Summary
The aim of our study was to identify and quantify spatiotemporal and kinematic gait parameters obtained by 3D gait analysis (GA) in a group of Parkinson’s disease (PD) patients compared with healthy subjects in order to investigate whether early PD patients could present an abnormal gait pattern.
Forty-four patients affected by early-stage PD compared with a control group were analyzed. All participants were evaluated with 3D GA in the gait laboratory.
The greatest significance in temporal parameters was found in cadence (102.46 ± 13.17 steps/min in parkinsonian patients vs 113.84 ± 4.30 steps/min in control subjects), followed by stride duration (1.19 ± 0.18 seconds right limb and 1.19 ± 0.19 seconds left limb in PD patients vs 0.426 ± 0.16 seconds right limb and 0.429 ± 0.23 seconds left limb in normal subjects) and stance duration.
Marked differences were also found in the swing phase and in swing duration (p<0.05), while the stance phase was not significantly different in patients compared with healthy subjects. A statistically different velocity in PD patients (0.082 ± 0.29 m/s) vs healthy subjects (1.33 ± 0.06 m/s) was shown by spatial parameter analysis. Step width, stride length and swing velocity were highly significant parameters, as was average velocity.
Our study highlighted some distinguishing characteristics of gait in early PD. Ambulation disorders may be present in the early stage of PD and their detection allows for early medical treatment and possible rehabilitation.
Keywords: gait disorders, Parkinson’s disease, rehabilitation
Introduction
Parkinson’s disease (PD) is a neurodegenerative disease caused by the loss of pigmented neurons in the substantia nigra pars compacta. Its main clinical manifestations are resting tremor, bradykinesia and rigidity. From the early stages of the disease, PD patients may present with an abnormal gait pattern characterized by a shortened stride length, increased stride variability, reduced walking speed and festinating gait (Buckley et al., 2008; Morris et al., 2001; Moore et al., 2008). Moreover, excessive hip and knee flexion is evident during the gait cycle, causing reduction in the movement excursion of the lower limb joints (Morris et al., 2001). Gait abnormalities can also be present in the elderly due to the age-associated decline in musculoskeletal and neurological functions, leading to a clinical profile ranging from fast short steps to a shuffling gait like that seen in PD (Plotnik et al., 2007; Rubino et al., 1993).
The spatial and temporal parameters of gait abnormalities in PD (Hausdorff et al., 2009) have already been studied using different techniques, such as fractal analysis, coefficient of variation analysis (Bello et al., 2010) and variability analysis based on complex wavelet analysis (Lakany et al., 2008). These techniques provide a single value for a spatiotemporal event that occurs during the gait cycle.
However, the pathognomonic characteristics of gait in early PD patient have not yet been defined; early identification is a key factor in establishing an effective therapy and reducing health care costs. Hence, the aim of our study was to identify and quantify spatiotemporal and kinematic gait parameters obtained by 3D gait analysis (GA) (Davis et al., 1991) in a group of PD patients, and to compare PD sufferers with a control group. Gait parameters were correlated with motor and non-motor impairment — features which had not been tested before — in order to determine the gait pattern disorder in the early stages of PD and its impact on daily activities.
Materials and methods
Forty-four patients, admitted to Santorso Hospital from May 2012 to September 2014, affected by early PD were analyzed. We observed 23 men and 21 women, with a mean age of 66.5 ± 9.11 years and a mean disease duration of 5.2 ± 3.07 months. The control group comprised 44 age- and sex-matched healthy subjects were included as controls. This group consisted of 22 men and 22 women aged 67.0 ± 9.42 years. Neither group had any kind of pain that could affect gait and all were able to walk over 10 meters without aids.
Exclusion criteria for the control group included a prior history of cardiovascular, neurological and musculoskeletal disorders. Therefore, they had normal flexibility and muscle strength and no obvious gait abnormalities.
