Abstract
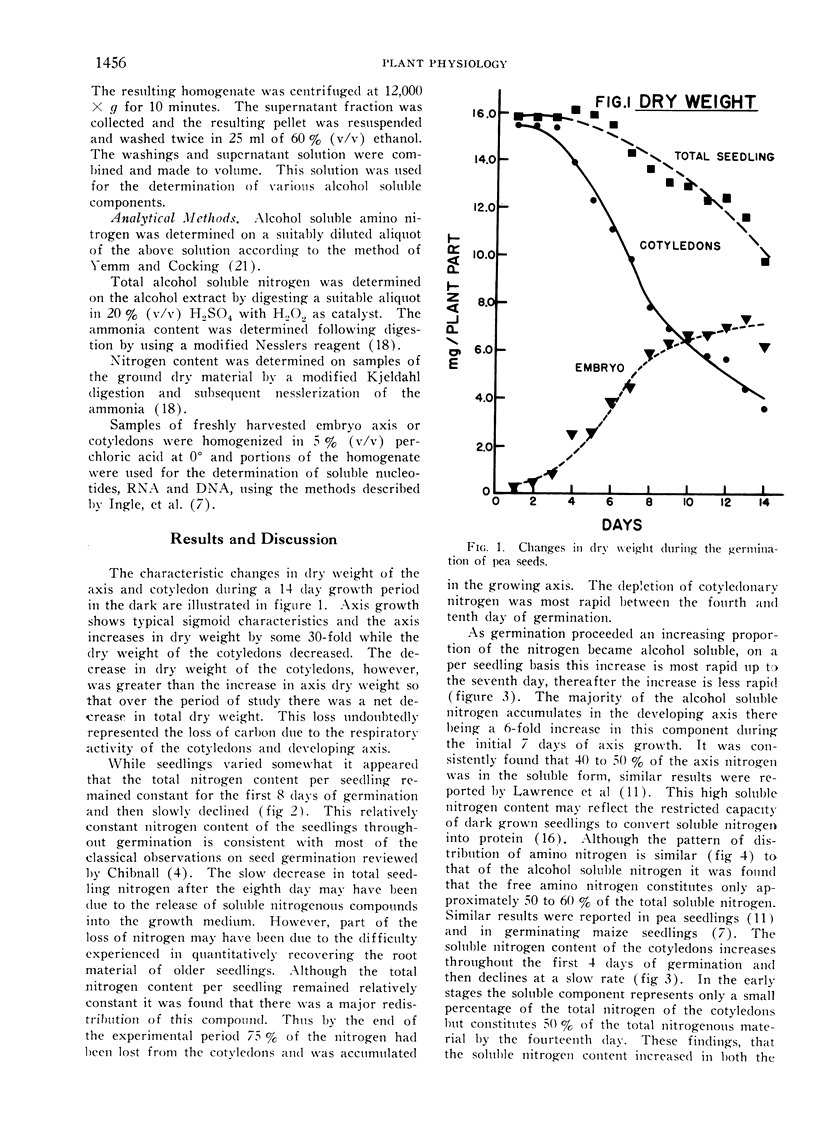
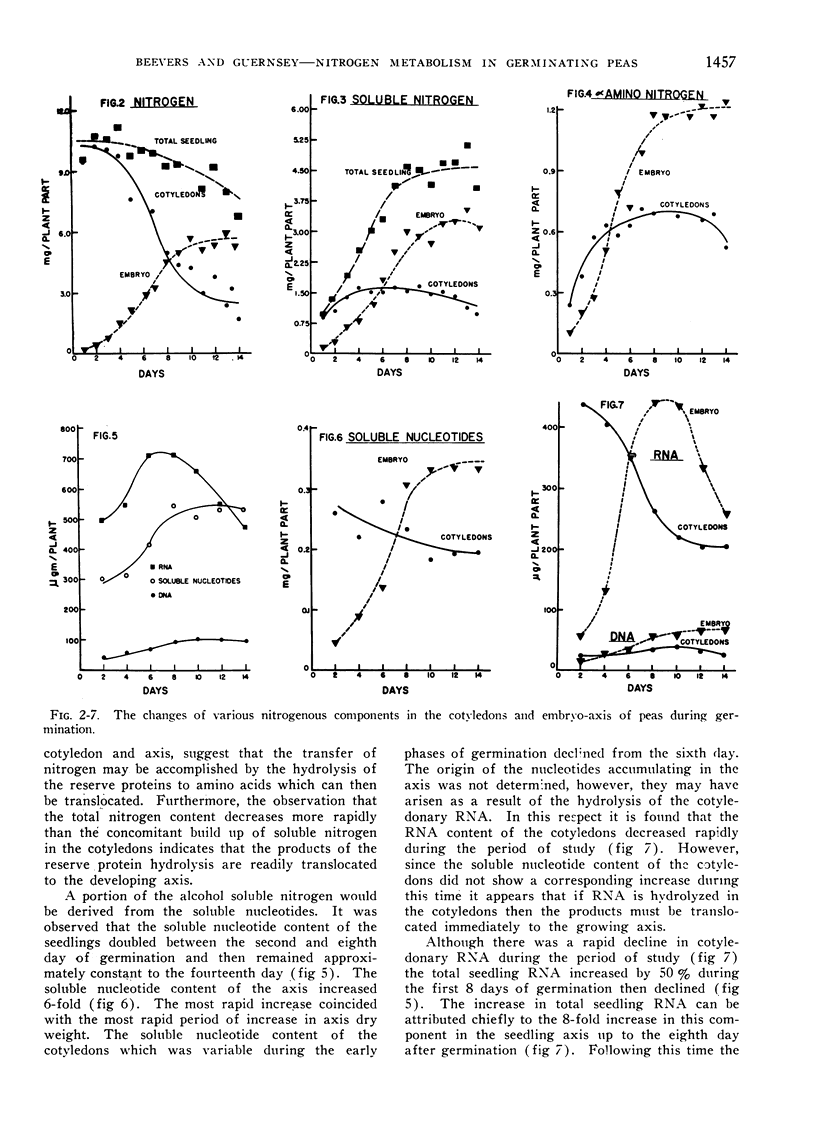
The changes in major nitrogenous components during the germination of pea seeds have been followed. During the period of rapid axis growth, 3 to 8 days following germination, the nitrogen content of the cotyledons declines rapidly with an accompanying increase of nitrogen in the developing axis. The accumulation of alcohol soluble nitrogen, primarily amino nitrogen, in the cotyledons and axis during germination indicates that the mobilization of nitrogen is facilitated by proteolysis and translocation of the products.
Pea cotyledons had an initially high RNA content which declined during germination while axis RNA increased. The increase in axis RNA was greater than the decline in cotyledonary RNA indicating a net nucleic acid synthesis. Thus some of initial nitrogen reserve is interconverted to provide for nucleic acid synthesis.
During the depletion of cotyledonary RNA there was no accumulation of nucleotides in the cotyledons, nucleotide content of the axis did, however, increase during the germination period.
The DNA content of the axis increased with growth of that organ. There was also an increase in DNA content of the cotyledons during the early stages of germination.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ingle J., Beevers L., Hageman R. H. Metabolic Changes Associated with the Germination of Corn. I. Changes in Weight and Metabolites and their Redistribution in the Embryo Axis, Scutellum, and Endosperm. Plant Physiol. 