Abstract
Background:
Insulin pens represent a significant technological advancement in diabetes management. While the vast majority have been designed with 1U-dosing increments, improved accuracy and precision facilitated by half-unit increments may be particularly significant in specific patients who are sensitive to insulin. These include patients with low insulin requirements and in those requiring more precise dose adjustments, such as the pediatric patient population. This review summarized functional characteristics of insulin half-unit pens (HUPs) and their effect on user experience.
Methods:
The literature search was restricted to articles published in English between January 1, 2000, and January 1, 2015. A total of 17 publications met the set criteria and were included in the review.
Results:
Overall, studies outlined characteristics for 4 insulin HUPs. Based on their functionality, the pens were generally similar and all met the ISO 11608-1 criteria for accuracy. However, some had specific advantageous features in terms of size, weight, design, dialing torque, and injection force. Although limited, the currently available user preference studies in children and adolescents with diabetes and their carers suggest that the selection of an HUP is likely to be influenced by a combination of factors such as these, in addition to the prescribed insulin and dosing regimen.
Conclusions:
Insulin HUPs are likely to be a key diabetes management tool for patients who are sensitive to insulin; specific pen features may further advance diabetes management in these populations.
Keywords: Half-unit insulin pen, HumaPen Luxura HD™, JuniorSTAR®, NovoPen Echo®, NovoPen Junior
Patients with diabetes who are sensitive to insulin (eg, children and the elderly) have low insulin requirements and so are more susceptible to dosing errors.1 A small volume error will represent a high additional percentage of total administered volume. Accuracy may be limited in low-dosage insulin users from rounding up or down to the nearest 1U.2 In this context, accuracy refers to the closeness of the administered value to the dialed/true value.3 Conversely, precision refers to the level of reproducibility of dosing facilitated by different insulin pens, that is, level of deviation between different doses administered.3 The improved accuracy and precision afforded by insulin half-unit pens (HUPs) compared with 1U pens and vial/syringe administration may potentially benefit patients sensitive to insulin through improved glycemic control.
Children and adolescents often use low doses of insulin and could therefore be vulnerable to dosing errors.1 Insulin requirements are determined based on body weight, age and pubertal status, and children with newly diagnosed type 1 diabetes mellitus (T1DM) may require an initial total daily dose as low as ~5-10 units/24 h administered in multiple low doses during the day.4-6 The high level of hypoglycemia observed in this population may be due to errors of administering small amounts of insulin1 or due to dosing limitations when adjusting to the nearest 1U dose with either mealtime or long-acting insulin.2 Similar to the pediatric population, elderly patients with diabetes who are sensitive to insulin may benefit from half-unit dosing accuracy.1 Half-unit dosing accuracy may also prove useful in patients who use carbohydrate counting to determine their mealtime insulin2,7 by precisely titrating their insulin dose to their dietary intake, and patients with concomitant Addison’s disease, hypopituitarism, chronic kidney disease, or chronic pancreatitis.2
The Diabetes Control and Complications Trial (DCCT) demonstrated the significance of intensified methods of blood glucose control in T1DM, as this was shown to delay the onset and slow the progression of diabetes-related complications.8-10 In TEENs, the largest worldwide study assessing T1DM management in youth aged 8-25 years, optimal glycemic control was only achieved by 28% of participants.11 Therapy adherence, central to optimal glycemic control, can impart significant treatment burden and worsen the quality of life (QoL) for people with T1DM.12 Adherence to therapy may be particularly challenging in the pediatric T1DM population, with lower compliance reported compared with adults.13 Traditionally, insulin has been administered subcutaneously by using a syringe manually filled from a vial.7 For many patients, this method presents barriers to treatment because of inaccuracy, pain, anxiety, inconvenience and problems with social acceptability.13-18 Alternative delivery methods, such as insulin pens, have been designed to help overcome these barriers,18-21 and HUPs may be particularly beneficial in achieving therapy adherence and optimal glycemic control in an insulin-sensitive population.
Since the first launch of a half-unit insulin pen (HUP) in 1992,15 other HUPs have been developed, for example, HumaPen Luxura HD™ (Eli Lilly and Company, Indianapolis, IN, USA; HPL), JuniorSTAR® (Sanofi, manufactured by Haselmeier, Stuttgart, Germany; JS), NovoPen Echo® (Novo Nordisk A/S, Bagsvaerd, Denmark; NPE), and NovoPen Junior (Novo Nordisk A/S; NPJ). The different functional characteristics and how these affect user experience and preference are outlined in the “Factors Affecting the User Preference for an HUP” section.
Objective
The objective of this review was to summarize the available literature on functional characteristics of HUPs and their effect on user experience and preferences.
