Abstract
Objective
Nonsteroidal anti-inflammatory drugs (NSAIDs) are metabolized by the cytochrome P450 enzymes (CYPs), predominantly CYP2C8 and CYP2C9. The aim of this study was to evaluate the possible association of polymorphisms in the CYP2C8*3 and CYP2C9 genes with the clinical efficacy of oral piroxicam (20 mg daily for 4 days) after lower third molar surgeries with regard to postoperative pain, swelling, trismus, adverse reactions, need for rescue medication and the volunteer’s overall satisfaction.
Materials and methods
For this purpose, 102 volunteers were genotyped for CYP2C8*3 and CYP2C9 polymorphisms. Briefly, genomic DNA was isolated from saliva collected from volunteers subjected to invasive lower third molar surgeries, and the preoperative, intraoperative and postoperative parameters were collected and analyzed.
Results
An equal amount of piroxicam sufficiently managed postoperative pain and inflammatory symptoms, with visual analog pain scores typically <40 mm for all genotypes investigated. Furthermore, only two out of 102 volunteers heterozygous for CYP2C8*3 and CYP2C9*3 reported adverse side effects.
Conclusion
In general, slow metabolizers of piroxicam, who were volunteers with mutant alleles, were indifferent from normal metabolizers with the wild-type alleles and therefore did not require specialized piroxicam doses to manage postoperative pain and inflammation.
Keywords: piroxicam, lower third molar surgery, P450, CYP2C8, CYP2C9, pharmacogenetics
Introduction
The variability in each individual’s response to the same dose of a drug is a major cause of side effects. In many cases, this variability is connected to polymorphisms in genes encoding enzymes responsible for metabolizing drugs.1 In particular, carriers of mutations in genes encoding drug-metabolizing enzymes tend to have higher plasma levels of the drug2,3 and an increase in the frequencies and severity of adverse reactions, when compared to nonmutants, when taking the usual doses of a drug that is metabolized by the affected enzyme.4–6 In other words, these mutations, which may consist of an alteration in one nucleotide, can cause absent, reduced or increased enzymatic activity.2,7 In particular, cytochrome P450 2C8 (CYP2C8) and 2C9 (CYP2C9) belong to one of the major families of enzymes involved in drug metabolism.
The genes encoding CYP2C8 and CYP2C9 are grouped in two consecutive clusters on chromosome 10.3 Briefly, a single-nucleotide polymorphism (SNP) is a variant DNA sequence wherein one nucleotide differs between common populations in a species.8 Furthermore, these SNPs are commonly found in exons and introns. The common nomenclature for the normal wild-type (wt) SNP for CYP2C9 is noted with a *1; two common polymorphic variants are allele *2, with the amino acid change R144C, and allele *3 with the amino acid change I359L; each individual commonly carries two alleles and may be either homozygous or heterozygous, in both cases, mutant (mt).9
Both CYP2C8 and CYP2C9 enzymes are associated with the metabolism of several drugs used clinically, some of which possess narrow therapeutic margins.10 The clinical importance of CYP2C8 and CYP2C9 polymorphisms with regard to nonsteroidal anti-inflammatory drugs (NSAIDs) mainly involves two factors: 1) these polymorphisms are often associated with an increased number of adverse reactions; and 2) these adverse reactions are often more serious. Notably, “slow” metabolizers of piroxicam (eg, people with CYP2C9*1/*3 and *3/*3 alleles), when compared to normal metabolizers (eg, people with CYP2C9*1/*1 wt alleles), might respond differently to the same dose of piroxicam, although it remains unknown whether piroxicam is metabolized differently by either CYP variant depending on whether these molecules are in ketonic or enolic tautomeric forms.
Data regarding the main role of CYP2C8 in the metabolism of piroxicam are inconsistent, but some studies suggest that SNPs of CYP2C8 tend to decrease the clearance of NSAIDs in general.9 Several studies have associated the genotypes of CYP2C8 and CYP2C9 with the pharmacokinetics and pharmacodynamics of piroxicam and other NSAIDs in healthy individuals.4,11–15 In contrast, this study aimed to evaluate the possible association of polymorphisms of CYP2C8*3, CYP2C9*2 and CYP2C9*3 with the clinical efficacy of oral piroxicam (20 mg daily for 4 days) after invasive lower third molar surgeries with regard to pain, swelling, trismus, need for rescue medication, volunteer satisfaction and adverse reactions to piroxicam. Briefly, the volunteers selected for this study required invasive surgeries for lower third molars with the following classifications: Pell and Gregory class IIB, IIC, IIIA, IIIB and IIIC and/or Winter horizontal, distal, inverted and transalveolar.16 It was hypothesized that postoperative pain would be effectively managed by a daily 20 mg oral dose of piroxicam for all genotypes, but that volunteers with mutant CYP2C8*3 and CYP2C9 genotypes would report less postoperative pain after lower third molar surgery.
