Abstract
Background:
Continuous glucose monitoring (CGM) remains underutilized in youth with type 1 diabetes (T1D). There is a need to investigate factors associated with CGM use.
Method:
In 61 T1D youth, CGM use was ascertained by downloads reflecting the 4-week periods preceding 3- and 6-month study visits. Demographic and clinical data were obtained from chart review and interview. Youth and parents completed validated psychosocial surveys at baseline and 6 months.
Results:
Youth (52% male, 93% Caucasian, 80% pump treated) were 12.7 ± 2.9 years old, with T1D for 6.3 ± 3.8 years; mean A1c was 7.9 ± 0.9%. Mean CGM use was 4.1 ± 2.1 days/week (median = 4.8) at 3 months and 3.4 ± 2.3 days/week (median = 3.9) at 6 months. At 3 and 6 months, 15% and 20% of youth, respectively, had stopped using CGM. At 6 months, youth using CGM 6-7 days/week had more frequent BG monitoring (P = .05), less insulin omission (P = .02), and greater probability of A1c < 7.5% (P = .01) than youth using CGM less often. Youth using CGM 6-7 days/week consistently over the 6 months demonstrated lower A1c at 3 months compared to baseline (P = .03) and the improvement was sustained at 6 months (P = .5, 3 vs 6 months); youth using CGM less often had no significant A1c change. Baseline BG monitoring ≥8 times/day or A1c within target (<7.5%) predicted greater CGM use (6-7 days/week) at 6 months (OR = 4.6, P = .02). There was no deterioration of psychosocial functioning with CGM use.
Conclusions:
Consistent and durable CGM use in youth with T1D is associated with treatment adherence and improved glycemic control without increasing psychosocial distress.
Keywords: adherence, continuous glucose monitoring, pediatrics, psychosocial factors, type 1 diabetes
Continuous glucose monitoring (CGM) can provide substantial glycemic benefits for patients with type 1 diabetes (T1D) of different ages.1 However, benefits are associated with the frequency of CGM use; patients who use CGM for the majority of time (generally considered to be 70% or more) have improved glycemic control in the absence of increased hypoglycemia.1-4
Although CGM accuracy, usability, and overall performance have improved over the past decade, consistent CGM use remains problematic for many patients, especially in the pediatric age group.5,6 Previous studies have shown that only a small proportion of youth with T1D use CGM consistently7-9 and that CGM use declines significantly over time among youth with T1D.5,10 Indeed, according to recent data from the Type 1 Diabetes Exchange Clinic Registry, a minority of patients in the registry were using CGM, and only 5 to 13% of pediatric patients were using CGM.7
Several barriers may affect consistent CGM use in pediatrics as the CGM places multiple demands on the patient, the family, and the clinical team.11-14 Physical barriers include pain associated with sensor insertion, skin reactions to sensor adhesive, and lack of “real estate” for sensor placement/insertion in young children, for example. Clinical barriers include multiple alerts and alarms that can lead to alarm fatigue and CGM discontinuation. Financial barriers include lack of insurance coverage and high copays/deductibles for CGM supplies. Demands placed on the clinical teams include the need to ensure proper training of patients and families along with the added time to review CGM tracings and provide clinical recommendations for diabetes management. Moreover, there are also a variety of psychosocial issues related to CGM use that can impact youth and family members, including diabetes burden, quality of life (QoL), diabetes-specific family conflict, parent involvement in diabetes management, and fear of hypoglycemia. Early generations of CGM devices with reduced accuracy and reliability also limited consistent use in the pediatric population, especially when the youth and their parents identified discrepancies between the CGM readings and glucose meter results, leading to CGM discontinuation. CGM devices with improved performance may mitigate some of these challenges,15 but CGM continues to place substantial demands on pediatric patients and their families, particularly with ongoing requirement for daily CGM calibrations based on blood glucose monitoring results. Thus, both biomedical and psychosocial factors related to CGM use remain an area for investigation in the pediatric population, especially as a means to predict uptake and durability of sensor use,16,17 given the low rates of CGM use in youth with T1D described in previous studies.
The first aim of the current study was to examine the associations of CGM use with biomedical and psychosocial characteristics of pediatric patients with type 1 diabetes at initiation of CGM and over a 6-month observation period. The second aim was to assess the impact of CGM use on glycemic control (hemoglobin A1c) over the 6-month observation period. We hypothesized that higher adherence to diabetes management tasks and lower perceived diabetes burden at baseline would be associated with more consistent CGM use over time. Furthermore, we hypothesized that greater CGM use would be related to improved glycemic control.