Literature Review Methodology
This systematic review adheres, where relevant, to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement on developing a systematic review. The search was restricted to articles published in English between January 1, 2000, and January 1, 2015. Key search inclusion terms included half-unit insulin pen, half-unit insulin dose, half-unit insulin injection device, glycemic control, diabetes management, injection force, accuracy, patient preference, clinical outcomes, comparison, outcomes, and insulin sensitivity. Additional references not listed on the PubMed database were added if known to the authors and applicable to the review objective. These also included the user guides of HUPs identified through the literature search. Retrieved references were evaluated for suitability. Exclusion criteria included studies not relating to diabetes or insulin delivery devices, those assessing suitability of injection needles, studies of alternative insulin delivery devices, comparison of vial/syringe–pen use and articles describing disposable/prefilled insulin pens or 1U insulin pens. A total of 17 publications met the criteria outlined above and are reviewed in detail below.
Results
The literature was split broadly into 2 categories: (1) functional characteristics of HUPs and (2) factors affecting the user preference for an HUP.
Functional Characteristics of HUPs
This section outlines the main features and technical characterization of HUPs (Table 1), their accuracy, and injection forces required to perform an injection.
Table 1.
Summary of Half-Unit Insulin Pen Characteristics.
| Company | NovoPen Echo |
NovoPen Junior |
JuniorSTAR |
HumaPen Luxura HD |
|
|---|---|---|---|---|---|
| Novo Nordisk | Novo Nordisk | Sanofi (manufactured by Haselmeier) | Eli Lilly and Company | ||
| Weight with cartridge, g (± SD) | With cap | 59.3 (± 0.17)22 | 61.3 (± 0.33)22 | 41.7 (± 0.16)22 | 64.2 (± 0.34)22 |
| Without cap | 44.1 (± 0.15)22 | 44.1 (± 0.20)22 | 27.4 g (± 0.04)22 | 44.3 (± 0.34)22 | |
| Injection volume range (U) | 0.5-30 U24,26 | 1-35 U37,56 | 1-30 U42,57 | 0.5-30 U43 | |
| Insulin type | Penfill® 3 mL insulin cartridges24 | Penfill 3 mL insulin cartridges56 | Sanofi insulin cartridges28,57 | Lilly insulin cartridges (Insulin lispro, Insulin lispro protamine suspension/insulin lispro injection)27,37,43 | |
| Cartridge replacement mechanism | A simple twist with auditory feedback once cartridge is secure24 | Several revolutions56 | Several revolutions57 | Several revolutions43 | |
| Lowest dose (U) | 0.524,40 | 137 | 157 | 143 (accuracy at 0.5 dose demonstrated)27 | |
| Memory function | Last dose and time elapsed (up to 12 h)24,40,53 | None14,56 | None57 | None40,43 | |
| Recommended in situ time (s) | 6s24 | 6s56 | 10s57 | 5s43 | |
| Dial back | Dial-without insulin loss24 | Requires partial disassembly37,56 | Dial-back without insulin loss42 | Dial back without insulin loss37,43 | |
Main Features and Technical Characterization of HUPs
Pen weight, dimensions, and design
The overall size and weight of an insulin pen can greatly affect the convenience of every day transportation and ease of operation.16 Of the 4 HUPs examined, JS had the lowest weight (Table 1), NPE was the shortest with the cap on, while JS was the shortest without the cap.22 The sizes of the dosing display and digits may be of particular significance for populations with impaired visual acuity.23 HPL had the largest dose display and JS had the largest digits.22 Dimensions of the injection button will be particularly significant in populations with limited manual dexterity. HPL and JS had the dose buttons of largest dimensions.22 JS, NPJ, and NPE are also available in multiple colors, which may be beneficial for users who require different insulin variants. Further individualization is offered for the NPE with different skins which may be particularly relevant for the pediatric population.24
Durability/robustness
Reusable insulin pens are designed to withstand everyday use, and lifetime simulation is required as part of the ISO 11608-1 criteria.25 Kristensen and Lilleore conducted a study of simulated lifetime use of NPE, which maintained dosing accuracy at all doses throughout the simulated lifetime use test.26 Clark et al investigated the accuracy of the initial half-unit dose dispensed using the HPL over 3 different temperatures as specified in the ISO 11608 criteria (5°C, room temperature, and 40°C).27 All delivered doses satisfied the ISO 11608-1 criteria for accuracy of needle-based injection systems.27
Dialing torque
Dialing torque of an insulin pen refers to the strength needed to adjust the dose-setting dial.23 Greater ease of use contributes to the confidence a user has in the dose setting and accuracy of a pen,23 and may be of particular significance in pediatric patients and patients with limited hand strength. Dialing torque has only been reported for JS, where the mean dialing torque for both dialing and correcting a dose was between 5 and 6 N cm.28 A dialing torque of this magnitude is expected to allow easy dose dialing and correction for pediatric patients.29
Accuracy
Dose accuracy is vital for maintaining glycemic control and minimizing the risk of complications in people with diabetes.30,31 All HUPs have been shown to meet ISO 11608-1 criteria for accuracy of needle-based injection systems, so fluctuations within these criteria are unlikely to be of clinical significance.26,27,31-35
Injection Force
Injection force is defined as the peak force reached when pushing the injection button during administration of an insulin dose.20,36 While not confirmed in a clinical trial, a lower injection force is expected to facilitate simpler operation and reduced injection-site pain.16,32,33,37-39 JS had the lowest mean injection force of the HUPs investigated (5.94 N for the flow rate 6.00U/s).22 All HUPs required a smaller mean injection force than the maximum force that can be exerted on such devices by the pediatric population (>21 N).29 Therefore all HUPs are suitable for use by this population.
