Abstract
Background:
Many individuals with type 1 diabetes (T1D) upload and review blood glucose data between clinic visits. Mobile phone applications that receive data from a “connected” glucometer and that support pattern management are available and have the capacity to make data upload and review less burdensome. Whether mobile apps can improve diabetes self-management among individuals with type 1 diabetes remains unknown.
Method:
We analyzed retrospective data on 81 youths with T1D who were trained to use a glucometer-connected mobile app in their self-management. To assess the effect of glucometer synchronization (“sync”) rate on hemoglobin A1c (HbA1c), mean blood glucose (mBG), and daily frequency of SMBG, we regressed those clinical outcomes on the frequency of glucometer syncs with the mobile app after controlling for other clinical care variables.
Results:
Median age was 14.0 (IQR 10.4-15.9) years, median duration of diabetes was 4.9 (2.7, 7.5) years, and median baseline HbA1c was 8.6% (7.9, 9.8). The sample was 49% male and 86% white. Youths with T1D synchronized glucometer data with the mobile app an average of 0.22 times per week (range 0-2.25). The glucometer sync rate did not have a statistically significant association with HbA1c or mean BG; in contrast, data sync frequency was associated with the frequency of self-monitoring of blood glucose (SMBG) such that each additional sync was associated with a 2.3-fold increase in SMBG frequency (P < .01).
Conclusion:
A glucometer-connected mobile app may increase an individual’s engagement with other aspects of care (eg, SMBG frequency). Whether diabetes device-connected mobile apps can improve glycemic control remains to be determined.
Keywords: adherence, children, glucometer, glycemic control, hemoglobin A1c, mobile application, type 1 diabetes
Despite advances in both insulin therapeutics and technology to improve insulin delivery and glucose monitoring, many children and adolescents with type 1 diabetes (T1D) fail to meet established targets for glycemic control.1 Recent data suggest that individuals with T1D who download and review their blood glucose data for patterns between clinic visits exhibit better glycemic control than those who do not.2 Unfortunately, the majority of individuals with T1D do not regularly download data between clinic visits or review those data for patterns to inform insulin adjustment.2-5 Continuous glucose monitors support real-time decision-making among individuals with T1D, but their associated mobile phone applications do not currently archive data across multiple days, incorporate software tools to support pattern management, or display complete glucometer or insulin pump information. Multiple mobile applications (ie, “apps”) to support diabetes self-management have been described; some of these also support direct data synchronization between glucometers and the mobile app on a mobile phone.6-9 Few studies have evaluated the effect of data synchronization to a mobile app on glycemic control or other self-care behaviors in T1D.
The Glooko software application for mobile phones (further referred to as “the mobile app”) supports data upload from many glucometers, as well as several continuous glucose monitors and insulin pumps.10 The software includes data visualizations designed to assist with the identification of patterns in blood glucose, insulin, carbohydrate, and exercise data. The data are also shared from the mobile app to a cloud data management system to facilitate review by caregivers and health care teams. In general, mobile apps to support diabetes self-management have the potential to ease the burden of data upload and review by individuals with T1D. But whether mobile apps can improve diabetes self-management remains unknown. It is important to answer this question, because there are a proliferation of mobile medical apps intended to support diabetes self-management. The impact of a mobile app on self-management, disease control, and quality of life must be examined before investigators can discern the mechanisms by which the mobile app impacts care, and before clinicians and patients can make informed decisions about incorporating mobile medical applications into diabetes self-care.
To begin to answer this question, we retrospectively reviewed the records of 81 youths who were provided access to the mobile app during routine clinical care from March 2015 to April 2016 and instructed to share data from their glucometer(s). The purpose of the present study was to assess the impact of the frequency of glucometer-based data synchronization events via the mobile app on HbA1c (primary endpoint). We also examined effects on two secondary endpoints: mean blood glucose and frequency of blood glucose monitoring.
Methods
Institutional review board approval was obtained, and the study was conducted according to the Declaration of Helsinki.
Data Source
Data were extracted from the Children’s Mercy database on Type One Diabetes in Pediatrics (the Children’s Mercy on TODP database).11,12 The Children’s Mercy on TODP database is a longitudinal database containing demographic, clinical, and laboratory data extracted from the electronic health records and medical device downloads of youth and young adults with T1D receiving care since June 1, 1993, at the Children’s Mercy Kansas City (Kansas City, MO, USA) diabetes center, which is a network of 13 clinic sites in two states in the United States.