Research Design and Methods
Study Population and Design
Youth with T1D and their caregivers were recruited from a pediatric diabetes clinic and were followed for a 6-month observation period to assess CGM use and outcomes. Eligibility criteria included willingness to use CGM, youth 8-17 years of age, type 1 diabetes duration of ≥1 year at enrollment, daily insulin dose of ≥0.5 units/kg, blood glucose (BG) monitoring frequency of ≥4 times per day, and A1c of 6.5-10%. All enrolled participants received the Dexcom SEVEN PLUS® CGM system or the Dexcom G4 PLATINUM® CGM system, when it became available, as part of the study participation.
Electronic medical records and joint parent-youth interviews provided demographic and clinical data. Data on insulin regimen, daily insulin dose, BG monitoring, and insulin omission were collected using meter and/or pump downloads as well as by participant and clinician reports. Youth and parents completed validated surveys, administered by trained research assistants, of parent involvement in diabetes management,18 diabetes-specific family conflict,19 fear of hypoglycemia,20,21 depressive symptoms,22,23 anxiety,24,25 diabetes-specific burden,26,27 and youth QoL (general and diabetes-specific)28,29 at baseline and 6 months (see below). At the baseline visit, youth/parents met with a pediatric nurse for CGM education, including device insertion and removal. The nurse, youth, or parent inserted the CGM device according to the family’s preference. Following the baseline visit, all youth underwent a 1-week run-in feasibility period to confirm the youth’s interest, ability, and tolerability of CGM.
At the 3- and 6-month study visits, CGM data were downloaded and youth/parents met with a pediatric nurse for CGM education and to review CGM data. CGM data were also made available to participants’ diabetes care providers. CGM usage (mean number of days and mean number of hours per week) was derived from the CGM download after the 1-week run-in period and at 3 months and 6 months of follow-up. At the 3- and 6-month visits, CGM use was based on the 4-week interval that preceded the visit. We grouped youth according to CGM use at 6 months, with infrequent CGM use categorized as 0-5 days/week and frequent CGM use as 6-7 days/week. We based these groups on results from the JDRF CGM RCT, in which CGM use ≥6 days/week was associated with significantly greater improvements in A1c than CGM use <6 days/week.10 As an additional way to describe CGM use over the entire 6-month observation period, we further categorized youth according to CGM use at both 3 and 6 months, with those who used CGM 0-5 days/week at both 3 and 6 months categorized as “minimal users,” those who used CGM 6-7 days/week at either 3 or 6 months categorized as “inconsistent users,” and those who used CGM 6-7 days/week at both 3 and 6 months categorized as “consistent users.”
Glycemic control was assessed by A1c, obtained at baseline, 3 months, and 6 months, measured in the same clinical laboratory using an assay standardized to the Diabetes Control and Complications Trial (reference range, 4.0-6.0% [20-42mmol/mol]) (Roche Diagnostics, Indianapolis, IN). The local Institutional Review Board approved the study protocol, and all youth/parents provided written informed assent/consent before beginning any study procedures. Youth received $20 compensation for time and effort for each completed study visit; this compensation was not dependent on CGM use.
Psychosocial Measures
The Diabetes Family Responsibility Questionnaire18 was used to assess youth self-report and parent self-report of parent involvement in diabetes management. Total scores on the 19-item survey can range from 0-100, with higher scores indicating more parent involvement.
The Diabetes Family Conflict Scale19 was used to assess youth self-report and parent self-report of family conflict related to diabetes management. Total scores on the 19-item survey can range from 0-100, with higher scores indicating more diabetes-specific family conflict.
The Hypoglycemia Fear Survey–Worry Scale20,21 was used to assess youth self-report and parent self-report of worry about low BG levels. Total scores on the 15-item scale can range from 0-100, with higher scores indicating more fear of hypoglycemia.
The Center for Epidemiologic Studies Depression Scale for Children22 and Center for Epidemiologic Studies Depression Scale23 were used to assess youth self-report and parent self-report of depressive symptoms. Total scores on the 20-item surveys can range from 0-60, with higher scores indicating more depressive symptoms.
The State-Trait Anxiety Inventory for Children24 and State-Trait Anxiety Inventory25 were used to assess youth self-report and parent self-report of current feelings of anxiety (state anxiety) and long-term characteristics of anxiety (trait anxiety). Total scores on each 20-item scale can range from 0-60, with higher scores indicating more anxiety.
The Problem Areas in Diabetes Survey–Pediatric26 and Problem Areas in Diabetes Survey–Parent Revised27 were used to assess youth self-report and parent self-report of diabetes burden. Total scores on the 18-item youth survey and the 20-item parent survey can range from 0-100, with higher scores indicating more burden.
