Abstract
The laryngeal chemoreflex (LCR), an airway protective reflex that causes apnea and bradycardia, has long been suspected as an initiating event in the sudden infant death syndrome (SIDS). Serotonin (5-HT) and 5-HT receptors may be deficient in the brainstems of babies who die of SIDS, and 5-HT seems to be important in terminating apneas directly or in causing arousals or as part of the process of autoresuscitation. We hypothesized that 5-HT in the brainstem would limit the duration of the LCR. We studied anesthetized rat pups between 7 and 21 days of age and made microinjections into the cisterna magna or into the nucleus of the solitary tract (NTS). Focal, bilateral microinjections of 5-HT into the caudal NTS significantly shortened the LCR. The 5-HT 1a receptor antagonist, WAY 100635, did not affect the LCR consistently, nor did a 5-HT2 receptor antagonist, ketanserin, alter the duration of the LCR. The 5-HT3 specific agonist, 1-(3-chlorophenyl)-biguanide, microinjected bilaterally into the caudal NTS significantly shortened the LCR. Thus, endogenous 5-HT released within the NTS may curtail the respiratory depression that is part of the LCR, and serotonergic shortening of the LCR may be attributed to activation of 5-HT3 receptors within the NTS. 5-HT3 receptors are expressed presynaptically on C-fiber afferents of the superior laryngeal nerve, and serotonergic shortening of the LCR may be mediated presynaptically by enhanced activation of inhibitory interneurons within the NTS that terminate during the LCR.
Keywords: Laryngeal chemoreflex, serotonin, 5-HT3 receptor
INTRODUCTION
The sudden infant death syndrome (SIDS) appears to occur when an apparently healthy sleeping infant experiences a challenge to cardiorespiratory homeostasis and fails to mount an effective response. In the United States in 2010, 2063 deaths were reported as SIDS, making it the leading cause of death in infants aged 1 month to 1 year (www.cdc.gov/sids/). Analyses of brain tissue from many babies who died of SIDS have consistently shown a high prevalence of abnormalities in the brainstem 5-HT system. These abnormalities include an increased number of serotonergic neurons, a higher proportion of serotonergic neurons displaying immature morphology, decreased tissue levels of 5-HT and its synthetic enzyme, tryptophan hydroxylase 2 (TPH2), and decreased 5-HT receptor binding intensity both in serotonergic nuclei themselves and in several projection sites known to be important in cardiorespiratory control (Kinney et al., 2003; Paterson et al., 2006; Duncan et al., 2010). Serotonin has widespread influences both on acute cardiorespiratory control and on central nervous system (CNS) development, where it plays important roles in differentiation, migration and other functions (Lauder, 1993). In addition, the activity of serotonergic neurons is strongly associated with the waking state (Heym et al., 1982; Veasey et al., 1995; Jacobs & Fornal, 1999) and may be associated with arousal responses in the transition from sleep to wakefulness (Leiter & Böhm, 2007; Buchanan & Richerson, 2010).
The nucleus of the solitary tract (NTS) is one region of the brainstem where 5-HT receptor binding deficiencies have been observed in infants who died of SIDS (Paterson et al., 2006; Machaalani et al., 2009). The NTS receives projections from serotonergic neurons in the caudal raphe nuclei, and neurons in the NTS express 5-HT 1A, 1B, 2A, 3, 4, and 7 receptors (Gustafson et al., 1996; Varnas et al., 2004; Dergacheva et al., 2009; Liu & Wong-Riley, 2010). The NTS integrates a variety of afferent projections involved in eliciting numerous inhibitory reflexes, including the laryngeal chemoreflex (LCR), which has been implicated as an initiating event in the pathogenesis of SIDS (Downing & Lee, 1975; Jeffery et al., 1995; Thach, 2001; Leiter & Böhm, 2007). The LCR is an airway protective reflex initiated when gastric contents, water or other solutions with a low pH or a low chloride concentration activate chemoreceptors in the perilaryngeal mucosa (Boggs & Bartlett, 1982). The physiological manifestations of the LCR change over the course of early postnatal life. In neonatal mammals, including human infants, the reflex is characterized by inhibitory responses such as apnea and bradycardia, which gradually wane and are largely replaced by coughing and swallowing as animals mature (Thach, 2001). The apneas that predominate early in life can be long lasting and result in repeated episodes of profound hypoxemia and hypercapnia. As hypoxemia is itself an inhibitor of respiratory drive in neonates (Saetta & Mortola, 1987), it is possible that LCR-induced apnea could lead to a downward spiral of increasing hypoxic inhibition that ultimately leads to death, especially if serotonergic arousal responses are deficient (Leiter & Böhm, 2007).
Serotonin generally stimulates or enhances respiratory activity in immature mammals. In genetically-altered mice lacking most serotoninergic neurons (Erikson & Sposato, 2009) or lacking the ability to synthesize 5-HT centrally (Chen et al., 2013a), recovery from anoxic apnea was delayed and less effective than in wild-type animals. The 5-HT deficient animals demonstrated gasping, but failed to arouse and failed to restore eupneic breathing (Leiter, 2009). Postnatal lesioning of brainstem serotoninergic neurons in rats also reduced the survival rate and delayed recovery from anoxic apnea (Cummings et al., 2011b). Thus, reduced 5-HT signaling in the NTS in infants may prolong the LCR, make hypoxia during the apnea more severe and reduce the likelihood of successful recovery from apnea. Therefore, we tested the hypothesis that augmenting levels of serotonergic signaling in the brainstem of immature rats, particularly in the caudal NTS, would reduce the duration of the LCR, reduce the duration of reflex apnea and lessen the bradycardia associated with respiratory inhibition. To test this hypothesis, we elicited the LCR in anesthetized neonatal rats before and after treatment with a variety of serotonergic agonists and antagonists.
METHODS
Ethical Approval
The Institutional Animal Care and Use Committee of Dartmouth College approved all surgical and experimental protocols. We included both male and female Sprague-Dawley rat pups ranging in age from post-natal day 7 (P7) to P21 in all sets of experiments.
Surgical preparation
We determined the sex and weight of each animal, and then administered 1.5 g/kg urethane (U2500, Sigma-Aldrich, St. Louis, MO) and 10 mg/kg chloralose (C0128, Sigma-Aldrich) in 0.9% saline by intraperitoneal injection. We maintained body temperature at ~34°C using a rectal temperature probe (RET-4, Physitemp, Clifton, NJ) connected to a Physitemp TCAT-2 DF servo-controller, which regulated a heat lamp positioned over the animal. After each animal reached a surgical plane of anesthesia (lack of withdrawal reflex or significant change in heart or respiratory rate following a toe pinch), we inserted two 30 gauge platinum needle electrodes (F-E2M, Grass Technologies, West Warwick, RI) subcutaneously over the chest to record EKG activity. We amplified (100X) and filtered (low cutoff 300 Hz, high cutoff 1 kHz) signals from the EKG leads using a DAM-80 AC Differential Amplifier (World Precision Instruments, Sarasota, FL). We inserted a second pair of needle electrodes into the intercostal muscles in the region of the ninth through eleventh ribs to monitor respiratory EMG activity. We secured both electrode pairs with small amounts of cyanoacrylate glue. We amplified (1000X) and filtered (low cutoff 300 Hz, high cutoff 3 kHz, 60 Hz notch) the EMG signals using Iso-DAM8A amplifiers (WPI, Sarasota, FL). We sampled both EKG and EMG signals at 1 kHz using a USB-6009 driven by a custom LabVIEW program (National Instruments, Austin, TX).
We placed the animal supine, exposed the trachea and larynx, incised the anterior wall of the trachea circumferentially between adjacent rings of cartilage and placed a polytetrafluoroethylene breathing tube (19–23 gauge) in the caudal segment. We inserted a length of PE-50 tubing into the rostral trachea and advanced it until its tip was near the caudal border of the larynx; this tubing was used to deliver microliter amounts of distilled water via a syringe pump to the larynx to elicit the LCR. We secured both tubes into the trachea using 4-0 silk sutures and cyanoacrylate adhesive. We then placed the animal in the prone position on the edge of an elevated platform and secured its head in a ventri-flexed position using surgical tape. We exposed the foramen magnum by removing the skin and bluntly dissecting the overlying muscle tissue. We used the tip of a 32 gauge needle to incise the membranes covering the foramen. This exposed the cerebrospinal fluid (CSF) and floor of the fourth ventricle to provide access for intracisternal or focal microinjections. We injected intracisternally using a 2.5 μL glass syringe with a removable 33 gauge needle (Hamilton, Reno, NV). In other animals, we made 30–50 nanoliter, focal injections through fine, pulled glass pipettes using a Picospritzer II (Parker Hannifin, Cleveland, OH). The microinjections were made just caudal and lateral (within 1 mm in both directions) of the termination of the floor of the fourth ventricle in the medial portion of the gracile nucleus. The glass pipettes were held in a micromanipulator perpendicular to the surface of the brainstem to allow precise control over the location and depth of microinjection. All microinjections included 0.02% of fluorescent 0.4 μ diameter carboxylated latex microspheres (Fluoresbrite, PolySciences, Inc., Warrington, PA) for verification of injection locations.
