Abstract
Background:
These studies investigated the accuracy of the new Contour®Next ONE blood glucose monitoring system (BGMS) that is designed to sync with the Contour™ Diabetes app on a smartphone or tablet.
Methods:
A laboratory study tested fingertip capillary blood samples from 100 subjects in duplicate using 3 test strip lots, based on ISO 15197:2013 Section 6.3 analytical accuracy standards. A clinical study assessed accuracy per ISO 15197:2013 Section 8 criteria. Subjects with (n = 333) or without (n = 43) diabetes and who had not used the BGMS previously were enrolled. Each subject performed a self-test using the BGMS, which was repeated by a site staff member. Alternate site tests and venipunctures were also performed for analysis. A questionnaire was provided to assess user feedback on ease of use.
Results:
In the laboratory study, 100% (600/600) of combined results for all 3 test strip lots met ISO 15197:2013 Section 6.3 accuracy criteria. In the clinical study, among subjects with diabetes, 99.4% (327/329) of subject self-test results, 99.7% (331/332) of results obtained by study staff, 97.2% (309/318) of subject palm results, and 100% (330/330) of venous results met ISO 15197:2013 Section 8 accuracy criteria. Moreover, 97.6% (321/329) of subject self-test results were within ±10 mg/dl (±0.6 mmol/L) or ±10% of the YSI reference result. Questionnaire results indicated that most subjects considered the system easy to use.
Conclusions:
The BGMS exceeded ISO 15197:2013 accuracy criteria in the laboratory and in a clinical setting.
Keywords: accuracy, blood glucose monitoring system, ISO 15197:2013, mobile app, self-monitoring of blood glucose
Self-monitoring of blood glucose (SMBG) is an important technique for assessing glycemic control in people with diabetes.1 SMBG can be useful for guiding therapy as it allows people with diabetes to evaluate their individual responses to treatment.1 When used for self-management of diabetes, SMBG has been correlated with lower glycated hemoglobin (HbA1c) levels,1 and is associated with positive effects on overall outcomes.1-4 Furthermore, SMBG has been associated with better glycemic control regardless of the type of diabetes or the therapy used.2
Emerging technologies, such as mobile apps, are helping people to manage their diabetes by providing personalized, real-time, data-driven support.5,6 Currently, there are many apps available to directly assist with diabetes management, as well as other apps that help track additional relevant information such as exercise, stress, diet, and medications. Many available diabetes apps involve manual logging of blood glucose and insulin data, which may be inconvenient for users and prone to errors.5 It is important that results obtained from SMBG are accurate because they are used to make critical decisions about diabetes management, and inaccurate results could lead to nutritional and drug dosing errors.7
Until recently, automatic wireless transmission of blood glucose data using smartphone technology was not available to people with diabetes.6 A new blood glucose monitoring system (BGMS), the Contour®Next ONE (Ascensia Diabetes Care, Parsippany, NJ, USA), has been developed for use with currently available Contour®Next test strips. The new BGMS features an easy-to-use blood glucose meter that can be wireless-enabled and link to a smart mobile device via Bluetooth® connectivity. The system is designed to sync with the Contour™ Diabetes app, which can be used on a smartphone or tablet.
To assess the accuracy of the new BGMS, 2 separate studies were undertaken. In the first study, the analytical accuracy of the BGMS was examined in the laboratory, based on the International Organization for Standardization (ISO) 15197:2013 Section 6.3 criteria.8 In the second study, BGMS performance and ease of use by subjects with and without diabetes in clinical settings were assessed based on the ISO 15197:2013 Section 8 protocol8 and the US Food and Drug Administration (FDA) draft guidance for BGMS accuracy,7 but only data related to ISO 15197:2013 accuracy criteria in subjects with diabetes (primary objective) will be presented in this article.
