Abstract
Objectives
We sought to understand how polymicrobial colonization varies during health, viral upper respiratory infection (URI) and acute upper respiratory bacterial infection to understand differences in infection-prone vs. non-prone patients.
Methods
Nasopharyngeal (NP) samples were collected from 74 acute otitis media (AOM) infection-prone and 754 non-prone children during 2094 healthy visits, 673 viral URI visits and 631 AOM visits. Three otopathogens Streptococcus pneumoniae (Spn), Nontypeable Haemophilus influenzae (NTHi), and Moraxella catarrhalis (Mcat) were identified by culture.
Results
NP colonization rates of multiple otopathogens during health were significantly lower than during viral URI, and during URI they were lower than at onset of upper respiratory bacterial infection in both AOM infection-prone and non-prone children. AOM infection-prone children had higher polymicrobial colonization rates than non-prone children during health, viral URI and AOM. Polymicrobial colonization rates of AOM infection-prone children during health were equivalent to that of non-prone children during viral URI, and during viral URI were equivalent to that of non-prone during AOM infection. Spn colonization was positively associated with NTHi and Mcat colonization during health, but negatively during AOM infection.
Conclusion
The infection-prone patients more frequently have multiple potential bacterial pathogens in the NP than the non-prone patients. Polymicrobial interaction in the NP differs during health and at onset of infection.
Keywords: polymicrobial colonization, acute otitis media, otitis prone children, viral upper respiratory infection
Introduction
The pathogenesis of upper and lower respiratory bacterial infections involves complex interactions among bacteria, respiratory viruses, and the host innate immune responses (1–4). Bacterial-viral interactions play a critical role in bacterial infection pathogenesis such as acute otitis media (AOM) (2, 5, 6). Viral upper respiratory tract infection (URI) is considered as the most important predictor for an upper respiratory bacterial infection such as AOM, unquestionably outweighing all other risk factors (1, 2, 5, 6). Approximately >90 % of upper respiratory bacterial infections have a symptomatic viral URI at the time of clinical presentation with an upper respiratory bacterial infection (4, 5, 7).
The most common pathogens that cause upper and lower respiratory bacterial infections are Streptococcus pneumonia (Spn), Nontypable Haemophilus influenzae (NTHi), and Moraxella catarrhalis (Mcat)(4, 8–12). During health, Spn, NTHi and Mcat nasopharyngeal (NP) colonization is asymptomatic and elicits mild NP epithelial inflammation that allows controlled immune surveillance in the NP, leading to the clearance of colonization without developing immunopathology(13–15). When a viral URI occurs, an inflammatory response induced by viral co-infection promotes increased growth of Spn, NTHi and/or Mcat if the organism is already colonized in the NP, or facilitates colonization and escalation of growth of new otopathogens (14–16).
Spn, NTHi and Mcat colonization in the NP is a necessary pre-requisite for infection due to these organisms. In a previous study we have shown that approximately 17% of healthy young children are colonized with two or more of the three major respiratory bacterial pathogens (Spn, NTHi, Mcat) (17). The major difference in NP colonization was seen in polymicrobial colonization, not single species colonization when comparing healthy children to children with AOM (17). Bacterial pathogens have different interaction patterns at onset of an upper respiratory bacterial infection and during health (17). Co-colonization influences antibody responses (18) and outcomes of infection (19). The objectives of this study were to characterize polymicrobial NP colonization during health, at onset of a viral URI and at onset of AOM as well as to determine if differences occurred in infection-prone vs. non-prone patients.
Material and Methods
Experimental Design
This study derives from a 10-year (2006–2015) prospective longitudinal evaluation of immunity to Spn and NTHi NP colonization and AOM in young children. Healthy children without any previous episodes of AOM were enrolled at 6 months of age from Rochester, NY. NP samples were collected at 6 healthy visits at 6, 9, 12, 15, 18 and 24 month of age, and classified into healthy and URI visits according to whether or not the children presented with viral URI symptoms such as sore throat, nasal congestion, runny nose, sneezing, cough, and low-grade fever. Whenever the children were diagnosed as having AOM, tympanocentesis was performed and MEF samples were collected to confirm the clinical diagnosis. When a child experienced 3 episodes of AOM infections within a 6 month time span or 4 episodes of AOM infections in a 12 month time span they were designated as AOM infection-prone and because the diagnosis was confirmed by tympanocentesis-derived middle ear fluid culture we have termed these children stringently-defined otitis prone in our prior publication (20). Three bacterial pathogens (Spn, NTHi and Mcat) were identified by culture. AOM diagnosis, sample collection and bacterial pathogen identifications were performed as described previously (17, 21). All children received the standard vaccination regimen in the United States, including the PCV-7 (before 2010) or PCV-13 (since 2010) vaccine (Prevnar, Pfizer Pharmaceuticals, Collegeville, PA) at the recommended ages. The study was approved by the Institutional Review Board of Rochester General Hospital. Written informed consent was obtained from parents and guardians of all children in the study.
Statistics
The rates of NP colonization among children of the same age at healthy, URI and AOM visits were compared using the Fisher exact test and GraphPad Prism software. Bacterial interactions were analyzed using repeated measures logistic regression models. Predicted outcomes of colonization with Spn, NTHi and Mcat were examined using multivariate logistic regression. Generalized estimating equations were used to model exchangeable correlation among subjects. Four logistic regression models (healthy, URI, AOM visits and overall) were calculated by using R version 2.13.2 (www.r-project.org/). To examine the effects of covariates on each of the 3 pathogens, we modeled NP colonization of each pathogen separately by using the remaining 2 pathogens as predictors and including an interaction term. For each model, we estimated odds ratios (ORs) for the response pathogen given the presence of each predictor pathogen alone, then jointly; synergistic associations between bacteria are indicated by OR>1; competitive association, by OR<1. The absence of both predictor pathogens was used as the reference condition. A p<0.05 was considered significant.