LCR protocol
After opening the foramen magnum, we delivered small volumes of water rostrally into the larynx via syringe pump to elicit the LCR. We started with 2–4 microliter and increased the volume until an LCR-associated apnea of a consistent, moderate duration (usually two to five seconds) was elicited. The majority of effective water stimulus volumes were between eight and twenty microliters. Once established, we held the stimulus volume constant for the duration of each experiment. We gave a minimum of three stimulations using the effective volume to establish a baseline response. Next, we administered a drug or vehicle by intracisternal or focal microinjection, after which we gave a minimum of three additional LCR stimulations. We waited ten to twenty minutes between stimulations to allow the animal to clear the water from the larynx, to allow breathing to recover and to minimize accommodation to the stimulus. At the conclusion of each experiment, we euthanized the animal by an anesthetic overdose. We decapitated each animal that received a microinjection and carefully removed its brain in order to determine the injection location.
Neuroanatomy
We fixed the brains of microinjected animals by immersion in approximately twenty volumes of 4% paraformaldehyde in 10 mM phosphate buffered saline (PBS) for a minimum of 48 hours, and cryoprotected them by immersion in tubes of 30% sucrose in PBS until they no longer floated. We blocked the brains, froze them in an aqueous cutting medium (Tissue-Tek O.C.T., Sakura Finetek, Torrance, CA) and sectioned them on a cryostat (CM 3050 S, Leica, Buffalo Grove, IL) at 40 or 50 microns. In some animals, we collected alternate slices: one set of slides was cleared, rehydrated and stained in a solution of 0.5% cresyl violet, and the other set was examined and photographed immediately using an E800 microscope (Nikon, Melville, NY) with a mercury arc light source with appropriate filters for the fluorescence profile of the beads used. We compared anatomical landmarks visible on each slice with those shown on atlas plates (Paxinos & Watson, 1998) to determine the location of each injection in the brainstem and plotted each location on the atlas plate that corresponded most closely with its position in the brain.
Data Analysis and Statistics
We measured the effect on respiration of each water stimulus in two ways. First, we measured the duration of the apnea, which we defined as the period from the onset of the last breath before the water stimulus was given until the start of the first obvious respiratory activity following the stimulus. Second, we measured the length of the period from the onset of the last breath before the water stimulus until the start of the first of five regular breaths after the stimulus (regular in rhythm, but not necessarily amplitude), a more comprehensive measure of respiratory disruption and instability that we refer to as the LCR. We found in previous studies (Curran et al., 2005; Xia et al., 2007) that this dual set of measurements provided a more robust measurement of respiratory disruption than either would alone. We also measured the respiratory rate of the ten breaths preceding each stimulus and immediately following the LCR, and the heart rate in each of these periods.
Data are presented as means ± the standard error of the mean (SEM). In general, we compared apnea and LCR durations before and after intracisternal or focal application of 5-HT, 5-HT receptor agonists/antagonists or vehicle. Therefore, we used a two-way ANOVA in which the particular drug was compared to a control treatment in different animals (a between subjects factor) and the responses were compared before and after treatment (a within subjects comparison). The variances of the durations of apnea and the LCR were not homogeneous, and the data were not normally distributed. Therefore, we log transformed these data and compared the data using two-way or three-way ANOVAs in Systat 9 (SPSS Inc., Chicago, IL). We incorporated age as a random effect in some of these analyses. When the ANOVA indicated that significant differences existed among treatments, specific, pre-planned comparisons were made using P-values adjusted by the Bonferroni method for multiple comparisons.
RESULTS
Intracisternal 5-HT shortens the LCR
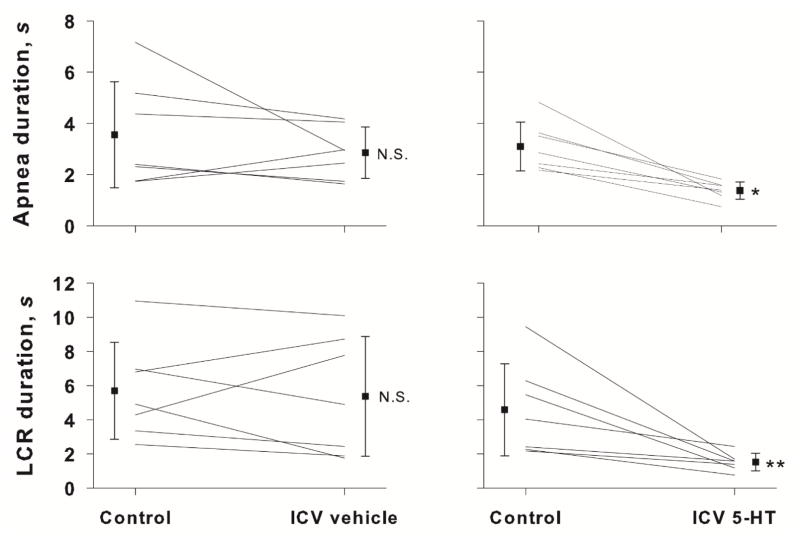
An example of the LCR before and after injection of 5-HT into the intracisternal space of an 11 day old female pup is shown in Figure 1. Before receiving 5-HT, water injected into the larynx caused apnea and obvious respiratory disruption. The average duration of the LCR in the control condition was 9.45 ± 3.06 sec. After receiving an intracisternal injection of 2 μL of 10 mM 5-HT, the LCR was substantially shortened. The average LCR duration in this animal diminished to 1.69 ± 2.13 sec. The average apnea and LCR durations in a group of seven rat pups, age P6 to P13, that received intracisternal injections of 5-HT are compared in Figure 2 to a group of seven control rat pups, age range P7 to P15 that received an intracisternal injection of vehicle alone. The apnea (P = 0.011) and LCR (P = 0.013) durations, were significantly shorter in the 5-HT-treated animals compared to those animals that were injected with vehicle. There was no effect of vehicle intracisternal injections on apnea duration or LCR duration. Moreover, the pre-intracisternal injection values of heart rate and respiratory rate were not different between treated and control animals. Thus, the animals had stable respiratory rates and heart rates over the entire time of the experiment.
Fig 1.
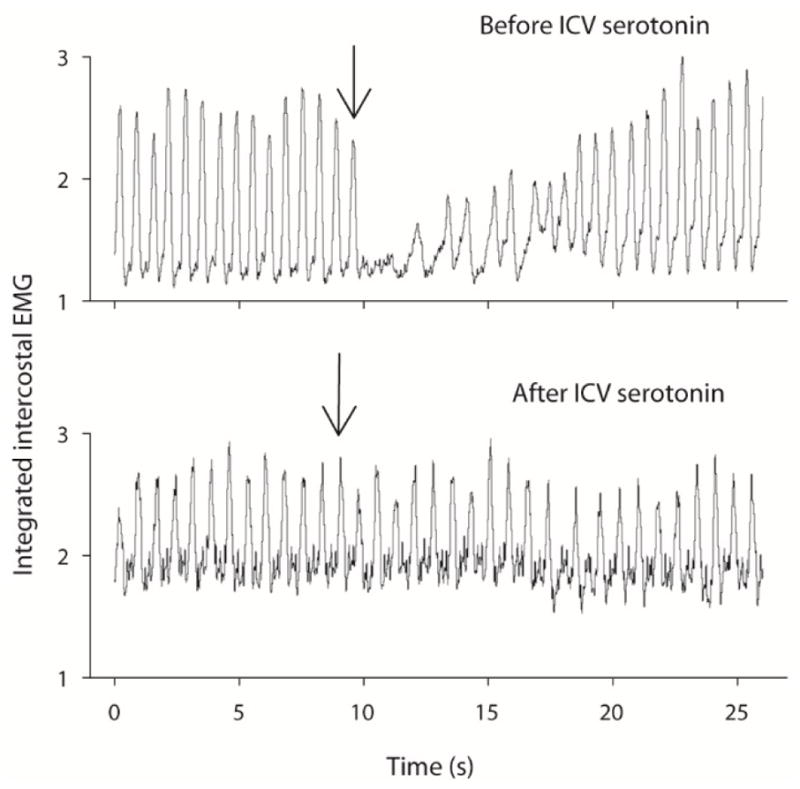
The inspiratory EMG responses to water stimuli of the larynx in a single P11 female rat pup are shown as functions of time. The control, pre-treatment response is shown in the top panel, and the duration and character of the LCR after intracisternal injection of 2 μL of 10 mM 5-HT is shown in the bottom trace: The arrows indicate the time of laryngeal injection, and the volume of injection in this animals was 8 μL.
Fig. 2.