Materials and methods
Registration and study design
The current study was performed in accordance with the Declaration of Helsinki and was approved by the ethics committee of Bauru School of Dentistry, University of São Paulo, Brazil National Research Ethics System (CAAE number: 20657913.7.0000.5417) in accordance with resolution 196/96 of the National Council of Health/Ministry of Health. This study was registered with Universal Trial (U1111-1155-3545); ClinicalTrials.gov (NCT02450487). All volunteers provided signed informed consent agreements after receiving all instructions and before undergoing any interventions.
Briefly, 105 volunteers (≥18 years old) visiting the Clinical Physiology and Pharmacology Laboratory (LAFFIC) requiring the extraction of at least one lower third molar that was included and/or impacted (as determined by panoramic radiography) participated in this study. Three volunteers did not return for postoperative measurements and were completely excluded from this study (Figure 1). Saliva samples were collected to genotype all volunteers, and postoperative data were used to judge the clinical efficacy of piroxicam (20 mg daily for 4 days) after lower third molar surgeries.
Figure 1.
CONSORT flowchart.
Eligibility criteria included adults without inflammation, infection and systemic diseases that could possibly interfere with the study. Exclusion criteria were as follows: any history of allergy, gastrointestinal bleeding/ulcers, kidney disease, asthma or any known allergic sensitivity to any NSAID. Pregnant women were also excluded from the study, as well as volunteers who used antidepressants, diuretics or NSAIDs 7 days prior to the start of the experiments. All surgeries were performed between December 2013 and October 2014, after ethical approval. The sample size calculation for the current study was based on the facial edema and trismus parameters from our previous research article.17 Taking into account the facial edema data with 94% power and effect size of 0.7, the number of patients required was 102; considering trismus with 80% power and effect size of 0.56, the number of patients remained 102.
All surgeries required bone removal, and, in some cases, tooth section. The positions of molars were determined with panoramic radiographs. No antibiotics were prescribed during the study to avoid possible bias in the drug protocol.17
Surgery intervention and assessments
All surgeries were performed by the same maxillofacial surgeon (PZG). One cartridge (1.8 mL) of local anesthetic (4% articaine with 1:200,000 epinephrine) was injected near the buccal, lingual and inferior alveolar nerves to achieve truncal anesthesia. After 3 minutes and when the patient confirmed a loss of sensation of the lower lip, a half cartridge (0.9 mL) of local anesthetic was injected to obtain complete and consistent anesthesia. A half cartridge (0.9 mL) of local anesthetic was also administered using the terminal infiltration technique to reduce bleeding and ensure mucosal anesthesia.16–24
The protocol used for postoperative pain medication after extraction of the third lower molars was a self-administered 20 mg tablet of piroxicam once every 24 hours for 4 days. Oral rescue analgesic medication was also available as needed throughout the study; for that, 750 mg tablets of paracetamol were provided to all volunteers, who were instructed to record the time of consumption while maintaining the use of piroxicam.16–19,21–26
The preoperative, intraoperative and postoperative parameters evaluated are reported in Table 1. Briefly, postoperative mouth opening (millimeters) between the mesial–incisal corners of the upper and lower right central incisors during maximum opening of the jaws was measured and recorded before surgery as well as during the second and seventh postoperative days, according to Ustun et al.16,24,27
Table 1.
Study parameters evaluated
| Parameters | Units |
|---|---|
| Total volume of local anesthetic | Milliliters |
| Onset of anesthetic agent action | Minutes |
| Surgery duration | Minutes |
| Overall experience of surgery reported by volunteer | 5-point scale: 1, poor; 2, fair; 3, good; 4, very good; 5, excellent |
| Quality of anesthesia | 3-point scale: 1, no discomfort during surgery; 2, any discomfort without anesthesia required; 3, any discomfort with anesthesia required |
| Surgery difficulty | 3-point scale: 1, no need for osteotomies without tooth sectioning; 2, need for osteotomies without tooth sectioning; 3, need for osteotomies as well as tooth sectioning complicated |
| Intraoperative bleeding | 3-point scale: 1, minimal bleeding; 2, normal bleeding; 3, excessive bleeding |
| Quality of wound healing | 7th day, 3-point scale: 1, normal healing without inflammation; 2, delayed healing; 3, healing complicated by inflammation or local infection with or without purulent material |
| Adverse reactions | Observed by the surgeon or reported by the volunteer, during the surgery in the first postoperative hour and during the 2nd and 7th days after surgery |
| Systolic, diastolic and mean arterial pressure; heart rate; and oxygen saturationa | Millimeters of mercury (mmHg); beats per minute (bpm); and percentage saturation of peripheral oxygen (% SpO2) |
| Body temperature | Preoperative period, 2nd, 7th postoperative day (degrees centigrade, °C) |
| Mouth opening | Preoperative period, 2nd, 7th postoperative day (millimeters) |
| Facial swelling | Preoperative period, 2nd, 7th postoperative day (millimeters) |
| Subjective evaluation of postoperative pain | VAS (0–100 mm) |
| Total amount of rescue medication | Number of tablets |
Note:
Dixtal® (model DX2021; Dixtal Biomédica Industria and Comércio Ltda, Marília, São Paulo, Brazil; ANVISA/MS 10293490035, model number 101503732).