Results
Study cohort
This analysis involved a total of 2094 healthy visits, 673 URI visits and 631 AOM visits. Samples were taken from 74 infection-prone children and 680 non-prone children at 6 to 24 month of age. The characteristics of the patients are summarized in Table 1.
Table 1.
Characteristics of study cohorts
| Overall | Infection-prone | Non-prone | P values (infection-prone vs. non-prone) |
||
|---|---|---|---|---|---|
| Total children | 754 (100%) | 74 (9.8%) | 680 (90.2%) | <0.0001 | |
|
| |||||
| Gender | |||||
| Female | 357 (47.3%) | 23(31.1%) | 334 (49.2%) | 0.003 | |
| Male | 397 (52.7%) | 51(68.9%) | 346 (50.8%) | ||
|
| |||||
| Race | |||||
| White | 520 (68.9%) | 61(82.4%) | 459 (67.5%) | 0.008 | |
| Non-white | 234 (32%) | 13 (17.6%) | 221 (32.5%) | ||
|
| |||||
| Brest feeding* | |||||
| > 6M | 104 (15.5%) | 3 (4.4%) | 101 (16.7%) | 0.52 | |
| < 6M | 181 (26.9%) | 23 (33.8%) | 158 (26.1%) | ||
| Formula | 387 (57.6%) | 42 (61.8%) | 345 (57.2%) | ||
|
| |||||
| Daycare attendance* | |||||
| Yes | 238 (34.2%) | 36 (62.1%) | 202 (31.7%) | <0.0001 | |
| No | 457 (65.8%) | 22 (37.9%) | 435 (68.3%) | ||
|
| |||||
| Sibling | |||||
| Yes | 472(62.6%) | 45 (61%) | 428 (62.9%) | 0.71 | |
| No | 282(37.4%) | 29 (39%) | 252 (37.1%) | ||
|
| |||||
| Home smoker | |||||
| Yes | 98(13.0%) | 5 (6.8%) | 93 (13.7%) | 0.10 | |
| No | 656 (90.2%) | 69 (93.2%) | 587 (86.3%) | ||
|
| |||||
| Total visits (samples) ** | 3398 (100%) | 542 (16.0%) | 2856 (84.0%) | <0.0001 | |
|
| |||||
| Visits of each healthy state group** | |||||
| Healthy | total visits | 2094 (61.6%) | 198 (36.5%) | 1896 (66.4%) | <0.0001 |
| visits per child | 2.78 | 2.67 | 2.79 | / | |
| URI | total visits | 673 (19.8%) | 95 (17.5%) | 578 (20.2%) | 0.15 |
| visits per child | 0.89 | 1.28 | 0.85 | / | |
| AOM | total visits | 631 (18.5%) | 249 (15.9%) | 382 (13.4%) | 0.12 |
| visits per child | 0.84 | 3.36 | 0.56 | / | |
daycare and breastfeeding date of some children were missing.
NP samples were collected at all visits
Comparison of NP colonization Among Health, URI and AOM visits
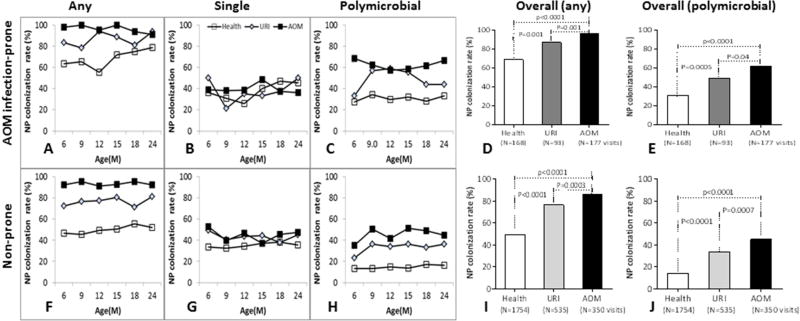
We compared NP colonization rates with single species, multiple species or any combination of the three potential bacterial pathogens (Spn, NTHi and Mcat) among three states (healthy, viral URI and AOM) in infection-prone and non-prone patients. We found that colonization at healthy visits was less frequent than at URI visits, and colonization at URI visits was less frequent than at a visit when AOM was diagnosed in both infection-prone and non-prone patients (Figure 1). In infection-prone children, overall NP colonization rates with any species of the three otopathogens at healthy visits (69.0% (116/168)) was significantly lower than URI visits (87.1% (81/93)) which was significantly lower than AOM visits (97% (229/236)) (all p <0.01, Fig 1A, 1D). The differences in NP colonization among the three states were in polymicrobial colonization two or more of the three otopathogens) (Fig. 1C, 1E), but not in single species colonization (Fig. 1B). The overall rates of polymicrobial colonization in infection-prone children were 31.0% (52/168) at healthy visits, 49.5% (46/93) at URI visits and 62.1% (110/177) at AOM visits (all P <0.01). Overall single species colonization rates were 38.1 % (64/168) at healthy visits, 37.6% (35/93) at viral URI visits and 41.8% (74/177) at AOM visits (all p>0.5) (Fig.1B). Similar results were obtained in non-prone children (Fig. 1F–1J). Overall colonization rates by any of the three otopathogens, as well as polymicrobial colonization rates at AOM visits were greater than at URI visits and rates at URI visits were greater than at healthy visits (all p<0.001) (Fig. 1I,1J).
Figure 1. Comparison of NP colonization of single and multiple species of otopathogens between healthy, URI and AOM visits.
