Abstract
Alveolar capillary dysplasia with misalignment of pulmonary veins (ACD/MPV) is a rare and lethal developmental disorder of the lung that affect both acinar structure and the intrinsic pulmonary vasculature. We report prenatal and postnatal imaging with histopathological findings of this rare condition. We, first, describe MR imaging features and discuss its role in prenatal imaging.
Keywords: pulmonary hypoplasia, fetal MRI, alveolar capillary dysplasia
Introduction
Prenatal Magnetic Resonance Imaging (MRI), nowadays, is considered an important complement to prenatal ultrasound (US) in the evaluation of fetal lung anomalies, in particular to confirm and quantify fetal pulmonary hypoplasia (1). In many cases pulmonary hypoplasia is secondary to extrathoracic compression of fetal lung (as in cases of maternal oligohydramnios), thoracic cage compression (as in cases of thoracic dystrophies), and intrathoracic fetal compression (as in cases of large congenital diaphragmatic hernias or large congenital cystic malformations). More rarely, pulmonary hypoplasia may be primary, with no obvious visible causes on prenatal imaging, since it is due to a diffuse disorder of lung development, as congenital alveolar dysplasia (CAD), alveolar capillary dysplasia (ACD) or acinar dysplasia (AD) (2).
We present the prenatal and postnatal radiological and clinical features of a full-term female neonate affected by alveolar capillary dysplasia with misalignment of pulmonary veins (ACD/MVP) (3); we compared prenatal findings with histological results, in order to assess the utility of prenatal imaging in the workup of diffuse developmental disorders of the infant lung and to evaluate possible advantages in term of early diagnosis.
To the best of our knowledge, this is the first report of prenatal (US and MR) imaging of proven histological diagnosis of ACD/MPV at autopsy.
Case report
A 36-year-old woman, gravida 3, para 2, livebirths 2, was referred to our prenatal unit at 34.6 weeks’ gestation with suspected small thorax, cardiomegaly and pericardial effusion detected at routine ultrasound in another hospital. Parents were non-consanguineous, with a non-contributory family history. The pregnancy was uncomplicated, with first-trimester screening for Down syndrome showing low risk. A normal fetal biometry and anatomy were observed at 20 weeks’ gestation. She underwent second level US and prenatal MR examination at our unit.
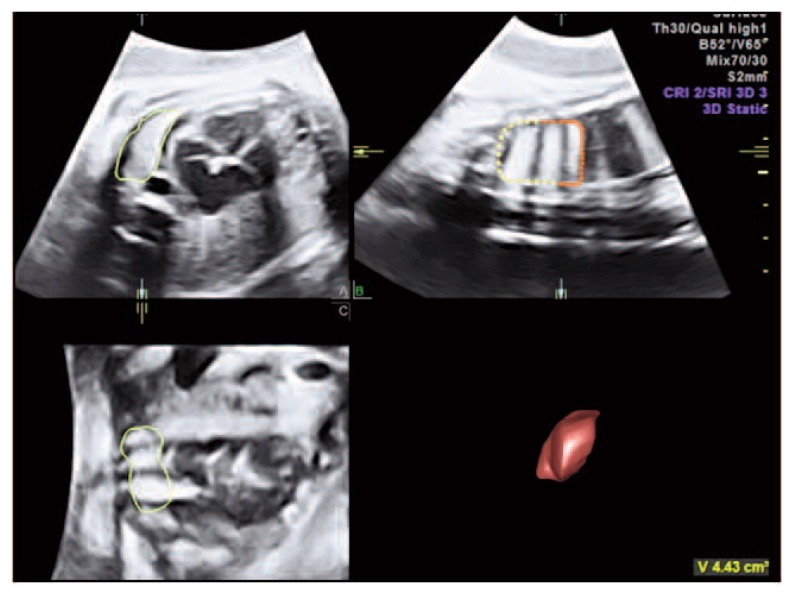
The second level US examination showed a female fetus growing at the 25° centile, with normal amniotic fluid, normal Doppler velocimetry and no signs of pericardial effusion. At echocardiography, heart anatomy and function appeared normal. There was no facial dysmorphism. The ribs were normal, with a thoracic circumference of 18.5 cm, below the 5° centile (normal ranges for gestational age: 5° centile 22.9 cm; 95° centile 28.6 cm) (4). The right and left lung volume were calculated using VOCAL technique (Virtual Organ Computer-aided AnaLysis, GE E8, 4–8 MHz probe - GE Healthcare, Zipf, Austria) (Figure 1). The left lung was severely reduced in volume (4.4 ml; normal ranges for gestational age: 5°centile 18.9 ml, 95° centile 48.0 ml), while the volume of the right lung was at the 5° centile (20 ml; normal ranges for gestational age: 5° centile 21.8 ml, 95° centile 68.0 ml). The US findings were suggestive of primitive pulmonary hypoplasia (5).