The mean apnea and LCR responses after intracisternal vehicle or 5-HT injection into the cisterna magna are shown. Filled squares indicate the mean and standard error. Individual responses are shown for each animal tested. Statistical comparisons were made between the control and treated condition within each drug or vehicle treat group. N.S. = not significant; * indicates P = 0.011; ** indicates P = 0.013.
5-HT1a and 5-HT2 antagonists do not consistently modify the LCR
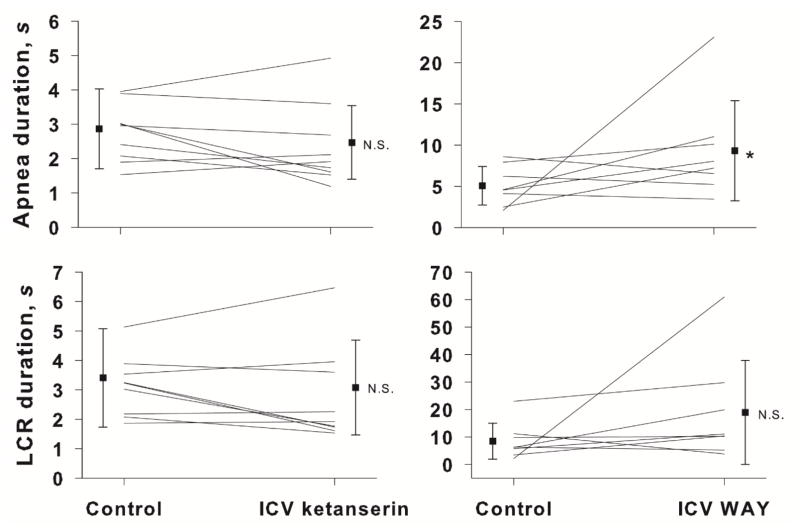
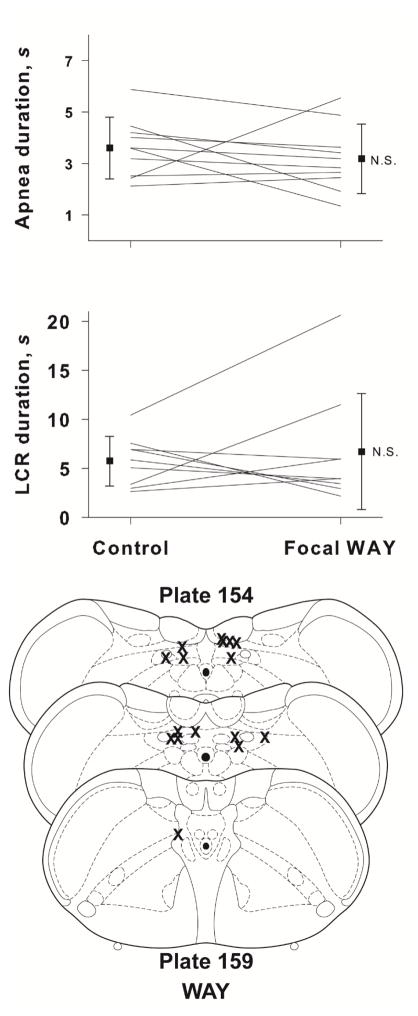
The number of 5-HT1a receptors in the brainstem is reduced in babies who died of SIDS (Paterson et al., 2006), and the NTS is one of the sites receiving serotonergic projections where the 5-HT1a receptor density is decreased (Duncan et al., 2010). Based on the results of our 5-HT experiments, we expected that blocking (presumably) postsynaptic 5-HT1a receptors in the NTS would prolong the LCR, and we tested the effect of WAY 100635, a specific 5-HT1a receptor antagonist, on the LCR duration. Intracisternal injection of WAY 100635 prolonged the average duration of apneas in eight neonatal rat pups, age P8 to P20 (P < 0.034), as shown in Figure 3. The average LCR duration also increased, but this change was not statistically significant. The small average changes in apnea and LCR durations and the variability of responses among animals indicated that shortening of the LCR by 5-HT is unlikely to act solely through a 5-HT1a receptor mechanism.
Fig. 3.
The mean and SEM of apnea and LCR durations along with individual animal responses are shown before (control) and after intracisternal ketanserin or intracisternal WAY 100635 injections. A statistical comparison was made between the control and treated conditions. NS = not significant; *P = 0.034.
Since the results of the WAY 100635 experiments did not adequately explain the serotonergic shortening of the LCR, we decided to investigate additional 5-HT subtypes. Serotonin 5-HT2a receptors are the most widely-expressed excitatory G-protein coupled 5-HT receptors in the brain. 5-HT2a receptors are ubiquitous in the brainstems of rat pups in the age range that we studied (Liu & Wong-Riley, 2010). Thus, we hypothesized that shortening of the LCR following administration of 5-HT might be mediated by this receptor, and blocking the receptor would unmask the serotonergic contribution to the termination of the LCR. Intracisternal injection of ketanserin did not, however, consistently modify the duration of the LCR in a group of nine rat pups, age P7 to P21 (Figure 3).
Focal microinjection of 5-HT into the NTS shortens the LCR
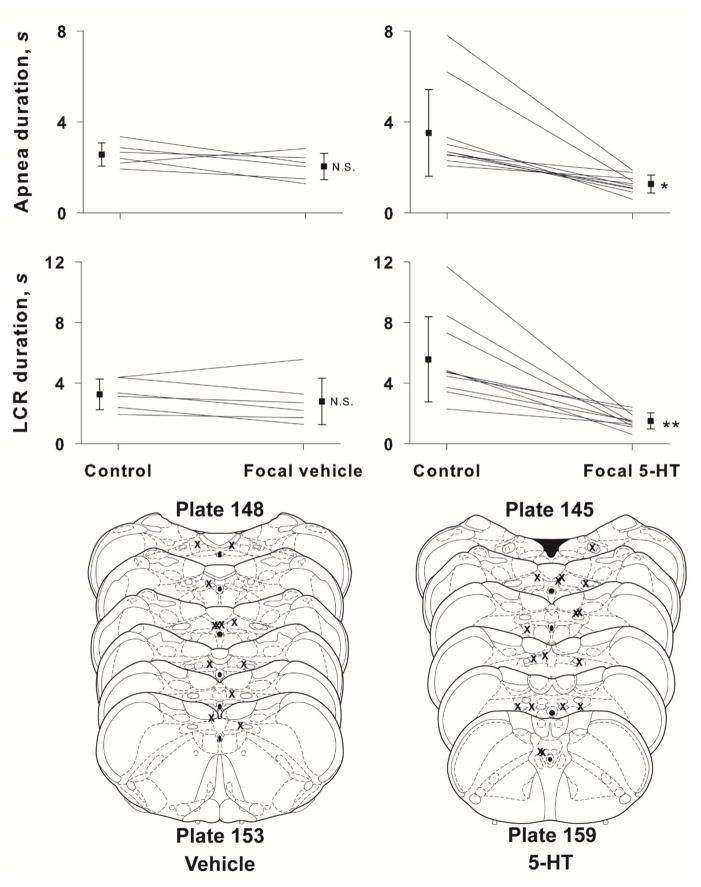
Given the sizable reductions in the LCR duration that resulted from intracisternal administration of 5-HT, the failures of the 5-HT1a and 5-HT2 antagonists to alter the LCR when injected intracisternally were surprising to us and gave us reason to be concerned about the anatomical specificity of the 5-HT effects on the LCR. Therefore, we decided to confirm that the effect of intracisternal 5-HT injection on the LCR was specific to receptors in the NTS. The average apnea and LCR responses to direct microinjection of 50 nL of 4 mM 5-HT into the NTS are shown in Figure 4. As in the case of the intracisternal injections, 5-HT injected directly into the NTS significantly shortened the LCR duration and shortened the duration of apnea within the LCR (P < 0.001 for both variables). Ten rat pups, age P7 to P16, were treated with 5-HT, and in all ten, the apnea and LCR durations diminished. In vehicle microinjected control animals (n = 7, age P9-P13), there was no effect of the microinjection on either apnea or LCR durations. The pre-injection heart rates and respiratory rates were not different between the vehicle-treated and the 5-HT-treated animals. The sites of injection are shown in Figure 6, and all injections were made within or near the NTS.
Fig. 4.
The mean and SEM of apnea and LCR durations along with individual animal responses are shown before (control) and after focal injection of either vehicle or 5-HT into the NTS. Statistical comparisons were made between the control and treated condition within each drug or vehicle treat group. N.S. = not significant; * indicates P < 0.001 for both within and between treatment group comparisons; ** indicates P < 0.001 for both within and between treatment group comparisons. Schematic diagrams of cross-sections of the brainstem show the approximate locations of focal injections of vehicle (left side, lower panel) and 5-HT (right side, lower panel). Focal microinjections were made bilaterally, so there are two ‘Xs’ for each microinjection, but they may not have been made in the same rostro-caudal location on both sides, and in some cases, we could identify only one injection site.
