Abstract
Neuroendocrine tumors (NET) are a very heterogeneous group of neoplasms; in recent years we have seen an increase in their incidence (3.65/100.000/year).
They can be associated with hereditary endocrine syndromes (MEN, Von Hippel Lindau); they can occur at any age and the incidence is slightly higher in men than women. The aetiology of the neuroendocrine tumors is unclear; in most cases, inflammation of the bile ducts may be the underlying cause and for this reason, the initial patient’s evaluation should be focused on the different aspects concerning the oncological one and the possible sequelae of the biliary obstructions that can evolve in biliary sepsis. All neuroendocrine tumors have malignant potential.
The most frequent sites of extrahepatic biliary NETs are the common hepatic duct and the distal common bile duct (19.2%), followed by the middle of the common bile duct (17.9%), the cystic duct (16.7%), and the proximal common bile duct (11.5%). We can divide them into: well-differentiated and poorly differentiated. Considering the clinical features, neuroendocrine tumors can be divided into functional and non-functional. As regards the staging, we distinguish localized, regional and metastatic tumors.
Tumors derived from the bile duct are difficult to diagnose preoperatively, mainly because of its low incidence and difficult diagnostic process. However since cholangiocarcinomas account for about 80% of all primary biliary tumors, it is important to think about other options despite their low frequency when a patient presents with abnormal characteristics.
The most sensitive immunohistochemical markers are expressing neuron-specific enolase, synaptophisin and chromogranin A.
Liver function tests, alkaline phosphatase and bilirubin are often high. Sometimes an anemia can appear in the presence of a chronic disease or in patients with more advanced disease. It is known that the measurement of chromogranin A is useful for the preoperative diagnosis of neuroendocrine tumors. Chromogranin A is elevated in 90% of neuroendocrine tumors of the intestine, and the levels correlate with tumor burden and the possibility of recurrence and, therefore, chromogranin A can be an effective biological marker for preoperative diagnosis of neuroendocrine tumors. Bile endocrine tumors remain silent until metastasizing or growing into neighboring organs, because of its uncommon diagnosis in early stages due to its low incidence, absence of serum markers and lack of symptoms related to the hormonal pattern.
Preoperative diagnosis of common bile duct carcinoma is extremely difficult, because it is foretold by non-specific symptoms that include pain or discomfort in the right upper quadrant level and weight loss. A 51- year-old woman presented a jaundice and severe bile duct dilatation. The enhanced CT scan showed a mass, approximately 15 mm in diameter, in the distal common biliary duct. The MRI and ERCP confirmed the mass. Cromogranin A value was negative. The diagnosis of well differentiated endocrine tumor of the biliary tract was done after its surgical resection was performed. The postoperative period was uneventful.
Extrahepatic biliary NETs are rare, and extrahepatic bile ducts reportedly account for only 0.32% of primary NET sites.
The prognosis for NET of the bile duct appears to be poor.
Keywords: Neuroendocrine tumor of the common hepatic duct, Chromogranin A
Introduction
Neuroendocrine tumors (NET) are a very heterogeneous group of neoplasms; in recent years we have seen an increase in their incidence (3.65/100.000/year) (1).
They can be associated with hereditary endocrine syndromes (MEN, Von Hippel Lindau); they can occur at any age and the incidence is slightly higher in men than women (2). The aetiology of the neuroendocrine tumors is unclear; in most cases, inflammation of the bile ducts may be the underlying cause and for this reason, the initial patient’s evaluation should be focused on the different aspects concerning the oncological one and the possible sequelae of the biliary obstructions that can evolve in biliary sepsis. All neuroendocrine tumors have malignant potential.
The most frequent sites of extrahepatic biliary NETs are the common hepatic duct and the distal common bile duct (19.2%), followed by the middle of the common bile duct (17.9%), the cystic duct (16.7%), and the proximal common bile duct (11.5%). We can distinguish them into: well-differentiated, poorly differentiated. Considering the clinical features, neuroendocrine tumors can be divided into functional and non-functional. As regards the staging, we distinguish localized, regional and metastatic tumors.
