Abstract
Spinal cord injury (SCI) is often accompanied by motor, vegetative and sensitive dysfunctions that can significantly decrease the chance of the complete recovery of the patients. The pathophysiological implication of these dysfunctions is represented by the increased production of the reactive species that are extremely aggressive to the surrounding tissue. The combination of massive production of free radicals, low concentration of antioxidants and the hypermetabolism present in patients with SCI leads to enhancement of the oxidative stress.
Current studies are focused on several biological active compounds that are able to reduce the effects of free radicals – tissue necrosis, inflammation, infection, apoptosis. In this paper, the mechanism of the action of several biological active compounds that are able to significantly reduce oxidative stress in critical patients with spinal cord injury is presented.
Keywords: oxidative stress, spinal cord injury, antioxidants, free radicals, critically ill patient
Rezumat
Leziunile măduvei spinării sunt adesea acompaniate de disfuncţii motorii, vegetative şi senzitive care conduc la scăderea semnificativă a şanselor de recuperare a pacienţilor cu traume severe. Implicaţiile fiziopatologice sunt reprezentate de biosinteza speciilor reactive cu efect extrem de agresiv asupra ţesutului din jur. Coroborarea producţiei masive de radicali liberi cu scăderea concentraţiei de antioxidanţi şi hipermetabolismul prezent la pacienţii critici conduce la instalarea stresului oxidativ.
Cercetarea actuală se concentrează asupra acţiunii unei serii de compuşi biologic activi capabili să reducă efectele radicalilor liberi asupra necrozei tisulare, inflamaţiilor sau apoptozei celulare. În această lucrare de actualizare este prezentat mecanismul de acţiune a compuşilor biologic activi capabili să reducă semnificativ stresul oxidativ la pacienţii critici cu leziuni medulare.
Introduction
Spinal cord injury (SCI) represents an aggression to the spinal cord resulting in a change, either temporary or permanent, of the cord’s normal motor, sensory, or autonomic function. Patients with spinal cord injury usually have permanent and often devastating neurologic deficits and disability [1]. The early management of a patient with an acute spinal cord injury is one of the most difficult tasks in trauma cases and the outcome depends upon the accuracy, adequacy, and speed of first aid management, diagnosis, and treatment within the first few hours.
Secondary lesions in severe spinal cord injuries are mainly due to the inflammatory cascade activation and overproduction of free radicals [2]. A complex cascade of pathophysiologic events related to free radicals, vasogenic edema, and altered blood flow accounts for clinical deterioration and poor overall outcome [3–5].
Although the etiology and pathogenesis of spinal cord injury remain to be fully understood, it has been suggested that reactive oxygen species and oxidative stress have a significant role in the pathophysiology of spinal cord injury. Hypermetabolism, physiological and metabolic imbalances, multiple organ dysfunction or generalized infection and inflammation lead to the increased synthesis of reactive species. Subsequently an aggressive oxidative stress follows which, combined with spinal cord injury, reduces the chance of survival considerably.
Biochemical and physiological aspects of oxidative stress in spinal cord injury
Reactive species significantly aggravate the clinical status of patients with SCI. These are represented by superoxide anion, hydrogen peroxide, hydroxyl radical, peroxynitrite, nitric oxide, lipid peroxyl and lipid alkoxyl radicals (Table 1) [6–9]. The most important modulator of oxidative stress is tripeptide glutathione (Glu-Cys-Gly) [10].
Table 1.