Fig. 6.
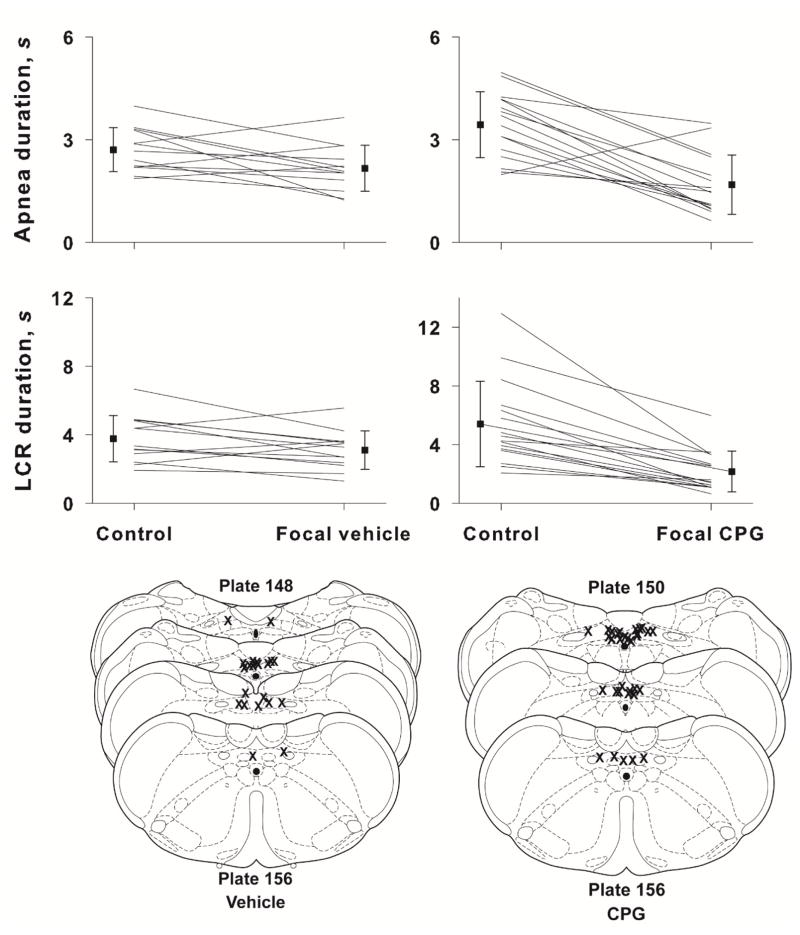
The mean and SEM of apnea and LCR durations along with individual animal responses are shown before (control) and after focal injection of either vehicle or CPG into the NTS. Statistical comparisons were made between the control and treated condition within each drug or vehicle treat group. N.S. = not significant; * indicates P < 0.001 for both within and between treatment group comparisons; ** indicates P < 0.001 for both within and between treatment group comparisons. Schematic diagrams of cross-sections of the brainstem show the approximate locations of focal injections of vehicle (left side, lower panel) and CPG (right side, lower panel). Focal microinjections were made bilaterally, so there are two ‘Xs’ for each microinjection, but they may not have been made in the same rostro-caudal location on both sides, and in some cases, we could identify only one injection site.
We analyzed heart rate responses in this group of animals before and after 5-HT microinjection into the NTS. The ANOVA indicated that heart rate diminished during the LCR (baseline heart rate during the 10 seconds preceding the LCR test = 291 ± 18; average heart rate during the LCR = 268 ± 19). But there was no significant difference in heart rate response during the LCR between vehicle and 5-HT treated animals. Thus, we conclude that there was a small bradycardia during the LCR in these rat pups, but 5-HT in the NTS did not seem to regulate or modulate the degree of the bradycardia.
In previous studies, we and others have noted that the LCR diminishes as animals mature (Thach, 2001; Xia et al., 2008; Xia et al., 2010). Therefore, we analyzed the effect of age on the effects of 5-HT injection in the NTS by incorporating age as a random effect variable in the ANOVA, and we found no effect of age on apnea or LCR durations in this group of animals.
Focal microinjection of WAY 100635 into the NTS does not prolong the LCR
Since intracisternal administration of WAY 100635 had prolonged apnea and LCR durations, we repeated this treatment using microinjection directly into the NTS to confirm the effect of WAY 100635 and to ask whether any effect of WAY 100635 originated from 5-HT1a receptors within the NTS where the initial integration of the LCR occurs. Microinjection of 50 nL of 20 mM WAY 100635 focally within the NTS failed to change either the apnea duration or the LCR duration in a group of nine rat pups, age P8 to P17 (Fig. 5). Therefore, activation of 5-HT1a receptors within the NTS and 5-HT2 receptors within the brainstem did not seem to be the source of the serotonergic shortening of the LCR shown in Figures 2 and 4.
Fig. 5.
The mean and SEM of apnea and LCR durations along with individual animal responses are shown before (control) or after focal microinjection into the NTS of WAY 100635. A statistical comparison was made between the control and treated conditions. Schematic diagrams of cross-sections of the brainstem show the approximate locations of focal injections of WAY-100635. Focal microinjections were made bilaterally, so there are two ‘Xs’ for each microinjection, but they may not have been made in the same rostro-caudal location on both sides, and in some cases, we could identify only one injection site.
Focal microinjection of a 5-HT3 agonist into the NTS shortens the LCR
5-HT3 receptors are known to be present in the NTS where they are found presynaptically on C-fiber afferents. The distribution of presynaptic receptors is segregated: A-fibers express presynaptic purinergic receptors, but not TRPV1 receptors; C-fibers express presynaptic TRPV1, 5-HT3 and cannabinoid 1 (CB1) receptors (to name only a subset of the presynaptic receptors present), but apparently not purinergic presynaptic receptors (Jin et al., 2004; Jeggo et al., 2005; Andresen et al., 2012). Activation of both A- and C-fibers seems to be important eliciting the LCR (Harding et al., 1978; Anderson et al., 1990; Mutoh et al., 2000; Roulier et al., 2003). Both A-fibers and C-fibers run in the internal branch of the superior laryngeal nerve and transmit sensory information from the larynx to the brainstem, where the afferents innervate second order neurons within the caudal NTS (Patrickson et al., 1991; Hayakawa et al., 2001), but to the extent that C-fiber afferents are activated, the 5-HT3 receptors may be likely candidates to mediate the serotonergic shortening of the LCR noted above. To test this hypothesis, we elicited the LCR in animals that received either bilateral 50 nL microinjections into the NTS of either 24 mM 1-(3-chlorophenyl)-biguanide HCl (CPG) in saline or saline alone. Over the course of these studies, we became concerned that the interval between tests of the LCR might modify the response – it seemed as if the 10 minute interval between tests were insufficient time for full restoration of baseline conditions. Therefore, we included two groups of animals in the study of CPG responses: one with 10 minutes between each test of the LCR and one group with 20 minutes between each test of the LCR. The individual and average results of these studies are shown in Figure 6. We studied 16 animals with CPG and 13 animals with saline vehicle alone. The animals ranged in age from P7 to P19 days. We found no effect of testing interval or age, and these variables were dropped from the analysis. In the simpler analysis of drug groups and treatment sequence, apnea duration did not change significantly in the vehicle treated group following bilateral microinjections into the NTS, but apnea duration was shortened significantly in the CPG treated group compared to the control condition within the CPG treatment and compared to the response to vehicle in that treatment group (P < 0.001 for both comparisons). In respect to the LCR duration, the slight decline in the duration of the LCR after vehicle microinjection was not significant, but the LCR was shortened significantly following CPG treatment compared to control condition within the CPG treatment group and compared to the response to vehicle treatment (P < 0.001 for both comparisons). As before, there were no differences in respiratory rate (P = 0.264) or heart rate (P = 0.588) preceding the pre- and post-microinjection tests of the LCR, and the respiratory rate and heart rate were not different in the groups with different intervals between tests of the LCR. The sites of CPG and vehicle microinjection are shown in Fig. 6, and there were no consistent differences among the microinjection sites between the two treatment conditions. All of the microinjections are within the caudal NTS or closely adjacent to the caudal NTS.