Figure 1.
Three dimensional fetal left lung measurement using the rotational technique with VOCAL (rotation step of 30°).
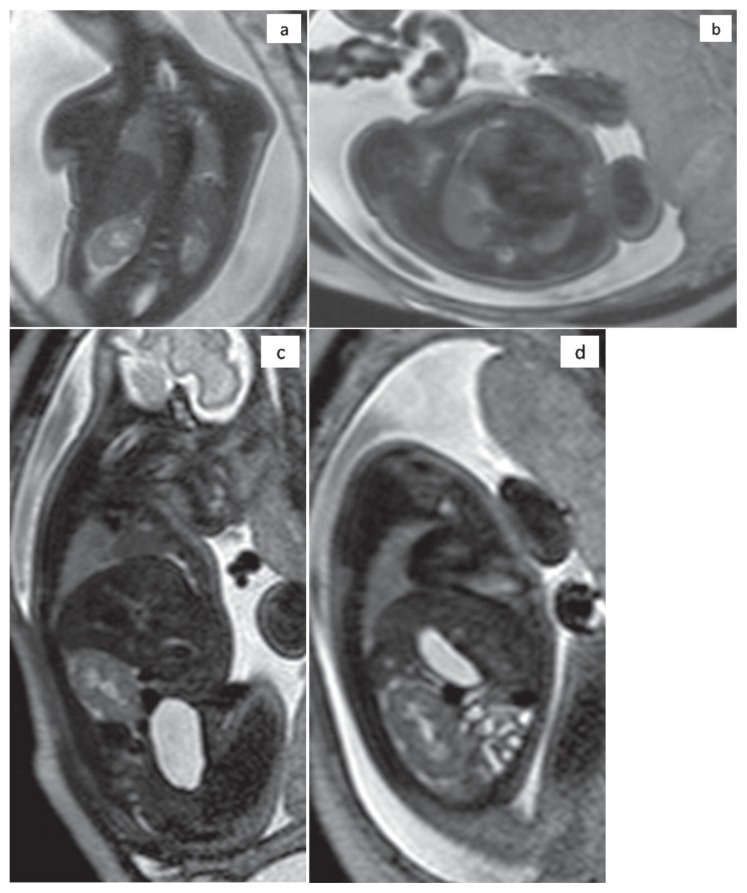
Prenatal MR imaging confirmed bilateral small lung volume with decreased signal intensity on single-shot fast spin-echo 4 mm thick contiguous T2-weighted sections (TR/TE 5000/120 ms) compared with expected lung signal intensity for gestational age (Figure 2). No other additional abnormal findings were found. The corresponding areas of lung were determined on each section by using freeform regions of interest on a picture archiving and communication system (Impax; Agfa-Gevaert, Mortsel, Belgium). The measured areas were added and multiplied by the section thickness to determine the entire volume of the right and left lung: the total fetal lung volume (FLV) was 3.6 ml for left lung and 7 ml for the right one.
Figure 2.
T2-weighted single-shot TSE MR images of the fetal thorax at 34.6 weeks’ gestation on three different planes: a) Coronal, b) Axial, c) Right sagittal. d) Left sagittal shows bilateral small lungs with decreased signal intensity.
The family was counselled extensively regarding the fatal prognosis. US examinations were subsequently repeated weekly, and no substantial changes were detected. Spontaneous delivery occurred at 39 weeks’ gestation and the full-term female neonate’s Apgar scores were 5 and 4 at 5 and 10 minutes, respectively. Her birth weight was 3300 g. The baby was immediately intubated after birth and mechanically ventilated. Array-comparative genomic hybridization (CGH) was performed on umbilical cord blood demonstrating a deletion of 1.1Mb in cr5p12, inherited form the mother.