Factors Affecting the User Preference for an HUP
This section summarizes 3 studies examining patient experience in the use of different HUPs (Table 2) and outlines how the pen features translate into usability preferences. Olsen et al compared the usability and functionality of NPE, NPJ, and HPL for children and adolescents with T1DM, as well as parents and health care professionals working as diabetes nurse educators or physician/pediatricians.40 Wong et al conducted a randomized, 2-period, cross-over, open-label study to compare functional usability and preference of HPL and NPJ in 65 adult caregivers of children aged 3-12 years with T1DM.41 Klonoff et al conducted a noncomparative assessment of JS in 168 HUP users from 5 European countries.42
Table 2.
Summary of Surveys Examining the Usability of Different Half-Unit Insulin Pens.
| Olsen et al40 | Wong et al41 | Klonoff et al42 | |
|---|---|---|---|
| Study type | Face-to-face interviews and functionality testing | Randomized 2-period cross-over, open-label simulated injection study | Face-to-face interviews |
| Location | Germany, France, Canada | Not reported | France, UK, Germany, Italy, Spain |
| Population | Children and adolescents aged 7-18 years with T1DM (n = 79), parents (n = 78), and health care professionals (n = 48) | Adult caregivers of children aged 3-12 with T1DM (n = 65) | Nurses working with children with T1DM (n = 109), parents of children with T1DM aged 0-5 years (n = 16), parents of children with T1DM aged 6-12 years (n = 20), adolescents with T1DM (aged 13-18 years (n = 22) |
| Method | Usability assessment: setting up the pen, adjusting, injection into a foam cushion and use of memory function Preference assessment: Rating scales on a standardized nonvalidated online questionnaire |
Each item was answered on a 7-point scale for specific pen features as well as proportions of caregivers with a pen preference were used to assess the overall ease of use, ease of changing the insulin cartridge and ease of correcting the insulin dose | The performance of JS was rated for 18 attributes on a 5-point scale depending on question type: 1 (very poor, very difficult, completely disagree), 2 (somewhat poor, somewhat difficult, somewhat disagree), 3 (neither poor nor good, neither easy or difficult, not sure), 4 (somewhat good, somewhat easy, somewhat agree), or 5 (very good, very easy or completely agree) |
| Pens included | NPE, NPJ, HPL | HPL, NPJ | JS |
| Selecting dose to be delivered | Successful setting of the pen: 84% NPE, 88% NPJ, 91% HPL Rated easy or very easy: 77% NPE, 75% NPJ, 87% HPL |
Preference of ease of use: 94.7% HPL, 5.3% NPJ |
Dialing the dose rated as easy by 87% of nurses and 66% of parents/adolescents (total 80%) Reading the dose rated as easy by 94% of nurses and 98% of parents/adolescents (total 96%) |
| Injecting a dose | Proportion of participants completing injections: 95% NPE, 97% HPL, 60% NPJ | Rated easy to inject by 87% of nurses and 97% of parents/adolescents (total 90%) | |
| Injection force | Rated as ideal: About 70% for NPE and HPL, 58% for NPJ |
Rated suitable by 84% of nurses and 93% of parents/adolescents (total 87%) | |
| Size | Ideal in size: 70% NPE, 54% NPJ, 20% HPL |
||
| Weight | Rated as ideal: 53% NPE, 42% NPJ, 36% HPL |
Weight rated as suitable by 71% of nurses and 67% of parents/adolescents (total 69%) Pen rated as easy to carry by 86% of nurses and 81% of parents/adolescents (total 84%) |
|
| Changing the cartridge | Preference: 94.1% HPL, 5.9% NPJ |
Rated easy to change by 65% of nurses and 83% of parents/adolescents (total 71%) | |
| Dose correction | Preference: 94% HPL, 6% NPJ |
Rated easy to dial back a dose by 92% of nurses and 78% of parents/adolescents (total 87%) Mechanism of dialing back was found to give flexibility in dialing the correct dose by 94% of nurses and 95% of parents/adolescents (total 94%) |
|
| Memory function | Rated as easy to use: 89% of pediatric subjected and 94% of parents |
||
| Overall | 80% favored NPE40 | 80% HPL | Rated as easy to use by 94% of nurses and 93% of parents/adolescents (total 93%) |
HPL, HumaPen Luxura; JS, JuniorSTAR; NPE, NovoPen Echo; NPJ, NovoPen Junior; T1DM, type 1 diabetes mellitus.