The PD patients were administered the Unified Parkinson’s Disease rating Scale (UPDRS) Part III (Fahn and Elton, 1987) and the Hoehn and Yahr (HY) scale (Hoehn and Yahr, 1967) to quantify motor symptoms, and the Italian version of a 19-item wearing-off questionnaire (WOQ-19) (Abbruzzese et al., 2012) to evaluate non-motor symptoms (Tab. I). Functional evaluation was based on Activities of Daily Living (ADL) and Instrumental Activities of Daily Living (IADL) measures (Katz et al., 1963) and cognitive profile was assessed using the Mini-Mental State Examination (MMSE) (Folstein et al., 1975). The inclusion criteria were: (1) idiopathic PD diagnosis; (2) HY scale score < 2.5 (bilateral, mild-symptoms with recovery on pull test); (3) stable medication use; (4) age between 50 and 75 years; (5) MMSE score > 24; (6) no visual acuity deficits (with or without lenses) and no audiometric deficits; (7) no other neurological disease history; (8) no musculoskeletal disorders affecting arm movement; (9) no pain which could affect gait. All the patients were evaluated by the same neurologist and the same physical therapist.
Table I.
Characteristics of PD patients and control group.
| PD GROUP | CONTROL GROUP | |||
|---|---|---|---|---|
|
| ||||
| Males | Females | Males | Females | |
| Sex | 23 | 21 | 22 | 22 |
| Age | 63.04 ± 10.43 | 70.28 ± 5.48 | 62.02 ± 9.21 | 71.22 ± 4.42 |
| L-Dopa equivalent dose (mg) | 252.17 ± 231.82 | 240.47 ± 193.40 | - | - |
| Disease Onset | 5.00 ± 3.38 | 5.42 ± 2.76 | - | - |
| UPDRS-III score | 15.00 ± 6.36 | 16.38 ± 4.94 | - | - |
| Hoehn & Yahr stage | 1.76 ± 0.78 | 1.57 ± 0.63 | - | - |
| WOQ-19 | 5.69 ± 3.40 | 6.19 ± 2.50 | - | - |
| ADL | 6.00 ± 0.00 | 6.00 ± 0.00 | 6.00 ± 0.00 | 6.00 ± 0.00 |
| IADL | 7.23 ± 0.69 | 7.17 ± 0.63 | 7.73 ± 0.21 | 7.44 ± 0.35 |
| MMSE | 27.67 ± 1.33 | 26.67 ± 1.30 | 27.5 ± 0.26 | 26.9 ± 1.33 |
Values are expressed as mean ± standard deviation
This evaluation included neurological and functional examination, videotaping and three-dimensional GA. All the patients were treated with levodopa and underwent evaluation one hour after the first daily drug administration (“on” phase). Exclusion criteria consisted of any other conditions that might affect gait.
Before being tested, each patient and control subject included in the study had an overall examination which included measurement of weight, height, pelvis width, leg length, knee and ankle circumference. Following an explanation given by the therapist, all the patients had to complete a walking test without help.
All participants were evaluated with 3D GA in the gait laboratory. The GA system included an optometric system, a dynamometric platform and dedicated software.
The optometric system (BTS S.p.A., Elite Clinic, Milan, Italy), also called an automatic video system, consisted of six infrared cameras able to detect signals reflected by passive markers positioned on the patient’s body. Each camera had an emission point of 100 Hz frequency and a central lens able to localize the signal reflected by each marker.
The passive markers consisted of 1.5-cm-diameter spheres made of plastic covered in reflective aluminum dust. Before starting recording, passive markers were positioned on specific landmarks on the patient performing the test, without this impairing their gait. 22 and 20 markers, for standing and walking kinematic evaluation respectively, were placed on each body segment following the DAVIS flow chart (Davis et al., 1991). The landmarks were the C7 apophysis, acromion-clavicular joint, S2 apophysis, anterior superior iliac spine, greater femoral trochanter, femoral lateral epicondyle, peroneal head, medial malleolus and heel (only for standing), fifth metatarsal head, middle third of the thigh (bar shaped marker), middle third of the calf (bar shaped marker).