Tumors derived from the bile duct are difficult to diagnose preoperatively, mainly because of its low incidence and difficult diagnostic process. However since cholangiocarcinomas account for about 80% of all primary biliary tumors, it is important to think about other options despite their low frequency when a patient presents with abnormal characteristics (3).
The most sensitive immunohistochemical markers are expressing neuron-specific enolase, synaptophisin and chromogranin A.
Liver function tests, alkaline phosphatase and bilirubin are often altered. Sometimes an anemia can appear in the presence of a chronic disease or in patients with more advanced disease. It is known that the measurement of chromogranin A is useful for the preoperative diagnosis of neuroendocrine tumors. Chromogranin A is elevated in 90% of neuroendocrine tumors of the intestine, and the levels correlate with tumor burden and the possibility of recurrence and, therefore, chromogranin A can be an effective biological marker for preoperative diagnosis of neuroendocrine tumors. Bile endocrine tumors remain silent until metastasize or invade neighboring organs, because of its uncommon diagnosis in early stages due to its low incidence, absence of serum markers and lack of symptoms related to the hormonal pattern (4).
Preoperative diagnosis of common bile duct carcinoma is extremely difficult, because it is foretold by nonspecific symptoms that include pain or discomfort in the right upper quadrant level and weight loss.
Extrahepatic biliary NETs are rare, and extrahepatic bile ducts reportedly account for only 0.32% of primary NET sites.
The prognosis for NET of the bile duct appears to be poor (5).
Case report
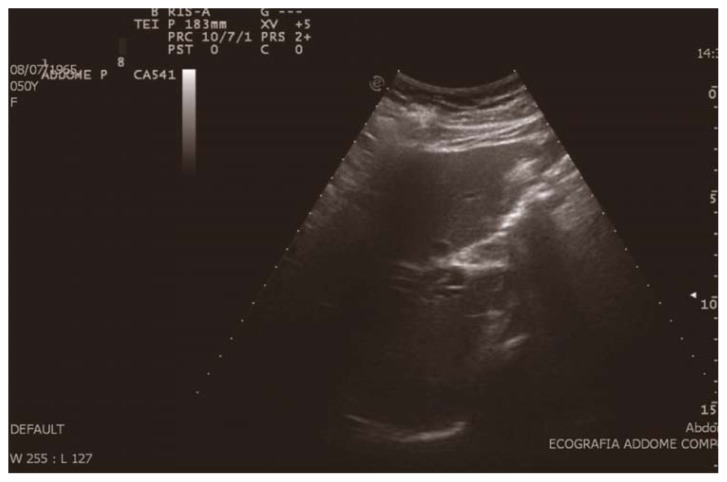
A 51-year-old woman was found to have a marked dilatation of the intrahepatic bile duct during an ultrasonography performed, as part of a medical examination, and was admitted to hospital for further investigation (Figure 1). The patient showed: jaundiced skin, itching all over the body, issuance of dark urine and feces hypocholic; the laboratory tests it was found increase of direct bilirubin values, GGT and alkaline phosphatase.
Fig. 1.
Ultrasonography examination: marked dilation of the in-trahepatic bile duct.
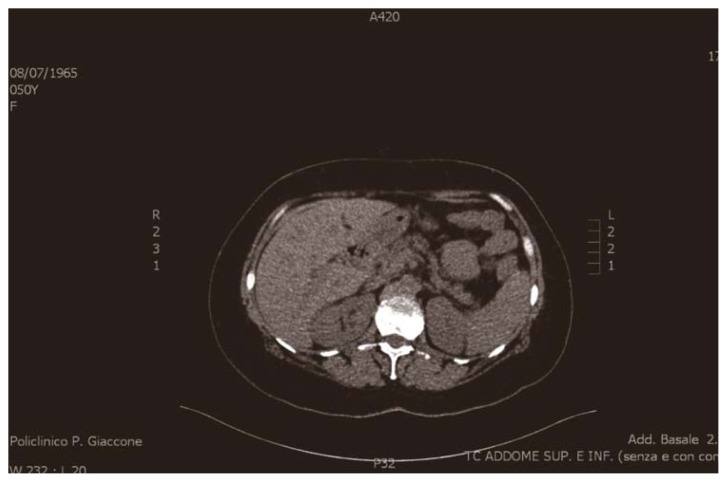
An abdominal ultrasonography examination, together with a contrast-enhanced abdominal computed tomography scan, showed a mild dilation of the biliary tree and a solid hypoechoic mass, approximately 15 mm in diameter, in the distal common bile duct (Figure 2).
