Abstract
Background:
We examined the reliability of trained dogs to alert to hypoglycemia in individuals with type 1 diabetes.
Methods:
Patients with type 1 diabetes who currently used diabetes alert dogs participated in this exploratory study. Subjects reported satisfaction, perceived dog glucose sensing ability and reasons for obtaining a trained dog. Reliability of dog alerts was assessed using capillary blood glucose (CBG) and blinded continuous glucose monitoring (CGM) as comparators in 8 subjects (age 4-48). Hypoglycemia was defined as CBG or CGM <70 mg/dL.
Results:
Dog users were very satisfied (8.9/10 on a Likert-type scale) and largely confident (7.9/10) in their dog’s ability to detect hypoglycemia. Detection of hypoglycemia was the primary reason for obtaining a trained dog. During hypoglycemia, spontaneous dog alerts occurred at a rate 3.2 (2.0-5.2, 95% CI) times higher than during euglycemia (70-179 mg/dL). Dogs provided timely alerts in 36% (sensitivity) of all hypoglycemia events (n = 45). Due to inappropriate alerts, the PPV of a dog alert for hypoglycemia was 12%. When there was concurrence of a hypoglycemic event between the dog alert and CGM (n = 30), CGM would have alerted prior to the dog in 73% of events (median 22-minute difference).
Conclusions:
This is the first study evaluating reliability of trained dogs to alert to hypoglycemia under real-life conditions. Trained dogs often alert a human companion to otherwise unknown hypoglycemia; however due to high false-positive rate, a dog alert alone is unlikely to be helpful in differentiating hypo-/hyper-/euglycemia. CGM often detects hypoglycemia before a trained dog by a clinically significant margin.
Keywords: continuous glucose monitor, dog, hypoglycemia, type 1 diabetes
The use of trained dogs to alert to changes in blood glucose in human companions with diabetes is increasing, yet their effectiveness is largely unknown.1 Limited in vitro studies of the ability of so-called diabetes alert dogs (DADs) to correctly differentiate between hypo- and euglycemia have yielded conflicting results.2,3 Survey-based studies of DAD users report improved clinical and psychosocial metrics; however they rely on owner accuracy in recalling events, thus potentially over-reporting success rates.1,4-6 Studies comparing capillary blood glucose reports and self-reported dog alerts suggest DADs may alert during times of hypo- or hyperglycemia more than would be expected by random chance.5,7 Scientifically rigorous studies using blinded continuous glucose monitors and detailed record keeping of dog alerts are needed to determine the accuracy of DADs compared to BG monitoring devices.1,6,8,9
Hypoglycemia is common and sometimes serious in patients with type 1 diabetes. Fear of hypoglycemia is a limiting factor to achieving tight glycemic management.10,11 DADs are potentially appealing for patients seeking a noninvasive option to assist with the recognition of hypoglycemia. The motivations for obtaining a DAD and the perceived benefits of their use are only partially understood.5,8
The physiological basis for how dogs sense hypoglycemia is unclear but postulated mechanisms include odor cues resulting from chemical changes in sweat or breath, behavioral alterations or response to more ethereal changes in electromagnetic fields.6 A recent study identified higher levels of isoprene in breath samples during hypoglycemia than euglycemia.12 Further proteomic studies of sweat, saliva, or breath during hypoglycemia versus euglycemia may be illustrative in identifying a biochemical signal available to dogs’ keen olfaction.
Although the volume of popular press stories and public interest in DADs continues to rise, diabetes providers are unable to provide informed advice regarding their use.8-11,13 We studied the reliability of DADs to alert to hypoglycemia in their human companions with type 1 diabetes under real-life conditions using capillary blood glucose (CBG) and blinded continuous glucose monitoring (CGM) as comparators.
Methods
Study Population
This exploratory study was conducted at a single center from 1 June 2015 to 1 September 2015. The study consisted of 2 visits, 1 week apart. Eligible subjects were 2-80 years of age with a prior diagnosis of type 1 diabetes and currently using a dog formally trained to alert to hypoglycemia in that individual. Subjects were excluded if they were unwilling to use only blinded CGM during the study, were pregnant or did not speak, read, write or understand English. The research protocol was approved by the Oregon Health & Science University Institutional Review Board (#10881) and enrolled subjects or parents for minors provided written informed consent.