Overall Preference
Noncomparative assessment of JS by both pediatric nurses and patients/parents (n = 167) found that the majority agreed JS was easy to use overall, convenient for everyday use and suitable for the lifestyle of a young person with T1DM.42 When the usability of HPL versus NPJ was tested, the majority of caregivers preferred HPL.41 Similarly, the majority of participants rated HPL easy/very easy to use, compared with NPE and NPJ.40
Injection Force
The majority of participants (both pediatric nurses and patients/parents) found the injection force of JS suitable for young people with diabetes.42 Olsen et al found that more participants using NPE and HPL found the injection force “ideal,” compared with users of NPJ (P = .005).40
Ease of Dose Dialing and Correction
To correct a dose that has been set higher than needed, NPJ requires the barrel and cartridge holder to be separated, followed by pressing the dial-up button back to 0, before the pen is assembled again and the correct dose can be dialed.19,37 In all other HUPs, an incorrect dose can be corrected by reversing the dial without any insulin loss.24,37,42,43 Overall, the majority of participants found it easy to dial and to dial back a dose using JS in terms of dial tactility and auditory feedback, and found that these pen features afford flexibility in dialing the correct dose.42 In terms of ease of correcting a dose, participants preferred HPL to NPJ.41 Similarly, NPE and HPL were rated better compared with NPJ.40 The lower preference of NPJ reported in these studies was likely due to the more complex dial-back mechanism compared with other HUPs. The studies indicate that a simple dial-back mechanism that does not waste insulin is preferable.
Ease of Reading Dose/Display
The majority of participants (children/adolescents/parents, 98%; nurses, 94%) rated JS as somewhat good/very good (top 2 options of a 5-point scale) in terms of ease of reading a dose, which may be because of the large dose digit measurement previously reported.42 The majority of participants also found the dose easy to read when comparing HPL, NPJ, and NPE in a pediatric population.40 This feature may be of particular significance for patients with sight impairments.
Changing of the Cartridge
Most HUPs require several revolutions to screw the cartridge holder onto the pen with the exception of NPE, which requires a single-movement twist. The majority of study participants (children/adolescents/parents, 83%; nurses, 65%) agreed that JS was somewhat good/very good for ease of changing the cartridge.42 In the direct comparison of HPL and NPJ, of the caregivers with a preference, the majority preferred HPL.41 No usability preference has been reported for NPE.40
Transportation and Handling
The majority of study participants (children/adolescents/parents, 81%; nurses, 86%) found JS easy to carry on a daily basis,42 which is important for insulin-sensitive patients who may need to keep the pen on them at all times and monitor insulin requirements regularly. A low weight and smaller size of the pen may be particularly important in light of smaller hands of children as well as for elderly patients with neuromuscular impairments, or hand fatigue.16,32,33,36,37,44
Memory Function
This feature has been developed as a safety feature to address user anxiety over a forgotten dose and risk of hypoglycemia through double-dosing.14,45-48 NPE is the only HUP with this feature and records the last dose volume and time (<12 h).24,40,49 The majority of pediatric users (89%) and parents (94%) rated this feature as very easy or easy to use.40 The memory function of NPE was also tested in the REMIND study, which included 2- to 18-year-old participants with T1DM.49 Overall, 83% of participants stated that they used the memory function during the study.49 Compared with the previously used device, forgotten injections were significantly reduced (51% vs 27%, respectively; P < 0.0001).49 However, switching from a previous device to an HUP with a memory function was not associated with improved glycemic control.49,50 Furthermore, only 42% of health care professionals rated this as a very important feature.46
Discussion
Clinical Significance
Inadvertent medication overdoses have been cited as “a common problem among children” by American Academy of Pediatrics.51 Accuracy to half-unit dose facilitated by HUPs may therefore be particularly significant in the pediatric population and adult patients who are sensitive to insulin.15,44 The outlined characteristics and features of the HUPs support a role for HUPs in improved adherence to treatment,37 leading to improved clinical outcomes and improved QoL.21,52 However, no interventional or comparative studies on clinical outcomes (glycemic control) using HUPs were identified in this literature search. Additional studies are required to investigate whether HUP use results in improved glycemic control, and how this feature translates into patient outcomes. Overall, while many of the benefits of HUPs may improve clinical outcomes for young patients with diabetes, real-world studies are required to determine whether this is the case.