Fig. 2.
Contrast-enhanced abdominal computed tomography scan: mild dilation of the biliary tree and a solid hypoechoic mass, approximately 15 mm in diameter, in the distal common bile duct.
The common bile duct, in his proximal segment, presents a distinctly hyperdense tissue in the arterial phase occupying entirely the lumen for a 1.5 cm longitudinal extension and 1.2 cm transverse. Respect to the possibility of a classic common bile duct carcinoma, it showed an atypical post contrastographic behaviour, placing the suspect for a neuroendocrine tumor of the same (0.2 % of the common bile duct tumors).
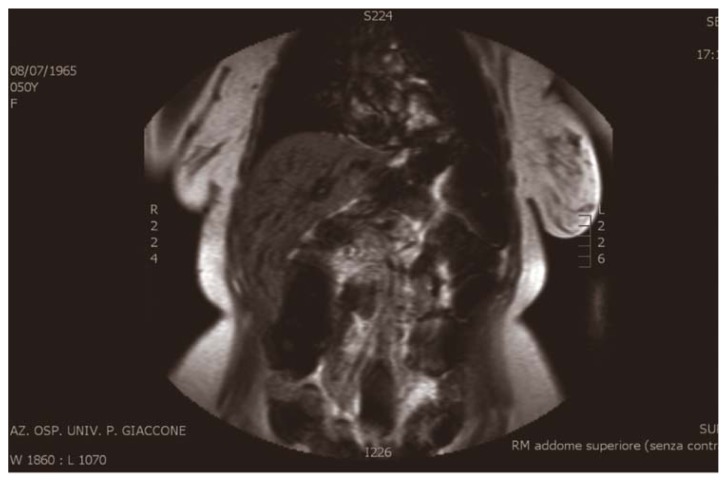
MRI showed dilatation of the intrahepatic bile ducts, more evident in the left sections, with a maximum caliber at the left hepatic duct (9 mm). Dilation of the common bile duct (DT Max 11 mm) presenting empty signal, not univocal interpretation (Figure 3).
Fig. 3.
MRI: dilatation of the intrahepatic bile ducts, more evident in the left sections, with a maximum caliber at the left hepatic duct (9 mm). Dilation of the common bile duct (DT Max 11 mm).
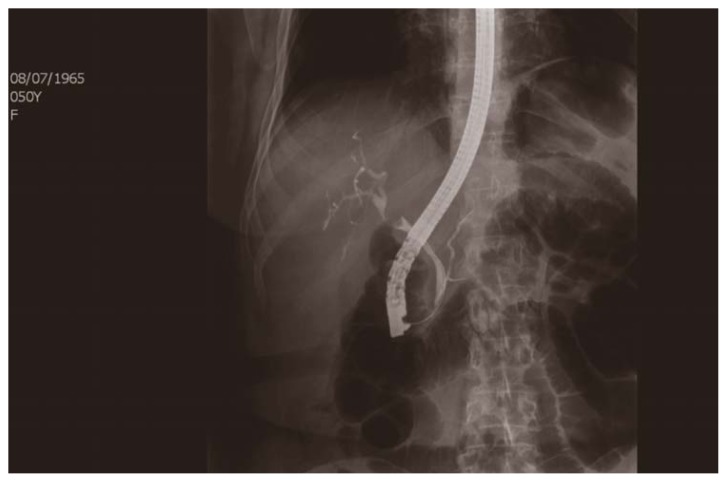
ERCP showed a lesion protruding into the lumen, at the level of the common hepatic duct, about 0.3 cm, irregularly oval, approximately 12 × 8 mm. The intrahepatic biliary ducts were moderately dilated and the gallbladder was not displayed. An endoscopic Papillotomy (PTe) and brushing of the lesion was made and it was positioned a 7 Fr nose-biliary drainage into the distal end of the right hepatic duct (Figure 4).