The characteristics of the free radicals
| Reactive species | Chemical structure | Synthesis / pathways |
|---|---|---|
| Hydroxyl Radical | HO | Fentan reaction The transformation of hydrogen peroxide by the action of iron ions Mitochondrial damage – leakage of electrons |
| Superoxide Anion | O2− | Xanthine activity Mitochondrial dysfunction Endoplasmic reticulum dysfunction Activation of lipoxygenase Activation of cyclooxygenase |
| Oxide Anion | O2− | Mitochondrial dysfunction Respiratory chain Denaturation of myoglobin, haemoglobin and cytochrome |
| Hydrogen Peroxide | H2O2 | Biochemical reactions catalyzed by superoxide dismutase |
| Nitric Oxide | NO | Biological and biochemical activity of endothelial cells Inhibition of cell surface receptors |
| Peroxynitrite | ONOO | The reaction between nitric oxide and superoxide anion |
| Lipid Peroxyl | LOO | The action of iron ions on lipid hydroperoxide The action of superoxide anion on cell membranes |
| Lipid Alkoxyl | LO | The action of iron ion on lipid peroxyl |
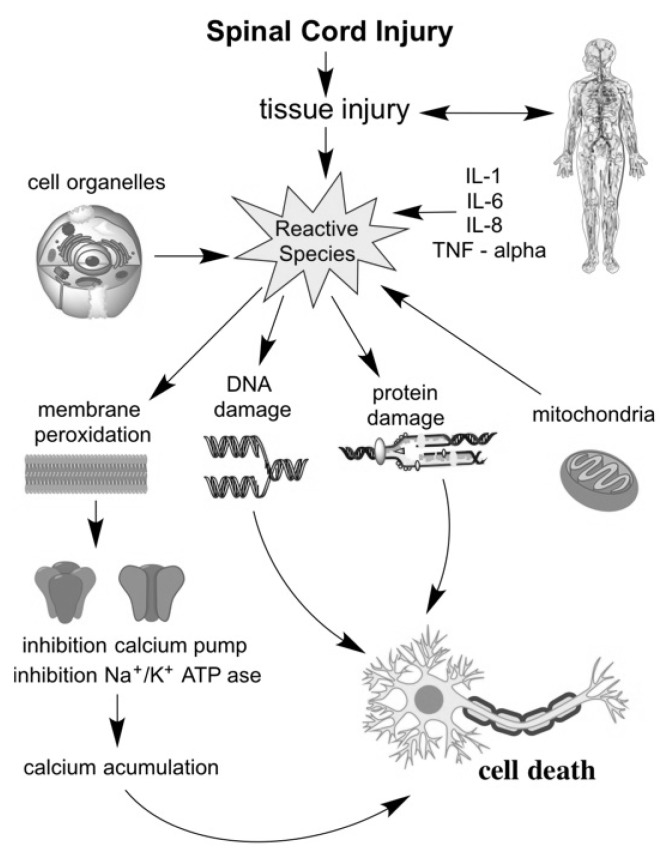
The pathophysiological consequence of oxidative stress is due to the interaction of the reactive species with lipids, proteins, DNA and other vital macromolecules (Figure 1).
Fig. 1.
Oxidative stress action in spinal cord injury
Reactive species have the ability to alter cell functions by blocking ion channels - calcium pump and Na + / K + ATPase [11], followed by an excessive accumulation of intracellular calcium ions and apoptosis [6, 12]. Neutrophils produce extremely potent modulators of inflammation. Proteases, elastases, pro inflammatory cytokines or myeloperoxidases are just some of the factors responsible for severe tissue inflammation [13, 14].
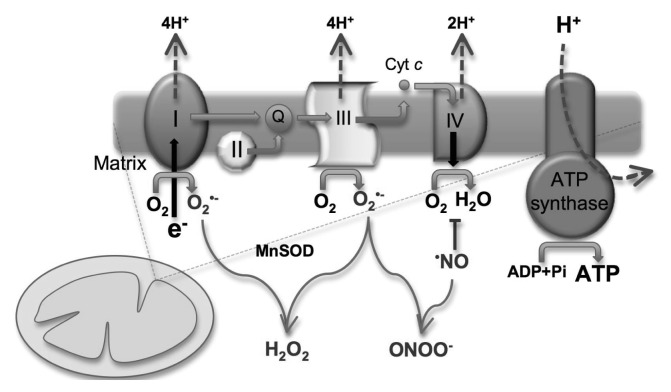
An increasing number of studies have concluded that mitochondria is responsible for the accelerated production of a significant number of reactive species, probably due to alterations in redox potential. The impaired electron transport chain leads to mitochondrial dysfunction, which furthermore blocks specific ion channels and inhibits specialized components in electron transport. Thus, highly reactive free radicals are produced. The most important step responsible for producing major quantities of superoxide radicals is represented by the transfer of electrons between channels I and III [8, 15–18] (Figure 2).
Fig. 2.
Biochemical pathway for the production of free radicals in the respiratory chain ([16] with Elsevier Agreement)
The imbalanced ratio of oxidant compounds and antioxidants leads to a severe tissue aggression, with severe consequences. Tissue destruction is produced by cell membrane injury, edema, axonal destructions, inflammation, hyperbiosynthesis of highly reactive free radicals, accumulation of calcium in cells, injury caused by ischemia – reperfusion, endothelial injury or poor vascularization caused by blood vessel damage and hypothension. It has been demonstrated that the level of reactive species increases sharply in about an hour after the spinal cord injury [6].
Reperfusion of ischemic tissue is often associated with microvascular dysfunction, enhanced fluid filtration and leukocyte plugging in capillaries. Activated endothelial cells in all segments of the microcirculation produce more oxygen radicals, but less nitric oxide, in the initial period following reperfusion. The resulting imbalance between superoxide and nitric oxide in endothelial cells leads to the production and release of inflammatory mediators and enhances the biosynthesis of adhesion molecules that mediate leukocyte-endothelial cell adhesion [19–23]. Moreover, reactive species induce mitochondrial dysfunctions [21], activate neuronal cell cyclooxygenase [24] and produce electrolyte imbalances at the cellular level [25].
Only cerebral endothelial cells are able to fight oxidative stress at a higher capacity than other tissues due to their higher concentrations of antioxidant enzymes [26–29].