Methods
Laboratory Study: Analytical Accuracy (ISO 15197:2013 Section 6.3)
Fingertip capillary blood samples were obtained from 100 subjects and tested in duplicate using 3 test strip lots (N = 600). Two sets of 6 blood glucose meters were alternated between subjects. After every 10 subjects, a new vial of test strips was used; a total of 12 vials per lot were included in the study. Most samples (n = 85) were tested fresh from the finger without modification. However, contrived samples at the low and high end of the distribution were obtained by allowing 10 samples to glycolyze to a lower blood glucose concentration (<50 mg/dl [<2.8 mmol/L], n = 3; 50-80 mg/dl [2.8-4.4 mmol/L], n = 7) and supplementing 5 specimens with a concentrated glucose solution (20%) to increase the glucose level (>300-400 mg/dl [>16.7-22.2 mmol/L], n = 3; >400 mg/dl [>22.2 mmol/L], n = 2). Before and after BGMS testing, capillary blood from the same fingertip lancing site was collected into a microcuvette and centrifuged to obtain plasma. The plasma was assayed in duplicate on a YSI 2300 STAT Plus™ laboratory glucose analyzer (YSI; YSI Life Sciences, Inc, Yellow Springs, OH, USA) to produce comparison laboratory method reference values. Fingertip blood was also collected into 2 micro-hematocrit capillary tubes (Drummond Scientific Company, Broomall, PA, USA) and centrifuged with a StatSpin I microcentrifuge (StatSpin, Inc, Norwood, MA, USA) to measure hematocrit. All results were obtained at 23±5ºC.
Clinical Trial: User Performance Evaluation
The clinical study was designed to satisfy the ISO 15197:2013 Section 8 User Performance Evaluation8 and the FDA SMBG Draft 2014 Guidance, Section C, Method Comparison/User Evaluation.7 Institutional review board approval was obtained for the protocol, informed consent forms, and all study documents requiring such approval. Each subject completed the informed consent process before participating in the study.
Subjects with or without diabetes who were aged ≥18 years and had never used the BGMS previously were enrolled at 2 clinical sites. In accordance with the FDA guidance, approximately 10% of enrolled subjects did not have diabetes (ie, naïve to SMBG devices). Each subject’s participation consisted of 1 study visit. To enable reference method comparison, day-of-study criteria required that, ≤2 hours prior to capillary blood testing, subjects had not eaten or drank liquids; taken bolus insulin, rapid-acting insulin, or oral diabetes medications; or vigorously exercised. Subjects were provided with the BGMS and instructional materials (User Guide and Quick Reference Guide) as the only form of training. Subject oversight by study staff during testing was limited to observing that tests were conducted as instructed in the instructional materials.
Three test strip lots were used in the study, with each subject randomized to a single lot. The test strip lots were provided to the investigator by the study sponsor. Each subject used a lancing device to puncture his/her finger and performed a self-test using the BGMS. The site staff member then lanced the subject’s fingertip (within 5 minutes of the subject test) using another lancing device to collect blood for the YSI reference assay. Immediately thereafter, each subject lanced his/her palm and performed an alternate site test using the BGMS, followed by a site staff member lancing the subject’s fingertip to perform a meter test. A venipuncture was performed only on subjects with diabetes, and blood was tested by study staff with the BGMS and the YSI analyzer. All capillary blood meter results were compared to the capillary blood YSI reference results, and venous meter results were compared to the venous blood YSI reference results. A hematocrit measurement was performed on all subjects and was required for the results to be considered evaluable.
After each subject completed the clinical testing portion of the study, the instructions for use and the messages displayed on the meter were evaluated by a questionnaire that was designed to assess whether the subjects understood how to use the device correctly. This type of user feedback is required per ISO 15197:2013 Section 8.8.2. All subjects (with and without diabetes) were asked to respond to 8 statements relating to the use of the BGMS. Responses were obtained via a 5-point ordinal (Likert-type) scale, as follows: (1) strongly disagree, (2) disagree, (3) neutral, (4) agree, (5) strongly agree. The protocol required that ≥90% of subjects must respond with a score of 3 or higher for 6 specified statements. Subjects with diabetes completed a second questionnaire on diabetes management behaviors; responses were based on the same Likert scale as the previous questionnaire, with the additional option of “no response.” Adverse events were monitored throughout the trial.