Fig. 4.
ERCP: a lesion protruding into the lumen, at the level of the common hepatic duct, about 0.3 cm, irregularly oval, approximately 12 × 8 mm. The intrahepatic biliary ducts are moderately dilated and the gallbladder is not displayed.
Furthermore, it was found a negative result to the pre-operative dosage of chromogranin A.
Based on these findings, a diagnosis of the distal common bile duct tumor was made, and the patient underwent a bile duct resection with the additional dissection of lymph nodes along the common bile duct, hepatic artery, and portal vein. The postoperative course was regular, the postoperative ileus resolved in 3rd postoperative day, no complications were observed during the course and the patient was discharged in the 10th postoperative day.
Histologic examination showed a well differentiated neuroendocrine tumor of the common hepatic duct (G1); with a maximum diameter of 1.8 cm, infiltrating the muscle layer; mitotic count index (10 HPF) <2, proliferative fraction (Ki67) ≤ 2% (IIC: CK7; CK20; CEA; chromogranin; synaptophysin; NSE, ki67; p53; EMA; S100; CD56; CK8-18).
The patient made an uneventful recovery even three weeks after the discharge.
Discussion and conclusion
The patient had a negative pre-operative result of chromogranin A and only after the histological examination a diagnosis of NET of common hepatic duct was made; in fact in this case a correct pre-operative diagnosis was more difficult, so it must be considered very rare.
Generally, the NET are diagnosed in the metastatic phase; their capability to metastasize is dependent on the site of occurrence of the primary tumor and the histological type. In particular, the most common site of metastasis is the liver (40–93%) followed by bone (12–20%) and lung (10.8%). It is estimated that approximately in 40% of patients with NET are present hepatic metastases at diagnosis.
NETs represent a very heterogeneous group of diseases, and for this reason it is not possible to define a unique treatment strategy. Also, being a quite rare tumor, it is imperative to consult a highly specialized center in which you can define a personalized therapy with a multidisciplinary medical approach. Among the different possible therapeutic options, surgery is the most used in potentially curative purpose: if the tumor is removed entirely, the surgery is on the whole the main step of the treatment and allows in many cases to cure it completely (6). For some neuroendocrine tumors it is possible to use local-regional therapies such as chemoembolization and the alcoholization (for metastases located in the liver areas difficult to reach by surgery) or cryosurgery and the high-frequency thermal ablation. The traditional radiotherapy is little used for the real cure of the tumor, but it is possible to use it with the aim of reducing the symptoms in more advanced stages. The adjuvant treatment should take into consideration the implications of the neuroendocrine system, especially the hypothalamic-pituitary-adrenal axis. In this perspective, the treatment with immunomodulators should be proposed in the future, similarly to other neoplastic as well as non-neoplastic diseases (7, 8). Targeted radiotherapy is based on the ability of some neuroendocrine tumors to concentrate in themselves radioactive substances. An example is the 131I-MIBG therapy. It is also possible to use octreotide, a somatostatin analogue.
In summary, neuroendocrine tumors are complex diseases and are an example of how a multidisciplinary approach has a favorable impact on diagnosis, treatment and control.
The appearance of a well-circumscribed hypervascular tumor during the arterial phase may help to differentiate NETs from other biliary neoplasms. The computed tomography images in the present case might be typical of biliary NET. The surgical treatment plays an important role in the treatment of NET, when to have healing purposes by excision a primary non metastatic lesion, both when the surgery interpreters the role of palliation in advanced disease. General criteria are needed for correct surgical approach to the disease; it’s necessary a radiological research of the primary tumor and a study of any metastases. When the primary tumor has no metastases, radical removal of the lesion appears as curative treatment. Surgical exploration, also with the aid of US intraoperative for digestive NET, has the task of locating the precise location of them. When the primary tumor is resectable, but coexist synchronous metastatic lesions, it is necessary to plan the type of treatment in the number, size and location of secondary localizations. In case of liver multifocal lesions, chemoembolization constitutes the best treatment; but if metastasis are confined to one of the two hepatic lobes may be taken into account the removal of one or more liver segments, after evaluation of anesthetic risk. Regarding debulking, we can use it for functional neoplasia, in order to reduce the amount of secreting tissue and then to control the symptoms of the disease, ensuring the patient a better quality of life but not a longer survival.