Dogs were considered “formally trained” if they had completed training specifically for the purpose of detecting hypoglycemia in their human companion. Training methods are rigorous, time-consuming and comprehensive, however there is some variation between trainers. Initial obedience and socialization training is followed by scent training that typically starts as a simple reward-based system and graduates to specific job skills (eg, successful alert to a cotton swab of sweat collected during hypoglycemia) performed first in focused scenarios and then in the presence of their human companion in various real-life conditions (at home, in public, in crowds). Subjects were instructed to go about their usual life in the presence of their trained dog while wearing a blinded CGM (Dexcom G4 using 505 algorithm; San Diego, CA) during the week between visits 1 and 2. Subjects kept a detailed diary of all dog alerts, and checked a CBG at the time of each alert, noting any corresponding hypoglycemia symptoms and the specific timing of the dog alert relative to symptoms and CBG. Subjects used their personal quality-controlled glucose monitors (Lifescan OneTouch Ultramini/Ultra 2/Ping, Milpitas, CA [4 subjects]; Bayer ContourNext, Parsippany, NJ [2 subjects]; Abbott Freestyle Lite, Alameda, CA [1 subject]; Roche Accu-Chek Nano, Basel, Switzerland [1 subject]). CGM measurements were downloaded at the final study visit and time-matched with subject-reported events in the detailed diary to determine the first signal of each event (dog, CGM or subject symptoms). Subjects (or parent for minors) completed surveys regarding hypoglycemia unawareness, perceived dog reliability, and value as well as reported detailed history of the dog, training regimen, and reasons for obtaining a DAD.
End Points and Assessments
The prespecified primary efficacy end point was the rate of correct identification and alert to hypoglycemia by the trained dog. Key secondary end points included timing of dog alert relative to other measures of hypoglycemia (subject symptoms, CBG, CGM) to calculate test characteristics (sensitivity, positive predictive value, false positive rate), mean and median time between dog alert and CGM <70 mg/dL, rate of change of CGM at the time of dog alert, proportion of time with CGM <70 mg/dL, subjective confidence in trained dogs’ ability to detect hypoglycemia, and proportion of hypoglycemia events for which the trained dog is unavailable.
Hypoglycemia was defined by CBG <70 mg/dL or CGM <70 mg/dL for ≥15 minutes, consistent with prior definitions for clinically meaningful outcomes research.14-18 To be eligible for a second hypoglycemia event, CGM was required to be ≥80 mg/dL for ≥30 minutes prior to a second excursion <70 mg/dL. Euglycemia was defined as 70-179 mg/dL and hyperglycemia as ≥180 mg/dL.
CGM downloads were reviewed to extract hypoglycemia event data and compared to the subject event diary to determine what the first sign of hypoglycemia was - subject symptoms, CBG, CGM, or dog alert. CGM successfully captured 93% of possible glucose values during the study CGM wear-time.
The established Clarke method (7 questions with composite score yielding binary outcome: hypoglycemia unawareness yes/no) was used as a standardized assessment of hypoglycemia unawareness.19 Perceived dog reliability and value were assessed using a survey developed for this study (Table 1).
Table 1.
Patient-Reported Satisfaction, Subjective Reliability, and Value of Trained Dog (n = 8).
| Question | Response range | Response mean |
|---|---|---|
| 1. How satisfied are you with your trained dog with 0 being “not at all satisfied” and 10 being “very satisfied?” | 7-10 | 8.9 |
| 2. If you have a low blood sugar, how often do you think your dog alerts you? | 70-90% | 79% |
| 3. When your dog alerts you, how often is it the first sign of a low blood sugar (before you feel symptoms and before you check a fingerstick blood sugar)? | 50-95% | 76% |
| 4. When your dog alerts you, how often is it correct (you are actually low)? | 50-100% | 83% |
| 5. For a trained dog to be “worth it,” what percent of low blood sugars should it alert you to? | 20-80% | 65% |
| 6. For a trained dog to be “worth it,” what percent of incorrect alerts is acceptable (dog alerts, but you are not actually low)? | 10-80% | 26% |
| If a dog could alert you 80% of the time you had a low blood sugar, realistically what would be a reasonable cost for this dog? | $2500-20 000 | $12 313 |
Statistical Analyses
Based on preliminary published data,2 and using methods described by Ahn et al,20 we determined the need to capture 45 hypoglycemia events. For a single-proportion sign test for clustered binary data, and assuming an intracluster correlation of .04, we estimate that we have 80% power to detect this 50% sensitivity with a 2-sided significance of 5% and a lower bound for sensitivity of 30%.