Abbreviation: VAS, visual analog scale.
For swelling measurements of the facial edema, methods were used according to Ustun et al, which sums the following measures: 1) the distance between the lateral canthal ligament and the gonion; 2) the distance between the tragus and the labial commissure of the mouth; and 3) the distance between the tragus and the soft tissue of pogonion.16,24,27 The preoperative sum of these three measures was considered the baseline. The percentage difference between the baseline value and the values of the second and seventh postoperative periods for swelling indicated the facial edema.
Genotyping CYP2C8*3 and CYP2C9
All volunteers were instructed to expectorate up to 4 mL of unstimulated saliva into a provided sterile polypropylene collection tube. Approximately 3 mL of the tube’s contents were then placed in a sterile microcentrifuge tube using a sterile pipette and immediately stored in a freezer at −20°C until the genomic DNA was extracted. Finally, a QIAamp DNA Mini Kit (catalog number 51104; Qiagen®, Hilden, Germany) was used to extract genomic DNA from the saliva.
Genomic DNA was genotyped using the Taqman® system for performing real-time polymerase chain reaction (qPCR) using a ViiA™ 7 system (Applied Biosystems®, Waltham, MA, USA). Each reaction was performed in duplicate. Specifically, manufactured assays were used and validated by Applied Biosystems® with the following catalog numbers: C_25628505_10, C_27104892_10, C_25625794_10 and C_256257782_20; CYP2C9 [CYP2C9*2-rs1799853 (C430→T) and CYP2C9*3-rs1057910 (A1075→C)] and CYP2C8 [CYP2C8*3-rs11572080 (G416→A) and rs10509681 (A1196→G), respectively]. DNA samples were quantified and qualified using TaqMan® Genotyping Master Mix (catalog number: 4381656; Applied Biosystems®) to determine the CYP genotypes.
Statistical analysis
The G*Power software v.3.0.10 was used for the sample calculation. Data were analyzed using Microsoft® Excel 2002 (version 10.6871.6870) and GraphPad Prism (version 4.0). Briefly, data were tested for normality using the Shapiro–Wilk test. When data were normally distributed, comparisons among and between groups were made using the unpaired t-test. For all nonnormally distributed data, statistical comparisons between two independent groups were performed using the Mann–Whitney U test, while the Kruskal–Wallis test by ranks was used for analyzing nonparametric data with more than two groups. Lastly, binary data (eg, the absence or presence of an adverse reaction) were compared using Fisher’s exact test.
Statistical significance was set at 0.05. Normally distributed data are reported as mean ± SD, whereas nonnormally distributed data are reported as median with the interquartile range (IQR).
Results
Table 2 reports the demographic distribution and intraoperative parameters of the 102 volunteers with lower third molar surgeries. Human subjects were between the ages of 14 and 58 years and came from the state of São Paulo, Brazil.
Table 2.