A series of methods for monitoring and assessing the level of oxidative stress in the body have been proposed. Biochemical markers identified in this regard are: glutathione-S-transferase (GST), glutathione-peroxidase (GPx), glutathione level (GSH), catalase (CAT), superoxide dismutase-(SOD), malonildial-dehyde (MDA) and total reactive antioxidant potential [30–32].
In addition, other conditions in the Intensive Care Unit (ICU) also increase the level of oxidative stress [33, 34]. Petronilho et al. have shown that sepsis is an extremely important factor in emphasizing side effects of oxidative stress [35]. Traumatic injuries resulting with complex surgery, general anaesthesia or long term/excessively administration of certain drugs in intensive therapy also produce high quantities of free radicals [9, 36, 37].
Methods of reducing oxidative stress
At the moment there are a lot of alternative treatments investigated in this respect - stem cell therapy, regulation of inflammatory response, Swann cell transplantation or administration of biologically active compounds that seem to be able to reduce oxidative stress [38, 39].
Antioxidants are reducing agents, participating in the redox reactions. Thus, they have the ability to protect cells from lesions induced by free radicals. Glutathione peroxidase, catalase, thioredoxin reductase and superoxide dismutase [40] represent endogenous antioxidants which in case of severe trauma are outnumbered by free radicals [41–43].
There are many studies that recommend administration of methylprednisolone in spine injuries [44]. Laboratory studies indicate that methylprednisolone inhibits lipid peroxidation chain reactions by blocking the production of lipid peroxyl radicals, the antioxidant action of methylprednisolone being enhanced by the inhibition of nuclear factor kappa B (NFκB) [45, 46].
Kamence et al. [44] demonstrated that administration of cysteine prodrug L-2-4-carboxylate oxothiozolidine has remarkable effects in reducing the level of free radicals in patients with spinal cord injury.
Vitamin E (α-tocopherol), a lipid-soluble antioxidant is also important for neural tissue integrity. Increased plasma concentrations of α-tocopherol leads to suppression of prostaglandin E2 production, which is responsible for severe inflammation. Morsy et al. showed that administration of 600 mg α-tocopherol, twice a week for 6 weeks, to laboratory mice with spinal cord injury significantly reduced plasma levels of free radicals [23].
Biochemically, guanosine is a nucleotide and is composed of a guanine attached to a ribose [47]. Recent studies suggest that guanosine is responsible for stopping the action of oxidative stress and stimulating cell proliferation. Moreover, it participates in the biosynthesis of a number of factors with neuroprotective effect – neuron growth factor, fibroblast growth factor and transforming growth factor. Jiang et al. confirmed the beneficial action of guanosine, reporting that administration of this compound stimulates oligodendrocytes, which significantly contributes to remyelination [48]. Dal-Cim et al. and also Pesch et al. confirmed the beneficial effects of guanosine [49, 50] reporting a significant decrease in oxidative stress induced by inflammation, destruction of mitochondria or endoplasmic reticulum [51].
Another compound used in order to reduce oxidative stress is montelukast. Biochemically and pharmacologically, this active compound is an antagonist of cysteinyl leukotriene receptors (CysLT) [52]. Currently in clinical practice it is used for its anti-inflammatory and bronchodilator effects. Cavus et al. demonstrated that the administration of montelukast in severe spinal cord injury significantly decreased the level of tissue injury produced by free radicals. Histopathological and biochemical studies have shown that tissues presented less inflammation markers and the pro-inflammatory mediators were found in lower concentrations as compared to the control groups when montelukast was administered [53]. Gokhan et al. also pointed out that the administration of montelukast significantly reduced the injuries produced by free radicals, tissue inflammation and ischemia-reperfusion on spinal cord injury in rats [54].
Other important antioxidant compounds are selegiline ((R) N-methyl-N-(1-phenylpropan-2yl) prop-1-yn-3-amine) [55] and edavarone (2-methyl 1-phenyl-2-one pyrazolin) [56]. Both compounds have significant neuroprotective effects by stimulating the activity of antioxidant enzymes – superoxide dismutase and catalase.
Chronidou et al. studied the effect of amifostine (S-2.3 aminopropylaminoethylphosphorothioic acid) on oxidative stress and production of free radicals [40]. The compound has remarkable protective effects on neuronal cells. Amifostine is used in clinical practice to reduce the oxidative effects produced by aggressive treatments such as chemotherapy or radiotherapy. Experimental studies have shown its neuroprotective effects in laboratory mice with spinal cord injury [40].
Docosahexaenoic acid, together with α-linolenic acid and eicosapentaenoic acid, is part of the physiological composition of cell membranes, constituting the structural composition of phospholipids. It has been reported that exogenous administration of docosahexaenoic acid increases axonal and neuronal regeneration capacity after spinal cord injury. Paterniti et al. have demonstrated its ability to modulate proinflammatory response and to decrease the oxidative stress in laboratory animals [57].