An effective palliative cytoreduction however involves removing of at least 90% of the neoplastic tissue, increasing substantially the risk of postoperative morbidity. When it is the primary tumor which can not be removed with the criterion of radicalism, surgical treatment will only aimed at correcting an altered intestinal transit and biliary adopting measures which may be surgical (biliary-digestive derivation, gastroenterostomy, intestinal bypass, ileo-colostomy), endoscopy (biliary prosthesis) or local (external biliary derivation). For pancreatic endocrine tumors localized in a single site of the head of pancreas, the surgical indication is for the pancreaticoduodenectomy (DCP) using the technique of Whipple or Longmire. Some exceptions are small tumors, which can be enucleated with the preservation of most pancreatic tissue (6, 9).
In general, the surgical resection should be completed with a lymph node dissection, likewise different surgical situations (10). Among the possible complications we can find: haemorrhage, both during surgery and in the first 24–48 postoperative hours; liver failure, if it is necessary to perform major hepatic resections, leaving insufficient residual liver to maintain its normal functions, then we might have jaundice or ascites; biliary fistulas; sepsis, and then fever or hypothermia, rapid breathing, fast heart rate, confusion, edema, up to the shock (11).
With the aim to prevent these complications and its fearful sequelae, the use of hemostatic patches, that could be helpful in other digestive anastomoses, has not been validated (12–15)
Complications such as intestinal ischemia or other abdominal complications have not been described (16–19). The laparoscopic approach, although possible, is not validated yet (20, 21).
References
- 1.Frilling A, et al. Recommendation for patients with neuroendocrine liver metastases. Lancet Oncol. 2014;15:e8–21. doi: 10.1016/S1470-2045(13)70362-0. [DOI] [PubMed] [Google Scholar]
- 2.Oberg K, et al. Neuroendocrine gastro-entero-pancreatic tumors: ESMO clinical practice guidelines for diagnosis, treatment and follow up. Annals of Oncology. 2012;23(Supplement 7):vii124–vii130. doi: 10.1093/annonc/mds295. [DOI] [PubMed] [Google Scholar]
- 3.Sánchez Cabús S, Pittau G, Sebagh M, Cherqui D. Primary non-functioning neuroendocrine tumor of the extrahepatic bile duct. Rev Esp Enferm Dig. 2016 Apr;29:109. doi: 10.17235/reed.2016.4071/2015. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.González-Chávez MA, Villegas-Tovar E, González Hermosillo-Cornejo D, Gutierrez-Ocampo A, López-Rangel JA, Athié-Athié A. Neuroendocrine small-cell carcinoma of the gallbladder. An unexpected finding after diagnostic laparoscopy. Cir Cir. 2016 Feb 23; doi: 10.1016/j.circir.2015.12.007. Epub ahead of print. pii: S0009-7411(15)00294-7. [DOI] [PubMed] [Google Scholar]
- 5.Kihara Y, Yokomizo H, Urata T, Nagamine M, Hirata T. A case report of primary neuroendocrine carcinoma of the perihilar bile duct. BMC Surg. 2015 Dec 10;15:125. doi: 10.1186/s12893-015-0116-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonventre S, Inviati A, Di Paola V, Morreale P, Di Giovanni S, Di Carlo P, Schifano D, Frazzetta G, Gulotta G, Scerrino G. Evaluating the efficacy of current treatments for reducing postoperative ileus: a randomized clinical trial in a single center. Minerva Chirurgica. 2014;69(1):47–55. [PubMed] [Google Scholar]