Preoperative, intraoperative and postoperative parameters
| Parameters | All (n=102) | |
|---|---|---|
| Age, years | Median | IQR |
|
| ||
| 24 | 7 | |
|
| ||
| Lower third molar position | n | % |
|
| ||
| Distoangular | 27 | 26 |
| Horizontal | 27 | 26 |
| Mesioangular | 19 | 19 |
| Transalveolar | 1 | 1 |
| Vertical | 28 | 27 |
|
| ||
| Surgery | Median | IQR |
|
| ||
| Duration, minutes | 10 | 7 |
| Difficulty (score assessed by surgeon) | 3.0 | 1.0 |
| Intraoperative bleeding (score assessed by surgeon) | 1.0 | 0.1 |
| Quality of surgery (score assessed by volunteer) | 3.5 | 1.0 |
| Quality of wound healing (score assessed by surgeon) | 1.0 | 0.0 |
|
| ||
| Local anesthetic, 4% articaine with 1:200,000 epinephrine | Median | IQR |
|
| ||
| Quantity, mL | 2.7 | 0.9 |
| Onset, minutes | 1.0 | 0.5 |
| Quality (score assessed by surgeon) | 1.5 | 2.0 |
|
| ||
| Mouth opening | Average | SD |
|
| ||
| Preoperative measurement, mm | 47 | 7 |
|
| ||
| Median | IQR | |
|
| ||
| 2-day postoperative measurement, percentage of preoperative | 42 | 18 |
| 7-day postoperative measurement, percentage of preoperative | 71 | 31 |
|
| ||
| Facial swelling | Median | IQR |
|
| ||
| Preoperative measurement, mm | 37 | 4 |
| 2-day postoperative measurement, percentage of preoperative | 104 | 4 |
| 7-day postoperative measurement, percentage of preoperative | 100 | 2 |
|
| ||
| Underarm temperature, °C | Median | IQR |
|
| ||
| Preoperative measurement | 36.2 | 0.7 |
| 2-day postoperative measurement | 36.3 | 0.7 |
| 7-day postoperative measurement | 36.1 | 0.6 |
|
| ||
| Paracetamol rescue medication | Median | IQR |
|
| ||
| Time between first consumption and surgery, hours | 3.5 | 3.5 |
|
| ||
| Average | SD | |
|
| ||
| VAS during first consumption, mm | 53 | 25 |
|
| ||
| Median | IQR | |
| Total quantity consumed, mg | 2250 | 3750 |
Abbreviations: IQR, interquartile range; VAS, visual analog scale.
Excluding the transalveolar position, the third molar position of the tooth to be extracted was evenly distributed (P=0.61). The transalveolar-positioned third molars overall were significantly less common (2.9%) than other molar positions (mesioangular, vertical, distoangular or horizontal) when compared to positions being equally distributed (chi-square test, P=0.00036).
Hemodynamic parameters obtained during surgery fluctuated slightly during the different phases of extraction, yet no unusual peaks in blood pressure, heart rate and oxygen saturation were observed (data not shown). Additionally, during and immediately following surgery, no adverse reactions were found in any of the volunteers. Throughout the entire study, only two volunteers reported adverse side effects; on the second day, one volunteer reported sleepiness (CYP2C8*3 mt and CYP2C9*1/*3 genotype) and one volunteer (CYP2C8*3 mt and CYP2C9*1/*3 genotype) reported stomachaches. In addition, as indicated by some of the postoperative parameters, a volunteer’s allele for CYP2C8*3 or CYP2C9 was not correlated with any variation in the anti-inflammatory effects of piroxicam.
The distribution of genotypes is reported in Table 3. Overall, all the volunteers grouped together were 72.5% homozygous for the wt CYP2C8*3 allele and 27.5% heterozygous or homozygous for the mt CYP2C8*3 allele (Table 3). Furthermore, volunteers grouped together were most commonly homozygous for the wt CYP2C9*1/*1 allele (66.7%), followed by the mt variants *1/*2 (19.6%), *1/*3 (7.8%) and *3/*3 (5.9%), with no *2/*2 or *2/*3 genotypes found in the study population (Table 3). Table 4 reports the frequencies of the CYP2C8*3 alleles compared to the CYP2C9 alleles found in the population of volunteers.
Table 3.
Genotype frequencies of CYP2C8*3 and CYP2C9
| Allele | All (n=120)
|
|
|---|---|---|
| n | % | |
| CYP2C8*3 | ||
| Wild type (wt) | 74 | 72.5 |
| Mutant (mt) | 28 | 27.5 |
| CYP2C9 | ||
| *1/*1 | 68 | 66.7 |
| *1/*2 | 20 | 19.6 |
| *2/*2 | 0 | 0.0 |
| *2/*3 | 0 | 0.0 |
| *1/*3 | 8 | 7.8 |
| *3/*3 | 6 | 5.9 |
Abbreviation: CYP, cytochrome P450.
Table 4.
Genotypic frequencies of CYP2C8 relative to CYP2C9 in a Brazilian population of volunteers with third molar surgeries
| Genotypes
|
CYP2C8*3
|
|||||||
|---|---|---|---|---|---|---|---|---|
| CYP2C9 | *1/*1
|
*1/*3
|
*3/*3
|
All
|
||||
| rs10509681 | rs11572080 | rs10509681 | rs11572080 | rs10509681 | rs11572080 | rs10509681 | rs11572080 | |
| *1/*1 | 63 (61.8%) | 63 (61.8%) | 5 (4.9%) | 5 (4.9%) | 0 (0.0%) | 0 (0.0%) | 68 (66.7%) | 68 (66.7%) |
| *1/*2 | 2 (2.0%) | 4 (3.9%) | 17 (16.7)% | 16 (15.7%) | 1 (1.0%) | 0 (0.0%) | 20 (19.6%) | 20 (19.6%) |
| *2/*2 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| *2/*3 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| *1/*3 | 6 (5.9%) | 7 (6.9%) | 2 (2.0%) | 1 (1.0%) | 0 (0.0%) | 0 (0.0%) | 8 (7.8%) | 8 (7.8%) |
| *3/*3 | 4 (3.9%) | 4 (3.9%) | 2 (2.0%) | 2 (2.0%) | 0 (0.0%) | 0 (0.0%) | 6 (5.9%) | 6 (5.9%) |
| All | 75 (73.5%) | 26 (25.5%) | 1 (1.0%) | 102 (100%) | ||||
Abbreviation: CYP, cytochrome P450.