The test was performed using a dynamometric platform equipped with piezoeletric sensors giving signals proportional to the weight applied. This platform (Kistler platform), rectangular in shape (40×60 cm) and covered in wood so as to avoid any distraction of the patient, was connected to a computer with dedicated software (BOX-ELITE-Elite system V5, BTS, Milan) able to guarantee image processing in real time, working with cross correlation algorithms. It analyzed three force components (x,y,z), pressure coordinates (Px, Py) and twisting movement (Mz), allowing three-dimensional data coordinates on three planes (frontal, sagittal and horizontal) and therefore the study of flexion/extension, abduction/adduction, rotation during gait, and any fall or rise of the patient (Fig. 1).
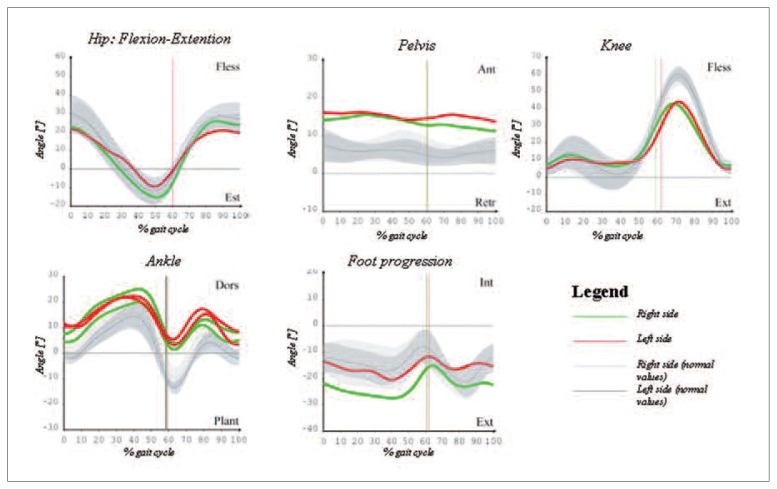
Figure 1.
Walking patterns of the lower limb joints in the sagittal plane in a typical PD subject from the study (age: 68 years) acquired under levodopa effect.
Data acquisition started by asking the patient to stand upright in front of the platform to allow acquisition of kinematic data on his/her standing position (with offset angles). After that, the patient, who was barefoot, was invited to walk back and forth along the platform at his/her own natural pace (self-selected speed), each time covering a distance of around 10 meters and reversing direction six times. At least five trials were carried out for each participant so as to guarantee the reproducibility of the results.
The measurements were taken by a single experienced operator so as to ensure reproducibility of the acquisition technique and avoid errors due to different operators.
Data acquisition was performed in “on” therapy; only patients with early/mild-severity PD were included to minimize variability due to clinical fluctuations and to avoid mistakes in multiple acquisitions.
Data consisted of temporal and spatial parameters. The temporal parameters were stance phase (%), swing phase (%), stance duration (seconds), stride duration (seconds), and cadence (steps/minute).
The spatial parameters were step length (meters), limb velocity (meters/second), swing velocity (meters/second), stride length (meters), step width (meters), and average velocity (meters/second).
For maximum accuracy, and also considering the frequent unilateral beginning and the prevalently unilateral involvement in PD, data regarding right and left side are shown separately.
In addition, information was gathered through the BOX-ELITE system with markers positioned on each side of the body at the upper and lower limbs, thus providing more specific details.
All graphs obtained from GA were normalized as a percentage of the gait cycle.
All defined bare parameters were computed for each participant, obtaining mean values and standard deviation of all indexes.
The data were analyzed through evaluation of the individual descriptive variables, contingency tables and graphical analysis.
Statistical Package for the Social Sciences (SPSS) 21.0 software (SPSS, Chicago, IL, USA) was used for data analysis. Differences between the participants’ performances and the values of control subjects, pre-analyzed and filed in BOX-ELITE software (TV image processing, Elite system V5, BTS, Milan), were examined using t test. A p value < 0.05 was considered statistically significant. Pearson’s correlation was used to assess relationships between scales of motor and non-motor disability (UPDRS part III, HY, WOQ-19) and space/time parameters, levodopa therapy, functional (ADL and IADL) and cognitive (MMSE) assessment scales.
The study was approved by the Research Ethics Committee of the Institute and written informed consent was obtained from the patients.
Results
Patients’ characteristics
Demographic data and features of motor and non-motor impairment and medical treatment in patients and the control group (divided by gender) are shown in Table I.