NP samples were collected from children at prospective healthy visits, URI and AOM at 6–24 months of age. The three bacterial potential pathogens Spn, NTHi and Mcat were identified by standard culture. The percentiles of NP colonization with any species of the three otopathogens, single species (B,G) and polymicrobial colonization (> two species) were compared by Fisher exact test between health, URI and AOM visits in AOM infection-prone and non-prone children. A–E, AOM infection-prone children; F-J, Nno-prone children: A and F, any species colonization rates over time; D and G, single species colonization rates over time; C and H polymicrobial colonization rates over time; D and I, overall colonization rates with any species; E and J, overall polymicrobial colonization rates.
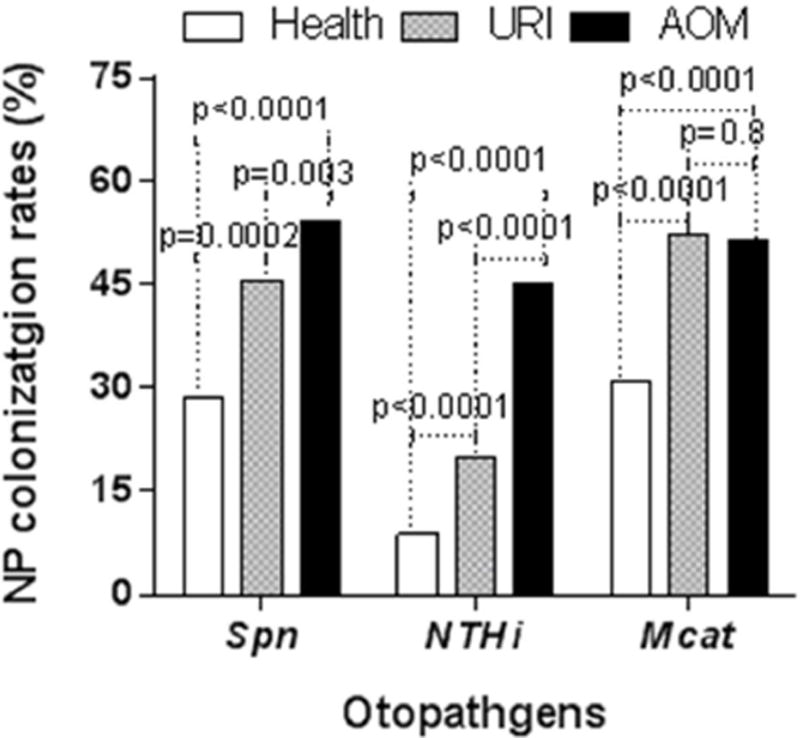
The overall colonization rates of Spn during health (28.5% (569/2094)) were less than at URI visits (45.6% (307/673)) and rates at URI visits were less than at AOM visits (53.8%(340/631)) (all p<0.01). Overall colonization rates of NTHi during health (8.8% (185/2094)) were less than at URI visits (19.8% (133/673)) rates at URI visits were less than at AOM visits (45.1% (285/631)) (all p< 0.0001). Overall colonization rates of Mcat during health (30.1% (643/2094)) were less than at viral URI (52% visits (350/673)) (p<0.0001), but there was no difference between viral URI and at AOM visits (p=0.8) (Figure 2).
Figure 2. Comparison of NP colonization rates of each otopathogens between healthy, URI and AOM visits.
NP samples were collected from children at prospective healthy visits, onset of URI and AOM at 6–24 months of age. The three bacterial potential pathogens Spn, NTHi and Mcat were identified by standard culture. The percentiles of NP colonization of Spn, NTHI, and Mcat by Fisher exact test between health, URI and AOM visits in all children.
Multivalent regression analysis showed that both viral URI and AOM were positively associated with NP colonization by each individual otopathogen (all p<0.05) except AOM states with Mcat colonization (p>0.05). The strongest association was identified between AOM and NTHi colonization (OR=3.45, z score =9.10, p<0.00001). Both viral URI and AOM were strongly and positively associated with polymicrobial colonization (all p≤0.0001) (Table 2).
Table 2.
Association of AOM infection-prone, URI and AOM with NP colonization of potential bacterial pathogens
| Outcomes | ||||||||
|---|---|---|---|---|---|---|---|---|
| Spn | NTHi | Mcat | Polymicrobial colonization | |||||
| OR (95%CI) |
P value | OR (95%CI) |
P value | OR (95%CI) |
P value | OR (95%CI) |
P value | |
| Infection-prone | 1.71 (1.30–2.24) | 0.0001 | 1.95 (1.42–2.67) | 0.00004 | 1.58 (1.23–2.04) | 0.00035 | 2.18 (1.67–2.84) | <0.00001 |
| URI | 1.67 (1.41–1.98) | <0.00001 | 2.09 (1.61–2.72) | <0.00001 | 2.20 (1.83–2.64) | <0.00001 | 2.46 (2.02–3.00) | <0.00001 |
| AOM | 1.32 (1.03–1.68 | 0.02 | 3.45 (2.64–4.51) | <0.00001 | 1.08 (0.87–1.35) | 0.49 | 1.58 (1.25–1.99) | 0.00011 |
Bold: statically significant difference. OR>1, positive association; OR<1, negative association.