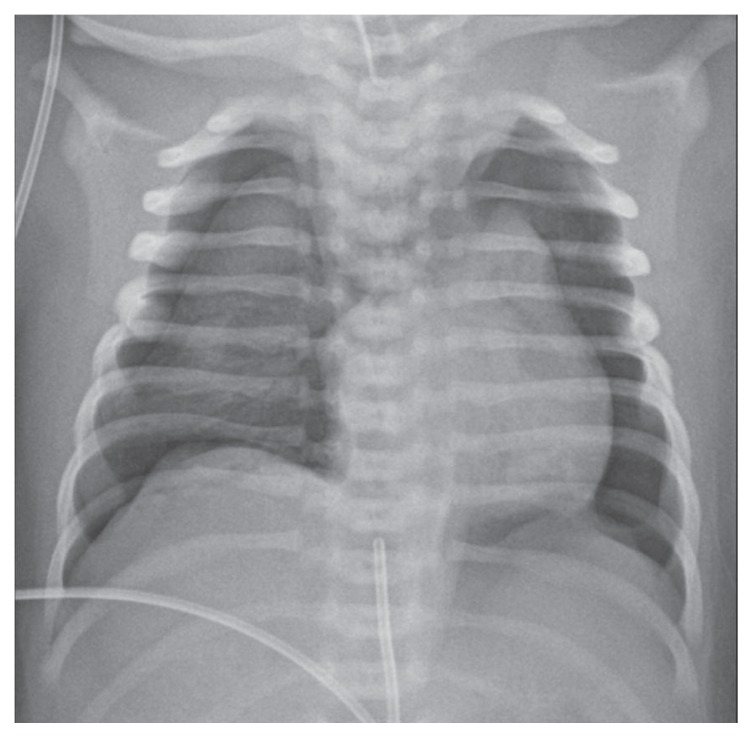
Her chest X-ray showed bilateral pneumothorax with reduced and poorly ventilated lung volume, in the presence of the endotracheal tube (Figure 3).
Figure 3.
Chest X-ray AP image shows bilateral pneumothorax with reduced and poorly ventilated lungs, in particular on the left hemithorax (note endotracheal tube and umbilical venous catheter).
The newborn’s condition deteriorated quickly with severe cardiorespiratory failure and pulmonary hypertension. She underwent to high frequency oscillatory ventilation and nitrous oxide therapy to treat progressive hypoxemia but she continued to be poorly oxygenated. Despite continuous treatment for severe pulmonary hypertension, she worsened 24 h later.
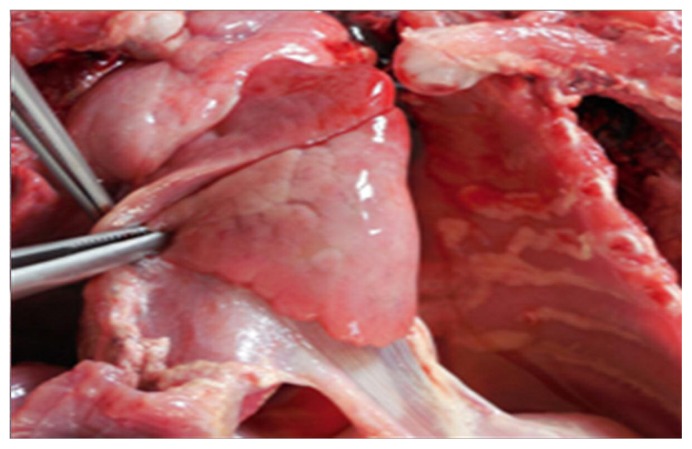
At autopsy, macroscopic examination revealed severe bilateral lung hypoplasia, especially on the left side (total lungs weight 17,2 g, expected weigh at term 44,6 +/− 22 g), with unexpanded, firm in consistency lungs. Right lung had also three incomplete lobes (Figure 4). Heart and great vessels inspection showed right ventricle hypertrophy (thickness 0,8 cm), patent foramen ovale and dilatation of the pulmonary trunk (diameter 1 cm).
Figure 4.
Severe lung hypoplasia was noted on gross examination, particularly of the left lung.
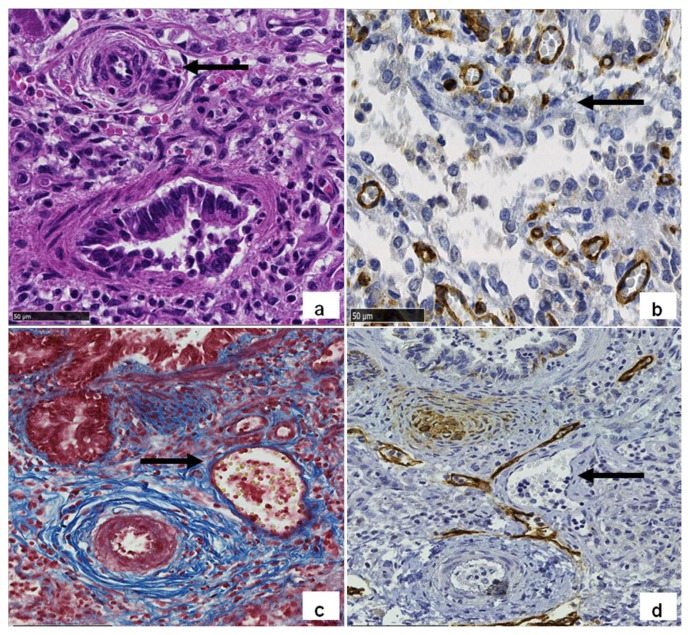
Moreover, mild hepatomegaly (liver weight 153 g, expected weight at term 127 g+/−35,8 g), diffuse visceral congestion and intrasplenic haemorrhages were present. Histological lung parenchyma characteristics were consistent with ACD/MPV with immature lobular development, reduction of alveolar capillaries number and increase in their distance from alveolar wall, anomalous position of dilated pulmonary veins, running in arteries and bronchi bundles, medial hypertrophy of small arteries (Figure 5).