We defined sensitivity as the mean proportion of CGM-captured hypoglycemic events where the dog alerted from 10 minutes before to 30 minutes after the first CGM measurement below 70 mg/dL. The lead-in time was chosen to approximate the known lag in CGM measurement relative to venous glucose. Similarly, we defined PPV as the proportion of dog signals that occurred at the start of a hypoglycemic event. We averaged over all dog-owner pairs using the predicted probabilities from a mixed-effects logistic model with a random intercept. Because non-hypoglycemia occurred as continuous time rather than as discrete events, we calculated a false positive rate (FPR) rather than specificity, defining FPR as the mean number of dog signals per person per week that occurred with CGM at or above 70 mg/dL, or after the first 30 minutes of a hypoglycemic event. The mean and standard error were calculated using a Poisson model with observation time as an offset. Analyses were performed using Stata version 13.
Results
Subjects
Twenty-three individuals underwent telephone or in-person screening; 8 enrolled and completed all study procedures. Reasons for not enrolling included “not interested or too busy” (7 individuals), sharing the same dog (2 individuals), and 1 individual each whose dog recently died, individual >80 years old, unwilling to be off un-blinded CGM, failed to return phone calls, “pretty sure dog no longer accurate,” and “dog too much of a burden, but now a great pet.”
The enrolled subjects (Table 2) were age 4-48 years (median 14 years), 6 female, 7 non-Hispanic/Latino and 1 Hispanic/Latino. Three subjects were of normal weight, 3 overweight, and 2 obese. Most recent glycated hemoglobin was 5.3-9.9% (34-85 mmol/mol). Self-reported frequency of hypoglycemia episodes per week was 2-21 (median 7). Two subjects used unblinded CGM either some or all of the time prior to the study; only blinded-CGM was used during the study.
Table 2.
Baseline Clinical/Demographic Data, Hypoglycemia Events, and Percentage Time of CGM Functionality.
| Subject | Age (y) | Gender | Diabetes duration (y) | Shot/pump | Hypo unaware (Clarke) | Prior hypo seizure | Diabetes complication | # hypo events | % time CGM functional |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 11 | M | 4 | Shot | N | Y | None | 4 | 89.2 |
| 2 | 21 | F | 14 | Pump | Y | Y | None | 5 | 94.3 |
| 3 | 8 | M | 2 | Shot | N | N | None | 3 | 74 |
| 4 | 14 | F | 9 | Shot | Y | N | None | 5 | 94.2 |
| 5 | 15 | F | 9 | Pump | Y | Y | None | 5 | 96.6 |
| 6 | 22 | F | 15 | Pump | Y | Y | None | 7 | 96.1 |
| 7 | 49 | F | 46 | Pump | Y | Y | Gastroparesis, retinopathy, CKD stage 2 | 6 | 100 |
| 8 | 4 | F | 2 | Shot | Y | Y | None | 10 | 97.6 |
Five enrolled subjects self-reported hypoglycemia unawareness. Six had hypoglycemia unawareness as determined by screening tool developed by Clarke et al.19 Five had concordant self-report and positive screening. Six subjects reported a history of hypoglycemia-related seizures and 2 of 6 reported at least 1 seizure both before and after obtaining a dog.
Dogs
Five of 8 dogs were Labrador or Labrador mixes; there were 1 each of Border Collie, Saint Bernard, and Golden Doodle. Age ranged from 1 year 9 months to 5 years 10 months and training duration was 6-24 months (median 10 months). Time since completion of training was 0 months to 3 years 4 months. Six different training programs were represented. All dogs were trained to detect hypoglycemia and 7 of 8 alerted to hyperglycemia with 6 of 7 having the same alert for both. Four of 8 reportedly alerted to rapid changes in blood glucose, though they were not necessarily trained to do so.
Reasons for obtaining a trained dog mentioned more than once included “extra protection against lows” (8/8), “companionship” (3/8), “accountability/reminder to check blood glucose” (3/8), “independence/scared to be alone” (2/8), and “so parents can sleep” (2/5 parents).
Median reported cost of pretrained dogs was $11 000 (range $5000-$15 000), though 2 subjects received their trained dog at no cost and 1 completed a home-based training program with weekly group classes over 2 years at a cost of $2000.