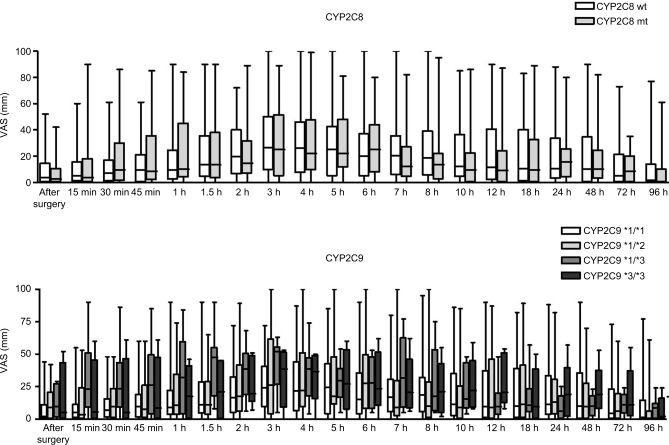
Piroxicam was effective in the control of pain regardless of the CYP haplotype (CYP2C8*3 and CYP2C9) carried by volunteers (Figure 2). There was a trend of lower levels of reporting pain (visual analog scale [VAS]) in volunteers carrying the CYP2C9*1/*1 wt allele, though a statistically significant difference was not found between groups. This trend is justified considering reports in the literature that ancient homozygous individuals for CYP2C9 are normal metabolizers of piroxicam.
Figure 2.
Postoperative pain scores reported by volunteers.
Notes: VAS of self-reported postoperative pain scores after lower third molar surgeries assessed at 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 5, 6, 7, 8, 10, 12, 18, 24, 48, 72 and 96 hours. Scores could range from 0 to 100 mm, with larger scores indicating increased pain. The top frame refers to the CYP2C8*3- and the lower frame to CYP2C9-bearing volunteers.
Abbreviations: CYP, cytochrome P450; VAS, visual analog scale; mt, mutant; wt, Wild type.
It is noted that the average pain scores reported by the volunteers is <44 mm, which demonstrates a very high pain control profile for piroxicam independent of the CYP haplotype carried by the volunteers.
Discussion
This is the first report focusing on the influence of pharmacogenetics of the P450 cytochrome family (CYP2C8*3 and CYP2C9) on the control of postoperative inflammatory signs after minor oral surgery. The vast majority of articles are related to pharmacokinetics, metabolism and adverse reactions of NSAIDs, such as gastrointestinal bleeding,2–6,10,12,13,28 but not the clinical control of inflammatory signs after surgical procedures. Some articles have shown pharmacogenetic impacts on large surgeries, such as the need for prior genetic analysis of patients before bariatric surgeries, regarding the lower use of opioids and lower rates of postoperative pain;29 The study of Murto et al30 shows the impact of CYP2C9*3 on lower postoperative pain rates after adenotonsillectomy surgeries in children taking celecoxib.
It was speculated that postoperative pain would be effectively managed by piroxicam (20 mg daily for 4 days) for all genotypes, but that all volunteers with CYP2C8*3 mt and CYP2C9 genotypes might have experienced less postoperative pain. It was also possible that adverse side effects would be decreased in volunteers with the wt alleles when compared to volunteers with the mutant alleles. In other words, individuals with mutant alleles for CY2C8*3 and CYP2C9 might have less pain or more side effects when compared to individuals with wt alleles for CY2C8*3 and CYP2C9. However, postoperative pain scores among the volunteers were not different when comparing the groups of both CYP2C8*3 and CYP2C9.
Jensen et al31 noted that patients undergoing postoperative pain reporting <44 mm (VAS) tend to describe their pain as mild or having minimal impact on their daily activities. In this study, volunteers reported pain scores on average <40 mm. Similarly, Rollason et al1 reported that the average postoperative pain score reported by their volunteers was <44 mm, demonstrating effective postoperative pain management that was independent of their CYP genotype. These data are clinically important since, for this type of surgery, with signs of acute pain and punctal inflammation that tend to cease within 2–3 days, patients presenting the ancestral version of CYP2C8*3 and CYP2C9 do not require piroxicam dose adjustment.