Choosing an HUP
Insulin pen characteristics will affect injection procedure and user experience, and may ultimately affect treatment outcome (Figure 1). As insulin pens are manufactured for use with specific insulins, the choice of an insulin pen could also be determined by the insulin prescribed.44 Since the main objective of using insulin pens is to facilitate optimization of a patient’s diabetes management,53 pen characteristics should be matched with a user’s requirements and lifestyle.
Figure 1.
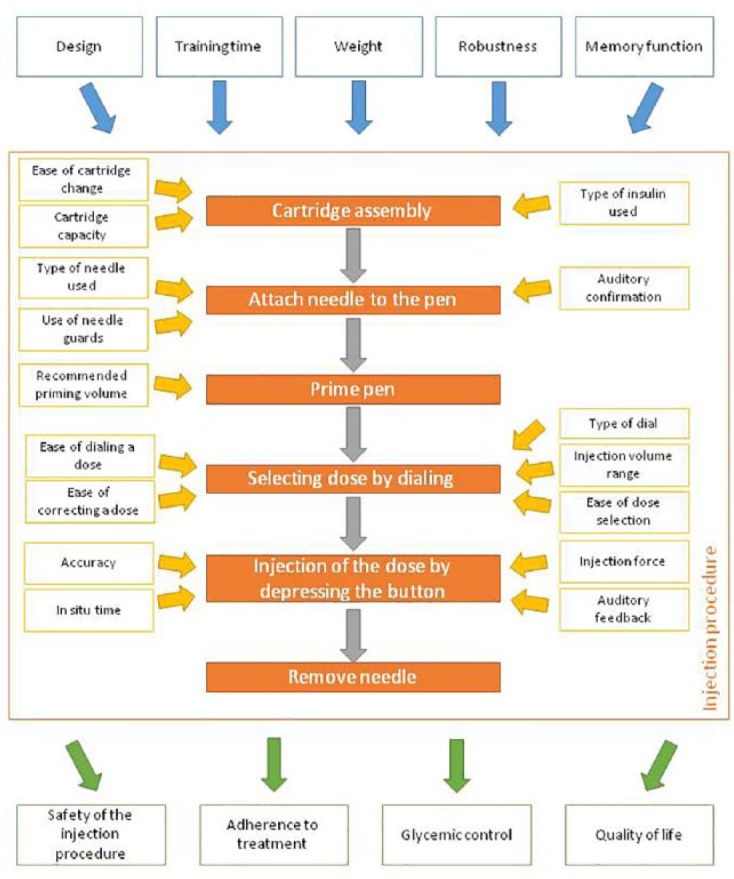
Summary of the different characteristics that may influence the user preference for an insulin pen and how these may impact the management of diabetes.
The choice of an insulin pen facilitating half-unit versus 1U dosing may be of particular significance to patients with T1DM who are more sensitive to insulin when accuracy at lower doses is crucial, as well as when requiring dose precision and accuracy to a half-unit. Other functional features of HUPs should be matched with user needs and preferences. HUP features affecting dose dialing, correction and dose reading may increase the confidence a user has in dose accuracy. Features facilitating ease and convenience of use, as well as discreetness, could improve treatment flexibility and social acceptance. The main objectives of HUPs are to enable accurate dose delivery to an insulin-sensitive patient, as well as reducing the physical, cognitive and emotional burden of diabetes management.53 Therefore, all the various features of HUPs and their impact on users’ lifestyle should be considered by health care professionals when prescribing insulin. The most recent development in insulin pens is the development of a “reusable wireless pen” facilitating half-unit increment for mealtime insulin for people with diabetes age 12 years and older, where a Bluetooth connection facilitates transfer of data from the insulin pen to a smartphone app.54 The app is designed to manage and catalog the insulin dose data, and provide a dose calculator to aid mealtime insulin dose calculations.54 It is likely that this type of technologic developments represent the future of insulin therapy in this patient population.
In the absence of head-to-head comparison studies across the available HUPs, no conclusion can be drawn on overall user preference.
Beyond Half-Unit Accuracy
U20 insulin preparations (20 units/mL) facilitated insulin dosing to 0.2U and were termed “toddler-friendly” insulins because of the small dose increments that could be administered.55 Since the standardization of U100 insulin preparations (100 units/mL), U20 (20 units/mL) have been withdrawn, leaving a gap in the market for insulin dosing accuracy below 1U.55 HUPs aid in addressing the lack of small dosing increments by enabling 0.5U dosing increments. Currently, precision and accuracy beyond 0.5U can only be achieved through the use of insulin pumps or by insulin dilution.55 However, these approaches have drawbacks, because the availability of pumps is limited and insulin dilution necessitates the use of a syringe,55 introducing the risk of contamination if a patient mixes their own parenteral medications. Pens delivering more accurate insulin dosing with 0.1U precision could benefit certain patient populations, such as insulin-sensitive patients using carbohydrate counting who may only require very low doses of insulin per day.