Spatiotemporal parameters
All the spatial and temporal parameters (mean values) were significantly different from the normative values acquired using the same methodology (Tabs. II, III).
Table II.
Spatial parameters of PD patients and control subjects.
| Right limb patients | Right limb Control subjects | p | Confidence interval | Left limb patients | Left limb Control subjects | p | Confidence interval | |
|---|---|---|---|---|---|---|---|---|
| Step lenght (m) | 0.48 ± 0.13 | 0.619 ± 0.04 | 0.0001* | From −0.170 to −0.089 | 0.49 ± 0.13 | 0.74 ± 0.19 | 0.0001* | From −3.319 to −0.189 |
| Velocity (m/s) | 0.84 ± 0.28 | 1.33 ± 0.06 | 0.0001* | From −0.575 to −0.402 | 0.83 ± 0.28 | 1.33 ± 0.06 | 0.0001* | From −0.573 to −0.402 |
| Swing velocity (m/s) | 2.17 ± 0.63 | 3.29 ± 1.37 | 0.0001* | From −1.573 to −0.668 | 2.13 ± 0.61 | 3.27 ± 0.184 | 0.0001* | From −1.330 to −0.946 |
| Stride lenght (m) | 0.98 ± 0.27 | 1.40 ± 0.74 | 0.0007* | From −0.656 to −0.183 | 0.95 ± 0.28 | 1.40 ± 0.62 | 0.0001* | From −0.687 to −0.212 |
| Step width (m) | 0.10 ± 0.10 | 1.40 ± 0.07 | 0.0001* | From −1.333 to −1-262 | 0.11 ± 0.09 | 1.40 ± 0.6 | 0.0001* | From −1.332 to −1.267 |
| Average velocity (m/s) | 0.082 ± 0.29 | 0.11 ± 0.26 | 0.63 | From 0.144 to 0.088 | 0.082 ± 0.29 | 0.12 ± 0.01 | 0.38 | From −0.124 to −0.048 |
Values are expressed as mean ± standard deviation (median); results of t-test are indicated (*p<0.05)
Table III.
Temporal parameters of PD patients and control subjects.
| Right limb patients | Right limb Control subjects | p | Confidence interval | Left limb patients | Left limb Control subjects | p | Confidence interval | |
|---|---|---|---|---|---|---|---|---|
| Stance phase (%) | 60.57 ± 9.97 | 59.6 ± 1.2 | 0.523 | From −2.03 to 3.979 | 61.24 ± 3.99 | 59.3 ± 1.8 | 0.01* | From −0.292 to −0.039 |
| Swing phase (%) | 37.9 ± 3.49 | 40.4 ± 1.2 | 0.0001* | From −3.606 to −1.394 | 38.7 ± 3.99 | 40.7 ± 1.8 | 0.0001* | From −7.746 to −7.373 |
| Stance duration (s) | 0.74 ± 0.14 | 13.4 ± 1.1 | 0.0001* | From −12.99 to −12.32 | 0.74 ± 0.16 | 8.3 ± 0.6 | 0.003* | From −3.34 to −0.688 |
| Swing duration(s) | 0.44 ± 0.04 | 0.63 ± 0.21 | 0.0001* | From −0.254 to −0.125 | 0.46 ± 0.05 | 0.626 ± 0.42 | 0.004* | From 0.628 to 3.251 |
| Stride duration(s) | 1.19 ± 0.18 | 0.426 ± 0.16 | 0.0001* | From 0.657 to 0.802 | 1.19 ± 0.19 | 0.429 ± 0.23 | 0.0001* | From 0.657 to 0.802 |
| Cadence (step/min) [Hz] | 102.4 ± 13.17 | 113.84 ± 4.30 | 0.0001* | From −15.55 to −7.24 | 102.4 ± 13.17 | 113.84 ± 4.30 | 0.0001* | From −15.55 to −7.24 |
Values are expressed as mean ± standard deviation (median); results of t-test are indicated (*p<0.05)
The most significant findings in temporal parameters concerned cadence (102.46 ± 13.17 steps/min in PD patients vs 113.84 ± 4.30 steps/min in control subjects), followed by stride duration (1.19 ± 0.18 seconds right limb and 1.19 ± 0.19 seconds left limb in PD patients vs 0.426 ± 0.16 seconds right limb and 0.429 ± 0.23 seconds left limb in control subjects) and stance duration (0.74 ± 0.14 seconds right limb and 0.74 ± 0.16 left limb in PD patients vs 13.4 ± 1.1 seconds right limb and 0.83 ± 0.6 seconds left limb in control subjects). Swing phase and swing duration differed considerably too (p<0.05), while the stance phase was not statistically significant in patients compared with healthy subjects.