Assessments and Analyses
The accuracy of BGMS results obtained in the laboratory study and in the clinical study was assessed in accordance with the ISO 15197:2013 Section 6.3 protocol and ISO 15197:2013 Section 8 protocol, respectively. BGMS results were compared with YSI reference results and assessed per ISO 15197:2013 accuracy criteria (ie, ≥95% of results within ±15 mg/dl [±0.8 mmol/L] of the reference result for samples with blood glucose concentrations <100 mg/dl [<5.6 mmol/L] or ±15% for samples with blood glucose concentrations ≥100 mg/dl [≥5.6 mmol/L]).8 In the clinical study, the primary objective was to assess the accuracy of the BGMS using fingertip self-test results in subjects with diabetes, based on ISO 15197:2013 accuracy criteria. Regression analysis was performed to compare BGMS results with YSI reference results. Parkes-Consensus Error Grid9 analyses were used to evaluate BGMS clinical accuracy in both the laboratory study and the clinical study. Radar Plots were constructed to compare BGMS results with YSI reference results in both the laboratory study and the clinical study.
Results
Laboratory Study: Analytical Accuracy (ISO 15197:2013 Section 6.3)
In the laboratory study, the observed range of plasma blood glucose concentrations was 36 mg/dl (2.0 mmol/L) to 643 mg/dl (35.7 mmol/L), and the hematocrit range was 31.5% to 51.5%. Overall, 100% (600/600) of combined results for all 3 test strip lots were within ±15 mg/dl (±0.8 mmol/L) or ±15% of the YSI reference result for samples with blood glucose concentrations <100 mg/dl (<5.6 mmol/L) or ≥100 mg/dl (≥5.6 mmol/L), respectively (Table 1). In addition, 98.3% (590/600) of combined results for all 3 test strip lots were within ±10 mg/dl (±0.6 mmol/L) or ±10% of the YSI reference result for samples with blood glucose concentrations <100 mg/dl (<5.6 mmol/L) or ≥100 mg/dl (≥5.6 mmol/L), respectively (Table 1). An ad hoc analysis was also conducted to determine the smallest error range within which at least 95% of meter inaccuracies (ie, differences between meter results and YSI reference results) fell. The results revealed that 95% of meter results were within 8.1 mg/dl (0.4 mmol/L) or 8.1% of the YSI reference result.
Table 1.
Summary of BGMS Results From the Laboratory Study (ISO 15197:2013 Section 6.3).
| Blood glucose concentration | Test strip lot | N | Number (%) of results within specified error limits | ||
|---|---|---|---|---|---|
| <100 mg/dl (<5.6 mmol/L) | ±5 mg/dl (±0.3 mmol/L) | ±10 mg/dl (±0.6 mmol/L) | ±15 mg/dl (±0.8 mmol/L)a | ||
| Lot 1 | 70 | 64 (91.4) | 70 (100.0) | 70 (100.0) | |
| Lot 2 | 70 | 62 (88.6) | 70 (100.0) | 70 (100.0) | |
| Lot 3 | 70 | 65 (92.9) | 70 (100.0) | 70 (100.0) | |
| Combined | 210 | 191 (91.0) | 210 (100.0) | 210 (100.0) | |
| ≥100 mg/dl (≥5.6 mmol/L) | ±5% | ±10% | ±15%a | ||
| Lot 1 | 130 | 96 (73.8) | 129 (99.2) | 130 (100.0) | |
| Lot 2 | 130 | 74 (56.9) | 124 (95.4) | 130 (100.0) | |
| Lot 3 | 130 | 87 (66.9) | 127 (97.7) | 130 (100.0) | |
| Combined | 390 | 257 (65.9) | 380 (97.4) | 390 (100.0) | |
| Total | ±5 mg/dl (±0.3 mmol/L) or ±5% | ±10 mg/dl (±0.6 mmol/L) or ±10% | ±15 mg/dl (±0.8 mmol/L)a or ±15%a | ||
| Lot 1 | 200 | 160 (80.0) | 199 (99.5) | 200 (100.0) | |
| Lot 2 | 200 | 136 (68.0) | 194 (97.0) | 200 (100.0) | |
| Lot 3 | 200 | 152 (76.0) | 197 (98.5) | 200 (100.0) | |
| Combined | 600 | 448 (74.7) | 590 (98.3) | 600 (100.0) | |
BGMS, blood glucose monitoring system; ISO, International Organization for Standardization.