Comparison of NP colonization between AOM infection-prone and non-prone patients
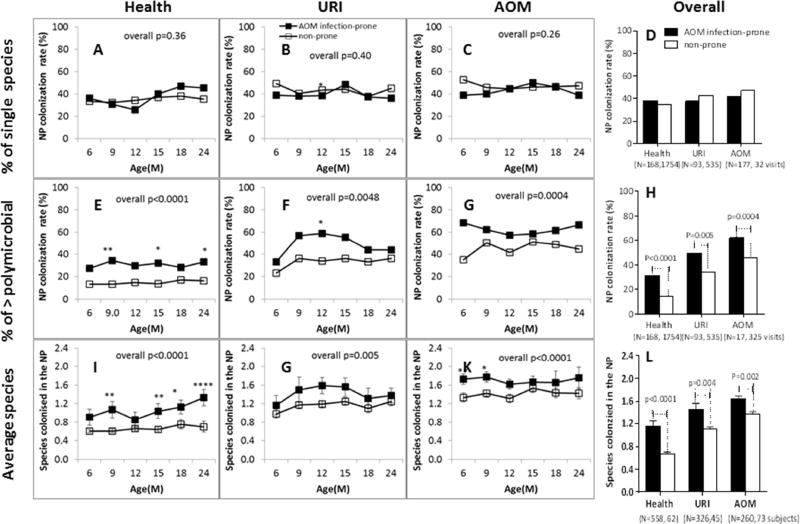
Infection-prone children had significantly higher rates of NP colonization with bacterial respiratory pathogens than non-prone children. The differences between infection-prone and non-prone were children in colonization with multiple species (Fig. 3E–3H) but not in single species colonization (Fig 3A–3D). The percentage of multiple species colonization in infection-prone children during health, URI and AOM visits were all higher than non-prone children across the ages (Fig. 3D–3G). The overall polymicrobial colonization rates in infection-prone children were significant higher than non-prone children during health (31.0% (52/168) vs. 14.4% (253/1754), p<0.0001), at URI visits (49.5% (46/93) vs. 33.6% (180/535), p=0.005) and at AOM visits (62.1% (110/177) vs. 45.5% (148/325), p=0.0004) (Fig.2H). Polymicrobial colonization rates in both infection-prone and non-prone during health were less than rates at URI visits and rates at URI visits were less than AOM visits. However, the polymicrobial colonization rates of infection-prone children during healthy visits were as high as the polymicrobial colonization rates in non-prone children at URI visits (31.0% (52/168) vs. 33.6% (180/535), p=0.57); and the polymicrobial colonization rates of infection-prone children at URI visits were as high as the polymicrobial colonization rates of non-prone children at the onset of AOM (49.5% (46/93) vs. 45.5% (148/325), p=0.56) (Fig 3G). Similar results were obtained when we observed average number of colonization events with bacterial respiratory pathogens (Fig. 3I–3L). There were no significant differences between infection-prone and non-prone children in single species colonization at healthy, at URI visits or AOM visits across ages (Fig.3A–3C) nor overall (Fig. 3D) (all p values >0.5).
Figure 3. Comparison of NP colonization between AOM infection-prone and non-prone children.
NP samples were collected from children at prospective healthy visits, URI and AOM at 6–24 months of age. The three potential bacterial otopathogens Spn, NTHi and Mcat were identified by standard culture. The percentiles of single species colonization (any of the three species), polymicrobial colonization (> two species) and average number of species colonizing in the NP were compared by Fisher exact test between AOM infection-prone and non-prone at health, URI and AOM visits. A-D, % single colonization; G-H, % polymicrobial colonization; I-L, average numbers of species colonized in the NP. *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001.
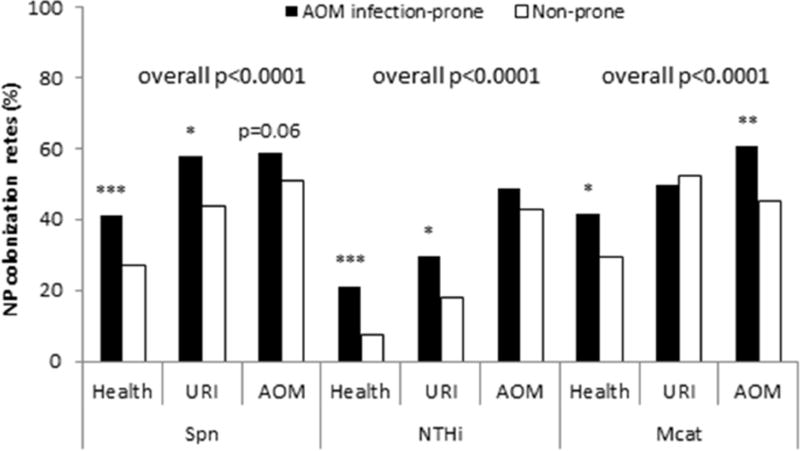
We previously reported that colonization rates of Spn, NTHi and Mcat in infection-prone children during healthy visits were significantly higher than non-prone children (22). Here we further compared the colonization rates at URI visits and AOM. We found that at URI visits, NP colonization rates of infection prone children were significantly higher than non-prone children for Spn (29.6% (67/95) vs. 43.6% (252/578), p=0.01) and NTHi (57.9% (55/95) vs. 18.2% (105/578), p=0.02), but not for Mcat (49.5% (151/249) vs. 52.4% (303/578), p=68). During AOM, infection-prone children had higher colonization rates with Mcat (60.6% (151/249) vs. 45.0% (172/382), p=0.0001), and trended higher for Spn, (58.6% (146/249) vs. 50.8% (194/382), p=0.06) and NTHi (48.6% (121/249) vs. 42.9% (164/28), p=0.17). Overall (all three states) infection-prone children had significantly higher colonization rates in Spn, NTHi, and Mcat (all p<0.0001) (Figure 4).
Figure 4. Comparison of NP colonization rates of each otopathogens between AOM infection-prone and non-prone children.
NP samples were collected from children at prospective healthy visits, URI and AOM at 6–24 months of age. The three bacterial potential pathogens Spn, NTHi and Mcat were identified by standard culture. The percentiles of NP colonization of Spn, NTHI, and Mcat by Fisher exact test between AOM infection-prone children and non-prone children during health, URI and AOM visits
Multivariate regression analysis showed that the infection-prone condition was strongly and positively associated with NP colonization of each individual respiratory bacterial pathogen as well as polymicrobial colonization (all OR>1, p<0.001) (Table 2).