1964 Sep;39(5):735–740. doi: 10.1104/pp.39.5.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingle J., Hageman R. H. Metabolic changes associated with the germination of corn. II. Nucleic acid metabolism. Plant Physiol. 1965 Jan;40(1):48–53. doi: 10.1104/pp.40.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KISLEV N., SWIFT H., BOGORAD L. NUCLEIC ACIDS OF CHLOROPLASTS AND MITOCHONDRIA IN SWISS CHARD. J Cell Biol. 1965 May;25:327–344. doi: 10.1083/jcb.25.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISMAN B., FUKUHARA H., DEMAILLY J., GENIN C. [Synthesis in vitro of beta-galactosidase and alkaline phosphatase by subcellular particulate fractions isolated from Escherichia coli]. Biochim Biophys Acta. 1962 May 14;55:704–718. doi: 10.1016/0006-3002(62)90848-x. [DOI] [PubMed] [Google Scholar]
- Shannon J. C., Hanson J. B., Wilson C. M. Ribonuclease Levels in the Mesocotyl Tissue of Zea mays as a Function of 2,4-Dichlorophenoxyacetic Acid Application. Plant Physiol. 1964 Sep;39(5):804–809. doi: 10.1104/pp.39.5.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varner J. E., Schidlovsky G. Intracellular Distribution of Proteins in Pea Cotyledons. Plant Physiol. 1963 Mar;38(2):139–144. doi: 10.1104/pp.38.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]