ISO 15197:2013 Section 6.3 accuracy criteria.
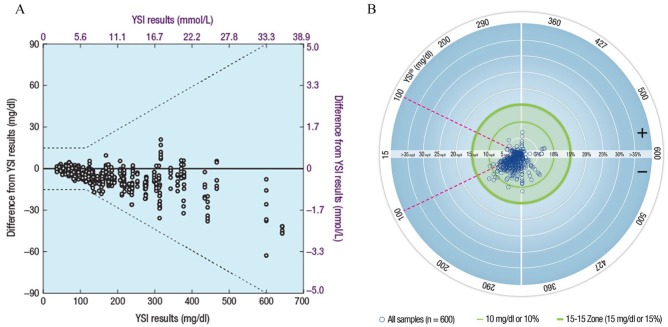
A plot of the differences of BGMS results from YSI reference results is shown in Figure 1A. A high degree of agreement between BGMS results and YSI reference results was demonstrated by regression analysis (y = 0.95x + 2.0 mg/dl [0.1 mmol/L]; R2 = 0.9961). All (100%; 600/600) results were within Zone A of the Parkes-Consensus Error Grid, satisfying the ISO 15197:2013 Section 6.3 accuracy criteria (ie, ≥99% of results shall fall within the combination of Zones A and B). A Radar Plot comparing BGMS results to YSI reference results is shown in Figure 1B. A Radar Plot is a recent way to plot the difference between BGMS values and reference instrument values. Points within the outer green circle (bolder line) satisfy ISO 15197:2013 Section 6.3 accuracy criteria. The outer green circle represents ±15 mg/dl (±0.8 mmol/L) or ±15% error for samples with YSI blood glucose concentrations <100 mg/dl (<5.6 mmol/L) or ≥100 mg/dl (≥5.6 mmol/L), respectively.
Figure 1.
Graphical representations of BGMS results from the laboratory study (ISO 15197:2013 Section 6.3). (A) Differences of BGMS results from YSI reference results.a (B) Radar Plot of BGMS results compared with YSI reference results.b
BGMS, blood glucose monitoring system; ISO, International Organization for Standardization; YSI, YSI analyzer.
aDashed lines represent ISO 15197:2013 Section 6.3 accuracy criteria.
bSingle points are representations of an error in units of mg/dl for YSI blood glucose values <100 mg/dl (<5.6 mmol/L; region of the plot within the magenta dashed lines) and in units of percentage for YSI blood glucose values ≥100 mg/dl (≥5.6 mmol/L; region of the plot outside the magenta dashed lines).
Clinical Trial: User Performance Evaluation in Subjects With Diabetes (ISO 15197:2013 Section 8)
Subjects
Of the 376 subjects enrolled in the study, 333 subjects had diabetes and were included in the ISO 15197:2013 Section 8 User Performance Evaluation. Demographic and baseline characteristics for subjects with diabetes are presented in Table 2. The mean age was 54 years (range, 18-81 years) and the ratio of female to male subjects was balanced for subjects with diabetes (51% female; 49% male). Of the subjects with diabetes, 35% had type 1 diabetes, 65% had type 2 diabetes, and 1% did not know their diabetes type. Most subjects with diabetes (67%) reported that they self-test their blood glucose ≥2 times daily.
Table 2.