The impact of animal age on the LCR
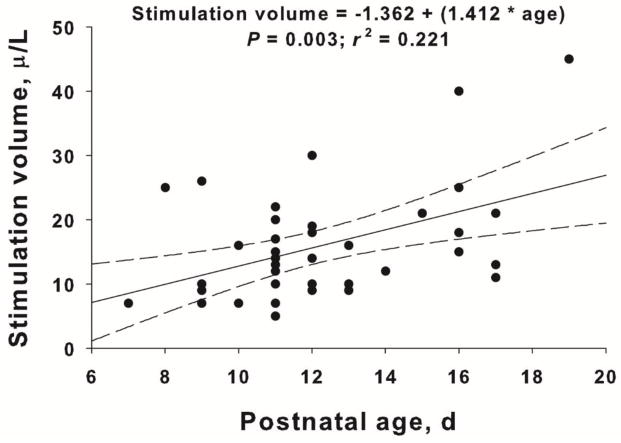
We and others have observed that the LCR diminishes as animals mature (Thach, 2001; Xia et al., 2008; Xia et al., 2010), and we wondered if the response to 5-HT3 receptor stimulation might change with age as well. Yet none of the analyses of individual data sets revealed any effect of age, but the numbers of animals were small within each study, and we also realized that there was a confounding variable. In each animal that we studied, we gave a small number of preliminary laryngeal injections, and we adjusted the volume of water injected to obtain apneas of about 2 seconds and LCR durations of about 5 seconds. Longer respiratory pauses tend to cause the animals to deteriorate over the study, and shorter durations make it difficult to reliably measure the apnea and LCR durations. It turns out that we unknowingly varied the laryngeal stimulation volume as a function of age, and this confounded our ability to see the maturational effect of age on the LCR. Stimulation volume has been plotted as a function of postnatal age from all the animals that received any kind of microinjections into the NTS in Figure 7. This larger pooled data set allows us to obtain a sufficient range of postnatal ages to see the relationship between stimulation volume and animal age, and it is clear that the laryngeal stimulation volume increased systematically by 1.4 μl per postnatal day; this means that a P7 animal would have received, on average, 8.5 μl whereas a P20 animal would have received 26.9 μl to achieve the same apnea and LCR durations. Thus, the sensitivity of the LCR declined as a function of postnatal age among the neonatal pups that we studied. Furthermore, when we included stimulation volume or age in the analysis of the response to serotonergic agents, we found no main effect or significant interaction of age or stimulation volume with any of the other independent variables in our model. This means that by changing the stimulation volume, we effectively normalized all apnea and LCR durations in the control conditions in all of the animals that we studied, and more importantly, the serotonergic effect to shorten the LCR was also thereby normalized. The apparent change in sensitivity of the LCR may reflect an actual diminution in the strength of the reflex as animals mature, but the size of the airway increases as animals mature, and it is possible that it simply takes more water to stimulate the same number of receptors as animals grow and airway size increases. We are currently using fixed stimulation volumes across postnatal age in other studies to determine if the magnitude of the serotonergic effect to shorten the LCR also varies as a function of postnatal age.
Fig. 7.
The relationship between laryngeal stimulation volume and neonatal postnatal age is shown above. All animals that received focal vehicle, focal 5-HT or focal CPG were included in the analysis. The line of least squares best fit linear regression is show as well along with the 95% confidence intervals.
DISCUSSION
The most important findings in this study are that first, increasing 5-HT in the brainstem, in the region of the NTS, shortened the apnea and respiratory disruption associated with the LCR, and second, the shortening of the LCR seems to be mediated by 5-HT3 receptors within the NTS since a 5-HT3-selective agonist microinjected bilaterally into the NTS replicated the effects of 5-HT on the LCR. Serotonin and CPG treatments did not have any effect on the bradycardic response to laryngeal stimulation, which was quite modest in any event.
Responses to Serotonin and Serotonin Agonists and Antagonists
In our initial experiments, we made intracisternal injections, which primarily affect tissues of the dorsal brainstem, including the NTS, but which may affect more ventral regions as well. We proceeded to make focal microinjections into the NTS of drugs that had been shown in the intracisternal injection experiments to influence the LCR. The shortening of the LCR duration associated with 5-HT given by focal microinjections into the NTS was similar to the effect seen following intracisternal administration of 5-HT. Within the focal 5-HT injection group, there was some variability in the baseline LCR responses between treatment and control groups, especially in the focal 5-HT injection group (Fig. 4). This variability is inherent to any experiment using intact animals. The selection of treatments was randomized, and the animals were, insofar as we can tell, in identical physiological states (the heart rates and respiratory rates in the initial pre-injection control were similar between treated and control animals). Moreover, when we dropped outliers from the analysis, the effect of focal 5-HT remained statistically significant. Thus, these outliers did not alter the fundamental finding – 5-HT in the NTS shortened the duration of the LCR whether 5-HT was administered focally within the NTS or by intracisternal injection. These results confirmed our initial hypothesis that 5-HT blunts or terminates the respiratory inhibition associated with the LCR.
Our efforts to determine the 5-HT receptor(s) responsible for mediating the shortening of the LCR were complicated by the fact that receptors of the 5-HT1, 2, 3, 4 and 7 families are all found in the NTS. Given 1) the large number of 5-HT receptor subtypes in the NTS, 2) the fact that different members of the 5-HT receptor family have opposing effects on neuron excitability, 3) that some 5-HT receptors are expressed pre-synaptically and some post-synaptically and 4) that they may have spatially distinct patterns of expression and temporally distinct activation by different groups of serotonergic neurons, isolating and identifying the contribution of a single receptor subtype to the 5-HT effect on the LCR may be difficult.
Given the interest in the 5-HT1a receptor in infants who died of SIDS (Paterson et al., 2006), we naturally thought that 5-HT1a receptors might mediate serotonergic shortening of the LCR. Moreover, the NTS is one of the sites receiving serotonergic projections where the 5-HT1a receptor density is decreased (Duncan et al., 2010). We expected that blocking postsynaptic 5-HT1a receptors in the NTS would prolong the LCR, and we tested the effect of WAY 100635, a specific 5-HT1a receptor antagonist, on the LCR duration. Intracisternal injection of WAY 100635 did prolong the average duration of apneas in eight neonatal rat pups, age P8 to P20 (P < 0.034), as shown in Figure 3, and we thought that postsynaptic 5-HT1a receptors were a likely candidate to mediate the serotonergic shortening of the LCR. However, when we administered WAY100635 by focal microinjection into the NTS, we found no consistent response. WAY100635 may also have had off target effects. It is a potent agonist at the D4 receptor, which is expressed in the intermediate and medial subnuclei of the NTS (Hyde et al., 1996), and in rat brainstem-spinal cord preparations, stimulation of the D4 receptor depressed the respiratory rhythm by inhibiting the activity of pre-I neurons (Fujii et al., 2004). The absence of a response to focally injected WAY100635 within the NTS indicates that any D4 receptors within the NTS are unlikely to modulate the duration of the LCR. Since WAY100635 actually increased the duration of the LCR (with some intersubject variability; Fig. 3 right panels), it seems unlikely that WAY 100635-mediated modulation of raphe neurons was responsible for shortening the LCR.
The 5-HT2a receptor also seemed a likely candidate to mediate the shortening of the LCR, as it is ubiquitous in the NTS, is frequently associated with neuronal excitation and plays a role in baroreflex function (Raul, 2003) and in the prolonged increase in respiration associated with carotid body stimulation (Millhorn et al., 1980). However, intracisternal administration of ketanserin had no consistent effect on the duration of the LCR in our experiments. For all the drugs tested, we selected doses in the midrange of values used in previous studies, and it is, therefore, unlikely that a larger dose of ketanserin (or any of the other drugs that we tested) would yield a different result. The lack of any effect of ketanserin may have been the result of off-target effects; ketanserin, while selective for the 5-HT2a receptor subtype, also has sub-micromolar affinities for 5-HT2c (282 nM), alpha-1 adrenergic (40 nM), histamine H1 (2 nM) and dopamine D1 (~300 nM) and D2 (78 nM) receptors (all ki values from the National Institute of Mental Health Psychoactive Drug Screening Program database). As we administered the ketanserin via intracisternal injection, it is also possible that effects of the drug in different parts of the brainstem opposed each other.
Despite the possibilities of off target effects of these different agonist and antagonist drugs, interactions with different receptors and diffusion to other brainstem nuclei, the simplest conclusion of these studies is that the shortening of the LCR caused by exogenous 5-HT treatment is not mediated by either the 5-HT1a or the 5-HT2 receptor. Therefore, we turned to studies of 5-HT3 receptors, and CPG, a 5-HT3 receptor agonist did shorten the LCR and apnea durations quite effectively. The NTS has a large complement of 5-HT3 receptors, and early 5-HT3 localization studies using autoradiography in human postmortem tissue found the receptor most densely in the area postrema (AP) and NTS, as well as in the substantia gelatinosa of the spinal cord (Waeber et al., 1989). In human tissue, the density of 5-HT3 receptors was also high in the AP, the NTS, hypoglossal nucleus and dorsal nucleus of the vagus (Barnes et al., 1990) A subsequent consensus statement indicated that the majority of the 5-HT3 receptors were actually in the NTS, in particular the subnucleus gelatinosus (also known as the area subpostrema) and the commissural subnucleus (Pratt et al., 1990). Studies in rat tissue confirmed that the majority of 5-HT3 receptors are expressed in the NTS, rather than the AP, and the receptors appear to be located presynaptically on afferents from the nodose ganglion, as unilateral ganglionectomy resulted in the loss of ~75% of 5-HT3 binding in the ipsilateral NTS (Pratt & Bowery, 1989). The microinjections in the neonatal rat pups that we studied were distributed in or close to the commissural nucleus of the NTS, where second order neurons targeted by the superior laryngeal nerve are found.
