Abstract
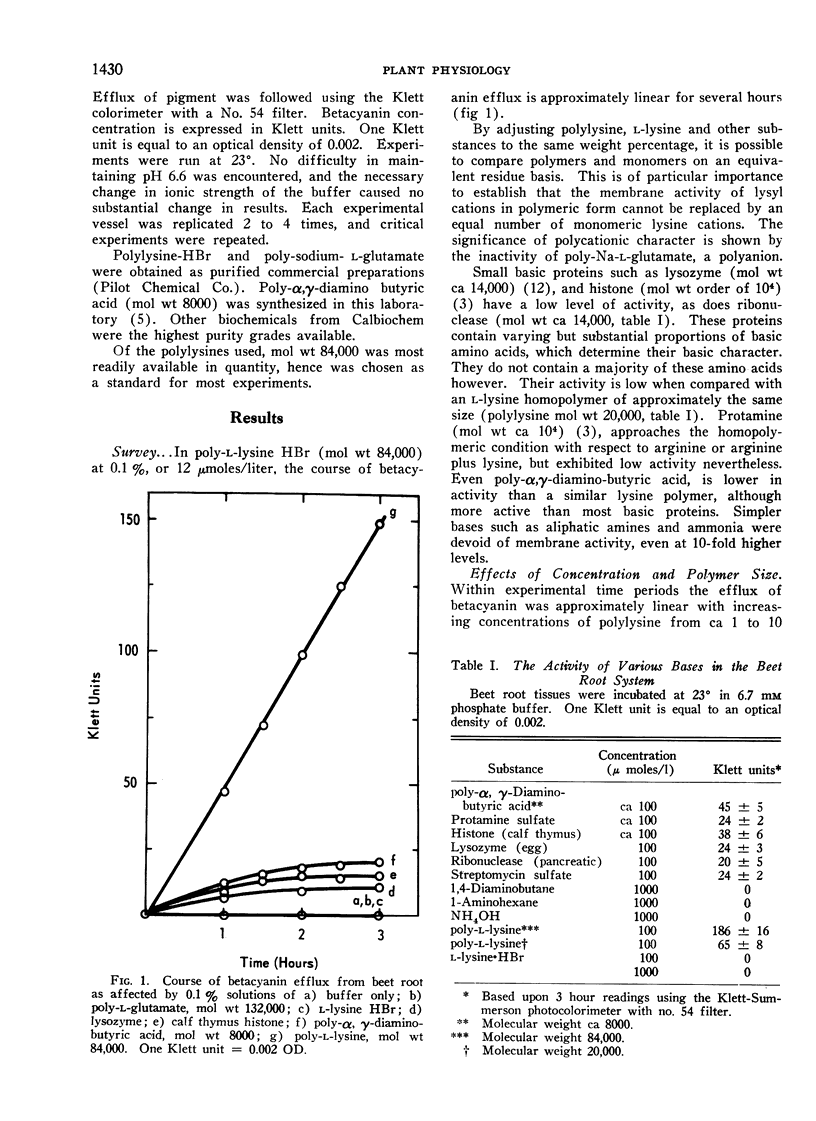
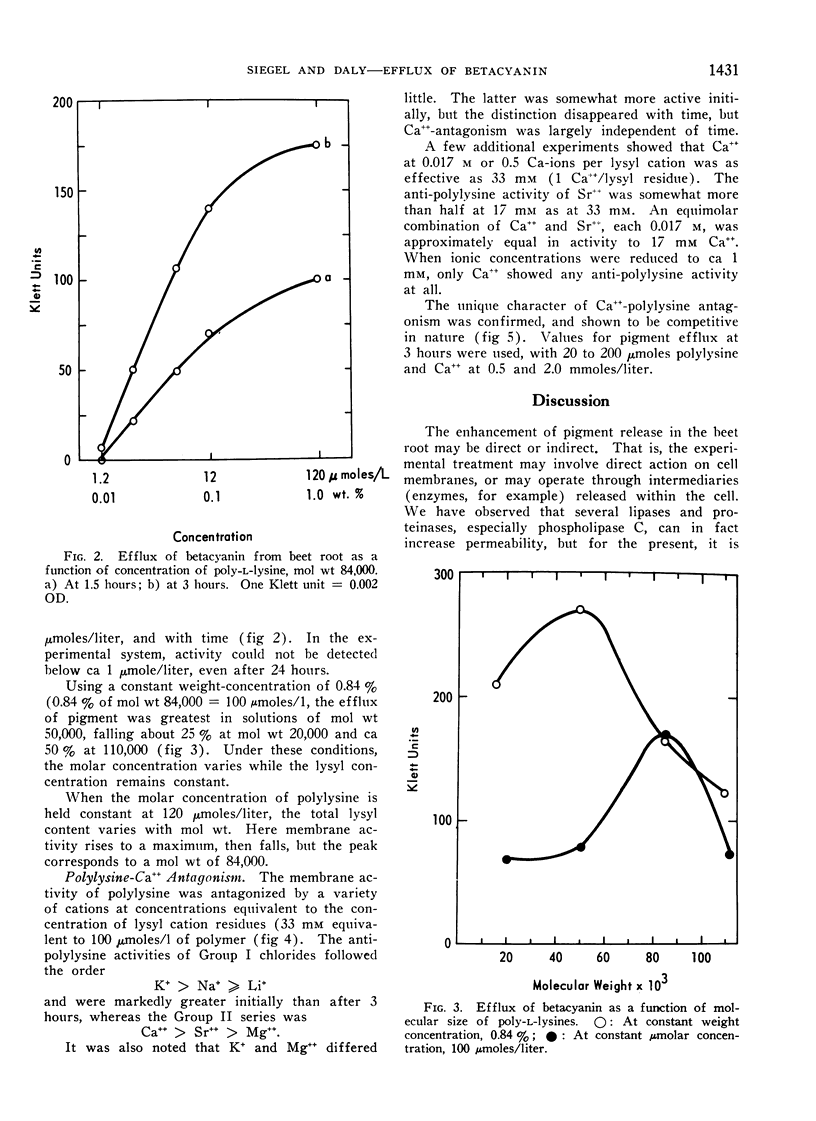
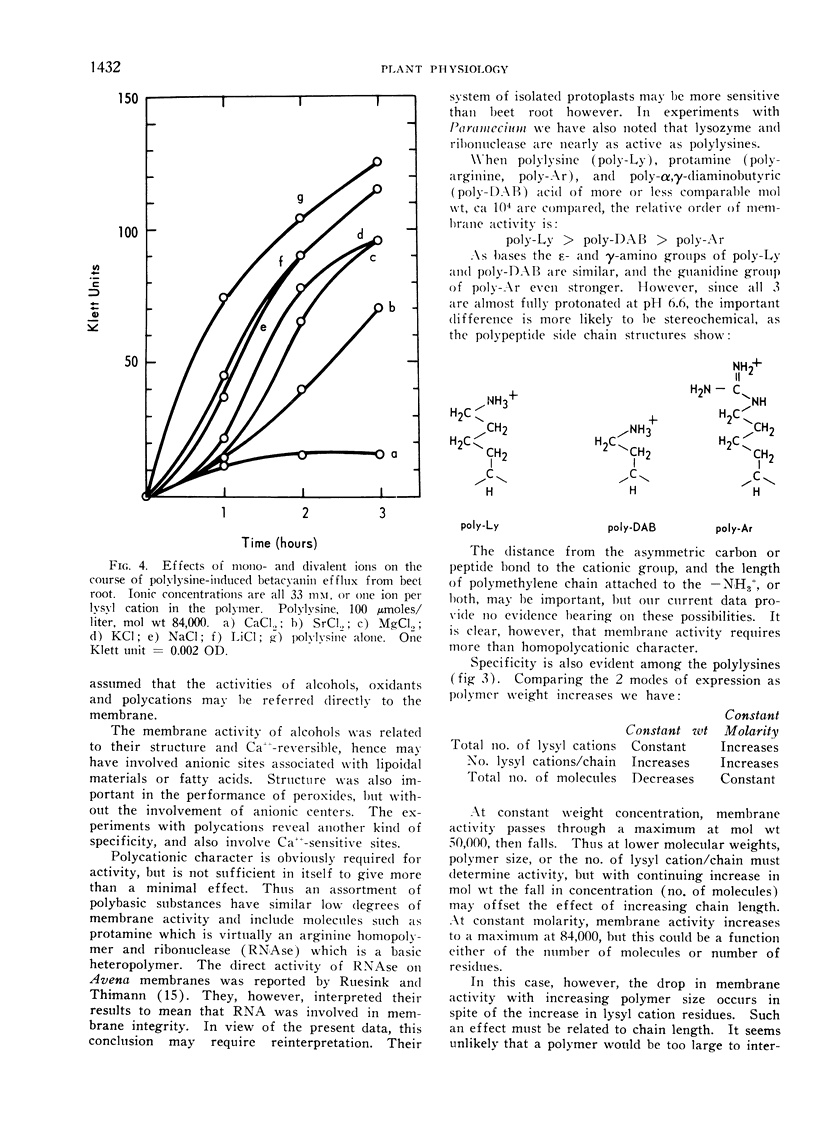
Poly-l-lysine, poly-α, γ-diaminobutyric acid and basic proteins cause efflux of betacyanin from beet root tissues to varying degrees. Membrane activities fall in the order: polylysine > poly-α, γ-diaminobutyric acid > polyarginine (protamine), suggesting the importance of steric factors in side-chain to backbone relations. It was also observed that homopolymer activity > heteropolymer activity, using ribonuclease and lysozyme as examples of the latter. Among polylysines, there appears to be an optimal chain length at a molecular weight equal to 50,000. Lowered activity of larger polymers is interpreted in terms of a diffusion barrier, the cell wall.
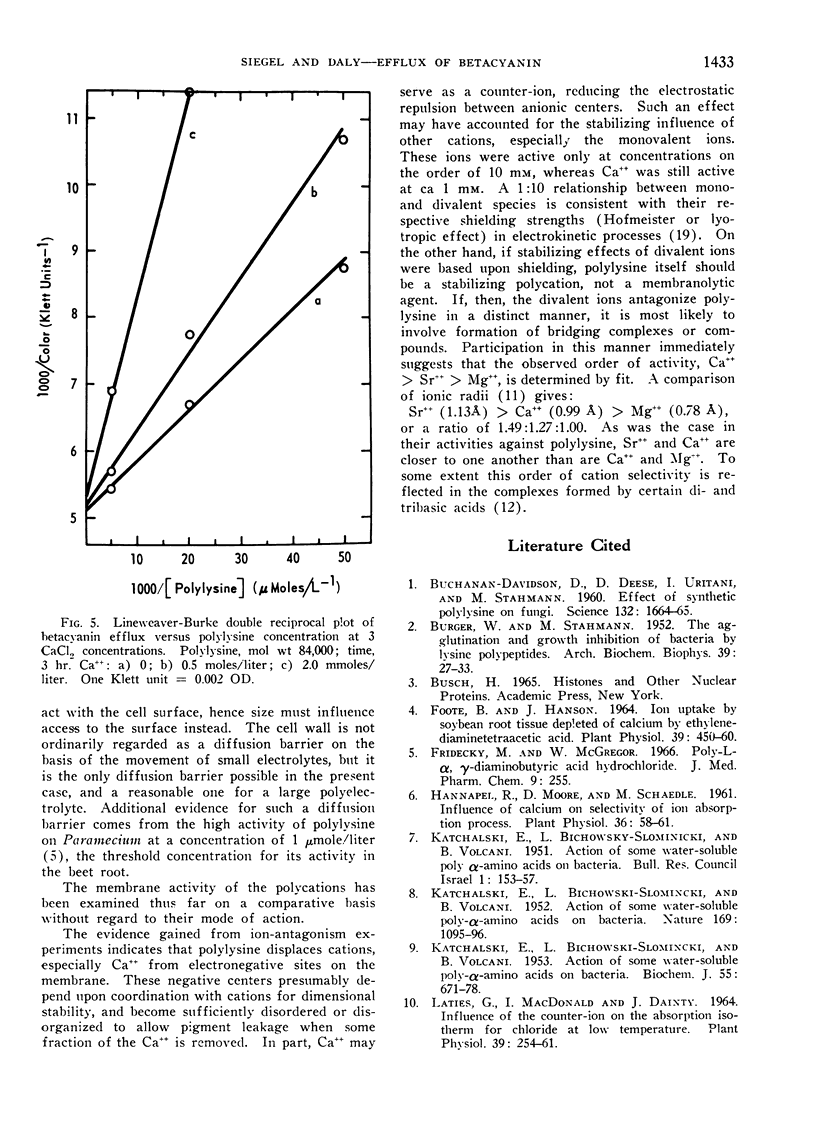