An important factor in regulating physiological neuronal tissue response is the nerve growth factor with remarkable antioxidant effects. Its mechanisms of action are based on the activation of specific receptors involved mainly in intracellular kinases pathways. Zhang et al. measured and quantified these effects on spinal cord, in rats, by the administration of exogenous nerve growth factor and showed that recovery from acute spinal cord injury was improved. They also observed an increased level of neural cells that have survived the trauma [58].
Tetramethylpyrazine is extracted from Chinese plants and possesses antioxidant properties. It blocks the accumulation of intracellular calcium and reduces the oxidative capacity of reactive species. Chen et al. showed that when administered intravenously tetramehylpyrazine alters oxidative stress [59]. Fan et al. also demonstrated the antioxidant effects of this compound in rabbits with SCI [60].
Numerous studies have shown that N-acetylcysteine has protective effects against free radicals [61] by increasing the production of glutathione peroxidase [62].
Biomacromolecules complexes that contain metal ions are also studied for their remarkable antioxidant properties [28]. Metalloporphyrin, a biologically active compound containing the manganese (III) ion, is able to block the transformation of superoxide anion to hydrogen peroxide and further to water. Liu et al. studied such a compound - Mn (III) -tetrakis- (4-benzoic acid) -porphyrin (MnTBAP), confirming its inhibitory effect on oxidative stress, in laboratory rats with spinal cord injury [18].
The extract from milk thistle Silybum Marianum, a European plant, silybin (CA No. 22888-79-6), is known for its anti-inflammatory effects [63]. Silybin has been used for a long time in liver disease management. Recent studies demonstrated its antioxidant activity in neuronal tissue damages from spinal cord injuries [64] while Perumal Vijayaraman et al. confirmed its beneficial effect in laboratory mice with spinal cord injury [65]. Cho et al. showed that it inhibited the oxidation of fatty acids and that it has anti-apoptotic and antiinflammatory effects [66]. Kumar et al. studied the effects of liposomal silymarin and concluded that this form has several advantages in fighting oxidative stress. Controlled release of the compound and the bioavailability provided by the liposomal matrix greatly increased the neuroprotective effects of the compound [63].
α-lipoic acid is known as a powerful antioxidant by blocking the activity of free radicals and studies conducted by Emmez et al. revealed its neuroprotective effects [67]. This drug also proved to be beneficial in multiple sclerosis, ischemia-reperfusion injury and peripheral nerve injury [68].
Administration of β-glucan significantly reduces oxidative aggression of free radicals and secondary lesions in SCI. Kayali et al. have demonstrated in a study on laboratory mice with spinal cord injury that its administration (250 mg/kg for 5 days) significantly decreased the level of biochemical markers specific for oxidative stress [69]. The action was confirmed by the measurement of plasma levels of malonyldialdehyde, superoxide dismutase and gluthatione peroxidase which were found significantly lower when tested in laboratory mice with spinal cord injury.
Salvianolic acid B as a biological active antioxidant extracted from Radix Salviae Miltiorrhizae showed its neuroprotective effects and confirmed the antioxidant and anti-inflammatory activity upon tissue affected by spinal cord injury [70].
Conclusion
Polytrauma often leads to damage of the spine and medulla and the repercussions arising from these lesions are devastating for patients in a critical condition. Cell physiological imbalances lead to accelerated biosynthesis of highly reactive chemical species. Current research pays more attention to this phenomenon, due to the complex pathophysiology involved. Critically ill patients with spinal cord injury are more prone to oxidative stress due to the multitude of the biological dysfunctions. There are many biologically active substances with antioxidant properties that have been studied in experimental models and have proved to be able to reduce oxidative stress, but further clinical trials are required. In conclusion, inhibition of oxidative aggression can significantly reduce the occurrence of inflammation, infection, apoptosis and thus severe organ dysfunction. Therefore, supplementation therapy with strong antioxidants may improve the outcome of these patients. Monitoring specific biomarkers for oxidative stress can guide antioxidant supplementation in patients with spinal cord injury.