The main importance of pharmacogenetic clinical studies after the consumption of NSAIDs is the occurrence of gene-related changes in pharmacokinetics producing side effects rather than a change in the efficacy profile. In general, carriers of mutations in genes encoding drug-metabolizing enzymes, when treated with the usual doses of a drug metabolized by the affected enzyme, tend to have increased plasma levels of the drug, faster rate of absorption relative to its elimination and increased frequency and severity of adverse reactions with the use of these drugs.4–7,13 Adverse side effects noted by the surgeon or the volunteers were rare in this study, with only two individuals reporting mild adverse reactions related to consuming their daily 20 mg dose of piroxicam. Thus, no correlations were found between genotype and adverse side effects in this regimen of prescription for the control of inflammatory signs after surgeries of lower third molars with piroxicam.
Other studies with large samples report increased risks of gastrointestinal bleeding when patients consuming NSAIDs carried the CYP2C9*2 and CYP2C9*3 mutant alleles,1,5,32 although they state that this risk of gastrointestinal bleeding is clearly dose related and the pharmacokinetics shows greater exposure to these drugs in carriers of the CYP2C9*2 and *3 alleles. Recommendations for dose adjustments in those patients are currently only for celecoxib and flurbiprofen.1 Martinez et al genotyped CYP2C9 in subjects who consumed various NSAIDs that are metabolized by CYP2C9 (ibuprofen, indomethacin, celecoxib, valdecoxib, lornoxicam, tenoxicam, meloxicam and piroxicam) and other NSAIDs not considered CYP2C9 substrates (salicylate and acetaminophen).5 These authors concluded that the association of the variant CYP2C9 alleles with the risk of gastrointestinal bleeding showed a gene–dose effect and was increased in patients receiving drugs metabolized mainly by CYP2C9 when compared to the wt allele, suggesting that genotyping CYP2C9 might identify subgroups of individuals potentially at risk of acute gastrointestinal bleeding.14,15,32,33 In the current study, only the patients’ reports were used as a parameter of gastrointestinal discomfort; no endoscopic examination was performed to investigate the asymptomatic gastrointestinal side effects, and thus, small lesions may have occurred that did not affect the daily routine of the patients.
Otherwise, within the postoperative parameters examined, the volunteers’ alleles for CYP2C8*3 or CYP2C9 were not correlated with any variation in the anti-inflammatory effects of piroxicam, indicating that piroxicam is capable of controlling inflammation regardless of their genotype. Other uninvestigated factors can interfere with inflammation such as postoperative dental hygiene around the extraction site.
Volunteers who underwent lower third molar surgeries, regardless of their CYP genotype, achieved satisfactory relief from postoperative pain and inflammation, with minimal risk of adverse side effects. Therefore, even for slow metabolizers of piroxicam, a daily 20 mg oral dose of piroxicam can be effective while minimizing adverse side effects after invasive oral surgery.
The impact of genetic variants on drug responses to control inflammatory signs and adverse effects of NSAIDs is clear since giving the “right drug” to the patient is a major goal in the era of medicine excellence. Further investigations with other NSAIDs used in the postoperative period of lower third molar surgeries are necessary because this group of drugs presents considerable variations regarding the genetic influence of CYP.
Conclusion
In general, slow metabolizers of piroxicam, namely, volunteers with mutant alleles, were indifferent from normal metabolizers with the wild-type alleles and therefore did not require specialized piroxicam doses to manage postoperative pain and inflammation after surgical removal of the lower third molar.