Interactions of bacterial otopathogens in the NP during health, URI and AOM
Since we previously found that bacterial interaction in the NP differed during health and at onset of AOM, we generated four different models with different datasets: during health, at URI visits, AOM and with all states together (Table 3). At healthy visits, Spn and NTHi colonization and Spn and Mcat colonization were positively associated with each other. When examining Spn colonization as the predicted outcome, Spn colonization was positively associated with Mcat (OR=1.77, p<0.0001) colonization, and trended toward a positive association with NTHi (OR=1.46, P=0.06). When examining NTHi colonization as the predicted outcome, NTHi colonization was positively associated with Spn (OR=1.52, P=0.04). When examining Mcat colonization as the predicted outcome, Mcat colonization was positively associated with Spn (OR=1.84, P<0.0001). The infection-prone condition was positively associated with all three respiratory bacterial pathogens colonizing the NP (all p<0.05). No significant association was identified between NTHi and Mcat colonization (P>0.05) (Table 3 Model I).
Table 3.
Interactions between potential pathogens of AOM in the NP
| Outcomes | |||||||
|---|---|---|---|---|---|---|---|
| Spn | NTHi | Mcat | |||||
| OR (95%CI) | P value | OR (95%CI) | P value | OR (95%CI) | P value | ||
| Model I (Health) | Spn | / | 1.52 (1.01–2.27) | 0.04 | 1.84 (1.48–2.3) | <0.0001 | |
| NTHi | 1.46 (0.98–2.18) | 0.06 | 0.87 (0.55–1.37) | 0.55 | 0.86 (0.54–1.368) | 0.54 | |
| Mcat | 1.77 (1.42–2.2) | <0.00001 | / | / | |||
| Spn X NTHi | / | / | 1.45 (0.76–2.78) | 0.26 | |||
| Spn X Mcat | / | 1.39 (0.72–2.7) | 0.33 | / | |||
| NTHi X Mcat | 1.41 (0.76–2.61) | 0.27 | / | ||||
| Infection-prone | 1.58 (1.08–2.31) | 0.020 | 3.22 (2.11–4.91) | <0.00001 | 1.46 (1.02–2.07) | 0.04 | |
| Model II (URI) | Spn | / | 1.48 (0.87–2.53) | 0.15 | 1.41(1.01–1.97) | 0.04 | |
| NTHi | 1.36 (0.8–2.31) | 0.25 | / | 0.58 (0.33–1.02) | 0.06 | ||
| Mcat | 1.33 (0.95–1.85) | 0.09 | 0.63 (0.36–1.09) | 0.09 | / | ||
| Spn X NTHi | / | / | 1.5 (0.7–3.19) | 0.30 | |||
| Spn X Mcat | / | 1.32 (0.62–2.77) | 0.48 | / | |||
| NTHi X Mcat | 1.61 (0.98–2.66) | 0.06 | / | / | |||
| Infection-prone | 1.44 (0.69–3.01) | 0.34 | 2.03 (1.14–3.61) | 0.02 | 0.80 (0.5–1.28) | 0.35 | |
| Model III (AOM) | Spn | / | 0.35 (0.22–0.56) | 0.00001 | 0.74 (0.48–1.16) | 0.192234. | |
| NTHi | 0.34 (0.21–0.55) | 0.00001 | / | 0.22 (0.13–0.37) | < 0.00001 | ||
| Mcat | 0.78 (0.5–1.21) | 0.28. | 0.24 (0.15–0.4) | <0.00001 | / | ||
| Spn X NTHi | / | / | 2.45 (1.26–4.74) | 0.00800 | |||
| Spn X Mcat | / | 2.17 (1.13–4.14) | 0.02 | / | |||
| NTHi X Mcat | 2.27 (1.17–4.39) | 0.02 | / | / | |||
| Infection-prone | 1.37 (0.96–1.95) | 0.09 | 1.6 (1.09–2.35) | 0.02 | 2.1 (1.53–2.89) | < 0.00001 | |
| Model IV (Overall) | Spn | / | 1.01 (0.78–1.31) | 0.95 | 1.57 (1.31–1.87) | <0.00001 | |
| NTHi | 0.93 (0.71–1.22) | 0.58820 | / | 0.52 (0.38–0.72) | 0.00006 | ||
| Mcat | 1.49 (1.25–1.76) | <0.00001 | 0.55 (0.41–0.74) | 0.00006 | / | ||
| Spn X NTHi | / | / | 1.32 (0.88–1.98) | 0.18 | |||
| Spn X Mcat | / | 1.12 (0.74–1.69) | 0.58 | / | |||
| NTHi X Mcat | 1.34 (0.92–1.97) | 0.13 | / | / | |||
| Infection-prone | 1.71 (1.3–2.24) | 0.0001 | 1.95 (1.42–2.67) | 0.00004 | 1.58 (1.23–2.04) | 0.0004 | |
Bold: statically significant difference. OR>1, positive association; OR<1, negative association.
At URI visits, Spn and Mcat colonization was positively associated each other when Mcat colonization was examined as the predicted outcome (OR=1.41, P=0.04) or trended toward a positive association when Spn colonization was examined as the predicted outcome (OR=1.33, P=0.09). NTHi and Mcat colonization trended toward a negative association with each other when Mcat (OR=0.58, p=0.06) and NTHI colonization (OR=0.63, p=0.09) was examined as the predicted outcomes. No significant association was identified between Spn and NTHi colonization when Spn (OR=1.36, p=0.25) and NTHi colonization (OR=1.48, p=0.15) was examined as the predicted outcomes (Table 3 Model II).
