottesman I. Power limitations in detecting heterogeneity of genetic effects: the case of sex-differences in alcoholism. Paper presented at the Society for Research on Psychopathology; Chicago, IL. 1993. [Google Scholar]
- Racine SE, Keel PK, Burt SA, Sisk CL, Neale M, Boker S, Klump KL. Exploring the relationship between negative urgency and dysregulated eating: etiologic associations and the role of negative affect. Journal of Abnormal Psychology. 2013;122:433–444. doi: 10.1037/a0031250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SH, Hewitt JK, Young SE, Corley RP, Crowley TJ, Stallings MC. Genetic and environmental influences on substance initiation, use, and problem use in adolescents. Archives of General Psychiatry. 2003;60:1256–1264. doi: 10.1001/archpsyc.60.12.1256. [DOI] [PubMed] [Google Scholar]
- Root TL, Pisetsky EM, Thornton L, Lichtenstein P, Pedersen NL, Bulik CM. Patterns of comorbidity of eating disorders and substance use in a large population-based sample of Swedish females. Psychological Medicine. 2010;40:105–115. doi: 10.1017/S0033291709005662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaumberg K, Earleywine M. Evaluating the acquired preparedness model for bulimic symptoms and problem drinking in male and female college students. Eating Behaviors. 2013;14:47–52. doi: 10.1016/j.eatbeh.2012.10.012. [DOI] [PubMed] [Google Scholar]
- Slane JD, Burt SA, Klump KL. Bulimic behaviors and alcohol use: shared genetic influences. Behavior Genetics. 2012;42:603–613. doi: 10.1007/s10519-012-9525-2. [DOI] [PubMed] [Google Scholar]
- Slane JD, Klump KL, McGue M, Iacono G. Genetic and environmental factors underlying comorbid bulimic behaviours and alcohol use disorders: a moderating role for the dysregulated personality cluster? European Eating Disorders Review. 2014a;22:159–169. doi: 10.1002/erv.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slane JD, Klump KL, McGue M, Iacono WG. Developmental trajectories of disordered eating from early adolescence to young adulthood: a longitudinal study. International Journal of Eating Disorders. 2014b;47:793–801. doi: 10.1002/eat.22329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillane NS, Boerner LM, Anderson KG, Smith GT. Comparability of the Eating Disorder Inventory-2 between women and men. Assessment. 2004;11:85–93. doi: 10.1177/1073191103260623. [DOI] [PubMed] [Google Scholar]
- Stautz K, Cooper A. Impulsivity-related personality traits and adolescent alcohol use: a meta-analytic review. Clinical Psychology Review. 2013;33:574–592. doi: 10.1016/j.cpr.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Stice E, Burton EM, Shaw H. Prospective relations between bulimic pathology, depression, and substance abuse: unpacking comorbidity in adolescent girls. Journal of Consulting and Clinical Psychology. 2004;72:62–71. doi: 10.1037/0022-006X.72.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickley A, Koyanagi A, Koposov R, McKee M, Murphy A, Ruchkin V. Binge drinking and eating problems in Russian adolescents. Alcoholism: Clinical and Experimental Research. 2015;39:540–547. doi: 10.1111/acer.12644. [DOI] [PubMed] [Google Scholar]
- Swanson SA, Crow SJ, Le Grange D, Swendsen J, Merikangas KR. Prevalence and correlates of eating disorders in adolescents: results from the national comorbidity survey replication adolescent supplement. Archives of General Psychiatry. 2011;68:714–723. doi: 10.1001/archgenpsychiatry.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson K, Roemer A, Leadbeater B. Impulsive personality, parental monitoring, and alcohol cutcomes from adolescence through young adulthood. Journal of Adolescent Health. 2015;57:320–326. doi: 10.1016/j.jadohealth.2015.05.005. [DOI] [PubMed] [Google Scholar]
- Tully EC, Iacono WG, McGue M. An adoption study of parental depression as an environmental liability for adolescent depression and childhood disruptive disorders. American Journal of Psychiatry. 2008;165:1148–1154. doi: 10.1176/appi.ajp.2008.07091438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhulst B. A Power Calculator for the Classical Twin Design. Behavior Genetics. 2016 doi: 10.1007/s10519-016-9828-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viken RJ, Kaprio J, Koskenvuo M, Rose RJ. Longitudinal analyses of the determinants of drinking and of drinking to intoxication in adolescent twins. Behavior Genetics. 1999;29:455–461. doi: 10.1023/a:1021631122461. [DOI] [PubMed] [Google Scholar]
- Visscher PM, Gordon S, Neale MC. Power of the classical twin design revisited: II detection of common environmental variance. Twin Research and Human Genetics. 2008;11:48–54. doi: 10.1375/twin.11.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Ranson K, Iacono W, McGue M. Disordered eating and substance use in an epidemiological sample: I. Associations within individuals. International Journal of Eating Disorders. 2002;31:389–403. doi: 10.1002/eat.10050. [DOI] [PubMed] [Google Scholar]