Acknowledgments
This study was supported by the following grants: São Paulo Research Foundation (FAPESP) grant numbers 2009/17851-8 and 2014/17803-1; and National Council for Scientific and Technological Development (CNPq) grant number 150845/2014-6.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Rollason V, Samer CF, Daali Y, Desmeules JA. Prediction by pharmacogenetics of safety and efficacy of non-steroidal anti-inflammatory drugs: a review. Curr Drug Metab. 2014;15(3):326–343. doi: 10.2174/1389200215666140202214454. [DOI] [PubMed] [Google Scholar]
- 2.Martinez C, Garcia-Martin E, Blanco G, Gamito FJ, Ladero JM, Agundez JA. The effect of the cytochrome P450 CYP2C8 polymorphism on the disposition of (R)-ibuprofen enantiomer in healthy subjects. Br J Clin Pharmacol. 2005;59(1):62–69. doi: 10.1111/j.1365-2125.2004.02183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia-Martin E, Martinez C, Ladero JM, Agundez JA. Interethnic and intraethnic variability of CYP2C8 and CYP2C9 polymorphisms in healthy individuals. Mol Diagn Ther. 2006;10(1):29–40. doi: 10.1007/BF03256440. [DOI] [PubMed] [Google Scholar]
- 4.Agundez JA, Garcia-Martin E, Martinez C. Genetically based impairment in CYP2C8- and CYP2C9-dependent NSAID metabolism as a risk factor for gastrointestinal bleeding: is a combination of pharmacogenomics and metabolomics required to improve personalized medicine? Expert Opin Drug Metab Toxicol. 2009;5(6):607–620. doi: 10.1517/17425250902970998. [DOI] [PubMed] [Google Scholar]
- 5.Martinez C, Blanco G, Ladero JM, et al. Genetic predisposition to acute gastrointestinal bleeding after NSAIDs use. Br J Pharmacol. 2004;141(2):205–208. doi: 10.1038/sj.bjp.0705623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanderson S, Emery J, Higgins J. CYP2C9 gene variants, drug dose, and bleeding risk in warfarin-treated patients: a HuGEnet systematic review and meta-analysis. Genet Med. 2005;7(2):97–104. doi: 10.1097/01.gim.0000153664.65759.cf. [DOI] [PubMed] [Google Scholar]
- 7.Meyer UA. Pharmacogenetics – five decades of therapeutic lessons from genetic diversity. Nat Rev Genet. 2004;5(9):669–676. doi: 10.1038/nrg1428. [DOI] [PubMed] [Google Scholar]
- 8.Pennisi E. A closer look at SNPs suggests difficulties. Science. 1998;281(5384):1787–1789. doi: 10.1126/science.281.5384.1787. [DOI] [PubMed] [Google Scholar]
- 9.Lopez-Rodriguez R, Novalbos J, Gallego-Sandin S, et al. Influence of CYP2C8 and CYP2C9 polymorphisms on pharmacokinetic and pharmacodynamic parameters of racemic and enantiomeric forms of ibuprofen in healthy volunteers. Pharmacol Res. 2008;58(1):77–84. doi: 10.1016/j.phrs.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Au N, Rettie AE. Pharmacogenomics of 4-hydroxycoumarin anticoagulants. Drug Metab Rev. 2008;40(2):355–375. doi: 10.1080/03602530801952187. [DOI] [PubMed] [Google Scholar]
- 11.Peiro AM, Novalbos J, Zapater P, et al. Pharmacogenetic relevance of the CYP2C9*3 allele in a tenoxicam bioequivalence study performed on Spaniards. Pharmacol Res. 2009;59(1):62–68. doi: 10.1016/j.phrs.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 12.Vianna-Jorge R, Perini JA, Rondinelli E, Suarez-Kurtz G. CYP2C9 genotypes and the pharmacokinetics of tenoxicam in Brazilians. Clin Pharmacol Ther. 2004;76(1):18–26. doi: 10.1016/j.clpt.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Perini JA, Vianna-Jorge R, Brogliato AR, Suarez-Kurtz G. Influence of CYP2C9 genotypes on the pharmacokinetics and pharmacodynamics of piroxicam. Clin Pharmacol Ther. 2005;78(4):362–369. doi: 10.1016/j.clpt.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 14.Rodrigues AD. Impact of CYP2C9 genotype on pharmacokinetics: are all cyclooxygenase inhibitors the same? Drug Metab Dispos. 2005;33(11):1567–1575. doi: 10.1124/dmd.105.006452. [DOI] [PubMed] [Google Scholar]
- 15.Samer CF, Lorenzini KI, Rollason V, Daali Y, Desmeules JA. Applications of CYP450 testing in the clinical setting. Mol Diagn Ther. 2013;17(3):165–184. doi: 10.1007/s40291-013-0028-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zupelari-Goncalves P, Weckwerth GM, Calvo AM, et al. Efficacy of oral diclofenac with or without codeine for pain control after invasive bilateral third molar extractions. Int J Oral Maxillofac Surg. 2017;46(5):621–627. doi: 10.1016/j.ijom.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 17.Calvo AM, Brozoski DT, Giglio FP, et al. Are antibiotics necessary after lower third molar removal? Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114(5 suppl):S199–S208. doi: 10.1016/j.oooo.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 18.Colombini BL, Modena KC, Calvo AM, et al. Articaine and mepivacaine efficacy in postoperative analgesia for lower third molar removal: a double-blind, randomized, crossover study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102(2):169–174. doi: 10.1016/j.tripleo.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Benetello V, Sakamoto FC, Giglio FP, et al. The selective and non-selective cyclooxygenase inhibitors valdecoxib and piroxicam induce the same postoperative analgesia and control of trismus and swelling after lower third molar removal. Braz J Med Biol Res. 2007;40(8):1133–1140. doi: 10.1590/s0100-879x2006005000123. [DOI] [PubMed] [Google Scholar]