The Role of NTS-Expressed 5-HT3 in Inhibitory Reflexes
Most of the central 5-HT3 expression appears to be presynaptic on glutamatergic vagal afferents, and it seems likely that their activation potentiates activity from these afferents. In urethane-anesthetized rats, microinjection of phenylbiguanide into the NTS significantly increased extracellular levels of L-glutamate, and this effect was blocked by i.c.v. administration of the 5-HT3 antagonist granisetron (Ashworth-Preece et al., 1995). Furthermore, experiments in anesthetized rats demonstrated that ionophoretic application of the 5-HT3 agonist CPG in the NTS increased the number of spikes recorded in nearly all second order NTS neurons receiving vagal afferent input, and of PBG into the right atrium either the 5-HT3 antagonist granisetron, or the combination of antagonists of non-NMDA and NMDA receptors (DNQX and AP-5, respectively) administered centrally were able to attenuate these potentiating effects of phenylbiguanide administration into the right atrium (Jeggo et al., 2005). These results, however, raise a question: if 5-HT3 activation in the NTS raises glutamate levels and activates second order neurons, how can activation of 5-HT3 receptors shorten the LCR?
The bradycardic effects of the Bezold-Jarish reflex and of activation of the carotid body can be inhibited by stimulation of 5-HT3 (Leal et al., 2001) and neurokinin 1 and GABAA receptors in the NTS (Netzer et al., 2009). Moreover, centrally administered 5-HT or CPG inhibit the bradycardia caused by activation of carotid chemoreceptors with potassium cyanide (KCN), an effect that can be blocked by both 5-HT3 antagonists and the GABAA antagonist bicuculline (Sevoz et al., 1997). This suggests that activation of 5-HT3 causes increased activity in second order GABAergic neurons within the NTS that then inhibit cardiovagal activity in the nucleus ambiguus. Baroreflex-initiated changes in blood pressure were similarly antagonized by microinjections of 5-HT or either of two 5-HT3 agonists (phenylbiguanide or 2-methyl-5-HT). These effects were also abolished when the GABAA antagonist bicuculline was injected into the NTS (Merahi et al., 1992).
It is our hypothesis that the intensity of the LCR varies as a function of the amount of glutamate released presynaptically onto second order neurons in the NTS, and that activation of these second order neurons then causes apnea by suspending the respiratory cycle in the post-inspiratory phase of respiration in what has been described as ‘post-inspiratory apneusis’ (Remmers et al., 1986). Since the post-inspiratory neurons are depolarized during this apneustic phase, we have assumed that the second order neurons in the NTS responsible for causing post-inspiratory apneustic apnea are excitatory and glutamatergic. However, the shortening of apnea and LCR durations by activation of presynaptic 5-HT3 receptors that increase glutamate release onto second order NTS neurons cannot explain the shortening LCR by the mechanism described above. On the other hand, GABAergic neurons are ubiquitous in the NTS, and many of these neurons are interneurons within the NTS – ideally suited, when activated, to inhibit the activity of other second order neurons within the NTS. Therefore, it is our hypothesis that C-fiber afferents are segregated into at least two classes: one population targets excitatory glutamatergic neurons and one population of C-fibers targets GABAergic neurons. Moreover, these excitatory and inhibitory populations of second order neurons likely express different subsets of presynaptic receptors. C-fiber afferents expressing presynaptic 5-HT3 receptors likely target GABAergic, inhibitory second order neurons, and C-fibers expressing presynaptic TRPV1 receptors likely target excitatory, glutamatergic second order neurons that project to ventral respiratory neurons controlling post-inspiratory activity of the respiratory cycle (Remmers et al., 1986; Czyzyk-Krzeska & Lawson, 1991), since activation of TRPV1 receptors prolongs the LCR (Xia et al., 2011). This is all speculative at this time and will require electrophysiological confirmation.
The Physiology of SIDS Risk Factors – an interaction between the LCR and 5-HT
Infants who died of SIDS may have abnormalities in the 5-HT and possibly other neurotransmitter systems in the brainstem (Kinney et al., 1995; Panigrahy et al., 1997; Panigrahy et al., 2000; Paterson et al., 2006). A number of animal experiments have found evidence that exposure to risk factors for SIDS has potentially detrimental effects on the brainstem 5-HT system. Piglets exposed postnatally to intermittent hypercapnic hypoxia (IHH) to mimic rebreathing showed reduced 5-HT1A receptor immunoreactivity in the NTS, hypoglossal nucleus, inferior olivary nucleus and dorsal motor nucleus of the vagus, and exposure to nicotine in the same study caused decreased 5-HT1A and 5-HT2A receptor immunoreactivity in the NTS (Say et al., 2007). Similar reductions in immunoreactivity of these receptors were seen in the NTS, dorsal nucleus of the vagus, and ventrolateral medulla in the brainstems of babies who died of SIDS, but not in those with congenital central hypoventilation syndrome (Ozawa & Okado, 2002), which suggests that the increased risk of SIDS associated with nicotine and IHH may result from serotonergic signaling deficiencies in these regions. Surprisingly, the distribution and density of 5-HT3 receptors has not been studied extensively in neonates or following hypoxic exposures or in infants who died of SIDS, though such information would be quite interesting in light of the effect of 5-HT3 receptor activation on LCR duration. Based on our study in neonatal rats, we would predict a marked reduction in 5-HT3 receptor density in babies who died of SIDS, and we would predict that any paucity of 5-HT3 receptors would be particularly marked in caudal and ventral regions of the NTS where laryngeal afferent fibers terminate on second order neurons in the NTS.
The activity of serotonergic neurons in the dorsal raphe is strongly correlated with wakefulness, diminishes with drowsiness and the onset of sleep, and all but disappears during REM sleep (Trulson & Jacobs, 1979). Pet1 knockout mice (Pet1−/−) have fewer serotonergic neurons than wild-type animals and have only 10–15% of normal 5-HT levels in key serotonergic projection sites (Hendricks et al., 2003). These animals exhibit delayed normalization of respiratory rhythm after birth, lower respiratory rates and more frequent long spontaneous apneas in P 4.5 pups and prolonged respiratory pauses upon termination of exposure to hypoxia (Erikson et al., 2007). When these mice were subjected to anoxic challenge at age P 4.5, they took four times longer to initiate gasping and took three times longer to successfully autoresuscitate than wild type controls (Erikson & Sposato, 2009). These results were confirmed in another study of Pet1−/− mice, but the age of particular susceptibility to anoxic apnea and the inability to autoresuscitate was slightly later (P 8) in this second study (Cummings et al., 2011a). In a similar study in rat pups, 5,7- dihydroxytryptamine, a toxin that kills serotonergic neurons, was injected into the cisterna magna to kill serotonergic cells in the brainstem. Rat pups treated in this way had a similarly reduced capacity to autoresuscitate following anoxia-induced apnea. The onset of gasping and the restoration of eupnea were both delayed in these animals (Cummings et al., 2011b).
The NTS receives serotonergic projections from the raphe nuclei in the brainstem, but also from neurons in the nodose ganglion, and possibly from serotonergic neurons within the NTS itself. The raphe pallidus and raphe obscurus send many projections to the NTS, and these projections enter the NTS near its rostral extent, but travel to projection sites caudally beyond the obex (Palkovits et al., 1986). We suspect that excitation of serotonergic neurons is part of an arousal process that terminates reflex apnea, fosters successful auto-resuscitation, augments eupneic respiration and causes a change in state to wakefulness to achieve stable and effective gas exchange and oxygenation (Leiter & Böhm, 2007), and we now believe that activation of 5-HT3 receptors in the NTS is part of the serotonergic arousal response that terminates the LCR and other reflex apneas.
The defects observed in 5-HT-deficient animals appear to be similar to problems seen in some babies who die of SIDS. Since mechanisms associated with arousal and restoration of regular breathing may be deficient in infants with reduced serotonergic signaling, these infants might be at enhanced risk for prolonged apneas and experience more profound hypoxia during apneas than babies with more normal levels of serotonergic signaling in the brainstem. Infants at risk for SIDS also experience unusually frequent and prolonged interruptions of cardiorespiratory activity (Meny et al., 1994) and are particularly prone to reflex apneas, such as the LCR (Menon et al., 1985; Thach, 2000). Furthermore, the LCR, which occurs in virtually all babies, may be unusually strong in infants at risk for SIDS, and profound apneas may be elicited by seemingly insignificant, normal pharyngeal and laryngeal stimuli (Page et al., 1996; Jeffery et al., 1999). Thus, infants at risk for SIDS may have a dual set of deficits: they may be unusually prone to reflex apnea due to non-serotonergic mechanisms that sensitize reflex apneic mechanisms (Xia et al., 2016); they may be particularly susceptible to hypoxic depression of breathing; and they may, due to deficient 5-HT and/or deficiencies of 5-HT receptor subtypes, have inadequate arousal responses capable of terminating apnea and restoring eupnea. The increased propensity for apneas, when combined with defects in arousal secondary to reduced serotonergic function, may make these infants susceptible to a downward spiral of apneic and hypoxic inhibition of eupnea from which they cannot arouse, ultimately ending in death. This sequence of events is compatible with the cardiorespiratory recordings made in infants who subsequently died of SIDS (Kelly et al., 1986).