- 7.Miller AH, Sonia Ancoli-Israel S, Bower JE, Capuronn L, Irwin MR. Neuroendocrine-Immune Mechanisms of Behavioral Comorbidities in Patients With Cancer. JCO. 2008;20:971–82. doi: 10.1200/JCO.2007.10.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cappello M, Bravatà I, Cocorullo G, Cacciatore M, Florena AM. Splenic littoral cell hemangioendothelioma in patient with Crohn’s disease previously treated with immunomodulators and anti-TNF agents: a rare tumor linked to deep immunodepression. Am J Gastroenterol. 2011;106(10):1863–5. doi: 10.1038/ajg.2011.204. [DOI] [PubMed] [Google Scholar]
- 9.Agrusa A, Romano G, Cucinella G, Cocorullo G, Bonventre S, Salamone G, Di Buono G, De Vita G, Frazzetta G, Chianetta D, Sorce V, Bellanca G, Gulotta G. Laparoscopic, three-port and SILS cholecystectomy: a retrospective study. G Chir. 2013 Sep-Oct;34(9–10):249–53. [PMC free article] [PubMed] [Google Scholar]
- 10.Scerrino G, Attard A, Melfa GI, Raspanti C, DI Giovanni S, Attard M, Inviati A, Mazzola S, Modica G, Gulotta G, Bonventre S. Role of prophylactic central neck dissection in cN0-papillary thyroid carcinoma: results from a high-prevalence area. Minerva Chir. 2016 Jun;71(3):159–67. Epub 2015 Jun 5. [PubMed] [Google Scholar]
- 11.Sartelli M, Abu-Zidan FM, Catena F, Griffiths EA, Di Saverio S, Coimbra R, Ordoñez CA, Leppaniemi A, Fraga GP, Coccolini F, Agresta F, Abbas A, Abdel Kader S, Agboola J, Amhed A, Ajibade A, Akkucuk S, Alharthi B, Anyfantakis D, Augustin G, Baiocchi G, Bala M, Baraket O, Bayrak S, Bellanova G, Beltràn MA, Bini R, Boal M, Borodach AV, Bouliaris K, Branger F, Brunelli D, Catani M, Che Jusoh A, Chichom-Mefire A, Cocorullo G, Colak E, Costa D, Costa S, Cui Y, Curca GL, Curry T, Das K, Delibegovic S, Demetrashvili Z, Di Carlo I, Drozdova N, El Zalabany T, Enani MA, Faro M, Gachabayov M, Giménez Maurel T, Gkiokas G, Gomes CA, Gonsaga RA, Guercioni G, Guner A, Gupta S, Gutierrez S, Hutan M, Ioannidis O, Isik A, Izawa Y, Jain SA, Jokubauskas M, Karamarkovic A, Kauhanen S, Kaushik R, Kenig J, Khokha V, Kim JI, Kong V, Koshy R, Krasniqi A, Kshirsagar A, Kuliesius Z, Lasithiotakis K, Leão P, Lee JG, Leon M, Lizarazu Pérez A, Lohsiriwat V, López-Tomassetti Fernandez E, Lostoridis E, Mn R, Major P, Marinis A, Marrelli D, Martinez-Perez A, Marwah S, McFarlane M, Melo RB, Mesina C, Michalopoulos N, Moldovanu R, Mouaqit O, Munyika A, Negoi I, Nikolopoulos I, Nita GE, Olaoye I, Omari A, Ossa PR, Ozkan Z, Padmakumar R, Pata F, Pereira Junior GA, Pereira J, Pintar T, Pouggouras K, Prabhu V, Rausei S, Rems M, Rios-Cruz D, Sakakushev B, Sánchez de Molina ML, Seretis C, Shelat V, Simões RL, Sinibaldi G, Skrovina M, Smirnov D, Spyropoulos C, Tepp J, Tezcaner T, Tolonen M, Torba M, Ulrych J, Uzunoglu MY, van Dellen D, van Ramshorst GH, Vasquez G, Venara A, Vereczkei A, Vettoretto N, Vlad N, Yadav SK, Yilmaz TU, Yuan KC, Zachariah SK, Zida M, Zilinskas J, Ansaloni L. Global validation of the WSES Sepsis Severity Score for patients with complicated intra-abdominal infections: a prospective multicentre study (WISS Study) World J Emerg Surg. 2015 Dec 16;10:61. doi: 10.1186/s13017-015-0055-0. eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chmelnik M, Lasch L, Weih S, Wink E, Romero P, Holland-Cunz S. Anastomotic sealing with a fibrin-coated collagen patch in small-diameter bowel. Langenbecks Arch Surg. 2011 Jun;396(5):685–91. doi: 10.1007/s00423-011-0750-6. Epub 2011 Mar 1. [DOI] [PubMed] [Google Scholar]