- 20.Calvo AM, Sakai VT, Giglio FP, et al. Analgesic and anti-inflammatory dose-response relationship of 7.5 and 15 mg meloxicam after lower third molar removal: a double-blind, randomized, crossover study. Int J Oral Maxillofac Surg. 2007;36(1):26–31. doi: 10.1016/j.ijom.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 21.Santos CF, Modena KC, Giglio FP, et al. Epinephrine concentration (1:100,000 or 1:200,000) does not affect the clinical efficacy of 4% articaine for lower third molar removal: a double-blind, randomized, crossover study. J Oral Maxillofac Surg. 2007;65(12):2445–2452. doi: 10.1016/j.joms.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 22.Gregorio LV, Giglio FP, Sakai VT, et al. A comparison of the clinical anesthetic efficacy of 4% articaine and 0.5% bupivacaine (both with 1:200,000 epinephrine) for lower third molar removal. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106(1):19–28. doi: 10.1016/j.tripleo.2007.11.024. [DOI] [PubMed] [Google Scholar]
- 23.Senes AM, Calvo AM, Colombini-Ishikiriama BL, et al. Efficacy and safety of 2% and 4% articaine for lower third molar surgery. J Dent Res. 2015;94(9 suppl):166S–173S. doi: 10.1177/0022034515596313. [DOI] [PubMed] [Google Scholar]
- 24.Weckwerth GM, Simoneti LF, Zupelari-Goncalves P, et al. Efficacy of naproxen with or without esomeprazole for pain and inflammation in patients after bilateral third molar extractions: a double blinded crossover study. Med Oral Patol Oral Cir Bucal. 2017;22(1):e122–e131. doi: 10.4317/medoral.21514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trindade PA, Giglio FP, Colombini-Ishikiriama BL, et al. Comparison of oral versus sublingual piroxicam during postoperative pain management after lower third molar extraction. Int J Oral Maxillofac Surg. 2011;40(3):292–297. doi: 10.1016/j.ijom.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 26.Trindade PA, Giglio FP, Colombini-Ishikiriama BL, et al. Sublingual ketorolac and sublingual piroxicam are equally effective for postoperative pain, trismus, and swelling management in lower third molar removal. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114(1):27–34. doi: 10.1016/j.tripleo.2011.05.027. [DOI] [PubMed] [Google Scholar]
- 27.Ustun Y, Erdogan O, Esen E, Karsli ED. Comparison of the effects of 2 doses of methylprednisolone on pain, swelling, and trismus after third molar surgery. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96(5):535–539. doi: 10.1016/S1079210403004645. [DOI] [PubMed] [Google Scholar]
- 28.Krasniqi V, Dimovski A, Domjanovic IK, Bilic I, Bozina N. How polymorphisms of the cytochrome P450 genes affect ibuprofen and diclofenac metabolism and toxicity. Arh Hig Rada Toksikol. 2016;67(1):1–8. doi: 10.1515/aiht-2016-67-2754. [DOI] [PubMed] [Google Scholar]
- 29.Senagore AJ, Champagne BJ, Dosokey E, et al. Pharmacogenetics-guided analgesics in major abdominal surgery: further benefits within an enhanced recovery protocol. Am J Surg. 2017;213(3):467–472. doi: 10.1016/j.amjsurg.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 30.Murto K, Lamontagne C, McFaul C, et al. Celecoxib pharmacogenetics and pediatric adenotonsillectomy: a double-blinded randomized controlled study. Can J Anaesth. 2015;62(7):785–797. doi: 10.1007/s12630-015-0376-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jensen MP, Martin SA, Cheung R. The meaning of pain relief in a clinical trial. J Pain. 2005;6(6):400–406. doi: 10.1016/j.jpain.2005.01.360. [DOI] [PubMed] [Google Scholar]
- 32.Carbonell N, Verstuyft C, Massard J, et al. CYP2C9*3 loss-of-function allele is associated with acute upper gastrointestinal bleeding related to the use of NSAIDs other than aspirin. Clin Pharmacol Ther. 2010;87(6):693–698. doi: 10.1038/clpt.2010.33. [DOI] [PubMed] [Google Scholar]
- 33.Blanco G, Martinez C, Ladero JM, et al. Interaction of CYP2C8 and CYP2C9 genotypes modifies the risk for nonsteroidal anti-inflammatory drugs-related acute gastrointestinal bleeding. Pharmacogenet Genomics. 2008;18(1):37–43. doi: 10.1097/FPC.0b013e3282f305a9. [DOI] [PubMed] [Google Scholar]