Primary Outcome
Rate of correct identification and alert to hypoglycemia by a trained dog
Figure 1 shows CGM value at the time of a dog alert (gray bars) and frequency of all CGM values (open bars). If dog alerts had been random, they would have occurred in equal distribution to overall CGM values and the histograms would be superimposable. There were 45 discrete episodes of hypoglycemia identified by CGM, of which 34 had concomitant hypoglycemia by CBG. The other 11 episodes were captured only on CGM download. During hypoglycemia, spontaneous dog alerts (alerts preceding subject symptoms or CBG) occurred at a rate 3.2 (2.0-5.2, 95% CI) times higher than the rate of spontaneous alerts during euglycemia. Trained dogs provided a timely alert (within 10 minutes before to 30 minutes after onset of hypoglycemia) in 36% (sensitivity) of all hypoglycemia events. Of all dog alerts, 12% (PPV) occurred during hypoglycemia. The false positive rate was 14.5 (12.1-17.5, 95% CI) false positive alerts per week, though there was a broad range among dogs of 2-30 false positive alerts per week.
Figure 1.
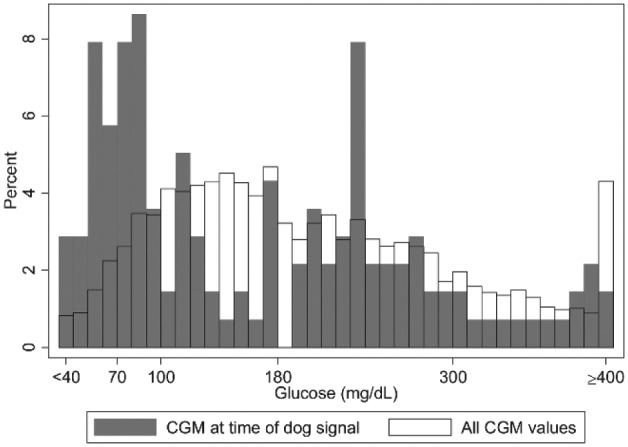
Histogram overlay of CGM at time of dog alert (gray bars) relative to frequency of all CGM values (open bars). If dog alerts were random, the 2 histograms would be superimposable. In the study, during hypoglycemia, trained dogs were 3.2 times as likely to alert than during euglycemia.
Secondary Outcomes
Trained dog versus CGM
In events when both the trained dog alerted and blinded-CGM reached the hypoglycemia threshold (n = 30), the first sign of hypoglycemia was subject symptoms in 12%, trained dog in 19%, and CGM in 70%. Compared to subject symptoms alone, the trained dog would have alerted to hypoglycemia before subject symptoms 62% of the time; however, when CGM is included, CGM would have alerted prior to the dog in 73% of events by a median difference of 22 minutes. An example of a trained dog in this study reliably alerting to otherwise unknown hypoglycemia is shown in Figure 2. In nearly all of the 11 hypoglycemia events that were detected only by CGM (never apparent to subject or dog), hypoglycemia was mild (CGM nadir 50-68 mg/dL) and of relatively short duration (<60 minutes), however 1 event had CGM nadir <40 mg/dL and lasted 7 hours during sleep.
Figure 2.
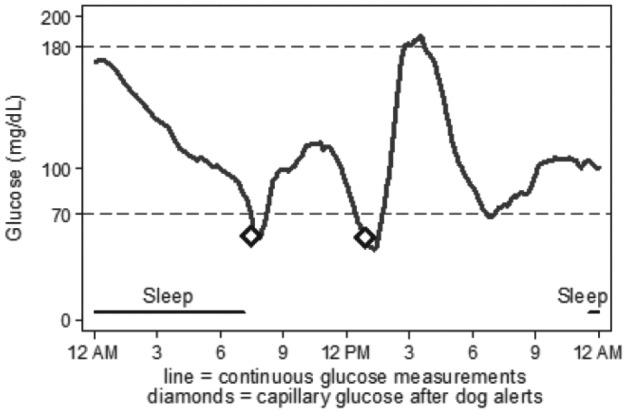
Blinded-CGM download and superimposed spontaneous dog alerts (diamonds) with corresponding CBG. In the first event, dog alert corresponded to CBG 55 mg/dL and preceded CGM threshold of <70 mg/dL by 7 minutes. In the second event, dog alert corresponded to CBG 54 mg/dL and came 24 minutes after CGM threshold of <70 mg/dL. By study definition, trained dog successfully alerted to both hypoglycemia events.