Inclusion/Exclusion Criteria for Data Source
All individuals diagnosed with T1D who had at least one appointment in the diabetes clinic were included. Those with other types of diabetes (such as type 2, monogenic, cystic-fibrosis-related, or iatrogenic diabetes) or with other comorbid diagnoses that might impact their diabetes care or complications (eg, sickle cell disease, leukemia, congenital syndromes and heart disease) were excluded. In total, 5923 individuals were included in the database.
Variable Definitions for Data Source
HbA1c
Youths’ HbA1c levels were measured on either the Tosoh G8 HPLC (Tosoh Bioscience Inc, San Francisco, CA, USA) or the Afinion AS100 Analyzer (Orlando, FL, USA). Both instruments have demonstrated traceability to the Diabetes Control and Complications Trial (DCCT) standard.13,14 Results were reported as percentages (%; NGSP standard). Conversions to SI units (mmol/mol; IFCC standard) can be performed using the NGSP’s HbA1c converter at http://www.ngsp.org/convert1.asp. Baseline HbA1c was the value obtained at the time the individual was first offered access to and educated to use the mobile app. End-of-study HbA1c was the subsequent HbA1c value obtained ≥60 days after the baseline HbA1c measurement.
Duration of diabetes
This was calculated as the age at baseline HbA1c for the study minus the age at diagnosis; it was expressed in decimal years. Age at diagnosis was calculated to the nearest one-hundredth year by counting the number of days between date of diagnosis and the date of birth. When not documented, date of diagnosis was determined as the first date at which (1) the patient met ADA criteria for a diagnosis of diabetes and (2) C peptide and/or auto-antibody screening indicated T1D.
Demographic characteristics
Sex (male/female) and race/ethnicity (Caucasian, African American, Hispanic, Asian, American Indian or Alaska Native, Native Hawaiian or Pacific Islander, Multiracial, or Other) were self-reported by the patient or family at the first encounter with the institution. Race (but not ethnicity) was used in the present analysis and was categorized as white/nonwhite. Age at baseline, BMI z-score, insulin pump use (yes/no), and continuous glucose monitoring (CGM) use (yes/no) were determined at the time each individual was first offered access to, and educated to use, the mobile app.
Synchronization rate
Each patient’s count of synchronizations during the observation period was divided by the length of the patient’s time in the observation period (age at end-of-study HbA1c minus age at baseline HbA1c) to yield the rate of synchronizations per week. The synchronization that occurred in clinic at the time the patient/family were trained to use the mobile app was not included in this calculation. Synchronization rate was the focal explanatory variable.
Mean blood glucose
This was calculated as the arithmetic mean of all blood glucose values obtained during the study period.
Blood glucose monitoring frequency
The count of blood glucose monitoring episodes using all available blood glucose values during the study period was recorded and used as a dependent variable in negative binomial regressions. The study day count was treated as an exposure variable in the regression model, allowing us to effectively model the blood glucose monitoring frequency (see the Statistical Analysis section).
Study Cohort
The study cohort represents a convenience sample of individuals introduced to a mobile app to support diabetes self-care. Only individuals who were introduced to, and educated to use, the mobile app (Glooko, Mountain View, CA, USA) between March 2015 and April 2016 were included in the present analyses. Of an initial sample of 95, 8 individuals with missing data on covariates were excluded, as were 5 individuals who also synchronized CGM data with the mobile app. An additional patient with a severely outlying synchronization count (8 standard deviations above the mean) was also excluded. The remaining 81 patients were included in the HbA1c analysis. Of these, data on blood glucose were available for 70 patients. The sample used in analyzing blood glucose monitoring frequency was further limited to the 59 patients for whom monitoring data were available for at least 14 days.
For comparison to the larger clinic population, information was also collected on all individuals with T1D receiving care from March 2015 and April 2016 (N = 2294).