Methodological limitations
The results of our study of the influence of 5-HT on the LCR must be evaluated in light of limitations of our study design. The primary caveats pertain to two aspects of the model. First, a neonatal rat cannot be said to be equivalent to a neonatal human in terms of brain development, anatomy or reflex development. Neonatal rats also have a much greater metabolic rate per weight than human infants and thus their respiratory drive must be correspondingly higher. Their sleep/wake, feeding, and other physiologic cycles also differ. Second, our animals were anesthetized in all experiments. We were compelled by both ethical and practical concerns to use anesthesia, especially since we were making microinjections directly into the brainstem. The urethane/chloralose doses that we used are more sparing of cardiorespiratory function and reflexes than most other anesthetics, and urethane has only modest effects on respiratory activity (Maggi & Meli, 1986). In addition, the anesthetics may have interfered with other airway clearing mechanisms, which would also tend to make the experimental apneas more severe. Nevertheless, reflex apneas in rats, pigs and dogs are remarkably similar to those in human infants (Kovar et al., 1979; Boggs & Bartlett, 1982; Lanier et al., 1983), and all of the respiratory and cardiovascular aspects of the LCR that are apparent in awake animals (van der Velde et al., 2003) are replicated in anesthetized animals. Thus, it seems likely that the reflex regulation of the LCR that we studied in anesthetized, neonatal rat pups accurately reflects the behavior of the LCR in sleeping animals.
Consideration must also be given to the limitations inherent to the drug delivery methods that we used. Drugs administered intracisternally can travel relatively long distances and thus have effects throughout the brainstem and into the spinal cord (Lee & Lim, 2010). Intracisternally-administered drugs likely take effect quickly in cells close to the dorsal brainstem and more slowly in ventral areas and areas deep inside the brainstem, as it probably takes some time for the drugs to diffuse to these locations (Chen et al., 2013b). Our focal drug administrations can only be considered in the region of the NTS. The fluorescent microspheres with which we mixed drugs for purposes of identifying our injection locations were typically found not in spherical volumes of tissue, but rather in an ellipsoidal volume shaped more like a rugby ball with the long axis of the football directed rostro-caudally. Moreover, the beads, while < 0.5 uM in diameter, probably did not diffuse as far as the drugs did. Thus, the shortening of the LCR after focal microinjection of 5-HT may have resulted from drug activity in locations outside, but adjacent to the NTS.
Summary
We tested the hypothesis that 5-HT, injected into the region of the NTS would shorten the LCR. This hypothesis was confirmed. We also determined that the serotonergic effect to shorten the LCR is likely mediated by 5-HT3 receptors, which are expressed presynaptically on C-fiber afferents within the NTS. Our finding that 5-HT shortens the LCR raises the possibility that infants at risk for SIDS have two deficits likely to promote and extend apneas. First, infants at risk for SIDS have a variety of risk factors that seem to increase the propensity for reflex apneas, especially the LCR (Leiter & Böhm, 2007; Xia et al., 2016). Second, infants at risk for SIDS seem to have deficient serotonergic function (Paterson et al., 2006). Increased serotonergic activity is associated with wakefulness and arousal, and to the extent that arousal mechanisms are deficient, apnea severity (both in terms of the degree of hypoxia and the duration of the apnea) is likely to be increased since based on the results of this study, 5-HT, acting through 5-HT3 receptors can shorten apnea and the LCR. Thus, infants at risk for SIDS may experience unusually frequent and severe apneas and, in addition, may have trouble terminating those apneic events and restoring and maintaining regular, eupneic breathing, especially during sleep when serotonergic activity is low, and they lack sufficient numbers of 5-HT3 receptors in the caudal NTS. Central to verifying this hypothesis will be an assessment of the density of 5-HT3 receptor expression in neonatal rat pups and in human infants who died of SIDS.
New Findings.
What is the central question of this study?
Failure to terminate apnea and arouse likely contributes to Sudden Infant Death Syndrome. Serotonin is deficient in the brainstems of babies who died of SIDS. Therefore, we tested the hypothesis that serotonin in the nucleus of the solitary tract (NTS) would shorten reflex apnea.
What is the main finding and its importance?
Serotonin microinjected into the NTS shortened the apnea and respiratory inhibition associated with the laryngeal chemoreflex. Moreover, this effect was achieved through a 5-HT3 receptor – a totally new insight that is likely relevant to the pathogenesis of SIDS.
Acknowledgments
Funding. This work was funded by grant 36379 from the NICHD.
Footnotes
Competing Interests: None.
Author Contributions
These experiemtns were conducted in laboratoies at the Geisel School of Medicine in Lebanon, NH, U.S.A. William T. Donnelly contributed to the conception and design of the work; the acquisition, analysis, and interpretation of data and contributed to the. drafting the work and revision of the manuscript for important intellectual content. Donald Bartlett, Jr. contributed to the conception and design of the work; the interpretation of data and contributed to the. drafting the work and revision of the manuscript for important intellectual content. J.C. Leiter contributed to the conception and design of the work; the analysis and interpretation of data and contributed to the. drafting the work and revision of the manuscript for important intellectual content.
All authors approved the final version of the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Moreover, all persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
References
- Anderson JW, Sant’Ambrosio FB, Mathew OP, Sant’Ambrogio G. Water-responsive laryngeal receptors in the dog are not specialized endings. Respir Physiol. 1990;79:33–43. doi: 10.1016/0034-5687(90)90058-7. [DOI] [PubMed] [Google Scholar]
- Andresen MC, Hofmann ME, Fawley JA. The unsilent majority-TRPV1 drives “spontaneous” transmission of unmyelinated primary afferents within cardiorespiratory NTS. Am J Physiol Regul Integr Comp Physiol. 2012;303:R1207–1216. doi: 10.1152/ajpregu.00398.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashworth-Preece MA, Jarrott B, Lawrence AJ. 5-Hydroxytryptamine3 receptor modulation of excitatory amino acid release in the rat nucleus tractus solitarius. Neurosci Lett. 1995;191:75–78. doi: 10.1016/0304-3940(95)11564-5. [DOI] [PubMed] [Google Scholar]
- Barnes JM, Barnes NM, Costall B, Deakin JF, Ironside JW, Kilpatrick GJ, Naylor RJ, Rudd JA, Simpson MD, Slater P, et al. Identification and distribution of 5-HT3 recognition sites within the human brainstem. Neurosci Lett. 1990;111:80–86. doi: 10.1016/0304-3940(90)90348-d. [DOI] [PubMed] [Google Scholar]
- Boggs DF, Bartlett D., Jr Chemical specificity of a laryngeal apneic reflex in puppies. J Appl Physiol. 1982;53:455–462. doi: 10.1152/jappl.1982.53.2.455. [DOI] [PubMed] [Google Scholar]
- Buchanan GF, Richerson GB. Central serotonin neurons are required for arousal to CO2. Proc Natl Acad Sci U S A. 2010;107:16354–16359. doi: 10.1073/pnas.1004587107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Magnusson J, Karsenty G, Cummings KJ. Time- and age-dependent effects of serotonin on gasping and autoresuscitation in neonatal mice. J Appl Physiol. 2013a;114:1668–1676. doi: 10.1152/japplphysiol.00003.2013. [DOI] [PubMed] [Google Scholar]
- Chen Y, Imai H, Ito A, Saito N. Novel modified method for injection into the cerebrospinal fluid via the cerebellomedullary cistern in mice. Acta Neurobiol Exp (Wars) 2013b;73:304–311. doi: 10.55782/ane-2013-1938. [DOI] [PubMed] [Google Scholar]