Rate of change
Median rate of change at the time of all spontaneous dog alerts for which rate of change data was computable (n = 83) was -0.6 mg/dL/min. Because of relatively frequent alerts found between CGM 70-99 mg/dL, a post hoc assessment at the time of these alerts showed median rate of change of -0.7 mg/dL/min and glucose trajectory that would have predicted hypoglycemia within 20 minutes in 11 of 21 (52%) alerts.
Responses to the survey of satisfaction, subjective reliability and value are shown in Table 1.
Other
CGM values were in hypoglycemia range for 5.5% of all CGM wear-time, representing approximately 80 minutes/day. This mean duration of hypoglycemia is similar to previous reports of hypoglycemia detected by blinded CGM.21,22 Frequency of dog alerts was 6-32 per week (mean 19, median 21). All dogs alerted to at least 1 hypoglycemia event (range 1-8) and all subjects experienced at least 3 hypoglycemia events (range 3-10) during the 1-week study period.
Nine of 45 hypoglycemia events occurred during sleep of which 6 were detected by the trained dog, though CGM would have detected 5 of these 6 prior to the dog. Two episodes of hypoglycemia occurred while the trained dog was not present.
Discussion
This is the first study to evaluate the reliability of trained dogs to alert to hypoglycemia using objective measures of glucose under real-life conditions. We demonstrate trained dogs often alert a human companion to otherwise unknown hypoglycemia; however due to high false-positive rate, a dog alert alone is unlikely to be helpful in differentiating hypo-/hyper-/euglycemia. CGM often detects hypoglycemia before a trained dog by a clinically significant margin.
Previous studies of trained dogs have not assessed test characteristics (sensitivity, positive predictive value, and false positive rate) using rigorous methodology or have been limited to in-vitro assessments which are less-generalizable. The current study helps define the clinical utility and limits of DADs and balances the often sensational reports of DADs in the popular press and social media.23,24 Surveys of DAD users suggest high levels of trust in their reliability and a recent survey of DAD users querying the subjective reliability to detect blood glucose <70 mg/dL reported identical findings (reported 79% detection) to our study.9
In the current study, DAD users cited the detection of hypoglycemia as the primary reason for having a trained dog and were very satisfied with and largely confident in their dog’s ability to detect hypoglycemia. Trained dogs alerted more frequently during hypoglycemia than euglycemia supporting the concept that dogs are capable of successfully detecting a hypoglycemia signal under real-life conditions. However the subjective views of reliability were overly optimistic and discordant with the measured performance of the trained dogs in the study. This has the potential to lead to over-reliance on a suboptimally performing diagnostic tool that has significant financial cost and time-investment.
In controlled environments, trained dogs have demonstrated success in biodetection of thyroid, breast, prostate, bladder and lung cancers.25-28 Methodologies for in-vitro training and testing of DADs for the detection of hypoglycemia are being refined.3
This is the first study to assess trained dog alerts concurrently with blinded CGM. While many trained dogs have a single alert for all possible glucose scenarios (hypoglycemia, hyperglycemia, rising glucose, falling glucose), CGM provides glucose values calibrated to a biochemical signal as well as dynamic trend data. In this study, CGM outperformed alert dogs in detection of hypoglycemia.
Trained dogs may provide psychosocial benefits that are difficult to measure. Previous studies of DAD users report decreased worry about hypoglycemia, hyperglycemia, improved quality of life, and ability to participate in physical activities,1 as well as increased independence and overall trust in and satisfaction with the dog.5 Several of these sentiments were reflected in the current study as well. Recently, 2 studies have reported improved glycemic control associated with responsible pet ownership.29,30 In a chronic health condition such as diabetes in which the daily tasks of management are rarely rewarded, perhaps a dog provides a positive partner and encouragement or may ease some of the burden of living with diabetes.8
Limitations of the current study include sensor failures (2), dropped CGM signal at the time of a hypoglycemia event (8) as well as inherent issues of using dogs as a diagnostic tool, namely that the diagnostic “device” is not standardized – variations in breed, age, training duration and methods, etc. likely play a role in reliability. A small sample size does not permit assessment of these variations, yet dog trainers rarely train more than a few dogs at a time so a large sample size of similar dogs is unlikely to be available.