Analysis of the spatial parameters revealed a statistically different velocity in PD patients (0.082 ± 0.29 m/s) vs healthy subjects (1.33 m/s ± 0.06). Step width, stride length and swing velocity were highly significant parameters, as was average velocity.
Motor and non-motor parameters
The motor impairment scales (HY and UPDRS-III) and non-motor impairment scale (WOQ-19) were statistically correlated directly with age, disease duration, mean speed, cadence, and levodopa therapy (p<0.05). The H&Y score showed a significant inverse correlation (p =−0.05) with mean speed (m/s). Similarly, a strong association was found between UPDRS-III and cadence (steps/min) (p=−0.021).
Disease duration was closely connected with mean speed (p<0.05), age (p=0.022), motor (HY, UPDRS-III p<0.05) and non-motor impairment (WOQ-19 p<0.05).
Some of the temporal and spatial parameter correlations were highly indicative of PD. Disease duration and stride duration were positively and inversely associated: the longer the disease duration, the shorter the stride duration (p =−0.01).
The spatial gait analysis revealed a significant inverse correlation between disease duration, stride length and step width (right/left), HY and UPDRS-III, but a linear association with levodopa dosage.
Drug administration (expressed in equivalent levodopa dose) showed a remarkable linear correlation with cadence (steps/minute) (p<0.05), and a significant inverse correlation with mean speed (p=−0.024). Levodopa equivalent dose correlated strongly with steps/minute (p<0.005), mean speed (p<−0.024), speed (p<0.05), stride length (p=−0.05), step width (p< 0.05), arm swing duration (p=−0.041), and stance duration (p<0.01).
The sample analyzed showed a good performance level concerning autonomy in basal and instrumental activities of daily living (ADL males 6.00 ± 0.00; females 7.23 ± 0.69 and IADL males 7.23 ± 0.69; females 7.17 ± 0.63) and on cognitive assessment (MMSE males 27.67 ± 1.33; females 26.67 ± 1.30), without significant differences between males and females or between groups (Tab. I).
Kinematic parameters
Kinematic data showed a particular gait pattern in these early PD patients compared with the healthy subjects. The PD patients showed bilateral extrarotation of the ankle joint, knee and foot.
The sagittal plane study showed a decrease of pelvic joint range of motion (ROM), ROM pelvic obliquity and ROM abduction in the PD patients. The PD patients showed slight flexion of the ankle joint during swing and minimum dorsiflexion in stance and gait, with slight reduction in flexion of knee joint (Fig. 2).
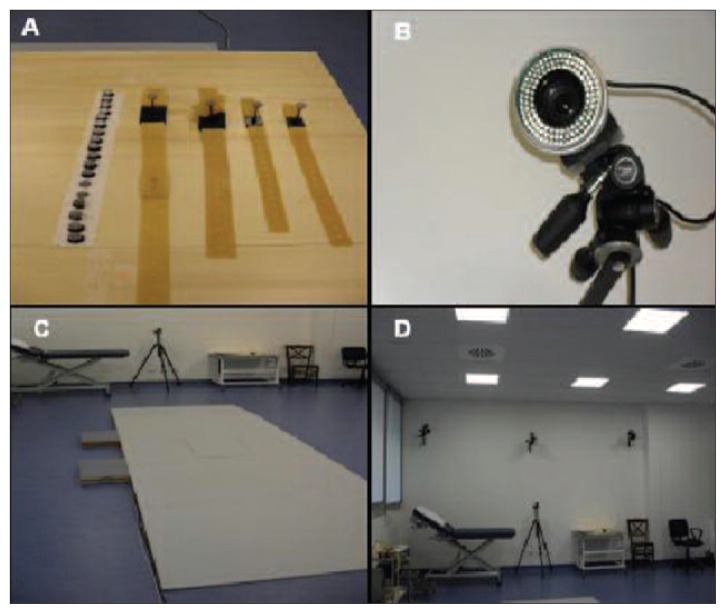
Figure 2.