Subject Demographic and Baseline Characteristics for Subjects With Diabetes in the Clinical Study.
| Characteristic | Subjects with diabetes (n = 333) |
|---|---|
| Gender, n (%) | |
| Female | 171 (51) |
| Male | 162 (49) |
| Type of diabetes, n (%)a | |
| Type 1 | 116 (35) |
| Type 2 | 215 (65) |
| Type unknown | 2 (1) |
| Do not have diabetes | — |
| Age, years | |
| Mean (range) | 54 (18-81) |
| Race, n (%)a | |
| White | 258 (78) |
| Black/African American | 46 (14) |
| Asian | 20 (6) |
| American Indian/Alaska Native | 6 (2) |
| Hawaiian/Pacific Islander | 2 (1) |
| No answer | 8 (2) |
| Duration of diabetes, n (%)a | |
| <1 month | 1 (0.3) |
| 1-3 months | 2 (1) |
| 4-6 months | 1 (0.3) |
| 7-12 months | 2 (1) |
| 13 months-2 years | 14 (4) |
| 3-5 years | 35 (11) |
| 6-10 years | 55 (17) |
| >10 years | 223 (67) |
| Frequency of daily SMBG, n (%) | |
| >4 | 84 (25) |
| 4 | 39 (12) |
| 3 | 50 (15) |
| 2 | 51 (15) |
| 1 | 65 (20) |
| <1 | 27 (8) |
| Does not test | 17 (5) |
SMBG, self-monitoring of blood glucose.
Percentages may not total 100% because of rounding. Also, some subjects provided more than 1 choice for race.
A total of 332 subjects with diabetes completed the study; the remaining 1 subject met enrollment criteria but did not meet day-of-study criteria. The observed range of plasma blood glucose concentrations for subjects with diabetes was 32 mg/dl (1.8 mmol/L) to 458 mg/dl (25.4 mmol/L) for capillary blood samples and 30 mg/dl (1.7 mmol/L) to 461 mg/dl (25.6 mmol/L) for venous blood samples; the hematocrit range was 33% to 59%. The range of plasma blood glucose concentrations for all subjects was 32 mg/dl (1.8 mmol/L) to 458 mg/dl (25.4 mmol/L) for capillary blood samples; the hematocrit range was 33% to 59%.
Accuracy
Evaluation of self-obtained capillary fingertip results from subjects with diabetes showed that 99.4% (327/329) of results met ISO 15197:2013 Section 8 accuracy criteria, and 97.6% (321/329) of results were within ±10 mg/dl (±0.6 mmol/L) or ±10% of the YSI reference result (Table 3). For subjects with diabetes, 99.7% (331/332) of fingertip results obtained by study staff, 97.2% (309/318) of subject palm results, and 100% (330/330) of venous results met ISO 15197:2013 Section 8 accuracy criteria (Table 3). An ad hoc analysis was conducted to determine the smallest error range within which at least 95% of meter inaccuracies (ie, differences between meter results and YSI reference results) fell. In this analysis, 95% of meter results for subjects with diabetes were within 8.4 mg/dl (0.5 mmol/L) or 8.4% of the YSI reference result for subject fingertip tests, 8.5 mg/dl (0.5 mmol/L) or 8.5% of the YSI reference result for study staff tests of subject fingertip blood, 11.8 mg/dl (0.7 mmol/L) or 11.8% of the YSI reference result for subject palm tests, and 6.4 mg/dl (0.4 mmol/L) or 6.4% of the YSI reference result for study staff tests of subject venous blood.
Table 3.
Summary of BGMS Accuracy for Subjects With Diabetes in the Clinical Study (ISO 15197:2013 Section 8).