Mobile App Training
Parents and youths were trained to use the mobile app during a routinely scheduled clinic visit by a physician or certified diabetes educator (CDE). The training protocol included guiding parents and youths through the processes of downloading the app to their mobile devices, creating an account in the mobile app, and connecting their account to the clinic’s professional account. They were provided a cable or Bluetooth adapter as needed to permit connection of the glucometer with their mobile phone. The teaching procedure used included (1) a demonstration of device synchronization by the physician or CDE, (2) reviewing each page of the mobile app with a talk-out loud-protocol to explain the function of and data presentations on each page, (3) a practice device synchronization by the parent or youth, and (4) teach-back by the parent or youth (if 13 years or greater). Parents and youths were instructed that the youth or either parent could be responsible for data synchronization. While synchronized data were potentially visible to the health care team, the present study did not record or evaluate whether synchronized data were reviewed for patterns by youths, their parents, or their health care team due to limited availability of such information.
Statistical Analysis
Covariates included patient sex, race (white or nonwhite), age at baseline, duration of diabetes, BMI z-score, and indicators for use of insulin pump (yes or no) and continuous glucose monitoring (yes or no). To evaluate how representative the cohort using the mobile app was of the entire clinic population, demographic variables of the study cohort were compared to demographic variables of the clinic population. Analyses were carried out in SAS 9.4 except as otherwise indicated.
We assessed the effect of synchronization frequency on HbA1c by regressing final HbA1c measurement on synchronizations per week, baseline HbA1c measurement, and the covariates listed above using the lm package in R. Model residuals were not Gaussian so confidence intervals and P values were computed by nonparametric bootstrapping using the R boot package with 15 000 bootstrap samples.
To assess the effect of synchronization frequency on mean blood glucose we regressed mean blood glucose on synchronization frequency and the set of covariates, again using the lm and boot packages in R.
We used negative binomial regression to assess the effect of synchronization frequency on the frequency of blood glucose monitoring, effectively modeling the blood glucose monitoring rate by modeling the count of monitoring episodes as the dependent variable and treating study day count as an exposure variable (taken into account by including the log of the study day count as an offset in the model). Synchronization frequency and the set of covariates were included as explanatory variables. The model was fit using the GLIMMIX Procedure in SAS.
Results
Cohort Characteristics
Characteristics of the study cohort and larger clinic population are provided in Table 1. In Wilcoxon two-sample tests for the four continuous variables, age (P = .06) and BMI z-score (P = .06) had P values suggesting differences that may not be attributable to chance. Fisher exact tests for the dichotomous variables yielded a statistically significant result only for insulin pump use (P = .02), which was higher in the sample (78%) than in the clinic population (65%).
Table 1.
Characteristics of Study Cohort and Clinic Population.
| Study cohort (n = 81) | Clinic population (n = 2294) | P value | |
|---|---|---|---|
| Age | 14.0 (10.4, 15.9)a | 14.4 (10.9, 17.0) | .06a |
| Duration of diabetes | 4.9 (2.7, 7.5) | 4.4 (2.0, 7.9) | .67a |
| Baseline HbA1c | 8.6 (7.9, 9.8) | 8.7 (7.8, 10.0) | .94a |
| BMI z-score | 0.46 (−0.26, 1.08) | 0.68 (0.00, 1.27) | .06a |
| Male (%) | 49 | 51 | .82b |
| White (%) | 86 | 82 | .38b |
| Insulin pump user (%) | 78 | 65 | .02b |
| CGM user (%) | 30 | 22 | .14b |
Values are median (interquartile range), unless otherwise noted.
Wilcoxon two-sample test. bFisher’s exact test.
Of the 70 patients with blood glucose data, the 11 without at least 14 days of monitoring data were slightly older than the 59 with at least 14 days of monitoring data (median 14.8 [IQR 14.0-16.2] vs 13.7 [8.8, 15.9] years) and had longer duration of diabetes (6.1 [3.1, 8.9] vs 4.7 [2.5, 7.3] years). They were also disproportionately male (64% vs 51%) and less likely to be users of an insulin pump (64% vs 80%) or CGM (9% vs 31%). None of these differences was statistically significant.
