Abstract
Severe sepsis and septic shock are associated with an inflammatory cascade that is primarily responsible for multiple organ dysfunction. To date, there are no specific treatments designed to modulate and rebalance inflammatory cytokines levels. We present a case of a 50 years old man with postoperative septic shock after undergoing cephalic pancreatectomy for a pancreatic cystic tumor. The use of a haemoadsorbtion device (CytoSorb®) in combination with continuous veno-venous haemofiltration was associated with a decrease in TNFα, IL-1β and IFNγ and an increase in IL-10 levels measured before and after two consecutive procedures. The effect of CytoSorb® on inflammatory cytokines translated into a more stable haemodynamic profile with a stable cardiac output and normalization of systemic vascular resistance index and decreased vasopressor requirements. Further prospective large clinical trials are required in order to determine the indications for CytoSorb® and to evaluate the overall outcome.
Keywords: sepsis, cytokines, CytoSorb, inflammation, haemodynamics, haemofiltration, pancreatectomy, xMAP assay
Rezumat
Răspunsul inflamator asociat sepsisului sever şi şocului septic este responsabil pentru disfuncţiile multiple de organ întâlnite în această patologie. Până în prezent, niciuna din terapiile existente nu şi-a dovedit eficienţa în modularea şi rebalansarea răspunsului imun. Prezentăm cazul unui pacient în vârstă de 50 ani cu şoc septic postoperator (duodeno-pancreatectomie cefalică pentru tumoră chistică pancreatică). Utilizarea filtrelor de hemoadsorbţie (CytoSorb®) în combinaţie cu hemofiltrarea continuă veno-venoasă a determinat scăderea valorilor plasmatice ale TNFα, IL-1β şi IFNγ şi creşterea valorilor IL-10, măsurate înainte şi după fiecare procedură. Efectele clinice ale utilizării CytoSorb® au constat în stabilitate hemodinamică: menţinerea constantă a indexului cardiac, creşterea rezistenţelor vasculare sistemice şi scăderea necesarului vasopresor. Studii prospective sunt necesare în vederea determinării indicaţiilor filtrelor de hemoadsorbţie şi efectele acestora asupra evoluţiei pacienţilor.
Introduction
Severe sepsis and septic shock have been associated with a high mortality and worst outcome among postoperative patients [1] and despite recent advances in both surgical and perioperative medical care, its incidence remains high among patients undergoing major abdominal surgery [2]. In clinical practice, such patients require both specialized intensive care unit (ICU) and surgical treatment at higher costs and for a longer period of time, making research on new specific therapies for the treatment of sepsis a priority among intensivists.
Recent studies [3–5] focused on the inflammatory cascade surrounding sepsis and ways to rebalance the inflammatory response. It is now believed that inflammation in sepsis is multimodal and starts with an intense systemic inflammatory response syndrome (SIRS) that is characterized by high levels of pro-inflammatory cytokines, especially tumor necrotizing factor alpha (TNFα) and interleukines (IL) IL-1, IL-6, that is progressively counterbalanced by the high levels of antiinflammatory cytokines, such as IL-10, a state known as MARS (mixed anti-inflammatory response syndrome) [6]. Studies focusing on different therapies have tried to modulate SIRS into a more compensated model of high anti-inflammatory and low proinflammatory cytokines (CARS – compensatory antiinflammatory response syndrome) with no therapy proving its benefits [7, 8].
Haemoadsorption columns, such as CytoSorb®, may help in treating such patients by establishing a balance between pro- and anti-inflammatory cytokines without causing immunosuppression. CytoSorb® is an extracorporeal cytokine filter that can be used in combination with different renal replacement therapies. The filter itself is made up out of a biocompatible, porous polymer bead that can remove molecules between 10–50 kD (molecular weight of most cytokines). Therefore it can remove most pro-inflammatory cytokines up to 30–50% more than standard therapy as demonstrated by Schadler et al [9].
Case presentation
We present the case of a 50 years old man who was admitted to the ICU in the fifth postoperative day, after cephalic pancreatectomy for a pancreatic cystic tumor, with hypotension, neurologic dysfunction (Glasgow Coma Scale GCS = 12 points) and lactic acidosis. On admission the patient was aphasic with a right-sided hemiparesis. An emergency head computer tomography (CT) was performed and no signs of acute stroke were found. Laboratory tests results showed elevated white blood cell count (WBC) 13560/μL, C reactive protein (CRP) of 75.5 mg/L, Procalcitonin (PCT) 0.529 Ng/ml, an elevated bilirubin level of 7.6 mg/dL and lactic acidosis (pH = 7.12, base excess – 8 mmol/L and lactate 3.5 mmol/L). Treatment was initiated promptly with fluid resuscitation, broad spectrum antibiotics (Meropenem, Linezolid) and anti-fungal drugs (Fluconazole), heparin, proton pomp inhibitors. The clinical state gradually improved over the next four days except for the neurologic deficit.