The Pediatric Quality of Life Inventory (PedsQL) Generic Core Scales28 and PedsQL Diabetes Module29 were used to assess youth self-report and parent proxy-report of youth generic and diabetes-specific QoL. Total scores on the 23-item PedsQL-Generic and the 28-item PedsQL-Diabetes can range from 0-100, with higher scores indicating better QoL.
Statistical Analysis
Demographic, clinical, and survey data are presented as mean ± SD or percentages. Statistical analyses were performed using SAS software (version 9.2; SAS Institute, Inc, Cary, NC) and included Spearman correlations, t-tests, ANOVA, chi-square tests, Fisher’s exact test, Wilcoxon rank sum tests, and Kruskal-Wallis tests. Use of parametric or nonparametric tests was determined by the distribution of the data. We used multivariate regression to examine the associations of baseline biomedical and baseline psychosocial factors with CGM use over the 6-month duration of the study. For the model assessing biomedical factors, predictor variables included a composite of baseline BG monitoring frequency (≥8 times/day) and target A1c attainment. We compared psychosocial survey scores at baseline and 6 months, as well as the change in survey scores, across CGM use groups. An alpha level of ≤.05 was used to determine statistical significance.
Results
Baseline Participant Characteristics
A total of 61 youth with T1D (52% male, 93% Caucasian) and their parents (89% mothers) participated in the study. At baseline, youth had a mean age of 12.7 ± 2.9 years, mean duration of T1D of 6.3 ± 3.8 years, and mean daily insulin dose of 0.9 ± 0.3 units/kg. The majority of participants were treated with an insulin pump (80%). The frequency of BG monitoring was 7.0 ± 2.6 times per day and the mean A1c level was 7.9 ± 0.9% (Table 1A). Most youth (79%) had at least 1 parent with a college degree and 84% of youth had private health insurance.
Table 1.
Biomedical Characteristics and Scores for Psychosocial Measures at Baseline.
| Table 1A. Biomedical Characteristics (N = 61). | ||
|---|---|---|
| Sex (% male) | 52 | |
| Age (years) | 12.7 ± 2.9 | |
| Diabetes duration (years) | 6.3 ± 3.8 | |
| z-BMI (SDS) | 0.5 ± 1.0 | |
| Insulin dose (U/kg/day) | 0.9 ± 0.3 | |
| Basal dose (% of total daily dose) | 41 ± 7 | |
| Insulin regimen (% pump users) | 80 | |
| Blood glucose monitoring (times/day) | 7.0 ± 2.6 | |
| Missing insulin doses (% yes) | 38 | |
| A1c (%) | 7.9 ± 0.9 | |
| A1c <7.5% (ADA target) (%) | 33 | |
| CGM use (hours/week, mean ± SD) | 128.7 ± 18.9 | |
| CGM use (hours/week, median) | 134.3 | |
| Table 1B. Psychosocial Characteristics. | ||
| Youth survey scores | Parent survey scores | |
| Parent involvement | 46.9 ± 15.9 | 57.4 ± 17.6 |
| Diabetes-specific family conflict | 13.0 ± 18.0 | 10.6 ± 9.4 |
| Fear of hypoglycemia | 25.1 ± 17.5 | 39.8 ± 16.9 |
| Depressive symptoms | 9.1 ± 8.2 | 9.0 ± 8.0 |
| State anxiety | 28.1 ± 3.9 | 31.1 ± 9.2 |
| Trait anxiety | 27.9 ± 5.4 | 34.0 ± 8.5 |
| Diabetes burden | 25.5 ± 19.1 | 41.4 ± 19.9 |
| Youth QoL (generic) | 86.5 ± 11.1 | 82.5 ± 11.6 |
| Youth QoL (diabetes-specific) | 79.9 ± 10.7 | 75.7 ± 11.2 |
Data are mean ± SD or %. A1c, hemoglobin A1c; CGM, continuous glucose monitoring; QoL, quality of life; SDS, SD score; z-BMI, body mass index z-score.
Baseline youth and parent reported survey scores are shown in Table 1B. Youth scores were significantly lower than parent scores for parent involvement (P < .0001), fear of hypoglycemia (P < .0001), state anxiety (P = .02), trait anxiety (P < .0001), and diabetes burden (P < .0001). Youth scores were significantly higher than parent proxy scores for youth generic QoL (P = .008) and youth diabetes-specific QoL (P = .003). Youth and parent scores were significantly positively correlated for parent involvement (r = .85, P < .0001), diabetes-specific family conflict (r = .53, P < .0001), diabetes burden (r = .38, P = .002), youth generic QoL (r = .57, P < .0001), and youth diabetes-specific QoL (r = .50, P < .0001).