(A) Position markers; (B) infrared camera; (C) dynamometric platform; (D) infrared cameras and position cameras.
Discussion
Resting tremor, bradykinesia and rigidity are the cardinal symptoms of PD; nevertheless abnormal posture and ambulation may be evident from the early stages of the disease.
Reduced step length has been reported as one of the key features of PD gait (Yang et al., 2007). Previous studies have suggested that variability analysis is more useful for monitoring and describing gait disorders than measurement of spatiotemporal walking parameters (Hausdorff et al., 1998). Conversely, the results of our study demonstrated that early moderate PD patients showed a reduction of spatiotemporal gait parameters (speed and cadence), which was confirmed by specific sub-item analysis.
Loss of balance, short stride and slowness have been found to be the most prevalent clinical features in PD, after exclusively parkinsonian signs (Morris et al., 1994; Morris et al., 1998; Chien et al., 2006; Sofuwa et al., 2005). Early PD gait may have different characteristics compared with other PD phases. We found a linear correlation between motor and non-motor impairment data and disease duration which confirmed this hypothesis. The more the motor damage increased (UPDRS-III and HY score), the slower gait became (steps/min). The spatial parameters showed a significant correlation with mean velocity, width and duration of step, while there emerged an inverse association with HY and UPDRS-III. These data could mean that in the early/moderate phase of the disease, starting gait is more involved than other gait phases. Step seems to be slower but narrower.
Stance phase was not statistically significant compared to stance duration. These data could suggest an early instability sign, more typical of medium-advanced phases of PD or parkinsonian syndromes than the early stage.
The WOQ-19 score showed a positive correlation with cadence and mean velocity (spatial and temporal parameters) and also with age and disease duration. Non-motor and motor symptoms were associated with disease progression and also with a specific abnormal pattern of the ambulation cycle.
Cadence correlated significantly and linearly with equivalent levodopa dosage; levodopa equivalent dosage correlated inversely with mean velocity: an increase in dopaminergic dosage resulted in improved gait. Levodopa also enhanced spatial and temporal parameters, so the medication dosage may be an indirect marker of disease progression which determines slow and shuffling steps (steps/min, mean velocity, stride length and width, swing and stance duration). It is unclear whether medication improves postural stability and balance in PD (Mancini et al., 2008; Ganesan et al., 2010; Colnat-Coulbois et al., 2011). No correlations could be observed with autonomy in activities of daily living and cognitive performance, probably because our patients were in the early stage of the disease and presented mild motor impairment. With regard to the kinematic parameters, the characteristics of PD gait in the elderly described in the literature can be summarized as a general reduction of lower limb joint ROMs and a shifted-towards-flexion profile of the hip and knee patterns. Notably, the ankle dorsi-plantar flexion pattern is shifted towards dorsiflexion (flat-footed landing). As well as PD patients, elderly healthy subjects display reduced ROMs at the lower limb joints and shifted-towards-flexion patterns.
In our study, excessive flexion was found at the lower limb joints, particularly the knee and the ankle. Patients affected by PD showed similar maximum dorsiflexion in stance and minimum plantarflexion values. They showed a more dorsiflexed attitude throughout the gait cycle compared with control subjects. The kinematic parameters showed a major knee ROM reduction, which was linked with PD progression — this finding has already been confirmed by other studies (Sale et al., 2013) — and not with the age of patients. Other typical features in the PD subjects were the reduction of knee flexion-extension ROM and intrarotation of the knee joint. PD sufferers adopt this flexed position for biomechanical reasons, too, for instance to offset augmented trunk flexion.
In conclusion, studies of gait and balance provide a rationale for physiotherapy strategies and their application in clinical practice improves evidence of therapeutic efficacy.