In the 5th ICU day an acute inflammatory response was noted: temperature of 40°C, CRP 390 mg/L and PCT 100 Ng/ml. Due to the alteration of the neurologic status (GCS = 5 points) and the development of Acute Respiratory Distress Syndrome (ARDS) – bilateral pulmonary opacities and PaO2/FiO2 140 (PaO2: partial pressure of arterial oxygen, FiO2: fraction of inspired oxygen) the patient was intubated and mechanically ventilated. Advanced haemodynamic monitoring (PiCCO Plus®) was installed and vasopressor support with noradrenalin at a rate of 4 mcg/kg/min was initiated for haemodynamic instability (mean arterial pressure < 60 mmHg despite adequate fluid resuscitation). After the diagnosis of septic shock (APACHE II score of 38 and SOFA score of 15) was established, renal replacement therapy (continuous veno-venous haemofiltration – CVVH) was initiated in combination with CytoSorb® (CytoSorbents Europe GmbH, Berlin, Germany) for acute kidney injury and severe lactic acidosis. Microbiological exam of the peritoneal fluid sample showed infection with Candida albicans and Klebsiella pneumoniae and antibiotics and anti-fungal drugs were administered starting the same day (Meropenem, Colistin, Fluconazole).
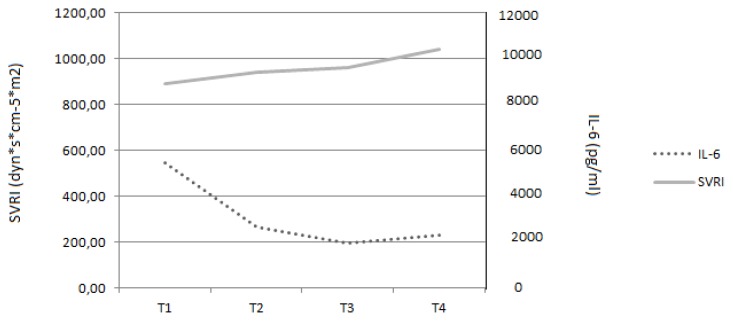
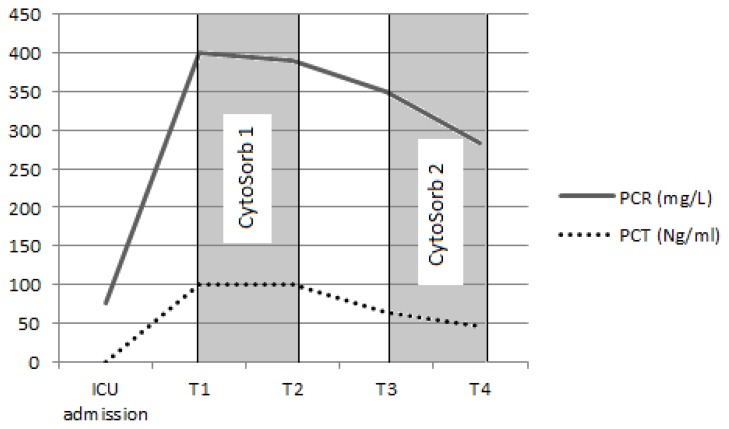
In total, two consecutive CVVH sessions with CytoSorb® were performed over a period of 64 hours (24 hours each). Inflammatory markers, haemodynamic parameters and serum cytokine levels were determined before and after each CytoSorb®. Cytokine levels were determined by an Immunology Multiplex Assay (Human Cytokine/Chemokine Immunoassay Panel, Merck Millipore, Billerica, MA, USA) on a Luminex-200™ System (Luminex, Austin, Texas) and the values are presented in Table 1. The haemoadsorption column was applied in combination with standard CVVH on Prismaflex® (Gambro, Lund, Sweden) using heparin anticoagulation (Figure 1). After each session an improvement in haemodynamic parameters was observed: stable cardiac output (4.7 L/min/m2), increase in systemic vascular resistance index (SVRI) from 890 to 1040 dyn*s*cm−5*m2 and a decrease in vasopressor dose from 4 to 0.4 mcg/kg/min (Figures 2A, 2B). Inflammatory markers also decreased: CRP from 400 mg/L to 283 mg/L, PCT from 100 Ng/mL to 46 Ng/mL and WBC count decreased from 16630/μL to 10310/μL (Figure 3).