Conclusions
The development of HUPs has provided improved precision and accuracy of insulin dosing. This feature of HUPs may be relevant to patients requiring lower doses of insulin (eg, pediatric and populations who are sensitive to insulin) as well as others requiring more precise insulin dose adjustments. Currently available HUPs are generally similar in terms of technical characteristics, although some have specific advantageous features and these should be carefully considered for any individual’s needs. Although limited, the currently available studies suggest that the selection of an HUP is likely to be influenced by a combination of these aforementioned factors as well as the insulin and dosing regimen prescribed to the user. In summary, HUPs fill a need in insulin dosing and are likely to be a key diabetes management tool for the foreseeable future.
Footnotes
Abbreviations: DCCT, Diabetes Control and Complications Trial; HPL, HumaPen Luxura HD; HUP, half-unit insulin pen; ISO, International Organization for Standardization; JS, JuniorSTAR; NPE, NovoPen Echo; NPJ, NovoPen Junior; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; QoL, quality of life; T1DM, type 1 diabetes mellitus.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: DK is a consultant for Insulet, Lifecare, SANOFI, and Voluntis. US, HO, and CD are employees of SANOFI. The other authors have no disclosures to report.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Editorial support was provided by Fishawack Communications, funded by Sanofi, Paris, France.
References
- 1. McCoy EK, Wright BM. A review of insulin pen devices. Postgrad Med. 2010;122(3):81-88. [DOI] [PubMed] [Google Scholar]
- 2. Magnotti MA, Rayfield EJ. An analysis of the HumaPen Luxura HD pen: what is the role of 0.5-unit insulin dosing? J Diabetes Sci Technol. 2010;4(2):357-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Accuracy (trueness and precision) of measurement methods and results—part 1: introduction and basic principles (ISO/WD 15725-1) 2011. Available at: https://www.google.co.uk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=5&cad=rja&uact=8&ved=0CEYQFjAEahUKEwjPx6PoqrLHAhXDZtsKHS6fDGs&url=http%3A%2F%2Fstandardsproposals.bsigroup.com%2FHome%2FgetPDF%2F830&ei=Af3SVY_DAcPN7QauvrLYBg&usg=AFQjCNHYcaxyS9jfF1qoX5a9fj-7aVM6Ew&sig2=PRSbb7EBPKLbReSOj-lM2Q&bvm=bv.99804247,d.ZGU. Accessed August 17, 2015.
- 4. Silverstein J, Klingensmith G, Copeland K, et al. Care of children and adolescents with type 1 diabetes: a statement of the American Diabetes Association. Diabetes Care. 2005;28(1):186-212. [DOI] [PubMed] [Google Scholar]
- 5. Mortensen HB, Robertson KJ, Aanstoot HJ, et al. Insulin management and metabolic control of type 1 diabetes mellitus in childhood and adolescence in 18 countries. The Hvidore Study Group on Childhood Diabetes. Diabet Med. 1998;15(9):752-759. [DOI] [PubMed] [Google Scholar]
- 6. Mortensen HB, Hougaard P. Comparison of metabolic control in a cross-sectional study of 2,873 children and adolescents with IDDM from 18 countries. The Hvidore Study Group on Childhood Diabetes. Diabetes Care. 1997;20(5):714-720. [DOI] [PubMed] [Google Scholar]
- 7. Kroon L. Overview of insulin delivery pen devices. J Am Pharm Assoc. 2009;49(5):e118-e131. [DOI] [PubMed] [Google Scholar]
- 8. Selam JL. Evolution of diabetes insulin delivery devices. J Diabetes Sci Technol. 2010;4(3):505-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977-986. [DOI] [PubMed] [Google Scholar]
- 10. Diabetes Control and Complications Trial Research Group. Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. J Pediatr. 1994;125(2):177-188. [DOI] [PubMed] [Google Scholar]
- 11. Laffel L, Domenger C, Dain MP, et al. Global assessment of factors associated with target glycemic control in youth with type 1 diabetes (T1D): the TEENs study. Diabetes. 2014;63(suppl 1 A1-A102):A9, abstract 32-OR. [Google Scholar]
- 12. Piscopo MA, Chiesa G, Bonfanti R, Viscardi M, Meschi F, Chiumello G. Quality of life and new devices in the management of type 1 diabetes in children and adolescents. Acta Biomed. 2003;74(Suppl 1):21-25. [PubMed] [Google Scholar]
- 13. Hyllested-Winge J, Jensen KH, Rex J. A review of 25 years’ experience with the NovoPen family of insulin pens in the management of diabetes mellitus. Clin Drug Investig. 2010;30(10):643-674. [DOI] [PubMed] [Google Scholar]
- 14. Hanas R, de Beaufort C, Hoey H, Anderson B. Insulin delivery by injection in children and adolescents with diabetes. Pediatr Diabetes. 2011;12(5):518-526. [DOI] [PubMed] [Google Scholar]