Polylysine and Ca++ exhibit competitive kinetics, and Ca++ otherwise is far more active than other cations. It is assumed that polylysine displaces Ca++ from anionic centers on the membrane, but cannot confer equivalent dimensional stability, rendering the membrane leaky. The possible role of cationic shielding in ionic stabilization of the membrane was also considered. The order of divalent ion activity against polylysine was Ca++ > Sr++ > Mg++, suggesting again a specific size-fit relationship.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURGER W. C., STAHMANN M. A. The agglutination and growth inhibition of bacteria by lysine polypeptides. Arch Biochem Biophys. 1952 Jul;39(1):27–36. doi: 10.1016/0003-9861(52)90257-9. [DOI] [PubMed] [Google Scholar]
- Buchanan-Davidson D. J., Deese D. C., Uritani I., Stahmann M. A. Effect of Synthetic Polylysine on Fungi. Science. 1960 Dec 2;132(3440):1664–1666. doi: 10.1126/science.132.3440.1664. [DOI] [PubMed] [Google Scholar]
- Foote B. D., Hanson J. B. Ion Uptake by Soybean Root Tissue Depleted of Calcium by Ethylenediaminetetraacetic Acid. Plant Physiol. 1964 May;39(3):450–460. doi: 10.1104/pp.39.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridecky M. J., McGregor W. H. Poly-L-alpha, gamma-diaminobutyric acid hydrochloride. J Med Chem. 1966 Mar;9(2):255–256. doi: 10.1021/jm00320a030. [DOI] [PubMed] [Google Scholar]
- Jacobson L., Hannapel R. J., Moore D. P., Schaedle M. Influence of calcium on selectivity of ion absorption process. Plant Physiol. 1961 Jan;36(1):58–61. doi: 10.1104/pp.36.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laties G. G., Macdonald I. R., Dainty J. Influence of the Counter-ion on the Absorption Isotherm for Chloride at Low Temperature. Plant Physiol. 1964 Mar;39(2):254–262. doi: 10.1104/pp.39.2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruesink A. W., Thimann K. V. Protoplasts from the Avena coleoptile. Proc Natl Acad Sci U S A. 1965 Jul;54(1):56–64. doi: 10.1073/pnas.54.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel S. M., Halpern L. A. Effects of peroxides on permeability and their modification by indoles, vitamin E, and other substances. Plant Physiol. 1965 Sep;40(5):792–796. doi: 10.1104/pp.40.5.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel S. M., Halpern L. A. THE EFFECT OF BRANCHING AT C-1 ON THE BIOLOGICAL ACTIVITY OF ALCOHOLS. Proc Natl Acad Sci U S A. 1964 May;51(5):765–768. doi: 10.1073/pnas.51.5.765. [DOI] [PMC free article] [PubMed] [Google Scholar]