Synchronization Frequency
Among individuals in the study cohort, the interval between baseline HbA1c and end-of-study HbA1c varied to some degree, with the average observation period being 0.3 years (3.6 months; mean age at end-of-study HbA1c minus mean age at baseline HbA1c), and the minimum observation period being 0.2 years (2.4 months). We thus calculated the weekly rate of data synchronization to the mobile app. Individuals shared data between their glucometers and the mobile app an average of 0.22 ± 0.40 times per week (range 0-2.25) during the observation period. Of the 81 individuals, 35 (43%) did not synchronize at all during the study period (see Figure 1); the mean among the 46 who synchronized at least once postbaseline was 0.39 ± 0.47.
Figure 1.
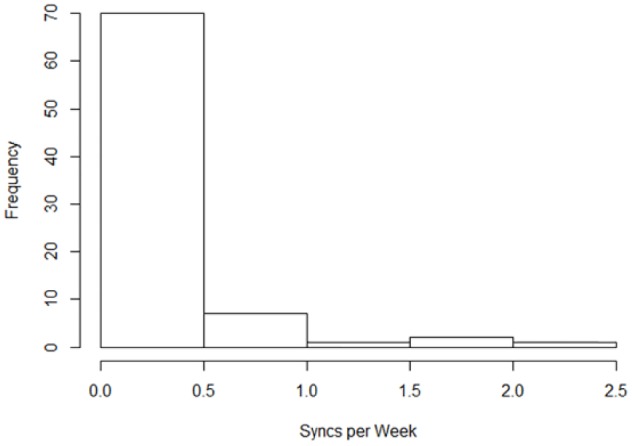
Synchronizations per week.
Main Outcomes
We first related synchronization frequency to end-of-study HbA1c while controlling for baseline HbA1c and covariates. The frequency of synchronizing glucometer data to the mobile app did not relate to end-of-study HbA1c in a statistically significant way, although modeling yielded an estimated 0.75 percentage point (0.43 SD) decrease in HbA1c per additional synchronization per week, after controlling for baseline HbA1c and covariates (Table 2). Next, we related data synchronization frequency to mean blood glucose while controlling for baseline HbA1c and covariates. Although we observed an estimated 20.3 mg/dL (0.40 SD) decrease in mean blood glucose concentration per additional synchronization per week was observed, this effect was similarly nonsignificant (Table 3). In contrast, there was a strong and statistically significant association between frequency of data synchronization to the mobile app and frequency of self-monitoring of blood glucose, which is a measure of treatment adherence. Each additional synchronization per week was associated with an estimated 2.3-fold increase in the rate of blood glucose checks per day (P < .01), assuming model covariates are held constant (Table 4).
Table 2.
Model for HbA1c.
| Estimate (95% CI) | P value | |
|---|---|---|
| Syncs per week | −0.75 (−1.72, 0.52) | .44 |
| Baseline HbA1c | 0.73 (0.47, 0.91) | .00 |
| Male | 0.41 (−0.17, 1.05) | .22 |
| White | 0.46 (−0.7, 1.24) | .27 |
| Age at baseline | −0.02 (−0.11, 0.05) | .57 |
| Duration | 0.06 (−0.05, 0.2) | .34 |
| Insulin pump | 0.15 (−0.65, 1.25) | .83 |
| CGM | −0.47 (−1.25, 0.13) | .26 |
| BMI Z Score | 0.08 (−0.24, 0.42) | .59 |
Table 3.
Model for Mean Blood Glucose.
| Estimate (95% CI) | P value | |
|---|---|---|
| Syncs per week | −20.3 (−56.0, 23.2) | .62 |
| Baseline HbA1c | 19.5 (10.4, 26.0) | .00 |
| Male | 3.3 (−14.6, 25.8) | .90 |
| White | 15.8 (−30.2, 47.5) | .29 |
| Age at baseline | −1.6 (−4.9, 0.9) | .32 |
| Duration | 2.9 (0.1, 6.6) | .11 |
| Insulin pump | 4.2 (−24.4, 40.6) | .90 |
| CGM | −11.2 (−40.5, 12.2) | .57 |
| BMI Z Score | 6.7 (−5.6, 17.9) | .23 |
Table 4.