At AOM visits, Spn and NTHi, as well as NTHi and Mcat colonization were negatively associated with each other (all OR<1, p<0.00001). However, Spn and NTHi co-colonization was positively associated with Mcat colonization (OR=2.45, p=0.008), and Spn and Mcat colonization positively associated with NTHi (OR=2.17, p=0.02), and NTHi and Mcat co-colonization positively associated with Spn (OR=2.27, p=0.02). Overall (all data together), Spn and Mcat colonization was positively associated each other (OR>1, P<0.00001), while NTHi and Mcat colonization were negatively associated each other (OR<1, p<0.0001) (Table 3 Model III).
Discussion
Microbial pathogens often coexist or compete with multiple other microbial species in their natural environments (23). The NP is a polymicrobial reservoir in which a variety of commensal bacteria and potential bacterial pathogens colonize (10, 24–27). Polymicrobial colonization is common in children with AOM (28, 29), and also other upper respiratory infections such as sinusitis (4, 30), and lower respiratory infections such as pneumonia (31–33). Polymicrobial infections are associated with increased infection severity and poorer patient outcomes (34). However, microbial pathogens are generally studied as individual manifestations (23, 34). To the best of our knowledge, this study is the first report to characterize polymicrobial colonization in the NP of infection-prone children. We found that differences in NP colonization between infection-prone and non-prone children most frequently involved increased multiple otopathogen colonization, not single species colonization. The infection-prone condition was very strongly associated with higher polymicrobial colonization rates during health, at URI and AOM visits.
The pathogenesis of respiratory bacterial infections begins in the NP and is triggered by a viral co-infection that facilitates the transition from asymptomatic bacterial carriage to an infection (3–5, 10, 27). During health, Spn, NTHi and Mcat are commensal bacteria (35, 36) that do not cause sufficient inflammation or breach epithelial barriers which would lead to an infection (23, 36). It is only during times of epithelial disruption or immune dysfunction that Spn, NTHi and Mcat cause infection (23, 36). Viral URI can enhance bacterial colonization by causing NP inflammation, changing physiological properties of NP epithelial cells, and modulating host innate and adaptive immune responses (5, 23, 36, 37). In this study, we found that the major differences between health, URI and AOM visits involved the rates of polymicrobial colonization, not single species colonization in the NP. URI and AOM were strongly associated with higher polymicrobial colonization. These results demonstrate that polymicrobial colonization plays a critical role in pathogenesis of AOM, especially in infection-prone children. Due to the increased frequency of polymicrobial colonization, infection-prone children are more susceptible than non-prone children to developing AOM from URI. Also due to immunological deficiency (20, 22), AOM infection-prone children have insufficient innate and adaptive immune responses after natural colonization with potential otopathogens and AOM to prevent recurrent colonization and infection. The etiological bacteria therefore persist in the NP of infection-prone children more so than non-prone children (38).
Polymicrobial interaction is critical for pathogenesis of respiratory bacterial infection (2, 5, 17, 23, 31, 34). Polymicrobes can interact synergistically or compete with each other to impact progress of infection by up- or down-regulating of virulence traits, altering the infected niche, or modulating the host immune response (5, 34). In this study we investigated the interaction of three bacterial pathogens (Spn, NTHi and Mcat) at three different health states. We found that Spn and NTHi, as well as Spn and Mcat interacted positively during health, but negatively during AOM. The results were consistent with our previous studies (17). We also found that at URI visits, Mcat colonization were positively associated with Spn colonization, but negatively with NTHi. Interestingly, during AOM, although the three respiratory bacterial pathogens had a strong negative association with each other, when two respiratory bacterial pathogens colonized in the NP, they were positively associated with colonization of the third respiratory bacterial pathogen. These results highlight the complexity of NP polymicrobial interactions in the NP.
AOM infection-prone children are a subset of the population (approximately 10%) of young children who have three or more episodes of AOM in six months, or four or more episodes in a 12 month period, all confirmed by tympanocentesis (20). Our previous studies have shown that AOM infection-prone children have immune deficiencies in systemic and mucosal antibody responses to NP colonization, as well as AOM, poor memory B-cell and T-helper cell generation, poor responses to routine pediatric vaccinations (diphtheria toxoid, tetanus toxoid, pertussis toxoid, filamentous hemagglutinin, pertactin and hepatitis B vaccines), and lower innate inflammatory responses to respiratory viruses than non-prone children (20, 39). Similarly, patients who experience recurrent acute sinusitis, recurrent acute exacerbations of chronic bronchitis and multiple episodes of community-acquired pneumonia may have immune deficiencies as we have discovered in otitis prone children. This would be an important area for future research.
This study has some limitations. NP microbial ecology includes a broad variety of microbiota (10, 24–27). We investigated only the three major potential bacterial respiratory pathogens that cause AOM. We did not prove that the symptoms during URI visits were caused by viruses and we did not evaluate the full microbiota or bacterial-viral interactions or host immunity-otopathogen interactions that have proved critical for clinical outcomes (6). Further studies are needed in those areas of research and role of respiratory microbiome in maintaining health and pathogenesis of infection.
In conclusion, in this study we have shown that polymicrobial NP colonization and bacterial-bacterial interaction in pathogenesis of upper respiratory bacterial infection differs during times of health, viral URI and at onset of AOM as well as between infection-prone and non-prone patients. The infection-prone patient more frequently has multiple potential bacterial pathogens in the NP than the non-prone patient.
Highlights.
NP polymicrobial colonization rates during health are < during URI< during AOM.
Otitis-prone child have higher polymicrobial colonization rates than non-prone child.
Polymicrobial interactions in the NP differ during health, URI and AOM.
Spn is associated with NTHi or Mcat positively during health, negatively during AOM.