Since dogs were trained to alert to individualized thresholds of hypoglycemia, a dog who correctly alerted to a trained threshold of 80 mg/dL may have been counted as a “false positive” in this study. Similarly, because the majority of dogs had the same alert for hypoglycemia and hyperglycemia, (unforeseen at the time of study design) the study calculation of PPV may be misleading if the dog correctly alerted to hyperglycemia. However, since the universal primary reason given for having a dog was detection of hypoglycemia, we focused our assessment on what is seen as their primary benefit. We recommend future studies account for hyperglycemia alerts in the study design. CGM offers the advantage that it clearly indicates what alert (high, low, rising, falling) is intended.
Reliance on subject-reported hypoglycemia event reporting may affect data capture; this was the primary reason why the study was 1 week in duration as the detailed diary required significant attention to detail that may not be sustained over longer studies. Special attention was given to careful training of subjects in event recording to attempt to minimize this risk of missing data.
Strengths of the current study include assessment of dog reliability compared to standardized measured of glucose (CBG, CGM) and the generalizability of the study results to clinical practice.
Conclusion
While using trained dogs to detect hypoglycemia appears feasible, providers and patients should be aware of the considerable limits of their utility. Strong subjective report of trust and satisfaction suggests the psychosocial benefits of DADs may be significant.
Acknowledgments
Parts of this study were presented at the 76th Scientific Sessions of the American Diabetes Association, New Orleans, LA, June 10-14, 2016.
Footnotes
Abbreviations: CBG, capillary blood glucose; CGM, continuous glucose monitoring; DAD, diabetes alert dog; FPR, false positive rate.
Author Contributions: EAL contributed to all aspects of the study. KLR contributed to study design and data analysis. IGB contributed to study design and editing of the manuscript. AJA contributed to study conception, design, initiation, and editing of the manuscript. All authors approved the manuscript before submission. EAL is the guarantor of this work and, as, such, had full access to all the data in the study and takes full responsibility for the integrity of the data and the accuracy of the data analysis.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: AJA has served on scientific advisory boards and received consulting fees and grants/research support from Dexcom. No other relevant conflicts of interest reported.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was supported by the Jaeb Center for Health Research (JCHR) grant 2015PG-T1D064. The content is solely the responsibility of the authors and does not necessarily represent the official views of the JCHR. The JCHR had no role in the design, conduct, or reporting of this work.
References
- 1. Gonder-Frederick L, Rice P, Warren D, Vajda K, Shepard J. Diabetic alert dogs: a preliminary survey of current users. Diabetes Care. 2013;36:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dehlinger K, Tarnowski K, House JL, et al. Can trained dogs detect a hypoglycemic scent in patients with type 1 diabetes? Diabetes Care. 2013;36:e98-e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hardin DS, Anderson W, Cattet J. Dogs can be successfully trained to alert to hypoglycemia samples from patients with type 1 diabetes. Diabetes Ther. 2015;6:509-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Seewoodhary J, Dacruz T, Lloyd E, Evans PJ. The role of diabetic alert dogs in the management of impaired hypoglycemia awareness. Pract Diabetes. 2014;31:323-325. [Google Scholar]
- 5. Rooney NJ, Morant S, Guest C. Investigation into the value of trained glycaemia alert dogs to clients with type I diabetes. PLOS ONE. 2013;8:e69921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Weber KS, Roden M, Müssig K. Do dogs sense hypoglycaemia? Diabet Med. 2016;33:934-938. [DOI] [PubMed] [Google Scholar]
- 7. Shepard J, Graham J, Ducar D, Tripathi A, McElgunn Z, Gonder-Frederick L. Accuracy of BG detection in diabetes alert dogs (DADs). Paper presented at: American Diabetes Association 74th Scientific Sessions; June 13-17, 2014; San Francisco, CA. [Google Scholar]