CGM Use
CGM use declined over the 6 months. At baseline, youth used CGM for a mean of 5.4 ± 0.8 days/week, median 5.6 (128.7 ± 18.9 hours/week, median 134.3); at 3 months, mean CGM use was 4.1 ± 2.1 days/week, median 4.8 (98.0 ± 50.2 hours/week, median 114.3); and at 6 months, mean CGM use was 3.4 ± 2.3 days/week, median 3.9 (82.5 ± 55.6 hours/week, median 93.7) (see Figure 1). By 3 months, 9 youth (15%) had discontinued CGM use and by 6 months, an additional 3 youth (12 youth total, 20%) had stopped using CGM. The most common reasons for stopping CGM cited by these 12 youth and their parents were pain/discomfort, concerns about appearance, number of devices, and challenges carrying the receiver. At 6 months, 22 youth (36%) were using CGM 6-7 days/week, while 39 (64%) were using CGM 0-5 days/week.
Figure 1.
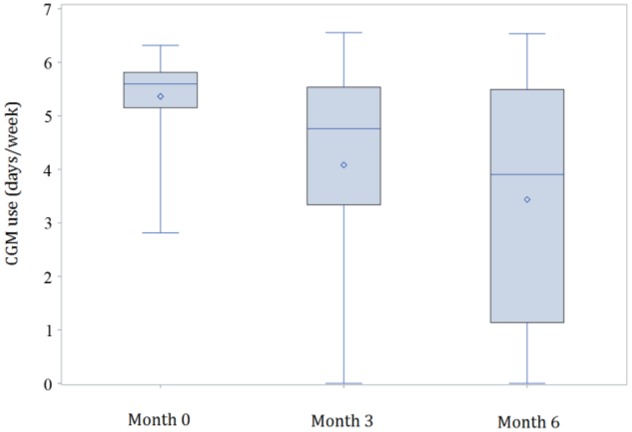
CGM use at baseline, 3 months, and 6 months in all participants. At baseline, youth used CGM a mean of 5.4 ± 0.8 days/week, median 5.6 (128.7 ± 18.9 hours/week, median 134.3); at 3 months, mean CGM use was 4.1 ± 2.1 days/week, median 4.8 (98.0 ± 50.2 hours/week, median 114.3); and at 6 months, mean CGM use was 3.4 ± 2.3 days/week, median 3.9 (82.5 ± 55.6 hours/week, median 93.7). At 3 months, 9 youth (15% of all participants) had stopped using CGM; at 6 months, a total of 12 youth (20% of all participants) had stopped using CGM. CGM, continuous glucose monitoring.
Factors Associated With CGM Use at 6 Months
There were no significant differences with respect to sex, age, duration of T1D, or pump use between youth using CGM 0-5 versus 6-7 days/week at 6 months (Table 2). However, youth using CGM more frequently (6-7 days/week) at 6 months checked their BG levels more often (P = .05), were less likely to omit insulin doses (P = .02), and were more than 4 times as likely to achieve target A1c <7.5% (P = .01) compared with youth using CGM less frequently. There were no significant differences for any of the youth and parent 6-month psychosocial survey scores between youth using CGM 0-5 versus 6-7 days/week at 6 months, controlling for baseline survey scores.
Table 2.
Biomedical Factors Associated With CGM Use at 6 Months.
| CGM use |
P | |||
|---|---|---|---|---|
| 0-5 days/week (n = 39) | 6-7 days/week (n = 22) | |||
| Sex (% male) | 56 | 45 | .4 | |
| Age (years) | 13.4 ± 3.0 | 12.9 ± 2.8 | .4 | |
| Diabetes duration (years) | 7.1 ± 3.9 | 6.5 ± 3.6 | .6 | |
| Insulin dose (U/kg/day) | 0.95 ± 0.25 | 0.89 ± 0.27 | .2 | |
| Insulin regimen (% pump users) | 85 | 91 | .7 | |
| Blood glucose monitoring (%) | 0-4 times/day | 21 | 18 | .05 |
| 5-7 times/day | 59 | 32 | ||
| ≥8 times/day | 21 | 50 | ||
| Missing insulin doses (% yes) | 47 | 18 | .02 | |
| A1c <7.5% (ADA target) (%) | 26 | 59 | .01 | |
Data are mean ± SD or %. Biomedical variables are from the 6-month visit. A1c, hemoglobin A1c.
Impact of CGM Use on Glycemic Control
To evaluate the impact of CGM use on glycemic control over time, baseline, 3-month, and 6-month A1c values were compared among minimal users (n = 32, 52%) inconsistent users (n = 10, 16%), and consistent users (n = 19, 31%) (Figure 2). Minimal users had the highest A1c at baseline (8.1 ± 0.9% compared to 7.8 ± 1.0% in inconsistent users and 7.8 ± 0.9% in consistent users). The consistent users improved their A1c values at 3 months compared with baseline (A1c 7.4 ± 0.7%, P = .03) and the improvement was sustained at 6 months (A1c 7.5 ± 0.6%, P = .5, 3 vs 6 months). The minimal user group and the inconsistent user group demonstrated no substantial change in A1c over the 6 months.