Our study highlighted some distinguishing characteristics of gait in early PD which may be considered the effects of three linked processes: aging, disease and therapy. This topic is still poorly investigated in the literature. An ambulation disorder may be present in early PD. Its identification in the correct clinical context of extrapyramidal symptoms and its distinction from other diseases in the elderly population allow for early medical treatment and rehabilitation, preventing the development of pathological locomotion and posture patterns, which can result from a prolonged incorrect gait pattern, and also reducing the possibility of falls.
An abnormal motor pattern is a common cause of impairment, and specific rehabilitation could ameliorate postural control and, of course, gait (Crizzle et al. 2006; Dibble et al. 2009). In this regard, some studies have evaluated the successful effects of physical exercises on strength, balance, mobility and quality of life in PD, but the literature is still limited in terms of quantity, quality and outcomes (Goodwin at al. 2008; Dibble et al. 2009).
The data presented in this study, not described elsewhere, may be useful for physiokinesitherapeutic approaches and rehabilitation practice. Longer follow-up is needed to establish whether benefits could last throughout the course of the disease and future studies might suggest a specific rehabilitation approach based on results of gait analysis.
Acknowledgment
The Authors would like to thank Santorso Hospital (Santorso, Italy) and Maria Grazia Pavei, English teacher, for providing English language assistance on this manuscript.
Footnotes
Ethical Standard
The study was approved by the Ethics Research Committee of the Istitute and written informed consent was obtained by the patients.
Conflict of interests
The Authors declare that they have no conflict of interest.
References
- Abbruzzese G, Antonini A, Barone P, et al. Linguistic, psychometric validation and diagnostic ability assessment of an Italian version of a 19-item wearing-off questionnaire for wearing-off detection in Parkinson’s disease. Neurol Sci Dec. 2012;33:1319–13227. doi: 10.1007/s10072-012-0943-y. [DOI] [PubMed] [Google Scholar]
- Bello O, Marquez G, Camblor M, et al. Mechanisms involved in treadmill walking improvements in Parkinson’s disease. Gait and Posture. 2010;32:118–123. doi: 10.1016/j.gaitpost.2010.04.015. [DOI] [PubMed] [Google Scholar]
- Buckley T, Pitsokoulis C, Hass C. Dynamic postural stability during sit-to-walk transitions in Parkinson disease patients. Movement Disorders. 2008;23:1274–1280. doi: 10.1002/mds.22079. [DOI] [PubMed] [Google Scholar]
- Chien A, Lin SZ, Liang CC, et al. The efficacy of qualitative gait analysis by the GAITRite system in evaluation of parkinsonian bradykinesia. Parkinsonism Relat Disord. 2006;12:438–442. doi: 10.1016/j.parkreldis.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Colnat-Coulbois S, Gauchard GC, Maillard L, et al. Management of postural sensory conflict and dynamic balance control in late-stage Parkinson’s disease. Neuroscience. 2011;193:363–369. doi: 10.1016/j.neuroscience.2011.04.043. [DOI] [PubMed] [Google Scholar]
- Crizzle AM, Newhouse IJ. Is physical exercise beneficial for persons with Parkinson’s disease? Clin J Sport Med Sep. 2006;16:422–425. doi: 10.1097/01.jsm.0000244612.55550.7d. [DOI] [PubMed] [Google Scholar]
- Davis RB, Ounpuu S, Tyburski DJ, et al. A gait analysis data collection and reduction technique. Hum Mov Sci. 1991;10:575–587. [Google Scholar]
- Dibble LE, Addison O, Papa E. The effects of exercise on balance in persons with Parkinson’s disease: a systematic review across the disability spectrum. J Neurol Phys Ther Mar. 2009;33:14–26. doi: 10.1097/NPT.0b013e3181990fcc. [DOI] [PubMed] [Google Scholar]