Footnotes
Conflict of interest
Nothing to declare
References
- 1.Wongkornrat W, Yamamoto S, Sekine Y, Ono M, Fujikawa T, Oshima S, et al. Predictors of paraplegia with current thoraco-abdominal aortic aneurysm repair. Asian Cardiovasc Thorac Ann. 2014;0(0):1–6. doi: 10.1177/0218492314549563. [DOI] [PubMed] [Google Scholar]
- 2.Szabo TA, Warters RD, Kadry B, Stroud RE, Matthews RG, DeSantis SM, et al. The effect of general vs spinal anesthesia on the inflammatory response in orthopedic surgery. J Rom Anest Terap Int. 2012;19:13–20. [Google Scholar]
- 3.Crimi E, Taccone FS, Infante T, Scolletta S, Crudele V, Napoli C. Effects of intracellular acidosis on endothelial function: an overview. J Crit Care. 2012;27:108–118. doi: 10.1016/j.jcrc.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Weil ZM, Gaier KR, Karelina K. Injury timing alters metabolic, inflammatory and functional outcomes following repeated mild traumatic brain injury. Neurobiol Dis. 2014;70:108–116. doi: 10.1016/j.nbd.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 5.Denton M, McKinlay J. Cervical cord injury and critical care. Contin Educ Anaesth Crit Care Pain. 2009;9:82–86. [Google Scholar]
- 6.Bains M, Hall ED. Antioxidant therapies in traumatic brain and spinal cord injury. Biochim Biophis Acta. 2012;1822(5):675–684. doi: 10.1016/j.bbadis.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ling X, Bao F, Qian H, Liu D. The temporal and spatial profiles of cell loss following experimental spinal cord injury: effect of antioxidant therapy on cell death and functional recovery. BMC Neurosci. 2013;14:146. doi: 10.1186/1471-2202-14-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quinlan CL, Perevoshchikova IV, Hey-Mogensen M, Orr AL, Brand MD. Sites of reactive oxygen species generation by mitochondria oxidizing different substrates. Redox Biol. 2013;1:304–312. doi: 10.1016/j.redox.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scheibmeir HD, Christensen K, Whitaker SH, Jegaethesan J, Clancy R, Pierce JD. A review of free radicals and antioxidants for critical care nurses. Intensive Crit Care Nurs. 2005;21:24–28. doi: 10.1016/j.iccn.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Lu S, Neoh KG, Huang C, Shi Z, Kang ET. Polyacrylamide hybrid nanogels for targeted cancer chemotherapy via co-delivery of gold nanoparticles and MTX. J Colloid Interface Sci. 2013;412:46–55. doi: 10.1016/j.jcis.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 11.Egnatchik RA, Leamy AK, Jacobson DA, Shiota M, Young JD. ER calcium release promotes mitochondrial dysfunction and hepatic cell lipotoxicity in response to palmitate overload. Mol Metab. 2014;3:544–553. doi: 10.1016/j.molmet.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karajibani M, Hashemi M, Montazerifar F, Dikshit M. Effect of vitamin E and C supplements on antioxidant defense system in cardiovascular disease patients in Zahedan, southeast Iran. J Nutr Sci Vitaminol (Tokyo) 2010;56:436–440. doi: 10.3177/jnsv.56.436. [DOI] [PubMed] [Google Scholar]
- 13.Gonca E. The effects of zileuton and montelukast in reperfusion-induced arrhythmias in anesthetized rats. Curr Ther Res Clin Exp. 2013;75:27–32. doi: 10.1016/j.curtheres.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tzekou A, Fehlings MG. Treatment of spinal cord injury with intravenous immunoglobulin G: preliminary evidence and future perspectives. J Clin Immunol. 2014;34(Suppl 1):S132–138. doi: 10.1007/s10875-014-0021-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Gubory KH. Mitochondria: omega-3 in the route of mitochondrial reactive oxygen species. Int J Biochem Cell Biol. 2012;44:1569–1573. doi: 10.1016/j.biocel.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Bolaños JP, Moro MA, Lizasoain I, Almeida A. Mitochondria and reactive oxygen and nitrogen species in neurological disorders and stroke: Therapeutic implications. Adv Drug Deliv Rev. 2009;61:1299–1315. doi: 10.1016/j.addr.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Bibi H, Vinokur V, Waisman D, Elenberg Y, Landesberg A, Faingersh A, et al. Zn/Ga-DFO iron-chelating complex attenuates the inflammatory process in a mouse model of asthma. Redox Biol. 2014;2:814–819. doi: 10.1016/j.redox.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu D, Shan Y, Valluru L, Bao F. Mn (III) tetrakis (4-benzoic acid) porphyrin scavenges reactive species, reduces oxidative stress, and improves functional recovery after experimental spinal cord injury in rats: comparison with methylprednisolone. BMC Neurosci. 2013;14:23. doi: 10.1186/1471-2202-14-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olson L. Combinatory treatments needed for spinal cord injury. Exp Neurol. 2013;248:309–315. doi: 10.1016/j.expneurol.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 20.Smith PD, Puskas F, Meng X, Lee JH, Cleveland JC, Jr, Weyant MJ, et al. The evolution of chemokine release supports a bimodal mechanism of spinal cord ischemia and reperfusion injury. Circulation. 2012;126(Suppl 1):S110–117. doi: 10.1161/CIRCULATIONAHA.111.080275. [DOI] [PubMed] [Google Scholar]