Acknowledgments
This study was supported by the NIH NIDCD R01 08671. We thank the nurses and staff of Legacy Pediatrics; the collaborating pediatricians from Sunrise Pediatrics, Westfall Pediatrics, Lewis Pediatrics and Long Pond Pediatrics and the parents who consented and the children who participated in this long and challenging study. We also thank Dr. Anthony Almudevar (Department of Biostatistics and Computational Biology, University of Rochester Medical Center) for data statistical analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bosch AA, Biesbroek G, Trzcinski K, Sanders EA, Bogaert D. Viral and bacterial interactions in the upper respiratory tract. PLoS pathogens. 2013 Jan;9(1):e1003057. doi: 10.1371/journal.ppat.1003057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pettigrew MM, Gent JF, Pyles RB, Miller AL, Nokso-Koivisto J, Chonmaitree T. Viral-bacterial interactions and risk of acute otitis media complicating upper respiratory tract infection. Journal of clinical microbiology. 2011 Nov;49(11):3750–5. doi: 10.1128/JCM.01186-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Purcell K, Fergie J. Concurrent serious bacterial infections in 912 infants and children hospitalized for treatment of respiratory syncytial virus lower respiratory tract infection. The Pediatric infectious disease journal. 2004 Mar;23(3):267–9. doi: 10.1097/01.inf.0000116759.21252.29. [DOI] [PubMed] [Google Scholar]
- 4.Marom T, Alvarez-Fernandez PE, Jennings K, Patel JA, McCormick DP, Chonmaitree T. Acute bacterial sinusitis complicating viral upper respiratory tract infection in young children. The Pediatric infectious disease journal. 2014 Aug;33(8):803–8. doi: 10.1097/INF.0000000000000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marom T, Nokso-Koivisto J, Chonmaitree T. Viral-bacterial interactions in acute otitis media. Current allergy and asthma reports. 2012 Dec;12(6):551–8. doi: 10.1007/s11882-012-0303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nokso-Koivisto J, Marom T, Chonmaitree T. Importance of viruses in acute otitis media. Current opinion in pediatrics. 2015 Feb;27(1):110–5. doi: 10.1097/MOP.0000000000000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lieberthal AS, Carroll AE, Chonmaitree T, Ganiats TG, Hoberman A, Jackson MA, et al. The diagnosis and management of acute otitis media. Pediatrics. 2013 Mar;131(3):e964–99. doi: 10.1542/peds.2012-3488. [DOI] [PubMed] [Google Scholar]
- 8.Qureishi A, Lee Y, Belfield K, Birchall JP, Daniel M. Update on otitis media - prevention and treatment. Infection and drug resistance. 2014 Jan 10;7:15–24. doi: 10.2147/IDR.S39637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monasta L, Ronfani L, Marchetti F, Montico M, Vecchi Brumatti L, Bavcar A, et al. Burden of disease caused by otitis media: systematic review and global estimates. PloS one. 2012;7(4):e36226. doi: 10.1371/journal.pone.0036226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chi DH, Hendley JO, French P, Arango P, Hayden FG, Winther B. Nasopharyngeal reservoir of bacterial otitis media and sinusitis pathogens in adults during wellness and viral respiratory illness. American journal of rhinology. 2003 Jul-Aug;17(4):209–14. [PubMed] [Google Scholar]
- 11.El-Saed A, Balkhy HH, Al-Dorzi HM, Khan R, Rishu AH, Arabi YM. Acinetobacter is the most common pathogen associated with late-onset and recurrent ventilator-associated pneumonia in an adult intensive care unit in Saudi Arabia. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases. 2013 Sep;17(9):e696–701. doi: 10.1016/j.ijid.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Peng Y, Shu C, Fu Z, Li QB, Liu Z, Yan L. [Pathogen detection of 1 613 cases of hospitalized children with community acquired pneumonia] Zhongguo dang dai er ke za zhi = Chinese journal of contemporary pediatrics. 2015 Nov;17(11):1193–9. [PubMed] [Google Scholar]
- 13.Zhang Z, Clarke TB, Weiser JN. Cellular effectors mediating Th17-dependent clearance of pneumococcal colonization in mice. The Journal of clinical investigation. 2009 Jul;119(7):1899–909. doi: 10.1172/JCI36731. Epub 2009/06/11. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King PT, Sharma R. The Lung Immune Response to Nontypeable Haemophilus influenzae (Lung Immunity to NTHi) Journal of immunology research. 2015;2015:706376. doi: 10.1155/2015/706376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brockson ME, Novotny LA, Jurcisek JA, McGillivary G, Bowers MR, Bakaletz LO. Respiratory syncytial virus promotes Moraxella catarrhalis-induced ascending experimental otitis media. PloS one. 2012;7(6):e40088. doi: 10.1371/journal.pone.0040088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richard AL, Siegel SJ, Erikson J, Weiser JN. TLR2 signaling decreases transmission of Streptococcus pneumoniae by limiting bacterial shedding in an infant mouse Influenza A co-infection model. PLoS pathogens. 2014 Aug;10(8):e1004339. doi: 10.1371/journal.ppat.1004339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu Q, Almudervar A, Casey JR, Pichichero ME. Nasopharyngeal bacterial interactions in children. Emerging infectious diseases. 2012 Nov;18(11):1738–45. doi: 10.3201/eid1811.111904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu Q, Pichichero ME. Co-colonization by Haemophilus influenzae with Streptococcus pneumoniae enhances pneumococcal-specific antibody response in young children. Vaccine. 2014 Feb 3;32(6):706–11. doi: 10.1016/j.vaccine.2013.11.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu Q, Casey JR, Chang A, Pichichero ME. When co-colonizing the nasopharynx haemophilus influenzae predominates over Streptococcus pneumoniae except serotype 19A strains to cause acute otitis media. The Pediatric infectious disease journal. 