Figure 2.
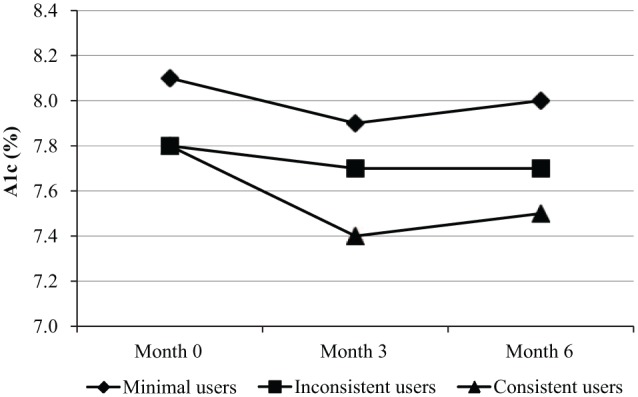
Impact of CGM use on glycemic control: trends in A1c according to CGM use over the 6-month study. CGM, continuous glucose monitoring; A1c, hemoglobin A1c. Youth are categorized as follows:
- “minimal users” (n = 32): those who used CGM 0-5 days/week at both 3 and 6 months; mean A1c (%) was 8.1 ± 0.9 at baseline; 7.9 ± 0.9 at 3 months; 8.0 ± 0.9 at 6 months.
- “inconsistent users” (n = 10): those who used CGM 6-7 days/week at either 3 or 6 months; mean A1c (%) was 7.8 ± 1.0 at baseline; 7.7 ± 0.9 at 3 months; 7.7 ± 0.7 at 6 months.
- “consistent users” (n = 19): those who used CGM 6-7 days/week at both 3 and 6 months; mean A1c (%) was 7.8 ± 0.9 at baseline; 7.4 ± 0.7 at 3 months; 7.5 ± 0.6 at 6 months.
Baseline Predictors of CGM Use Over the 6-Month Follow-Up Period
Among the baseline biomedical factors, only a composite of baseline BG monitoring frequency and target A1c attainment significantly predicted CGM use at 6 months. In a multivariate logistic regression model controlling for baseline age and diabetes duration, youth who were checking BG levels ≥8 times/day at baseline or who had A1c within target (<7.5%) at baseline were 4.6 times more likely (P = .02, 95% CI 1.3-16.1) to use CGM 6-7 days/week at 6 months than youth who were checking BG levels <8 times/day at baseline and had baseline A1c above target. Among the baseline psychosocial factors, only parent proxy-report of youth generic QoL was significantly related to CGM use over the 6 months. For youth using CGM 6-7 days/week at 3 months and 6 months, baseline parent proxy-report of youth generic QoL was 87 compared to 80 (out of 100) for youth using CGM less often (P = .03).
Change in Psychosocial Survey Scores
To investigate the impact of CGM use on psychosocial outcomes, we assessed the change in both youth and parent reported survey responses from baseline to the 6-month follow-up visit in the minimal users, inconsistent CGM users, and consistent CGM users. There was no decline in psychosocial functioning in any of the 3 CGM groups.
Discussion and Conclusions
Despite availability of new technologies for diabetes management, suboptimal glycemic control remains common among youth with T1D.30 CGM has been shown to improve outcomes, particularly when used on a near-daily basis.1,3,4 However, data from previous studies have shown that only a small proportion of patients with T1D use CGM consistently, especially in the pediatric age group.7-10 To promote CGM initiation and consistent use over time, we investigated biomedical and psychosocial factors as possible predictors of CGM use over a 6-month observation period.
In this study of 61 young persons with T1D who expressed a willingness to start CGM, use of CGM declined over time, with 20% of participants discontinuing CGM entirely by 6 months. Indeed, only slightly more than 1/3 (36%) of youth were using CGM 6-7 days/week at 6 months while slighter fewer than 1/3 (31%) used CGM 6-7 days/week at both 3 and 6 months. In this study, CGM use predicted glycemic outcomes as those who used CGM consistently (6-7 days/week at both 3 and 6 months) had better glycemic control over time, with A1c of 7.5% compared with 7.7% in the inconsistent users and 8.0% in the minimal users. Youth using CGM consistently at 6 months also demonstrated more frequent BG monitoring, lower likelihood of missing insulin bolus doses, and greater likelihood of attaining A1c target levels than youth using CGM less often. These observations suggest that the youth demonstrating consistent CGM use were, overall, more adherent to aspects of the diabetes treatment program. Alternatively, these youth may have had supportive parents/families that encouraged and supported the consistent use of CGM in their children. Compatible with the latter speculation, the parents of youth using CGM consistently at 6 months reported significantly higher general QoL for their children compared with the parents of youth using CGM less often.