- 21.Kalogeris T, Bao Y, Korthuis RJ. Mitochondrial reactive oxygen species: a double edged sword in ischemia/reperfusion vs preconditioning. Redox Biol. 2014;2:702–714. doi: 10.1016/j.redox.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kauvar DS, Baer DG, Dubick MA, Walters TJ. Effect of fluid resuscitation on acute skeletal muscle ischemia-reperfusion injury after hemorrhagic shock in rats. J Am Coll Surg. 2006;202:888–896. doi: 10.1016/j.jamcollsurg.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Morsy MD, Mostafa OA, Hassan WN. A potential protective effect of α-tocopherol on vascular complication in spinal cord reperfusion injury in rats. J Biomed Sci. 2010;17:55. doi: 10.1186/1423-0127-17-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boerma EC, Ince C. The role of vasoactive agents in the resuscitation of microvascular perfusion and tissue oxygenation in critically ill patients. Intensive Care Med. 2010;36:2004–2018. doi: 10.1007/s00134-010-1970-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahler SA, Conrad SA, Wang H, Arnold TC. Resuscitation with balanced electrolyte solution prevents hyperchloremic metabolic acidosis in patients with diabetic ketoacidosis. Am J Emerg Med. 2011;29:670–674. doi: 10.1016/j.ajem.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 26.Wu J, Hecker JG, Chiamvimonvat N. Antioxidant enzyme gene transfer for ischemic diseases. Adv Drug Deliv Rev. 2009;61:351–363. doi: 10.1016/j.addr.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freeman LR, Keller JN. Oxidative stress and cerebral endothelial cells: regulation of the blood-brain-barrier and antioxidant based interventions. Biochim Biophys Acta. 2012;1822:822–829. doi: 10.1016/j.bbadis.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vlascici D, Pruneanu S, Olenic L, Pogacean F, Ostafe V, Chiriac V, et al. Manganese(III) porphyrin-based potentiometric sensors for diclofenac assay in pharmaceutical preparations. Sensors (Basel) 2010;10:8850–8864. doi: 10.3390/s101008850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552(Pt 2):335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abdel-Wahab WM. Protective effect of thymoquinone on sodium fluoride-induced hepatotoxicity and oxidative stress in rats. J Basic Appl Zool. 2013;66:263–270. [Google Scholar]
- 31.Vargas HO, Vargas Nunes SO, de Castro MRP, Vargas MM, Barbosa DS, Bortolasci CC, et al. Oxidative stress and inflammatory markers are associated with depression and nicotine dependence. Neurosci Lett. 2013;544:136–140. doi: 10.1016/j.neulet.2013.03.059. [DOI] [PubMed] [Google Scholar]
- 32.You H, Chen S, Mao L, Li B, Yuan Y, Li R, et al. The adjuvant effect induced by di-(2-ethylhexyl) phthalate (DEHP) is mediated through oxidative stress in a mouse model of asthma. Food Chem Toxicol. 2014;71:272–281. doi: 10.1016/j.fct.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 33.Andrades M, Ritter C, de Oliveira MR, Streck EL, Fonseca Moreira JC, Dal-Pizzol F. Antioxidant treatment reverses organ failure in rat model of sepsis: role of antioxidant enzymes imbalance, neutrophil infiltration, and oxidative stress. J Surg Res. 2011;167:e307–313. doi: 10.1016/j.jss.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 34.Quoilin C, Mouithys-Mickalad A, Lécart S, Fontaine-Aupart MP, Hoebeke M. Evidence of oxidative stress and mitochondrial respiratory chain dysfunction in an in vitro model of sepsis-induced kidney injury. Biochim Biophys Acta. 2014;1837:1790–1800. doi: 10.1016/j.bbabio.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 35.Petronilho F, Périco SR, Vuolo F, Mina F, Constantino L, Comim CM, et al. Protective effects of guanosine against sepsis-induced damage in rat brain and cognitive impairment. Brain Behav Immun. 2012;26:904–910. doi: 10.1016/j.bbi.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 36.Hassler SN, Johnson KM, Hulsebosch CE. Reactive oxygen species and lipid peroxidation inhibitors reduce mechanical sensitivity in a chronic neuropathic pain model of spinal cord injury in rats. J Neurochem. 2014 doi: 10.1111/jnc.12830. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Erbas M, Demiraran Y, Ak Yildirim H, Sezen G, Iskender A, Karagoz I, et al. Comparison of effects on the oxidant/antioxidant system of sevoflurane, desflurane and propofol infusion during general anesthesia. Rev Bras Anestesiol. 2014 doi: 10.1016/j.bjan.2014.05.002. (in press) DOI: http://dx.doi.org/10.1016/j.bjane.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 38.Hall AP, Davies MJ. Diabetic emergencies in acute/critical care. Found Years. 2008;4:230–233. [Google Scholar]