| Blood glucose concentration | Test | Number (%) of results within specified error limits | |||
|---|---|---|---|---|---|
| <100 mg/dl (<5.6 mmol/L) | ±5 mg/dl (0.3 mmol/L) | ±10 mg/dl (0.6 mmol/L) | ±15 mg/dl (0.8 mmol/L)a | ±20 mg/dl (1.1 mmol/L) | |
| Subject fingertip (n = 74) | 65 (87.8) | 72 (97.3) | 73 (98.6) | 74 (100) | |
| Staff fingertip (n = 75) | 57 (76.0) | 73 (97.3) | 74 (98.7) | 75 (100) | |
| Subject palm (n = 61) | 45 (73.8) | 52 (85.2) | 58 (95.1) | 59 (96.7) | |
| Venous (n = 82) | 74 (90.2) | 82 (100) | 82 (100) | 82 (100) | |
| ≥100 mg/dl (≥5.6 mmol/L) | ±5% | ±10% | ±15%a | ±20% | |
| Subject fingertip (n = 255) | 214 (83.9) | 249 (97.6) | 254 (99.6) | 255 (100) | |
| Staff fingertip (n = 257) | 219 (85.2) | 253 (98.4) | 257 (100) | 257 (100) | |
| Subject palm (n = 257) | 188 (73.1) | 238 (92.6) | 251 (97.7) | 255 (99.2) | |
| Venous (n = 248) | 219 (88.3) | 247 (99.6) | 248 (100) | 248 (100) | |
| Total | ±5 mg/dl (0.3 mmol/L) or ±5% | ±10 mg/dl (0.6 mmol/L) or ±10% | ±15 mg/dl (0.8 mmol/L) or ±15%a | ±20 mg/dl (1.1 mmol/L) or ±20% | |
| Subject fingertip (n = 329) | 279 (84.8) | 321 (97.6) | 327 (99.4) | 329 (100) | |
| Staff fingertip (n = 332) | 276 (83.1) | 326 (98.2) | 331 (99.7) | 332 (100) | |
| Subject palm (n = 318) | 233 (73.3) | 290 (91.2) | 309 (97.2) | 314 (98.7) | |
| Venous (n = 330) | 293 (88.8) | 329 (99.7) | 330 (100) | 330 (100) | |
BGMS, blood glucose monitoring system; ISO, International Organization for Standardization.
ISO 15197:2013 Section 8 accuracy criteria.
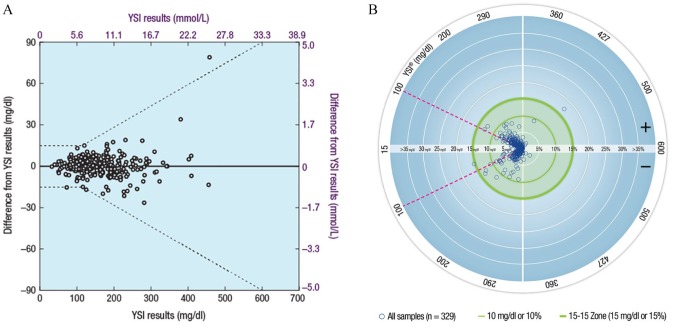
A plot of the difference of subject-obtained capillary fingertip results from YSI reference results for subjects with diabetes is shown in Figure 2A. A regression analysis of clinical results for subjects with diabetes demonstrated a strong correlation between BGMS and YSI reference results (adjusted R2 > 0.96 for all regressions). The R2 value was 0.9899 for subject-obtained capillary fingertip results, while the R2 values for study staff–obtained fingertip, subject palm, and venous results were 0.9899, 0.9697, and 0.9939, respectively. A Radar Plot comparing subject-obtained capillary fingertip results to YSI reference results for subjects with diabetes is shown in Figure 2B.
Figure 2.
Graphical representations of BGMS results from the clinical study in subjects with diabetes (ISO 15197:2013 Section 8). (A) Differences of subject-obtained capillary fingertip results from YSI reference results.a (B) Radar plot of subject-obtained capillary fingertip results compared with YSI reference results.b
BGMS, blood glucose monitoring system; ISO, International Organization for Standardization; YSI, YSI analyzer.
aDashed lines represent ISO 15197:2013 Section 8 accuracy criteria.
bSingle points are representations of an error in units of mg/dl for YSI blood glucose values <100 mg/dl (<5.6 mmol/L; region of the plot within the magenta dashed lines) and in units of percentage for YSI blood glucose values ≥100 mg/dl (≥5.6 mmol/L; region of the plot outside the magenta dashed lines).
By Parkes-Consensus Error Grid analysis, 100% (329/329) of subject-obtained capillary fingertip results for subjects with diabetes were within Zone A. For subjects with diabetes, all (100%) results were also within Zone A for study staff–obtained fingertip (332/332) and venous (330/330) testing; 98.7% (314/318) of results were within Zone A for subject palm testing, with the remainder (1.3%; 4/318) in Zone B.