- 8. Petry NM, Wagner JA, Rash CJ, Hood KK. Perceptions about professionally and non-professionally trained hypoglycemia detection dogs. Diabetes Res Clin Pract. 2015;109:389-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shepard JA, Grabman JH, Tripathi AV, Gonder-Frederick L. Diabetes alert dogs (DADs) vs. technology: patient experiences and perceived accuracy. Paper presented at: American Diabetes Association 76th Scientific Sessions; June 10-14, 2016; New Orleans, LA. [Google Scholar]
- 10. Diabetes Control and Complications Trial Research Group. Hypoglycemia in the Diabetes Control and Complications Trial. Diabetes. 1997;46:271-286. [PubMed] [Google Scholar]
- 11. Cryer PE. Hypoglycemia is the limiting factor in the management of diabetes. Diabetes Metab Res Rev. 1999;15:42-46. [DOI] [PubMed] [Google Scholar]
- 12. Neupane S, Peverall R, Richmond G, et al. Exhaled breath isoprene rises during hypoglycemia in type 1 diabetes. Diabetes Care. 2016; 39:e97-e98. [DOI] [PubMed] [Google Scholar]
- 13. Howsmon D, Bequette BW. Hypo- and hyperglycemic alarms: devices and algorithms. J Diabetes Sci Technol. 2015;9:1126-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ahmet A, Dagenais S, Barrowman NJ, Collins CJ, Lowson ML. Prevalence of nocturnal hypoglycemia in pediatric type 1 diabetes: a pilot study using continuous glucose monitoring. J Pediatr. 2011;159:297-302. [DOI] [PubMed] [Google Scholar]
- 15. Muchmore DB, Heinemann L, Tamborlane W, Wu XW, Fleming A. Assessing rates of hypoglycemia as an end point in clinical trials. Diabetes Care. 2015;38:e160-e161. [DOI] [PubMed] [Google Scholar]
- 16. Beck RW, Calhoun P, Kollman C. Use of continuous glucose monitoring as an outcome measure in clinical trials. Diabetes Technol Ther. 2012;14:877-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Beck RW, Kollman C, Xing D, Buckingham BA, Chase HP. Outcome measures for outpatient hypoglycemia prevention studies. J Diabetes Sci Technol. 2011;5:999-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Seaquist ER, Anderson J, Childs B, Cryer P, Dagogo-Jack S, Fish L. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. 2013;36:1384-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Clarke WL, Cox DJ, Gonder-Frederick LA, Julian D, Schlundt D, Polonsky W. Reduced awareness of hypoglycemia in adults with IDDM. A prospective study of hypoglycemic frequency and associated symptoms. Diabetes Care. 1995;18:517-522. [DOI] [PubMed] [Google Scholar]
- 20. Ahn C, Hu F, Schucany WR. Sample size calculation for clustered binary data with sign tests using different weighting schemes. Stat Biopharm Res. 2011;3:65-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Battelino T, Phillip M, Bratina N, Nimri R, Oskarsson P, Bolinder J. Effect of continuous glucose monitoring on hypoglycemia in type 1 diabetes. Diabetes Care. 2011;34:795-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. The effect of continuous glucose monitoring in well-controlled type 1 diabetes. Diabetes Care. 2009;32:1378-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Frellick M. Blood glucose hounds: dogs alert vulnerable diabetes patients. Available at: http://www.medscape.com/viewarticle/852127. Accessed June 15, 2016.
- 24. Glu. Available at: https://myglu.org/searches/dog?filter=poll. Accessed June 15, 2016.
- 25. Ferrando AA, Hinson AM, Wilkerson BM, Stack BC, Bodenner DL. Canine detection of metastatic thyroid cancer. J Video Endocrinology. 2014;1. doi: 10.1089/ve.2014.0015. [DOI] [Google Scholar]
- 26. McCulloch M, Jezierski T, Broffman M, Hubbard A, Turner K, Janecki T. Diagnostic accuracy of canine scent detection in early- and late-stage lung and breast cancers. Integrative Cancer Therapies.2006;5:30-39. [DOI] [PubMed] [Google Scholar]
- 27. Taverna G, Tidu L, Grizzi F, et al. Olfactory system of highly trained dogs detects prostate cancer in urine samples. J Urol. 2015;193:1382-1387. [DOI] [PubMed] [Google Scholar]
- 28. Willis CM, Britton LE, Harris R, Wallace J, Guest CM. Volatile organic compounds as biomarkers of bladder cancer: sensitivity and specificity using trained sniffer dogs. Cancer Biomark. 2010;8:145-153. [DOI] [PubMed] [Google Scholar]
- 29. Maranda L, Gupta OT. Association between responsible pet ownership and glycemic control in youths with type 1 diabetes. PLOS ONE. 2016;11:e0152332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Maranda L, Lau M, Stewart S, Grupta O. A novel behavioral intervention in adolescents with type 1 diabetes mellitus improves glycemic control: preliminary results from a pilot randomized control trial. Diabetes Educ. 2015;41:224-230. [DOI] [PMC free article] [PubMed] [Google Scholar]