There were a modest number of baseline characteristics related to durable CGM use. While additional research is warranted in more diverse samples, the study highlighted a few factors related to CGM use. More frequent BG monitoring or A1c target attainment at baseline was related to consistent CGM use of 6-7 days per week at 6 months of observation. In addition, the parents of youth using CGM consistently at both 3 and 6 months reported higher baseline general QoL for their children than the parents of youth using CGM less often.
As previously reported, adherence to CGM appears to be particularly challenging for youth with T1D. This observational study allows us to assess trends in CGM use over 6 months, a period of time generally reported in the literature, among youth with T1D and their parents who agreed to initiate CGM use. Despite the initial enthusiasm for CGM following the 1-week trial period, the minority of these pediatric patients maintained consistent and durable CGM use for the 6 months. The T1D Exchange Clinic Registry recently showed that only a small proportion of patients with T1D, specifically 11% overall, are using CGM in clinical practice, with the lowest rates in the school age, adolescent, and young adult patients, with rates of 8%, 5%, and 7%, respectively.7 Among youth in the T1D Exchange, those using CGM were more likely to use an insulin pump and have lower A1c levels than those not using CGM, suggesting greater adherence overall.31 Our findings of more frequent BG monitoring and a greater proportion of youth at target A1c at baseline are consistent with the suggestion that greater adherence overall is associated with CGM use, akin to the associations observed in the T1D Exchange.
Barriers to consistent CGM use in the pediatric population reflect multiple areas, including physical issues, financial concerns, emotional burdens, and device inadequacies.11-14,31,32 Physical problems include insertions, trouble with adhesive tape, discomfort wearing the sensor, and challenges carrying the receiver. The latter problem has potentially been eliminated with the CGM transmitter connecting directly to cell phones. Financial problems arise with lack of insurance coverage or high copays/deductibles. Emotional burdens can stem from inaccuracies of CGM readings compared with BG monitoring results and frequent alerts and alarms that can overwhelm pediatric patients and family members. Device inadequacies have been reduced with more recent CGM tools that demonstrate substantially improved performance characteristics.15 Despite the lower accuracy and performance of earlier CGM devices, consistent use that amounted to 6-7 days per week along with durable CGM use for 6 months or longer had a positive impact on glycemic control as reported previously.4,5,9,32-35 It is likely that the “noise” or inaccuracies of the earlier generations of CGM devices could be overcome by the “signals” generated with consistent and durable CGM use, leading to the improvements in glycemic control without severe hypoglycemia, as witnessed in these earlier studies.
The use of CGM demands extra effort on the part of the young persons with T1D and their parents, therefore, it is important to assess if this extra burden leads to psychosocial distress.36 This observational study included survey evaluations of psychosocial factors at baseline and after 6 months of observation. Notably, there was no decline in any of the psychosocial factors over this time frame, even among those using CGM most consistently. Others have reported similar findings with potential benefits to perceived QoL.37
CGM devices continue to require human input. Therefore, it is critical that health care providers offer realistic expectations regarding CGM to their patients, including discussions of limitations (eg, ongoing need for calibration). In addition to the potential benefits of CGM, patients and families need to understand such potential challenges. Thus, there is a need for the design, implementation, and evaluation of family-based behavioral interventions aimed at providing realistic expectations for CGM uptake and deriving problem-solving strategies to overcome barriers to sustained CGM use in the pediatric population.
The unique contributions of this study relate to the integrated evaluation of both biomedical and psychosocial factors associated with consistent and durable CGM use over time. Nonetheless, this study has limitations, related to the modest sample size and the relatively short follow-up period of 6 months. In addition, the study sample was unique in many aspects due to the large proportion of participants treated with insulin pump therapy, the high frequency of BG monitoring at baseline, and the relatively low mean A1c compared to other samples of youth with T1D,7 and, therefore, the results may not be generalizable to the general population of youth with T1D. However, the characteristics of our study sample are similar to published data that describe higher rates of pump use and BG monitoring among youth using CGM compared to youth not using CGM.31,38 In addition, the observations demonstrate the difficulty in maintaining consistent CGM use over time even among youth with relatively high adherence and confirm a glycemic benefit for youth with T1D who maintain consistent and durable CGM use over time. Additional research in more diverse pediatric samples to confirm these findings is warranted.