Model for Blood Glucose Monitoring Frequency.
| Exp(B)a | 95% lower for exp(B) | 95% upper for exp(B) | P value | |
|---|---|---|---|---|
| Syncs per week | 2.30 | 1.82 | 2.90 | <.01 |
| Baseline HbA1c | 0.96 | 0.90 | 1.01 | .13 |
| Male | 0.99 | 0.79 | 1.23 | .89 |
| White | 0.92 | 0.68 | 1.24 | .56 |
| Age at baseline | 0.93 | 0.90 | 0.96 | <.01 |
| Duration | 1.03 | 1.00 | 1.06 | .03 |
| Insulin pump | 0.85 | 0.64 | 1.13 | .26 |
| CGM | 0.95 | 0.75 | 1.20 | .64 |
| BMI Z Score | 1.06 | 0.95 | 1.19 | .31 |
The exponentiated regression coefficient is the estimated multiplicative effect on the rate of blood glucose checks per day associated with a 1-unit increase in the explanatory variable.
Discussion
In the present study, we discovered that the frequency of daily SMBG changed as a function of the rate with which youths with T1D or their parents synchronized data from their glucometers to a mobile phone app. While a causal relationship cannot be determined from these data, the results support the possibility that engagement in one aspect of T1D self-care (data synchronization between visits) could have a positive effect on engagement in another aspect of T1D self-care (self-monitoring of blood glucose). In contrast, we did not find a statistically significant relationship between the rate of glucometer-based data synchronization and glycemic control in this small convenience sample. Specifically, although model estimates suggest meaningful effect sizes, neither mean blood glucose nor HbA1c was significantly related to synchronization frequency. To our knowledge, the present study is the first to examine the frequency of data synchronization to a commercially available mobile app in relation to both adherence and glycemic control.
While there was insufficient evidence in the present study to conclude that an association exists between frequent data synchronization to a mobile app and glycemic control, previous studies have found that individuals who frequently upload and review data between clinic visits using any upload method exhibit lower HbA1c values than those who do not. In one study that included 340 adults and children with T1D, “routine reviewers” were found to have a significantly lower HbA1c than nonroutine reviewers (7.2 vs 8.1%).2 There are several potential explanations for these disparate findings. First, the present study captured information only on data synchronization events with the mobile app; there is no way to know from the available data whether parents or youth actually performed data review in conjunction with the data synchronization episodes. Second, the present study captured observations on individuals for only a short period (for 0.3 years, on average) after introduction to, and training on, the mobile app, while the prior study evaluated individuals’ behaviors over a year. Third, the prior study was larger (N = 340) and thus had greater statistical power. Finally, despite the fact that the diabetes care team trained families on using the mobile app and provided encouragement to share glucometer data with the app, the rate of data synchronization remained relatively low, with a significant proportion of individuals failing to synchronize data during the observation period. Previous studies have evaluated the impact of mobile apps for diabetes self-management on HbA1c; however, those studies also included telehealth or text messaging from the health care team15,16 or incorporated incentives for using the app;6 these features of the prior studies make it impossible to isolate the effect of data synchronization or data visualization within the mobile app from the effect of the associated interventions. Notably, one of those studies was a nonrandomized prospective pilot study (N = 20) which identified a relationship between mobile app use and adherence to SMBG. That study also failed to see an effect of mobile app use on HbA1c.6
The present study did identify a correlation between data synchronization rate and daily frequency of SMBG. No prior studies in the literature evaluate potential associations between the frequency of data synchronization with a mobile device and the frequency of other adherence behaviors, including frequency of SMBG.