Subject questionnaires
Ease-of-use questionnaire responses among subjects with diabetes (n = 332) demonstrated that most subjects “strongly agree,” “agree,” or are “neutral” that (1) it is easy to do a fingerstick blood test with this meter (97.9%), (2) the meter display is easy to see and read (98.2%), (3) it is easy to understand the test results (99.7%), (4) the instructions are easy to understand (97.6%), (5) the instructions clearly explain how to run a test (98.5%), and (6) the instructions clearly explain what to do if an error message is displayed by the meter (99.4%).
Diabetes management questionnaire responses among subjects with diabetes (n = 332) demonstrated that the majority of subjects “strongly agree,” “agree,” or are “neutral” that accuracy is important: (1) to help with their ability to manage their diabetes (99.4%), (2) to help with understanding how food or exercise affects low blood sugars (99.7%), (3) to help with preventing low blood sugars (99.7%), and (4) to help with using their results to gain better control of their diabetes (99.4%). In addition, 85.6% of subjects responded “strongly agree,” “agree,” or “neutral” that they use their current meter because their insurance companies cover the strips, and 92.1% of subjects responded “strongly agree,” “agree,” or “neutral” that they preferred the meter used in this study to their regular meters.
Safety
There were 8 mild, anticipated, non–device-related adverse events; all were classified as hypoglycemia and all occurred in subjects with diabetes. All adverse events resolved prior to the subjects leaving the testing site.
Discussion
Considering the importance of SMBG for the self-management of diabetes, it is crucial that a BGMS demonstrates a high degree of accuracy. We performed 2 separate studies to investigate the accuracy of the new BGMS. In the laboratory study, ISO 15197:2013 Section 6.3 accuracy criteria were used as the benchmark for assessing the results; while, in the clinical study, we employed ISO 15197:2013 Section 8 accuracy criteria. In our laboratory study, 100% of results with the BGMS met the accuracy criteria and 98.3% of results were within 10 mg/dl or 10% of the YSI reference values. In the clinical study, the BGMS exceeded the accuracy criteria. The BGMS demonstrated a high level of accuracy regardless of whether testing was performed by study staff, or by subjects who never used this BGMS previously and had no training other than access to the product instruction manual. The BGMS also demonstrated ease of use, as nearly all subjects with diabetes rated the ease of use of the BGMS favorably on a questionnaire in the clinical study.
Use of computer-based diabetes self-management interventions has shown generally positive results with regard to blood glucose control, with an increased effect when using mobile smartphone apps.10 A recent meta-analysis of 22 trials (N = 1,657) on the effect of mobile phone interventions in diabetes showed a 6-mmol/mol (0.5%) reduction in HbA1c values over 6 months.11 Several additional reviews and meta-analyses have evaluated similar data, all showing generally positive results in diabetes management among people with diabetes using mobile phone apps.10,12-14
Although studies have shown various clinical benefits of using mobile apps for diabetes, including reductions in HbA1c levels, further research is needed in this area.10,12-14 Studies with larger sample sizes and longer durations are needed to adequately assess the true impact of mobile phone apps on diabetes management.13 In addition, the reasons underlying this increased effectiveness with smartphone apps—whether it be convenience, frequency, prompting, feedback, or behavioral change—are still undifferentiated.10,14 Investigation into the types of individuals who are most likely to benefit from electronic monitoring may also help us better understand which aspects of these interventions are most effective and why, and these learnings could potentially be extended to other chronic conditions.
Conclusion
The results of these studies demonstrate the analytical and clinical accuracy of the new BGMS as well as its ease of use in a clinical setting among subjects who had never used the BGMS previously.