Figure 5.
Lung histology showed features of ACD/MPV (Original magnification 40X). (a) Arteriolar muscular hyperplasia (arrow) on hematoxylin and eosin stained section; (b) CD31 antibody highlights the relative paucity of capillaries (brown stained) and their abnormal distance from respiratory spaces (arrow); c and d) serial sections stained with Masson trichrome; (c) and lymphatic endothelium marker D2-40; (d) demonstrates abnormal thin walled vessels (arrow in c) along with arteries and bronchi in the same adventitial sheath (MPV), and confirms its non-lymphatic origin (arrow in d).
Alveolar septa thickening was difficult to observe because of the panlobar acute pneumonitis, obscuring alveolar and interstitial spaces. Initial hyaline membrane disease was also present.
Discussion
ACD/MPV (OMIM #265380) is a rare cause of severe respiratory distress, usually fatal in the neonatal period as affected newborns present persistent and untreatable pulmonary hypertension. Histologically it is characterized by typical vascular anomalies involving alveolar capillaries, small pulmonary arterioles and pulmonary veins. Up to date FOXF1 is the only gene known to be associated with ACD/MPV, as heterozygous mutations/deletions have been identified in many affected infants (6). In our case, before having the histological confirmation of ACD/MPV, array-CGH analysis was performed on the umbilical cord blood and revealed a 1,1 Mb deletion on the short arm of chromosome 5, of familial origins. This region contains only one known disease-causing gene, NNT (OMIM#607878), whose mutations in homozygosity or compound heterozygosity are associated with Glucocorticoid deficiency-4 (OMIM #614736). Considering this observation and considering that the microdeletion identified in our case has been inherited from the healthy mother, likely it has no role on the pathogenesis of ACD/MPV. However, a case of lung hypoplasia have been described in association with a larger chromosomal deletion (about 2,3 Mb) in the same region (patient id 4542 on DECIPHER - Database of Chromosomal Imbalance and Phenotype in Humans using Ensembl Resources; https://decipher.sanger.ac.uk); thus, a possible modulating role cannot be excluded, even if current knowledge is too limited.
FOXF1 molecular analysis has not been carried out in our case because the parents did not agree to perform further genetic investigations.
While other Authors already described US prenatal imaging features of histologically confirmed ACD/MPV (7, 8), in our report, with the aim of implementing US findings, we investigated the prenatal MR characteristics of a diffuse disorder of lung development.
In our case prenatal US and MR findings of serious bilateral pulmonary hypoplasia, not associated with other abnormalities (such as congenital diaphragmatic hernia, congenital lung masses, giant omphalocele, severe oligohydramnios and skeletal dysplasia), suggested a diffuse disorder of lung development. A large number of studies, predominantly with regard to fetuses with congenital diaphragmatic hernia, has shown the correlation between FLV and neonatal pulmonary hypoplasia (9–11). We evaluated FLV, both with US and MR studies. Our data showed a discrepancy between lung volume measured at US examination and MRI, with evidence of overestimation at US. Also considering the discrepancy between these values, both imaging modalities demonstrated significantly lower lung volumes than normal for the gestational age, as predictor of severe pulmonary hypoplasia. In our case, according to Cannie et al. (12), FLV has proven to be a valuable biometric variable in predicting lung volume, with a good accordance with autopsy volume lung measurements. At MR imaging, we also evaluated lung signal intensity (SI), observing a significantly decreased SI on T2-weighted sequences in affected lungs compare with expected lung SI for gestational age. This result, as demonstrated in the literature in cases of congenital diaphragmatic hernia, is suggestive of pulmonary hypoplasia. In contrast to Langenstroer et al. (13), which demonstrated that there are no apparent changes in the echogenic properties of the lung that might suggest a prenatal diagnosis of CAD, the SI changes we reported suggest the feasibility of prenatal MR diagnosis of a diffuse disorder of lung development as CAD and ACD. In this specific case MRI allowed also the exclusion of secondary hypoplasia thanks to its well known ability in diagnosing related congenital intra or extrathoracic pathologies (such as congenital diaphragmatic hernia or other malformative conditions).