- 39.Song L, Chen L, Zhang X, Li J, Le W. Resveratrol ameliorates motor neuron degeneration and improves survival in SOD1(G93A) mouse model of amyotrophic lateral sclerosis. Biomed Res Int. 2014;2014:483501. doi: 10.1155/2014/483501. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Chronidou F, Apostolakis E, Papapostolou I, Grintzalis K, Georgiou CD, Koletsis EN, et al. Beneficial effect of the oxygen free radical scavenger amifostine (WR-2721) on spinal cord ischemia / reperfusion injury in rabbits. J Cardiothorac Surg. 2009;4:50. doi: 10.1186/1749-8090-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gottfredsen RH, Larsen UG, Enghild JJ, Petersen SV. Hydrogen peroxide induce modifications of human extracellular superoxide dismutase that results in enzyme inhibition. Redox Biol. 2013;1:24–31. doi: 10.1016/j.redox.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kondo Y, Masutomi H, Noda Y, Ozawa Y, Takahashi K, Handa S, et al. Senescence marker protein-30/superoxide dismutase 1 double knockout mice exhibit increased oxidative stress and hepatic steatosis. FEBS Open Bio. 2014;4:522–532. doi: 10.1016/j.fob.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rahman I, Biswas SK, Kode A. Oxidant and antioxidant balance in the airways and airway diseases. Eur J Pharmacol. 2006;533:222–239. doi: 10.1016/j.ejphar.2005.12.087. [DOI] [PubMed] [Google Scholar]
- 44.Kamencic H, Griebel RW, Lyon AW, Paterson PG, Juurlink BHJ. Promoting glutathione synthesis after spinal cord trauma decreases secondary damage and promotes retention of function. FASEB J. 2001;15:243–250. doi: 10.1096/fj.00-0228com. [DOI] [PubMed] [Google Scholar]
- 45.Bhalla A, Singhal M, Suri V, Malhotra S, Shafiq N, Varma S. Methylprednisolone in dengue patients with alarm signs: The MIDWAS study. Int J Infect Dis. 2014;21(Suppl 1):323. [Google Scholar]
- 46.Nonato FR, Santana DG, de Melo FM, dos Santos GGL, Brustolim D, Camargo E, et al. Anti-inflammatory properties of rose oxide. Int Immunopharmacol. 2012;14:779–784. doi: 10.1016/j.intimp.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 47.Pietrangelo T, Guarnieri S, Fulle S, Fanň G, Mariggiò MA. Signal transduction events induced by extracellular guanosine 5′ triphosphate in excitable cells. Purinergic Signal. 2006;2:633–636. doi: 10.1007/s11302-006-9021-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang S, Ballerini P, Buccella S, Giuliani P, Jiang C, Huang X, et al. Remyelination after chronic spinal cord injury is associated with proliferation of endogenous adult progenitor cells after systemic administration of guanosine. Purinergic Signal. 2008;4:61–71. doi: 10.1007/s11302-007-9093-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dal-Cim T, Molz S, Egea J, Parada E, Romero A, Budni J, et al. Guanosine protects human neuroblastoma SH-SY5Y cells against mitochondrial oxidative stress by inducing heme oxigenase-1 via PI3K/Akt/GSK-3β pathway. Neurochem Int. 2012;61:397–404. doi: 10.1016/j.neuint.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 50.Pesch B, Lotz A, Koch HM, Marczynski B, Casjens S, Käfferlein HU, et al. Oxidatively damaged guanosine in white blood cells and in urine of welders: associations with exposure to welding fumes and body iron stores. Arch Toxicol. 2014;2014 doi: 10.1007/s00204-014-1319-2. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Z, Gao L, Cheng Y, Jiang J, Chen Y, Jiang H, et al. Resveratrol, a natural antioxidant, has a protective effect on liver injury induced by inorganic arsenic exposure. Biomed Res Int. 2014;2014:617202. doi: 10.1155/2014/617202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Al Saadi MM, Meo SA, Mustafa A, Shafi A, Tuwajri AS. Effects of Montelukast on free radical production in whole blood and isolated human polymorphonuclear neutrophils (PMNs) in asthmatic children. Saudi Pharm J. 2011;19:215–220. doi: 10.1016/j.jsps.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cavus G, Altas M, Aras M, Özgür T, Serarslan Y, Yilmaz N, et al. Effects of montelukast and methylprednisolone on experimental spinal cord injury in rats. Eur Rev Med Pharmacol Sci. 2014;18:1770–1777. [PubMed] [Google Scholar]