Footnotes
Abbreviations: BGMS, blood glucose monitoring system; FDA, US Food and Drug Administration; HbA1c, glycated hemoglobin; ISO, International Organization for Standardization; SMBG, self-monitoring of blood glucose; YSI, YSI glucose analyzer.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: MC has nothing to disclose. CG, SP, MEWW, and BH are employees of Ascensia Diabetes Care. RM was an employee of Ascensia Diabetes Care at the time of the study. TSB has received research support from Abbott, ACON, Ascensia Diabetes Care, Bristol-Myers Squibb, Dexcom, GlaxoSmithKline, Halozyme, Insulet, Janssen, Lexicon, LifeScan, Lilly, Medtronic, Merck, Novo Nordisk, Orexigen, and Sanofi; has received consulting honoraria from Ascensia Diabetes Care, AstraZeneca, BD, Lilly, Medtronic, Novo Nordisk, and Sanofi; and has received speaking honoraria from Abbott, Insulet, Novo Nordisk, and Sanofi.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: These studies were supported by Bayer HealthCare, the predecessor-in-interest of Ascensia Diabetes Care, Parsippany, NJ, USA. Medical writing assistance was provided by Allison Michaelis, PhD, of MedErgy, and was funded in part by Ascensia Diabetes Care, and in part by Bayer HealthCare as Ascensia’s predecessor-in-interest.
References
- 1. American Diabetes Association. Standards of medical care in diabetes—2016. Diabetes Care. 2016;39(suppl 1):S1-S108. [DOI] [PubMed] [Google Scholar]
- 2. Karter AJ, Ackerson LM, Darbinian JA, et al. Self-monitoring of blood glucose levels and glycemic control: the Northern California Kaiser Permanente Diabetes Registry. Am J Med. 2001;111(1):1-9. [DOI] [PubMed] [Google Scholar]
- 3. Polonsky WH, Fisher L, Schikman CH, et al. A structured self-monitoring of blood glucose approach in type 2 diabetes encourages more frequent, intensive, and effective physician interventions: results from the STeP study. Diabetes Technol Ther. 2011;13(8):797-802. [DOI] [PubMed] [Google Scholar]
- 4. Franciosi M, Lucisano G, Pellegrini F, et al. ROSES: role of self-monitoring of blood glucose and intensive education in patients with type 2 diabetes not receiving insulin. A pilot randomized clinical trial. Diabet Med. 2011;28(7):789-796. [DOI] [PubMed] [Google Scholar]
- 5. Heintzman ND. A digital ecosystem of diabetes data and technology: services, systems, and tools enabled by wearables, sensors, and apps. J Diabetes Sci Technol. 2015;10(1):35-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Klonoff DC. The current status of mHealth for diabetes: will it be the next big thing? J Diabetes Sci Technol. 2013;7(3):749-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. US Food and Drug Administration. Blood glucose monitoring test systems for prescription point-of-care use: draft guidance for industry and Food and Drug Administration staff. Available from: http://www.fda.gov/downloads/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm380325.pdf. Accessed June 10, 2016.
- 8. International Organization for Standardization. ISO 15197:2013(E): in vitro diagnostic test systems—requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus. Geneva, Switzerland: International Organization for Standardization; 2013. [Google Scholar]
- 9. Parkes JL, Slatin SL, Pardo S, Ginsberg BH. A new consensus error grid to evaluate the clinical significance of inaccuracies in the measurement of blood glucose. Diabetes Care. 2000;23(8):1143-1148. [DOI] [PubMed] [Google Scholar]
- 10. Pal K, Eastwood SV, Michie S, et al. Computer-based interventions to improve self-management in adults with type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2014;37(6):1759-1766. [DOI] [PubMed] [Google Scholar]
- 11. Liang X, Wang Q, Yang X, et al. Effect of mobile phone intervention for diabetes on glycaemic control: a meta-analysis. Diabet Med. 2011;28(4):455-463. [DOI] [PubMed] [Google Scholar]
- 12. Baron J, McBain H, Newman S. The impact of mobile monitoring technologies on glycosylated hemoglobin in diabetes: a systematic review. J Diabetes Sci Technol. 2012;6(5):1185-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Holtz B, Lauckner C. Diabetes management via mobile phones: a systematic review. Telemed J E Health. 2012;18(3):175-184. [DOI] [PubMed] [Google Scholar]
- 14. El-Gayar O, Timsina P, Nawar N, Eid W. Mobile applications for diabetes self-management: status and potential. J Diabetes Sci Technol. 2013;7(1):247-262. [DOI] [PMC free article] [PubMed] [Google Scholar]