- 15. Rex J, Jensen KH, Lawton SA. A review of 20 years’ experience with the NovoPen family of insulin injection devices. Clin Drug Investig. 2006;26(7):367-401. [DOI] [PubMed] [Google Scholar]
- 16. Cuddihy RM, Borgman SK. Considerations for diabetes: treatment with insulin pen devices. Am J Ther. 2013;20(6):694-702. [DOI] [PubMed] [Google Scholar]
- 17. Brunton S. Insulin delivery systems: reducing barriers to insulin therapy and advancing diabetes mellitus treatment. Am J Med. 2008;121(6 suppl):S35-S41. [DOI] [PubMed] [Google Scholar]
- 18. Asakura T, Seino H, Jensen KH. Patient acceptance and issues of education of two durable insulin pen devices. Diabetes Technol Ther. 2008;10(4):299-304. [DOI] [PubMed] [Google Scholar]
- 19. Tschiedel B, Almeida O, Redfearn J, Flacke F. Initial experience and evaluation of reusable insulin pen devices among patients with diabetes in emerging countries. Diabetes Ther. 2014;5(2):545-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Baruah MP. Insulin pens: the modern delivery devices. J Assoc Physicians India. 2011;59(suppl):38-40. [PubMed] [Google Scholar]
- 21. Oyer D, Niemeyer M, Moses A. Empowering people with diabetes: improving perceptions and outcomes with technical advances in insulin pens. Postgrad Med. 2012;124(5):110-120. [DOI] [PubMed] [Google Scholar]
- 22. Klonoff D, Nayberg I, Rabbone I, et al. Functional characterization of four different half-unit reusable insulin pens. Diabetes Technol Ther. 2015;17(S1):230. [Google Scholar]
- 23. Asakura T. Comparison of clinically relevant technical attributes of five insulin injection pens. J Diabetes Sci Technol. 2011;5(5):1203-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lakshmanadoss U, Ayyappan S, Jain A, Konezny M, Rajamani K. The broken pen penalty. Diabetes Technol Ther. 2010;12(3):241-243. [DOI] [PubMed] [Google Scholar]
- 25. Needle-based injection systems for medical use—requirements and test methods—part 1: needle-based injection systems. 2012. Available at: http://www.iso.org/iso/home/store/catalogue_ics/catalogue_detail_ics.htm?csnumber=52525. Accessed October 9, 2013.
- 26. Kristensen CM, Lilleore SK. Dose accuracy and durability of a durable insulin pen before and after simulated lifetime use. Curr Med Res Opin. 2011;27(10):1877-1883. [DOI] [PubMed] [Google Scholar]
- 27. Clark PE, Okenfuss CR, Campbell M. Half-unit dose accuracy with HumaPen Luxura HD: an insulin pen for patients who need precise dosing. J Diabetes Sci Technol. 2010;4(2):353-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Klonoff D, Nayberg I, Rabbone I, et al. Functional evaluation of the reusable JuniorSTAR® half-unit insulin pen. J Diabetes Sci Technol. 2015;9(3):625-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Department of Trade and Industry. Strength data for design safety—phase 1. Research commissioned by Consumer Affairs Division; 2000. Available at: http://webarchive.nationalarchives.gov.uk/+/http:/www.dti.gov.uk/files/file21830.pdf. Accessed February 19, 2014. [Google Scholar]
- 30. Friedrichs A, Basso N, Adler S. Dose accuracy of the ClikSTAR, NovoPen 4, and Luxura insulin pens: results of laboratory and field studies. J Diabetes Sci Technol. 2011;5(5):1179-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hanel H, Weise A, Sun W, Pfutzner JW, Thome N, Pfutzner A. Differences in the dose accuracy of insulin pens. J Diabetes Sci Technol. 2008;2(3):478-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Friedrichs A, Bohnet J, Korger V, Adler S, Schubert-Zsilavecz M, Abdel-Tawab M. Dose accuracy and injection force of different insulin glargine pens. J Diabetes Sci Technol. 2013;7(5):1346-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Friedrichs A, Korger V, Adler S. Injection force of reusable insulin pens: Novopen 4, Lilly Luxura, Berlipen, and ClikSTAR. J Diabetes Sci Technol. 2011;5(5):1185-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Xue L, Mikkelsen KH. Dose accuracy of a durable insulin pen with memory function, before and after simulated lifetime use and under stress conditions. Expert Opin Drug Deliv. 2013;10(3):301-306. [DOI] [PubMed] [Google Scholar]
- 35. Clarke A, Dain MP. Dose accuracy of a reusable insulin pen using a cartridge system with an integrated plunger mechanism. Expert Opin Drug Deliv. 2006;3(5):677-683. [DOI] [PubMed] [Google Scholar]
- 36. Toraishi K, Yuizono Y, Nakamura N, et al. Force requirements and insulin delivery profiles of four injection devices. Diabetes Technol Ther. 2005;7(4):629-635. [DOI] [PubMed] [Google Scholar]