This 6-month observational study identified an association of consistent and durable CGM use in pediatric patients with type 1 diabetes who perform frequent BG monitoring and achieve target A1c levels. Improved CGM technologies may offer an opportunity to reach a larger group of pediatric patients with T1D.15 Ongoing education and discussions between health care providers and families can help families to remain engaged and up-to-date with emerging diabetes technologies. It is also reassuring that use of CGM in these pediatric patients was not associated with any negative impact on psychosocial factors. As there remains a need to improve glycemic control in the majority of pediatric patients with type 1 diabetes, encouraging implementation of CGM along with its consistent and durable use can likely help increase the proportion of pediatric patients achieving target A1c levels.
Acknowledgments
The authors thank the patients and their families for their study participation.
Footnotes
Abbreviations: A1c, hemoglobin A1c; BG, blood glucose; CGM, continuous glucose monitoring; QoL, quality of life; T1D, type 1 diabetes.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: LML reports consultancy for Johnson & Johnson, Eli Lilly, Sanofi, NovoNordisk, Roche Diagnostics, Dexcom, Astra-Zeneca, and Boehringer Ingelheim. EG, RS, and LKV declare no competing financial interests exist.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by funding from the National Institutes of Health (grants R01DK089349, P30DK036836, and T32DK007260), the Katherine Adler Astrove Youth Education Fund, the Maria Griffin Drury Pediatric Fund, and the Eleanor Chesterman Beatson Fund. The content is solely the responsibility of the authors and does not represent the official views of these organizations.
References
- 1. Pickup JC, Freeman SC, Sutton AJ. Glycaemic control in type 1 diabetes during real time continuous glucose monitoring compared with self monitoring of blood glucose: meta-analysis of randomised controlled trials using individual patient data. BMJ. 2011;343:d3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chiang JL, Kirkman MS, Laffel LM, Peters AL. Type 1 diabetes through the life span: a position statement of the American Diabetes Association. Diabetes Care. 2014;37:2034-2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Floyd B, Chandra P, Hall S, et al. Comparative analysis of the efficacy of continuous glucose monitoring and self-monitoring of blood glucose in type 1 diabetes mellitus. J Diabetes Sci Technol. 2012;6:1094-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359:1464-1476. [DOI] [PubMed] [Google Scholar]
- 5. Mauras N, Beck R, Xing D, et al. A randomized clinical trial to assess the efficacy and safety of real-time continuous glucose monitoring in the management of type 1 diabetes in young children aged 4 to <10 years. Diabetes Care. 2012;35:204-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tsalikian E, Fox L, Weinzimer S, et al. Feasibility of prolonged continuous glucose monitoring in toddlers with type 1 diabetes. Pediatr Diabetes. 2012;13:301-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miller KM, Foster NC, Beck RW, et al. Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange clinic registry. Diabetes Care. 2015;38:971-978. [DOI] [PubMed] [Google Scholar]
- 8. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Factors predictive of use and of benefit from continuous glucose monitoring in type 1 diabetes. Diabetes Care. 2009;32:1947-1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Effectiveness of continuous glucose monitoring in a clinical care environment: evidence from the Juvenile Diabetes Research Foundation Continuous Glucose Monitoring (JDRF-CGM) trial. Diabetes Care. 2010;33:17-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chase HP, Beck RW, Xing D, et al. Continuous glucose monitoring in youth with type 1 diabetes: 12-month follow-up of the Juvenile Diabetes Research Foundation continuous glucose monitoring randomized trial. Diabetes Technol Ther. 2010;12:507-515. [DOI] [PubMed] [Google Scholar]
- 11. Tansey M, Laffel L, Cheng J, et al. Satisfaction with continuous glucose monitoring in adults and youths with Type 1 diabetes. Diabet Med. 2011;28:1118-1122. [DOI] [PubMed] [Google Scholar]
- 12. Rodbard D. Continuous glucose monitoring: A review of successes, challenges, and opportunities. Diabetes Technol Ther. 2016;18(suppl 2):S2-3-S2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pickup JC, Ford HM, Samsi K. Real-time continuous glucose monitoring in type 1 diabetes: a qualitative framework analysis of patient narratives. Diabetes Care. 2015;38:544-550. [DOI] [PubMed] [Google Scholar]