- 54.Lafci G, Gedik HS, Korkmaz K, Erdem H, Cicek OF, Nacar OA, et al. Efficacy of iloprost and montelukast combination on spinal cord ischemia/reperfusion injury in a rat model. J Cardiothorac Surg. 2013;8:64. doi: 10.1186/1749-8090-8-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Citrome L, Goldberg JF, Portland KB. Placing transdermal selegiline for major depressive disorder into clinical context: number needed to treat, number needed to harm, and likelihood to be helped or harmed. J Affect Disord. 2013;151:409–417. doi: 10.1016/j.jad.2013.06.027. [DOI] [PubMed] [Google Scholar]
- 56.Cerón-Carrasco JP, Roy HM, Cerezo J, Jacquemin D, Laurent AD. Theoretical insights on the antioxidant activity of edaravone free radical scavengers derivatives. Chem Phys Lett. 2014;599:73–79. [Google Scholar]
- 57.Paterniti I, Impellizzeri D, Di Paola R, Esposito E, Gladman S, Yip P, et al. Docosahexaenoic acid attenuates the early inflammatory response following spinal cord injury in mice: in-vivo and in-vitro studies. J Neuroinflammation. 2014;11:6. doi: 10.1186/1742-2094-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang H, Wu F, Kong X, Yang J, Chen H, Deng L, et al. Nerve growth factor improves functional recovery by inhibiting endoplasmic reticulum stress-induced neuronal apoptosis in rats with spinal cord injury. J Transl Med. 2014;12:130. doi: 10.1186/1479-5876-12-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen S, Xiong L, Wang Q, Sang H, Zhu Z, Dong H, et al. Tetramethylpyrazine attenuates spinal cord ischemic injury due to aortic cross-clamping in rabbits. BMC Neurol. 2002;2:1. doi: 10.1186/1471-2377-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fan L-H, Wang K-Z, Cheng B, Wang C-S, Dang X-Q. Anti-apoptotic and neuroprotective effects of Tetramethylpyrazine following spinal cord ischemia in rabbits. BMC Neurosci. 2006;7:48. doi: 10.1186/1471-2202-7-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gundersen Y, Vaagenes P, Thrane I, Sterri SH, Opstad PK. N-Acetylcysteine administered as part of the immediate posttraumatic resuscitation regimen does not significantly influence initiation of inflammatory responses or subsequent endotoxin hyporesponsiveness. Resuscitation. 2005;64:377–382. doi: 10.1016/j.resuscitation.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 62.Kim EC, Meng H, Jun AS. N-Acetylcysteine increases corneal endothelial cell survival in a mouse model of Fuchs endothelial corneal dystrophy. Exp Eye Res. 2014;127:20–25. doi: 10.1016/j.exer.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kumar N, Rai A, Reddy ND, Raj PV, Jain P, Deshpande P, et al. Silymarin liposomes improves oral bioavailability of silybin besides targeting hepatocytes, and immune cells. Pharmacol Rep. 2014;66:788–798. doi: 10.1016/j.pharep.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 64.Tsai MJ, Liao JF, Lin DY, Huang MC, Liou DY, Yang HC, et al. Silymarin protects spinal cord and cortical cells against oxidative stress and lipopolysaccharide stimulation. Neurochem Int. 2010;57:867–875. doi: 10.1016/j.neuint.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 65.Perumal Vijayaraman K, Muruganantham S, Subramanian M, Shunmugiah KP, Kasi PD. Silymarin attenuates benzo(a)pyrene induced toxicity by mitigating ROS production, DNA damage and calcium mediated apoptosis in peripheral blood mononuclear cells (PBMC) Ecotoxicol Environ Saf. 2012;86:79–85. doi: 10.1016/j.ecoenv.2012.08.031. [DOI] [PubMed] [Google Scholar]
- 66.Cho SI, Lee JE, Do NY. Protective effect of silymarin against cisplatin-induced ototoxicity. Int J Pediatr Otorhinolaryngol. 2014;78:474–478. doi: 10.1016/j.ijporl.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 67.Emmez H, Yildirim Z, Kale A, Tönge M, Durdađ E, Börcek A, et al. Anti-apoptotic and neuroprotective effects of alpha-lipoic acid on spinal cord ischemia-reperfusion injury in rabbits. Acta Neurochir (Wien) 2010;152:1591–1601. doi: 10.1007/s00701-010-0703-9. [DOI] [PubMed] [Google Scholar]
- 68.Smith AR, Shenvi SV, Widlansky M, Suh JH, Hagen TM. Lipoic acid as a potential therapy for chronic diseases associated with oxidative stress. Curr Med Chem. 2004;11:1135–1146. doi: 10.2174/0929867043365387. [DOI] [PubMed] [Google Scholar]
- 69.Kayali H, Ozdag MF, Kahraman S, Aydin A, Gonul E, Sayal A, et al. The antioxidant effect of beta-Glucan on oxidative stress status in experimental spinal cord injury in rats. Neurosurg Rev. 2005;28:298–302. doi: 10.1007/s10143-005-0389-2. [DOI] [PubMed] [Google Scholar]
- 70.Fu J, Fan HB, Guo Z, Wang Z, Li XD, Li J, et al. Salvianolic acid B attenuates spinal cord ischemia-reperfusion-induced neuronal injury and andoxidative stress by activating the extracellular signal-regulated kinase pathway in rats. J Surg Res. 2014;188:222–230. doi: 10.1016/j.jss.2013.11.1118. [DOI] [PubMed] [Google Scholar]