- 37. Pearson TL. Practical aspects of insulin pen devices. J Diabetes Sci Technol. 2010;4(3):522-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Martin JM, Llewelyn JA, Ristic S, Bates PC. Acceptability and safety of a new 3.0 ml re-usable insulin pen (HumaPen) in clinical use. Diabetes Nutr Metab. 1999;12(5):306-309. [PubMed] [Google Scholar]
- 39. Pfutzner A, Asakura T, Sommavilla B, Lee W. Insulin delivery with FlexPen: dose accuracy, patient preference and adherence. Expert Opin Drug Deliv. 2008;5(8):915-925. [DOI] [PubMed] [Google Scholar]
- 40. Olsen BS, Lilleore SK, Korsholm CN, Kracht T. Novopen Echo® for the delivery of insulin: a comparison of usability, functionality and preference among pediatric subjects, their parents, and health care professionals. J Diabetes Sci Technol. 2010;4(6):1468-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wong M, Abdulnabi R, Fu H. Ease of use of two reusable, half-unit increment dosing insulin pens by adult caregivers of children with type 1 diabetes: a randomized, crossover comparison. J Diabetes Sci Technol. 2013;7(2):582-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Klonoff D, Nayberg I, Rabbone I, Domenger C, Danne T. Evaluation of the JuniorSTAR® half-unit insulin pen in young people with type 1 diabetes—user perspectives. Eur Endocrinol. 2013;9(2):82-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. HumaPen Luxura HD insulin delivery device user manual. 2013. Available at: http://pi.lilly.com/us/HumaPen_Luxura_HD_um.pdf. Accessed January 15, 2014.
- 44. Thurman JE. Analysis of insulin pen devices for the treatment of diabetes mellitus. J Diabetes Sci Technol. 2008;2(3):482-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Klausmann G, Hramiak I, Qvist M, Mikkelsen KH, Guo X. Evaluation of preference for a novel durable insulin pen with memory function among patients with diabetes and health care professionals. Patient Prefer Adherence. 2013;7:285-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Guo X, Sommavilla B, Vanterpool G, Qvist M, Bethien M, Lilleore SK. Evaluation of a new durable insulin pen with memory function among people with diabetes and healthcare professionals. Expert Opin Drug Deliv. 2012;9(4):355-356. [DOI] [PubMed] [Google Scholar]
- 47. Breslin SD, Ignaut DA, Boyd DE. Lessons learned during the development of HumaPen memoir, an insulin pen with a memory feature. J Diabetes Sci Technol. 2010;4(3):540-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ignaut DA, Venekamp WJ. HumaPen memoir: a novel insulin-injecting pen with a dose-memory feature. Expert Rev Med Devices. 2007;4(6):793-802. [DOI] [PubMed] [Google Scholar]
- 49. Adolfsson P, Veijola R, Huot C, Hansen HD, Lademann JB, Phillip M. Safety and patient perception of an insulin pen with simple memory function for children and adolescents with type 1 diabetes—the REMIND study. Curr Med Res Opin. 2012;28(9):1455-1463. [DOI] [PubMed] [Google Scholar]
- 50. Danne T, Forst T, Deinhard J, Rose L, Moennig E, Haupt A. No effect of insulin pen with memory function on glycemic control in a patient cohort with poorly controlled type 1 diabetes: a randomized open-label study. J Diabetes Sci Technol. 2012;6(6):1392-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Metric units and the preferred dosing of orally administered liquid medications. Pediatrics. 2015;135(4):784-787. [Google Scholar]
- 52. Lee IT, Liu HC, Liau YJ, Lee WJ, Huang CN, Sheu WH. Improvement in health-related quality of life, independent of fasting glucose concentration, via insulin pen device in diabetic patients. J Eval Clin Pract. 2009;15(4):699-703. [DOI] [PubMed] [Google Scholar]
- 53. Fry A. Insulin delivery device technology 2012: where are we after 90 years? J Diabetes Sci Technol. 2012;6(4):947-953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. FDA—InPen System. Available at: https://www.accessdata.fda.gov/cdrh_docs/pdf16/K160629.pdf. Accessed October 17, 2016.
- 55. Abul-Ainine SA, Abul-Ainine AA. Toddlers’ choice: Yo-Yoing diabetes control or deci-unit insulin dosing? World J Diabetes. 2012;3(2):35-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. NovoPen Junior instruction manual. 2001. Available at: http://www.i-wish.com/images/diabetes/pdf/novopen_junior_instruction_manual.pdf. Accessed February 10, 2014.
- 57. JuniorSTAR instructions for use. 2014. Available at: http://www.mystarsanofi.com/web/products/insulin-pens/juniorstar. Accessed July 2, 2015.