- Fahn S, Elton RL. Members of the UPDRS Development Committee Unified Parkinson’s Disease Rating Scale. In: Fahn S, Marsden CD, Calne DB, Lieberman A, editors. Recent Developments in Parkinson’s Disease. Florham Park, NJ: MacMillan Health Care Information; 1987. pp. 153–163. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Minimental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Ganesan M, Pal PK, Gupta A, et al. Dynamic posturography in evaluation of balance in patients of Parkinson’s disease with normal pull test: concept of a diagonal pull test. Parkinsonism Relat Disord. 2010;16:595–599. doi: 10.1016/j.parkreldis.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Goodwin VA, Richards SH, Taylor RS, et al. The effectiveness of exercise interventions for people with Parkinson’s disease: a systematic review and meta-analysis. Mov Disord Apr. 2008;23:631–640. doi: 10.1002/mds.21922. [DOI] [PubMed] [Google Scholar]
- Hausdorff JM. Gait dynamics in Parkinson’s disease: common and distinct behavior among stride length, gait variability, and fractal-like scaling. Chaos Jun. 2009;19:026113. doi: 10.1063/1.3147408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausdorff JM, Cudkowicz ME, Firtion R, et al. Gait variability and basal ganglia disorders: stride-to-stride variations of gait cycle timing in Parkinson’s disease and Huntington’s disease. Movement Disorders. 1998;13:428–437. doi: 10.1002/mds.870130310. [DOI] [PubMed] [Google Scholar]
- Hoehn MM, Yahr MD. Parkinsonism: onset, progression, and mortality. Neurology May. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Katz S, Ford AB, Moskowitz RW, et al. Studies of illness in the aged. The index of ADL: A standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- Lakany H. Extracting a diagnostic gait signature. Pattern Recognition. 2008;41:1627–1637. [Google Scholar]
- Mancini M, Rocchi L, Horak FB, et al. Effects of Parkinson’s disease and levodopa on functional limits of stability. Clin Biomech. 2008;23:450–458. doi: 10.1016/j.clinbiomech.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris M, Iansek R, Matyas T, et al. The pathogenesis of gait hypokinesia in Parkinson’s disease. Brain. 1994;117:1169–1181. doi: 10.1093/brain/117.5.1169. [DOI] [PubMed] [Google Scholar]
- Morris M, Iansek R, Matyas T, et al. Abnormalities in the stride length-cadence relation in parkinsonian gait. Mov Disord. 1998;13:61–69. doi: 10.1002/mds.870130115. [DOI] [PubMed] [Google Scholar]
- Morris ME, Huxham F, McGinley J, et al. The biomechanics and motor control of gait in Parkinson disease. Clin Biomech Jul. 2001;16:459–470. doi: 10.1016/s0268-0033(01)00035-3. Review. [DOI] [PubMed] [Google Scholar]
- Moore ST, MacDougall HG, Gracies JM, et al. Locomotor response to levodopa in fluctuating Parkinson’s disease. Exp Brain Res Feb. 2008;184:469–478. doi: 10.1007/s00221-007-1113-y. Epub 2007 Sep. [DOI] [PubMed] [Google Scholar]
- Plotnik M, Giladi N, Hausdorff JM. A new measure for quantifying the bilateral coordination of human gait: effects of aging and Parkinson’s disease. Exp Brain Res Aug. 2007;181:561–570. doi: 10.1007/s00221-007-0955-7. [DOI] [PubMed] [Google Scholar]
- Rubino FA. Gait disorders in the elderly. Distinguishing between normal and dysfunctional gaits. Postgrad Med May. 1993;93:185–190. doi: 10.1080/00325481.1993.11701693. [DOI] [PubMed] [Google Scholar]
- Sale P, De Pandis MF, Vimercati SL, et al. The relation between Parkinson’s disease and ageing. Comparison of the gait patterns of young Parkinson’s disease subjects with healthy elderly subjects. Eur J Phys Rehabil Med Apr. 2013;49:161. [PubMed] [Google Scholar]
- Sofuwa O, Nieuwboer A, Desloovere K, et al. Quantitative gait analysis in Parkinson’s disease: comparison with a healthy control group. Arch Phys Med Rehabil. 2005;86:1007–1013. doi: 10.1016/j.apmr.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Yang Y, Lee Y, Cheng S, et al. Relationships between gait and dynamic balance in early Parkinson’s disease. Gait & Posture. 2007;27:611–615. doi: 10.1016/j.gaitpost.2007.08.003. [DOI] [PubMed] [Google Scholar]