- 13.Pantelis D, Beissel A, Kahl P, Wehner S, Vilz TO, Kalff JC. The effect of sealing with a fixed combination of collagen matrix-bound coagulation factors on the healing of colonic anastomoses in experimental high-risk mice models. Langenbecks Arch Surg. 2010 Nov;395(8):1039–48. doi: 10.1007/s00423-010-0703-5. [DOI] [PubMed] [Google Scholar]
- 14.Fingerhut A, Uranues S, Ettorre GM, Felli E, Colasanti M, Scerrino G, Melfa GI, Raspanti C, Gulotta G, Meyer A, Oberhoffer M, Schmoeckel M, Weltert LP, Vignolini G, Salvi M, Masieri L, Vittori G, Siena G, Minervini A, Serni S, Carini M. European Initial Hands-On Experience with HEMOPATCH, a Novel Sealing Hemostatic Patch: Application in General, Gastrointestinal, Biliopancreatic, Cardiac, and Urologic Surgery. Surg Technol Int. 2014 Nov;25:29–35. [PubMed] [Google Scholar]
- 15.Scerrino G, Paladino NC, Di Paola V, Morfino G, Amodio E, Gulotta G, Bonventre S. The use of haemostatic agents in thyroid surgery: efficacy and further advantages. Collagen-Fibrinogen-Thrombin Patch (CFTP) versus Cellulose Gauze. Ann Ital Chir. 2013 Sep-Oct;84(5):545–50. [PubMed] [Google Scholar]
- 16.Cocorullo G, Mirabella A, Falco N, Fontana T, Tutino R, Licari L, Salamone G, Scerrino G, Gulotta G. An investigation of bedside laparoscopy in the ICU for cases of non-occlusive mesenteric ischemia. World J Emerg Surg. 2017;12:4. doi: 10.1186/s13017-017-0118-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mascolino A, Scerrino G, Gullo R, Genova C, Melfa GI, Raspanti C, Fontana T, Falco N, Porrello C, Gulotta G. Large retroperitoneal abscess extended to the inferior right limb secondary to a perforated ileal Crohn’s disease: the importance of the multidisciplinary approach. G Chir. 2016 Jan-Feb;37(1):37–41. doi: 10.11138/gchir/2016.37.1.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paladino NC, Inviati A, Di Paola V, Busuito G, Amodio E, Bonventre S, Scerrino G. Predictive factors of mortality in patients with acute mesenteric ischemia. A retrospective study. Ann Ital Chir. 2014 May-Jun;85(3):265–70. [PubMed] [Google Scholar]
- 19.Carroccio A, Mansueto P, Morfino G, D’Alcamo A, Di Paola V, Iacono G, Soresi M, Scerrino G, Maresi E, Gulotta G, Rini G, Bonventre S. Oligo-antigenic diet in the treatment of chronic anal fissures. Evidence for a relationship between food hypersensitivity and anal fissures. Am J Gastroenterol. 2013;108(5):825–32. doi: 10.1038/ajg.2013.58. [DOI] [PubMed] [Google Scholar]
- 20.Melfa G, Coccorullo G, Raspanti C, Falco N, Porrello C, Gullo R, Rotolo G, Genova C, Gulotta G, Scerrino G. Laparoscopic treatment of a large pedunculated hemangioma of the liver: a case report. G Chir. 2016;37(4):162–166. doi: 10.11138/gchir/2016.37.4.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cocorullo G, Falco N, Tutino R, Fontana T, Scerrino G, Salamone G, Licari L, Gulotta G. Open versus laparoscopic approach in the treatment of abdominal emergencies in elderly population. G Chir. 2016;37(3):108–112. doi: 10.11138/gchir/2016.37.3.108. [DOI] [PMC free article] [PubMed] [Google Scholar]