The present findings are clinically important because multiple mobile apps have been developed or are currently under development to support diabetes self-management; some of these also support data synchronization between glucometers and a mobile device.6-8 In the present study, 43% of individuals failed to synchronize glucometer data to the mobile app at all after receiving training, indicating that either additional support/training may be needed, or patients/families may not be convinced of a significant “payoff” for their effort. As such, families might need additional motivators, (like attention from the diabetes care team or financial incentives) to drive synchronization behavior. Alternatively, efforts by manufacturers to further reduce the burden of data synchronization by allowing passive data synchronization between glucometers and mobile apps may be warranted. The key features that support improvements in patient engagement and glycemic control must be rigorously evaluated. If the frequency of SMBG is found to be increased during mobile app use in prospective interventional studies, that could have a positive impact on glycemic control, especially among adolescents. Prior studies have demonstrated that among children and adolescents with T1D, an increase of one blood glucose check per day is associated with a 0.19% decrease in HbA1c.17 Whether engagement with data synchronization is associated with an increase other self-management behaviors such as mealtime insulin bolusing should also be determined in future prospective studies. Among youths who miss at least one mealtime insulin bolus per day, increasing the frequency of mealtime insulin boluses by one has been associated with a 1.5% decrease in HbA1c.18 Reductions in HbA1c, in turn, are associated with decreased T1D-related risk for micro- and macrovascular disease.19-22
The present study should be interpreted within the context of certain significant limitations. First, the cohort of individuals utilizing the mobile app was a convenience sample and was not randomized. Individuals were offered the opportunity to use the mobile app if their blood glucose meter and mobile phone were compatible according to the mobile app manufacturer. Sampling bias could therefore influence the outcomes. Second, the cohort was small and the observation period was short, essentially consisting of the interval between two routine clinical care visits. A longer observation period may have revealed a significant relationship between data synchronization and glycemic control. This is especially true considering the nonsignificant trends toward lower HbA1c and mean blood glucose observed during the short observation period for the present sample. The size of the estimated effects of synchronization frequency on HbA1c and mean blood glucose suggest associations that might be statistically significant in a larger sample of youths. Third, the frequency of data synchronization with the mobile app by individuals with T1D remained relatively low during the observation period. This has been observed in other studies, with one recent study reporting that only 12% of adults and 27% of children’s caregivers routinely reviewed data from diabetes self-management devices.2 Furthermore, we were unable to measure whether (1) individuals reviewed their blood glucose data, (2) reviewed data and also took action (eg, adjusting basal insulin doses or mealtime insulin:carbohydrate ratios), or (3) shared data with their health care team and received feedback. Future studies of the efficacy of mobile medical apps in diabetes care should be prospective and should include a randomized clinical trial of the mobile app. Prior work in adults with type 2 diabetes suggests that long-term engagement with mobile apps that support diabetes self-management can be problematic.23 Investigators should therefore consider incentivizing frequent data synchronization as part of initial trials to allow evaluation of the technology’s full potential to improve glycemic control. In future prospective studies, investigators should also obtain other objective measures of patient engagement with the mobile app (ie, app-specific screen time on data visualization screens), patient-reported measures of the frequency of data review, and the frequency of action taken (eg, insulin adjustment). Fourth, the present study would have been improved if it had included a comparative analysis to a matched cohort form the clinic who were not exposed to the mobile app. The study would similarly have been improved if the available data had allowed a comparison of baseline frequency of SMBG to the frequency of SMBG at the end of the observation period. Future prospective studies should address these two design limitations. Finally, in the present study we only evaluated the frequency of data synchronization from a glucometer as a predictive variable; data synchronization frequency from insulin pumps and continuous glucose monitors was not evaluated. Whether data synchronization and review of data from those devices relates to the frequency of SMBG, mean blood glucose, or HbA1c remains an unanswered question.
Several strengths of the present study are also notable. First, the study cohort appears to be generally representative of the larger clinic population, although there were more insulin pump and CGM users in the study cohort. Second, the study included multiple covariates collected from the electronic health record. Third, the study employed multivariable analyses to control for potential confounding effects of different patient characteristics. Finally, the study included a small but reasonably sized and reasonably diverse sample of individuals who were introduced to the mobile app.
Conclusions
These results add to the literature by providing initial evidence that use of a mobile app to capture glucometer data, which should allow more frequent pattern management, is associated with increased adherence to blood glucose monitoring, an important aspect of self-management. The present study also provides foundational knowledge to power future prospective studies evaluating the efficacy of mobile apps designed support pattern management in T1D.
Acknowledgments
The authors acknowledge Mitchell Barnes.