- 14. Ramchandani N, Arya S, Ten S, Bhandari S. Real-life utilization of real-time continuous glucose monitoring: the complete picture. J Diabetes Sci Technol. 2011;5:860-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Laffel L. Improved accuracy of continuous glucose monitoring systems in pediatric patients with diabetes mellitus: results from two studies. Diabetes Technol Ther. 2016;18:S2-23-S2-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Validation of measures of satisfaction with and impact of continuous and conventional glucose monitoring. Diabetes Technol Ther. 2010;12:679-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Telo GH, Volkening LK, Butler DA, Laffel LM. Salient characteristics of youth with type 1 diabetes initiating continuous glucose monitoring. Diabetes Technol Ther. 2015;17:373-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Anderson BJ, Auslander WF, Jung KC, Miller JP, Santiago JV. Assessing family sharing of diabetes responsibilities. J Pediatr Psychol. 1990;15:477-492. [DOI] [PubMed] [Google Scholar]
- 19. Hood KK, Butler DA, Anderson BJ, Laffel LMB. Updated and revised Diabetes Family Conflict Scale. Diabetes Care. 2007;30:1764-1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Green LB, Wysocki T, Reineck BM. Fear of hypoglycemia in children and adolescents with diabetes. J Pediatr Psychol. 1990;15:633-641. [DOI] [PubMed] [Google Scholar]
- 21. Clarke WL, Gonder-Frederick A, Snyder AL, Cox DJ. Maternal fear of hypoglycemia in their children with insulin dependent diabetes mellitus. J Pediatr Endocrinol Metab. 1998;11(suppl 1):189-194. [DOI] [PubMed] [Google Scholar]
- 22. Fendrich M, Weissman MM, Warner V. Screening for depressive disorder in children and adolescents: validating the Center for Epidemiologic Studies Depression Scale for Children. Am J Epidemiol. 1990;131:538-551. [DOI] [PubMed] [Google Scholar]
- 23. Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psych Meas. 1977;1:385-401. [Google Scholar]
- 24. Spielberger CD. Manual for the State-Trait Anxiety Inventory for Children. Palo Alto, CA: Consulting Psychologists Press; 1973. [Google Scholar]
- 25. Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Test Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 26. Markowitz JT, Volkening LK, Butler DA, Laffel LM. Youth-perceived burden of type 1 diabetes: Problem Areas in Diabetes Survey–Pediatric version (PAID-Peds). J Diabetes Sci Technol. 2015;9:1080-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Markowitz JT, Volkening LK, Butler DA, Antisdel-Lomaglio J, Anderson BJ, Laffel LM. Re-examining a measure of diabetes-related burden in parents of young people with type 1 diabetes: the Problem Areas in Diabetes Survey–Parent Revised version (PAID-PR). Diabet Med. 2012;29:526-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39:800-812. [DOI] [PubMed] [Google Scholar]
- 29. Varni JW, Burwinkle TM, Jacobs JR, Gottschalk M, Kaufman F, Jones KL. The PedsQL in type 1 and type 2 diabetes: reliability and validity of the Pediatric Quality of Life Inventory Generic Core Scales and type 1 Diabetes Module. Diabetes Care. 2003;26:631-637. [DOI] [PubMed] [Google Scholar]
- 30. McKnight JA, Wild SH, Lamb MJ, et al. Glycaemic control of type 1 diabetes in clinical practice early in the 21st century: an international comparison. Diabet Med. 2015;32:1036-1050. [DOI] [PubMed] [Google Scholar]
- 31. Wong JC, Foster NC, Maahs DM, et al. Real-time continuous glucose monitoring among participants in the T1D Exchange Clinic Registry. Diabetes Care. 2014;37:2702-2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. The effect of continuous glucose monitoring in well-controlled type 1 diabetes. Diabetes Care. 2009;32:1378-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mauras N, Fox L, Englert K, Beck RW. Continuous glucose monitoring in type 1 diabetes. Endocrine. 2013;43:41-50. [DOI] [PubMed] [Google Scholar]
- 34. Tubiana-Rufi N, Riveline JP, Dardari D. Real-time continuous glucose monitoring using GuardianRT: from research to clinical practice. Diabetes Metab. 2007;33:415-420. [DOI] [PubMed] [Google Scholar]
- 35. Triolo TM, Maahs DM, Pyle L, et al. Effects of frequency of sensor-augmented pump use on HbA1c and C-peptide levels in the first year of type 1 diabetes. Diabetes Care. 2016;39:e61-e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Markowitz JT, Pratt K, Aggarwal J, Volkening LK, Laffel LM. Psychosocial correlates of continuous glucose monitoring use in youth and adults with type 1 diabetes and parents of youth. Diabetes Technol Ther. 2012;14:523-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Polonsky WH, Hessler D. What are the quality of life-related benefits and losses associated with real-time continuous glucose monitoring? A survey of current users. Diabetes Technol Ther. 2013;15:295-301. [DOI] [PubMed] [Google Scholar]
- 38. Rachmiel M, Landau Z, Boaz M, et al. The use of continuous glucose monitoring systems in a pediatric population with type 1 diabetes mellitus in real-life settings: the AWeSoMe Study Group experience. Acta Diabetol. 2015;52:323-329. [DOI] [PubMed] [Google Scholar]