Table 1.
Cytokine values during the use of CytoSorb®
| GM-CSF | IFNγ | IL-1β | IL-2 | IL-4 | IL-5 | IL-6 | IL-7 | IL-8 | IL-10 | IL-12p70 | IL-13 | MCP-1 | TNFα | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference range | < 5,38 | < 0,5 | < 2,62 | < 21,81 | < 13,91 | < 12,94 | < 7,25 | < 26,54 | < 20,2 | < 14,23 | < 12,79 | < 18,07 | < 281,61 | < 16,09 |
| T1 | 21,14 | 17,05 | 14,00 | 25,62 | 21,25 | 2,02 | 5440,67 | 53,08 | 897,17 | 89,89 | 13,69 | 21,54 | 10112,48 | 155,45 |
| T2 | 16,63 | 15,16 | 8,73 | 22,11 | 13,23 | <2.00 | 2653,45 | 51,31 | 857,52 | 112,21 | 13,68 | 18,84 | 5589,22 | 102,53 |
| T3 | 32,65 | 27,45 | 7,79 | 25,78 | 20,68 | <2.00 | 1965,46 | 51,31 | 279,04 | 311,02 | 12,96 | 18,45 | 14966,42 | 265,49 |
| T4 | 14,49 | 14,48 | 6,62 | 23,07 | 12,66 | <2.00 | 2294,89 | 50,86 | 347,16 | 350,05 | 13,69 | 18,45 | 4884,68 | 252,59 |
T1 – before the first CytoSorb®, T2 – after the first CytoSorb®, T3 – before the second CytoSorb®, T4 – after the second CytoSorb® IL – interleukin, GM-CSF – granulocyte-macrophage colony-stimulating factor, IFNγ – interferon gamma, MCP-1 – monocyte chemotactic protein 1, TNFα – tumor necrosis factor alpha
Values are expresed in pg/mL
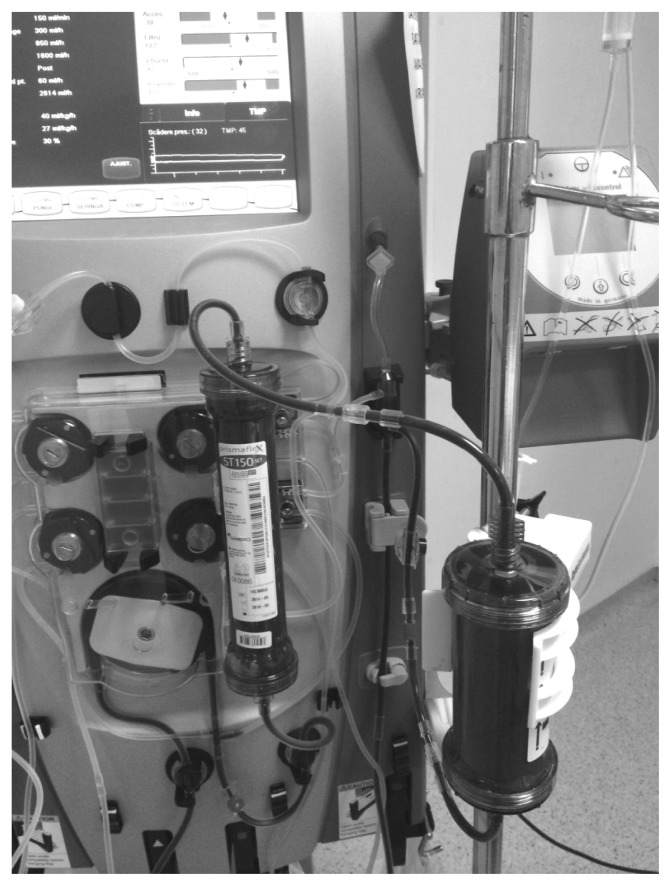
Fig. 1.
CytoSorb® was used in combination with continuous veno-venous hemofiltration (CVVH). Note that the CytoSorb® cartridge was placed before the hemofilter for CVVH
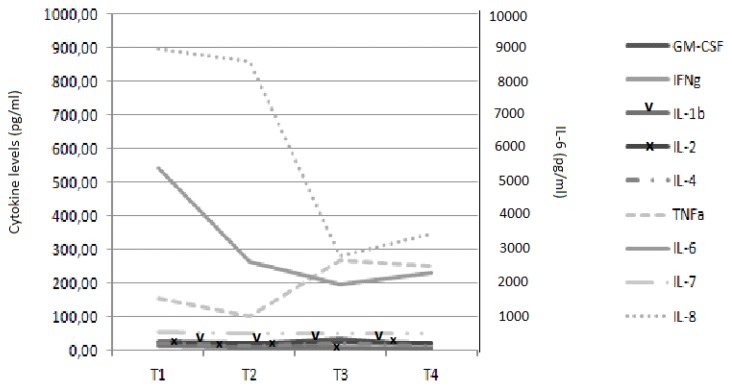
Fig. 2A.