2012 Jun;31(6):638–40. doi: 10.1097/INF.0b013e31824ba6f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pichichero ME. Ten-Year Study of the Stringently Defined Otitis-prone Child in Rochester, NY. The Pediatric infectious disease journal. 2016 Sep;35(9):1033–9. doi: 10.1097/INF.0000000000001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Casey JR, Adlowitz DG, Pichichero ME. New patterns in the otopathogens causing acute otitis media six to eight years after introduction of pneumococcal conjugate vaccine. The Pediatric infectious disease journal. 2010 Apr;29(4):304–9. doi: 10.1097/INF.0b013e3181c1bc48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu Q, Casey JR, Newman E, Pichichero ME. Otitis-prone Children Have Immunologic Deficiencies in Naturally Acquired Nasopharyngeal Mucosal Antibody Response after Streptococcus pneumoniae Colonization. The Pediatric infectious disease journal. 2016 Jan;35(1):54–60. doi: 10.1097/INF.0000000000000949. [DOI] [PubMed] [Google Scholar]
- 23.Peters BM, Jabra-Rizk MA, O'May GA, Costerton JW, Shirtliff ME. Polymicrobial interactions: impact on pathogenesis and human disease. Clinical microbiology reviews. 2012 Jan;25(1):193–213. doi: 10.1128/CMR.00013-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allen EK, Koeppel AF, Hendley JO, Turner SD, Winther B, Sale MM. Characterization of the nasopharyngeal microbiota in health and during rhinovirus challenge. Microbiome. 2014;2:22. doi: 10.1186/2049-2618-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cremers AJ, Zomer AL, Gritzfeld JF, Ferwerda G, van Hijum SA, Ferreira DM, et al. The adult nasopharyngeal microbiome as a determinant of pneumococcal acquisition. Microbiome. 2014;2:44. doi: 10.1186/2049-2618-2-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosas-Salazar C, Shilts MH, Tovchigrechko A, Chappell JD, Larkin EK, Nelson KE, et al. Nasopharyngeal Microbiome in Respiratory Syncytial Virus Resembles Profile Associated with Increased Childhood Asthma Risk. American journal of respiratory and critical care medicine. 2016 May 15;193(10):1180–3. doi: 10.1164/rccm.201512-2350LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teo SM, Mok D, Pham K, Kusel M, Serralha M, Troy N, et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell host & microbe. 2015 May 13;17(5):704–15. doi: 10.1016/j.chom.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kilpi T, Herva E, Kaijalainen T, Syrjanen R, Takala AK. Bacteriology of acute otitis media in a cohort of Finnish children followed for the first two years of life. The Pediatric infectious disease journal. 2001 Jul;20(7):654–62. doi: 10.1097/00006454-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Ruohola A, Meurman O, Nikkari S, Skottman T, Heikkinen T, Ruuskanen O. The dynamics of bacteria in the middle ear during the course of acute otitis media with tympanostomy tube otorrhea. The Pediatric infectious disease journal. 2007 Oct;26(10):892–6. doi: 10.1097/INF.0b013e31812e4b6c. [DOI] [PubMed] [Google Scholar]
- 30.Brook I. Microbiology of sinusitis. Proceedings of the American Thoracic Society. 2011 Mar;8(1):90–100. doi: 10.1513/pats.201006-038RN. [DOI] [PubMed] [Google Scholar]
- 31.Cilloniz C, Civljak R, Nicolini A, Torres A. Polymicrobial community-acquired pneumonia: An emerging entity. Respirology. 2016 Jan;21(1):65–75. doi: 10.1111/resp.12663. [DOI] [PubMed] [Google Scholar]
- 32.Cilloniz C, Ewig S, Polverino E, Marcos MA, Esquinas C, Gabarrus A, et al. Microbial aetiology of community-acquired pneumonia and its relation to severity. Thorax. 2011 Apr;66(4):340–6. doi: 10.1136/thx.2010.143982. [DOI] [PubMed] [Google Scholar]
- 33.Holter JC, Muller F, Bjorang O, Samdal HH, Marthinsen JB, Jenum PA, et al. Etiology of community-acquired pneumonia and diagnostic yields of microbiological methods: a 3-year prospective study in Norway. BMC infectious diseases. 2015;15:64. doi: 10.1186/s12879-015-0803-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tay WH, Chong KK, Kline KA. Polymicrobial-Host Interactions during Infection. Journal of molecular biology. 2016 Aug 28;428(17):3355–71. doi: 10.1016/j.jmb.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 35.Pettigrew MM, Laufer AS, Gent JF, Kong Y, Fennie KP, Metlay JP. Upper respiratory tract microbial communities, acute otitis media pathogens, and antibiotic use in healthy and sick children. Appl Environ Microbiol. 2012 Sep;78(17):6262–70. doi: 10.1128/AEM.01051-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bakaletz LO. Immunopathogenesis of polymicrobial otitis media. J Leukoc Biol. 2010 Feb;87(2):213–22. doi: 10.1189/jlb.0709518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wren JT, Blevins LK, Pang B, King LB, Perez AC, Murrah KA, et al. Influenza A virus alters pneumococcal nasal colonization and middle ear infection independently of phase variation. Infection and immunity. 2014 Nov;82(11):4802–12. doi: 10.1128/IAI.01856-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yokota S, Harimaya A, Sato K, Somekawa Y, Himi T, Fujii N. Colonization and turnover of Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis in otitis-prone children. Microbiology and immunology. 2007;51(2):223–30. doi: 10.1111/j.1348-0421.2007.tb03904.x. [DOI] [PubMed] [Google Scholar]
- 39.Pichichero ME, Casey JR, Almudevar A. Nonprotective responses to pediatric vaccines occur in children who are otitis prone. The Pediatric infectious disease journal. 2013 Nov;32(11):1163–8. doi: 10.1097/INF.0b013e31829e887e. [DOI] [PMC free article] [PubMed] [Google Scholar]