In conclusion, we believe that MRI and US measured FLV together with MRI SI assessment may have important clinical applications in confirming and quantifying fetal pulmonary hypoplasia in diffuse disorders of lung development. The suspect of such entity can be raised even at an early gestational stage (13), when genetic and grief antenatal counseling could be implemented, options given, and possibly operative delivery and expensive neonatal intensive care unit stays avoided.
References
- 1.Cannie M, Jani J, De Keyzer F, et al. T2 quantifications of fetal lungs at MRI-normal ranges. Prenat Diagn. 2011;31:705–711. doi: 10.1002/pd.2746. [DOI] [PubMed] [Google Scholar]
- 2.Dishop MK. Paediatric interstitial lung disease: classification and definitions. Paediatr Respir Rev. 2011;12:230–237. doi: 10.1016/j.prrv.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Bishop NB, Stankiewicz P, Steinhorn RH. Alveolar capillary dysplasia. Am J Respir Crit Care Med. 2011;184:172–179. doi: 10.1164/rccm.201010-1697CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chitkara U, Rosenberg J, Chervenak FA, et al. Prenatal sonographic assessment of the fetal thorax: normal values. Am J Obstet Gynecol. 1987;156:1069. doi: 10.1016/0002-9378(87)90112-8. [DOI] [PubMed] [Google Scholar]
- 5.Gerards FA, Engels MAJ, Twisk JWR, et al. Normal fetal lung measured with three-dimensional ultrasound. Ultrasound Obstet Gynecol. 2006;27:134–144. doi: 10.1002/uog.2672. [DOI] [PubMed] [Google Scholar]
- 6.Sen P, Yang Y, Navarro C, et al. Novel FOXF1 mutations in sporadic and familial cases of alveolar capillary dysplasia with misaligned pulmonary veins imply a role for its DNA binding domain. Hum Mutat. 2013;34(6):801–11. doi: 10.1002/humu.22313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prothro SL, Plosa E, Markham M, et al. Prenatal diagnosis of alveolar capillary dysplasia with misalignment of pulmonary veins. J Pediatr. 2016;170:317–318. doi: 10.1016/j.jpeds.2015.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parris T, Nik AM, Kotecha S, et al. Inversion upstream of FOXF1 in a case of lethal alveolar capillary dysplasia with misalignment of pulmonary veins. Am J Med Genet A. 2013;161A:764–770. doi: 10.1002/ajmg.a.35832. [DOI] [PubMed] [Google Scholar]
- 9.Walleyo A, Debus A, Kehl S, et al. Periodic MRI lung volume assessment in fetuses with congenital diaphragmatic hernia: prediction of survival, need for ECMO, and development of chronic lung disease. AJR Am J Roentgenol. 2013;201:419–426. doi: 10.2214/AJR.12.8655. [DOI] [PubMed] [Google Scholar]
- 10.Barros CA, Rezende GC, Araujo E, Júnior, et al. Prediction of lethal pulmonary hypoplasia by means fetal lung volume in skeletal dysplasias: a three-dimensional ultrasound assessment. J Matern Fetal Neonatal Med. 2015;29:1–6. doi: 10.3109/14767058.2015.1064887. [DOI] [PubMed] [Google Scholar]
- 11.Zamora IJ, Sheikh F, Cassady CI, et al. Fetal MRI lung volumes are predictive of perinatal outcomes in fetuses with congenital lung masses. J Pediatr Surg. 2014;49:853–858. doi: 10.1016/j.jpedsurg.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 12.Cannie M, Jani JC, De Keyzer F, et al. Fetal body volume: use at MR imaging to quantify relative lung volume in fetuses suspected of having pulmonary hypoplasia. Radiology. 2006;241:847–853. doi: 10.1148/radiol.2413051228. [DOI] [PubMed] [Google Scholar]
- 13.Langenstroer M, Carlan SJ, Fanaian N, et al. Congenital acinar dysplasia: report of a case and review of literature. AJP Rep. 2013;3:9–12. doi: 10.1055/s-0032-1329126. [DOI] [PMC free article] [PubMed] [Google Scholar]