Trend of cytokine levels before and after the use of CytoSorb®. T1 – before the first CytoSorb®, T2 – after the first CytoSorb®, T3 – before the second CytoSorb®, T4 – after the second CytoSorb®. IL – interleukin, GM-CSF – granulocyte-macrophage colony-stimulating factor, IFNγ – Interferon gamma, TNFα – tumor necrosis factor alpha
Fig. 2B.
The use of CytoSorb® was associated with a more stable haemodynamic profile with near normal values for both cardiac output and systemic vascular resistance. 1 – before the first CytoSorb®, 2 – after the first CytoSorb®, 3 – before the second CytoSorb®, 4 – after the second CytoSorb®. IL-6 – interleukin-6, SVRI – systemic vascular resistance index
Fig. 3.
Dynamics of sepsis markers (PCR, PCT) at the time of ICU admission, before (T1) and after (T2) the first CytoSorb® and before (T3) and after (T4) the second CytoSorb®. ICU – intensive care unit, PCT – procalcitonin, PCR – C reactive protein
The clinical effects associated with CytoSorb® correlated with a rebalance in cytokine levels. We observed a decrease in pro-inflammatory cytokines, especially IL-1β and TNFα, while anti-inflammatory cytokine levels increased, especially IL-10 and IL-8. Also, a marked reduction in Interferon gamma (IFNγ) was noted. The reduction in WBC count correlated with a marked decrease in both MCP-1 (monocyte chemoattractant protein-1) and GM-CSF (granulocyte macrophage colony stimulating factor).
Decision to control the septic source was made, but the patient died 24 hours after the second CytoSorb® was dismounted due to a cardiac arrhythmia (ventricular fibrillation) unresponsive to resuscitation manoeuvres.
Discussion
It is now known that sepsis causes death by an excessive release of cytokines that attack the organs of the body leading to multiple organ failure. The effects of a rebalanced immunological response between pro- and anti-inflammatory cytokines associated with the use of CytoSorb® translated into a more stable haemodynamic profile and decreased vasopressor support. In our case, CytoSorb acted by lowering proinflammatory cytokine levels (especially IL-1β, IL-6 and MCP-1) while maintaining anti-inflammatory cytokines within normal-high range (IL-10). The compensated immune response that was observed during and after CytoSorb® was most likely responsible for the improvement in haemodynamics, and it might prove beneficial to other cytokine-targeted therapies that focus only on one specific cytokine, unable to rebalance the whole process of inflammation.
The recent “Surviving sepsis campaign guidelines” [10] recommend only fluid resuscitation, antibiotics, source control and supportive measures (haemodynamic optimization) for the treatment of severe sepsis and septic shock. There are no recommendations made for modulating the immune response in order to prevent and minimize organ damage during the early cytokine storm. Treating subsequent organ dysfunction consists of specific measures for organ support. Research in the field of immunological modulation of inflammatory response in septic patients has only focused until now on targeting specific cytokines [11], such as TNFα and IL-1β. A prospective control study [12] in septic patients with acute renal failure attempted to demonstrate the effect of renal replacement therapy (CVVH) on cytokine levels, especially TNFα, but failed to do so. Up until this moment no specific therapy for cytokine removal has demonstrated its benefits. The use of CytoSorb® in patients with severe sepsis or septic shock is a new area of research with insufficient data to promote large prospective randomized control trials. Nevertheless, clinical cases and small clinical trials published to date have showed promising results. Hetz et al. [13] reported a case of a woman with septic shock and multi-organ failure due to necrotizing fasciitis successfully treated with CVVH with CytoSorb® with a reduction of IL-6 levels and improved patient condition until surgical control of the source was achieved. Mitzner et al [14] reported a case of a 80 years patient with a pneumogenic septic shock. After 24 hours of CVVH with CytoSorb®, the need for vasopressor dramatically decreased, as well as IL-6 levels, leucocytes and inflammation markers (CRP, PCT). In a small prospective clinical trial, Born et al. [15] showed a decrease in IL-6 levels, CRP and WBC during cardiac surgery with conventional extracorporeal circulation.