Footnotes
Abbreviations: CDE, certified diabetes educator; CGM, continuous glucose monitoring; HbA1c, hemoglobin A1c; mobile app, mobile phone software application; SMBG, self-monitoring of blood glucose; T1D, type 1 diabetes.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: MAC is a volunteer (unpaid) member of the advisory board for Glooko; Glooko has provided travel support to advisory board meetings. VSS has none.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Miller KM, Foster NC, Beck RW, et al. Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange clinic registry. Diabetes Care. 2015;38(6):971-978. [DOI] [PubMed] [Google Scholar]
- 2. Wong JC, Neinstein AB, Spindler M, Adi S. A minority of patients with type 1 diabetes routinely downloads and retrospectively reviews device data. Diabetes Technol Ther. 2015;17(8):555-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shalitin S, Ben-Ari T, Yackobovitch-Gavan M, et al. Using the Internet-based upload blood glucose monitoring and therapy management system in patients with type 1 diabetes. Acta Diabetol. 2014;51(2):247-256. [DOI] [PubMed] [Google Scholar]
- 4. Wong JC, Foster NC, Maahs DM, et al. Real-time continuous glucose monitoring among participants in the T1D Exchange Clinic Registry. Diabetes Care. 2014;37(10):2702-2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Corriveau EA, Durso PJ, Kaufman ED, Skipper BJ, Laskaratos LA, Heintzman KB. Effect of Carelink, an internet-based insulin pump monitoring system, on glycemic control in rural and urban children with type 1 diabetes mellitus. Pediatr Diabetes. 2008;9(4 pt 2):360-366. [DOI] [PubMed] [Google Scholar]
- 6. Cafazzo JA, Casselman M, Hamming N, Katzman DK, Palmert MR. Design of an mHealth app for the self-management of adolescent type 1 diabetes: a pilot study. J Med Internet Res. 2012;14(3):e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lifescan Inc. 2016. http://www.onetouch.com/veriosync. Accessed October 4, 2016.
- 8. Diasend. 2016. https://www.diasend.com/us/. Accessed October 4, 2016.
- 9. Diabeto Medtech India Pvt Ltd. 2016. http://diabe.to/. Accessed October 7, 2016.
- 10. Glooko Inc. 2016. Glooko: Remote patient monitoring for diabetes. https://www.glooko.com/. Accessed October 4, 2016.
- 11. Clements MA, Lind M, Raman S, et al. Age at diagnosis predicts deterioration in glycaemic control among children and adolescents with type 1 diabetes. BMJ Open Diabetes Res Care. 2014;2(1):e000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Raman S, Dai H, DeLurgio SA, et al. High hemoglobin A1c variability is associated with early risk of microalbuminuria in children with T1D. Pediatr Diabetes. 2015;17(6):398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lenters-Westra E, Slingerland RJ. Three of 7 hemoglobin A1c point-of-care instruments do not meet generally accepted analytical performance criteria. Clin Chem. 2014;60(8):1062-1072. [DOI] [PubMed] [Google Scholar]
- 14. NGSP. List of NGSP certified methods. http://www.ngsp.org/docs/methods.pdf.
- 15. Rossi MC, Nicolucci A, Di Bartolo P, et al. Diabetes Interactive Diary: a new telemedicine system enabling flexible diet and insulin therapy while improving quality of life: an open-label, international, multicenter, randomized study. Diabetes Care. 2010;33(1):109-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kirwan M, Vandelanotte C, Fenning A, Duncan MJ. Diabetes self-management smartphone application for adults with type 1 diabetes: randomized controlled trial. J Med Internet Res. 2013;15(11):e235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rausch JR, Hood KK, Delamater A, et al. Changes in treatment adherence and glycemic control during the transition to adolescence in type 1 diabetes. Diabetes Care. 2012;35(6):1219-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Patton SR, Clements MA, Fridlington A, Cohoon C, Turpin AL, Delurgio SA. Frequency of mealtime insulin bolus as a proxy measure of adherence for children and youths with type 1 diabetes mellitus. Diabetes Technol Ther. 2013;15(2):124-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977-986. [DOI] [PubMed] [Google Scholar]
- 20. Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Diabetes Control and Complications Study Group. Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA. 2003;290(16):2159-2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Diabetes Control and Complications Trial Research Group. Effect of intensive diabetes management on macrovascular events and risk factors in the Diabetes Control and Complications Trial. Am J Cardiol. 1995;75(14):894-903. [DOI] [PubMed] [Google Scholar]
- 23. Tatara N, Arsand E, Bratteteig T, Hartvigsen G. Usage and perceptions of a mobile self-management application for people with type 2 diabetes: qualitative study of a five-month trial. Stud Health Technol Inform. 2013;192:127-131. [PubMed] [Google Scholar]