Conclusion
In the presented case, the use of CytoSorb® in combination with CVVH managed to re-establish a balance between pro- and anti-inflammatory cytokines that lead to a stable haemodynamic profile. The technology is simple to use and can be added on conventional CVVH machines and the therapy is well tolerated with no adverse effects.
The timing of Cytosorb®, whether early (after onset of SIRS) or late (after onset of organ dysfunction) use of this novel therapy, has still to be established.
Footnotes
Conflict of interest
Nothing to declare
References
- 1.Vogel TR, Dombrovskiy VY, Carson JL, Graham AM, Lowry SF. Postoperative sepsis in the United States. Ann Surg. 2010;252:1065–1071. doi: 10.1097/SLA.0b013e3181dcf36e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore LJ, Moore FA, Todd SR, Jones SL, Turner KL, Bass BL. Sepsis in general surgery: the 2005–2007 national surgical quality improvement program perspective. Arch Surg. 2010;145:695–700. doi: 10.1001/archsurg.2010.107. [DOI] [PubMed] [Google Scholar]
- 3.Aziz M, Jacob A, Yang WL, Matsuda A, Wang P. Current trends in inflammatory and immunomodulatory mediators in sepsis. J Leukoc Biol. 2013;93:329–342. doi: 10.1189/jlb.0912437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13:862–874. doi: 10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Venet F, Lukaszewicz AC, Payen D, Hotchkiss R, Monneret G. Monitoring the immune response in sepsis: a rational approach to administration of immunoadjuvant therapies. Curr Opin Immunol. 2013;25:477–483. doi: 10.1016/j.coi.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oberholzer A, Oberholzer C, Moldawer LL. Sepsis syndromes: understanding the role of innate and acquired immunity. Shock. 2001;16:83–96. doi: 10.1097/00024382-200116020-00001. [DOI] [PubMed] [Google Scholar]
- 7.Zisman DA, Kunkel SL, Strieter RM, Gauldie J, Tsai WC, Bramson J, et al. Anti-interleukin-12 therapy protects mice in lethal endotoxemia but impairs bacterial clearance in murine Escherichia coli peritoneal sepsis. Shock. 1997;8:349–356. doi: 10.1097/00024382-199711000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Fisher CJ, Dhainaut JFA, Opal SM, Pribble JP, Balk RA, Slotman GJ, et al. Recombinant human interleukin 1 receptor antagonist in the treatment of patients with sepsis syndrome. Results from a randomized, double-blind, placebo-controlled trial. JAMA. 1994;271:1836–1843. [PubMed] [Google Scholar]
- 9.Schadler D, Porzelius C, Jörres A, Marx G, Meier-Hellmann A, Putensen C, et al. A multicenter randomized controlled study of an extracorporeal cytokine hemoadsorption device in septic patients. Crit Care. 2013;17(Suppl 2):P62. [Google Scholar]
- 10.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Intensive Care Med. 2013;39:165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abraham E, Wunderink R, Silverman H, Perl TM, Nasraway S, Levy H, et al. Efficacy and safety of monoclonal antibody to human tumor necrosis factor alpha in patients with sepsis syndrome. A randomized, controlled, double-blind, multicenter clinical trial. TNF-alpha MAb Sepsis Study Group. JAMA. 1995;273:934–941. [PubMed] [Google Scholar]
- 12.Heering P, Morgera S, Schmitz FJ, Schmitz G, Willers R, Schultheiss HP, et al. Cytokine removal and cardiovascular hemodynamics in septic patients with continuous venovenous hemofiltration. Intensive Care Med. 1997;23:288–296. doi: 10.1007/s001340050330. [DOI] [PubMed] [Google Scholar]
- 13.Hetz H, Berger R, Recknagel P, Steltzer H. Septic shock secondary to β-hemolytic streptococcus-induced necrotizing fasciitis treated with a novel cytokine adsorption therapy. Int J Artif Organs. 2014;37:422–426. doi: 10.5301/ijao.5000315. [DOI] [PubMed] [Google Scholar]
- 14.Mitzner SR, Gloger M, Henschel J, Koball S. Improvement of hemodynamic and inflammatory parameters by combined hemoadsorption and hemodiafiltration in septic shock: a case report. Blood Purif. 2013;35:314–315. doi: 10.1159/000351206. [DOI] [PubMed] [Google Scholar]
- 15.Born F, Pichlmaier M, Peterss S, Khaladj N, Hagl C. Systemic Inflammatory Response Syndrome in heart surgery: New possibilities for treatment through the use of a cytokine adsorber during ECC? Kardiotechnik. 2014;2 [Google Scholar]