- Cummings KJ, Commons KG, Hewitt JC, Daubenspeck JA, Li A, Kinney HC, Nattie EE. Failed heart rate recovery at a critical age in 5-HT-deficient mice exposed to episodic anoxia: implications for SIDS. J Appl Physiol. 2011a;111:825–833. doi: 10.1152/japplphysiol.00336.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings KJ, Hewitt JC, Li A, Daubenspeck JA, Nattie EE. Postnatal loss of brainstem serotonin neurones compromises the ability of neonatal rats to survive episodic severe hypoxia. J Physiol. 2011b;589:5247–5256. doi: 10.1113/jphysiol.2011.214445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran AK, Xia L, Leiter JC, Bartlett D., Jr Elevated body temperature enhances the laryngeal chemoreflex in decerebrate piglets. J Appl Physiol. 2005;98:780–786. doi: 10.1152/japplphysiol.00906.2004. [DOI] [PubMed] [Google Scholar]
- Czyzyk-Krzeska MF, Lawson EE. Synaptic events in ventral respiratory neurones during apnoea induced by laryngeal nerve stimulation in neonatal piglet. J Physiol. 1991;436:131–147. doi: 10.1113/jphysiol.1991.sp018543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dergacheva O, Kamendi H, Wang X, Pinol RM, Frank J, Jameson H, Gorini C, Mendelowitz D. The role of 5-HT3 and other excitatory receptors in central cardiorespiratory responses to hypoxia: implications for sudden infant death syndrome. Pediatr Res. 2009;65:625–630. doi: 10.1203/PDR.0b013e3181a16e9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing SE, Lee JC. Laryngeal chemosensitivity: A possible mechanism of sudden infant death. Pediatrics. 1975;55:640–649. [PubMed] [Google Scholar]
- Duncan JR, Paterson DS, Hoffman JM, Mokler DJ, Borenstein NS, Belliveau RA, Krous HF, Haas EA, Stanley C, Nattie EE, Trachtenberg F, Kinney HC. Brainstem serotonergic deficiency in sudden infant death syndrome. JAMA. 2010;303:430–437. doi: 10.1001/jama.2010.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson JT, Shafer G, Rossetti MD, Wilson CG, Deneris ES. Arrest of 5HT neuron differentiation delays respiratory maturation and impairs neonatal homeostatic responses to environmental challenges. Respir Physiol Neurobiol. 2007;159:85–101. doi: 10.1016/j.resp.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson JT, Sposato BC. Autoresuscitation responses to hypoxia-induced apnea are delayed in newborn 5-HT-deficient Pet-1 homozygous mice. J Appl Physiol. 2009 doi: 10.1152/japplphysiol.90729.2008. (in press) [DOI] [PubMed] [Google Scholar]
- Fujii M, Umezawa K, Arata A. Dopaminergic modulation on respiratory rhythm in rat brainstem-spinal cord preparation. Neurosci Res. 2004;50:355–359. doi: 10.1016/j.neures.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Gustafson EL, Durkin MM, Bard JA, Zgombick J, Branchek TA. A receptor autoradiographic and in situ hybridization analysis of the distribution of the 5-ht7 receptor in rat brain. Br J Pharmacol. 1996;117:657–666. doi: 10.1111/j.1476-5381.1996.tb15241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding R, Johnson P, McClelland ME. Liquid-sensitive laryngeal receptors in the developing sheep, cat and monkey. J Physiol (London) 1978;277:409–422. doi: 10.1113/jphysiol.1978.sp012281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa T, Takanaga A, Maeda S, Seki M, Yajima Y. Subnuclear distribution of afferents from the oral, pharyngeal and laryngeal regions in the nucleus tractus solitarii of the rat: a study using transganglionic transport of cholera toxin. Neurosci Res. 2001;39:221–232. doi: 10.1016/s0168-0102(00)00218-2. [DOI] [PubMed] [Google Scholar]
- Hendricks TJ, Fyodorov DV, Wegman LJ, Leluth NB, Pehek EA, Yamamoto B, Silver J, Weeber EJ, Sweet JD, Deneris ES. Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron. 2003;37:233–247. doi: 10.1016/s0896-6273(02)01167-4. [DOI] [PubMed] [Google Scholar]
- Heym J, Steinfels GF, Jacobs BL. Activity of serotonin-containing neurons in the nucleus raphe pallidus of freely moving cats. Brain Res. 1982;251:259–276. doi: 10.1016/0006-8993(82)90743-0. [DOI] [PubMed] [Google Scholar]
- Hyde TM, Knable MB, Murray AM. Distribution of dopamine D1–D4 receptor subtypes in human dorsal vagal complex. Synapse. 1996;24:224–232. doi: 10.1002/(SICI)1098-2396(199611)24:3<224::AID-SYN4>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Fornal CA. Activity of serotonergic neurons in behaving animals. Neuropsychopharnacology. 1999;21:9S–15S. doi: 10.1016/S0893-133X(99)00012-3. [DOI] [PubMed] [Google Scholar]
- Jeffery HE, Megevand A, Page M. Why the prone position is a risk factor for sudden infant death syndrome. Pediatrics. 1999;104:263–269. doi: 10.1542/peds.104.2.263. [DOI] [PubMed] [Google Scholar]
- Jeffery HE, Page M, Post EJ, Wood AK. Physiological studies of gastro-oesophageal reflux and airway protective responses in the young animal and human infant. Clin Exp Pharmacol Physiol. 1995;22:544–549. doi: 10.1111/j.1440-1681.1995.tb02064.x. [DOI] [PubMed] [Google Scholar]
- Jeggo RD, Kellett DO, Wang Y, Ramage AG, Jordan D. The role of central 5-HT3 receptors in vagal reflex inputs to neurones in the nucleus tractus solitarius of anaesthetized rats. J Physiol. 2005;566:939–953. doi: 10.1113/jphysiol.2005.085845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y-H, Bailey TW, Li B, Schild JH, Andresen MC. Puringergic and vanilloid receptor activation releases glutamate from separate cranial afferent terminals in nucleus tractus solitarius. J Neurosci. 2004;24:4709–4717. doi: 10.1523/JNEUROSCI.0753-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DH, Golub H, Carley D, Shannon DC. Pneumograms in infants who subsequently died of sudden infant death syndrome. J Pediatr. 1986;109:249–254. doi: 10.1016/s0022-3476(86)80380-8. [DOI] [PubMed] [Google Scholar]
- Kinney HC, Filiano JJ, Sleeper LA, Mandell F, Valdes-Dapena M, White WF. Decreased muscarinic receptor binding in the arcuate nucleus in Sudden Infant Death Syndrome. Science. 1995;269:1446–1450. doi: 10.1126/science.7660131. [DOI] [PubMed] [Google Scholar]
- Kinney HC, Randall LL, Sleeper LA, Willinger M, Belliveau RA, Zec N, Rava LA, Dominici L, Iyasu S, Randall B, Habbe D, Wilson H, Mandell F, McClain M, Welty TK. Serotonergic brainstem abnormalities in Northern Plains Indians with the Sudden Infant Death Syndrome. J Neuropath Exp Neurol. 2003;62:1178–1191. doi: 10.1093/jnen/62.11.1178. [DOI] [PubMed] [Google Scholar]
- Kovar I, Selstam U, Catterton WZ, Stahlman MT, Sundell HW. Laryngeal chemoreflex in newborn lambs: Respiratory and swallowing response to salts, acids, and sugars. Pediatr Res. 1979;13:1144–1149. doi: 10.1203/00006450-197910000-00013. [DOI] [PubMed] [Google Scholar]
- Lanier B, Richardson MA, Cummings C. Effect of hypoxia on laryngeal reflex apnea - implications for sudden infant death. Otolaryngol Head Neck Surg. 1983;91:597. doi: 10.1177/019459988309100602. [DOI] [PubMed] [Google Scholar]
- Lauder JM. Neurotransmitters as growth regulatory signals: role of receptors and second messengers. Trends Neurosci. 1993;16:233–240. doi: 10.1016/0166-2236(93)90162-f. [DOI] [PubMed] [Google Scholar]
- Leal DM, Callera JC, Bonagamba LG, Nosjean A, Laguzzi R, Machado BH. Microinjection of a 5-HT3 receptor agonist into the NTS of awake rats inhibits the bradycardic response to activation of the von Bezold-Jarisch reflex. Brain Res Bull. 2001;54:7–11. doi: 10.1016/s0361-9230(00)00408-1. [DOI] [PubMed] [Google Scholar]
- Lee IO, Lim ES. Intracisternal or intrathecal glycine, taurine, or muscimol inhibit bicuculline-induced allodynia and thermal hyperalgesia in mice. Acta Pharmacol Sin. 2010;31:907–914. doi: 10.1038/aps.2010.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiter JC. Serotonin, gasping, autoresuscitation and SIDS - a contrarian view. J Appl Physiol. 2009:106. doi: 10.1152/japplphysiol.00329.2009. [DOI] [PubMed] [Google Scholar]
- Leiter JC, Böhm I. Mechanisms of pathogenesis in the Sudden Infant Death Syndrome (SIDS) Respir Physiol Neurobiol. 2007;159:127–138. doi: 10.1016/j.resp.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MTT. Postnatal changes in the expressions of serotonin 1A, 1B, and 2A receptors in ten brain stem nuclei of the rat: Implication for a sensitive period. Neurosci. 2010;165:61–78. doi: 10.1016/j.neuroscience.2009.09.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machaalani R, Say M, Waters KA. Serotonergic receptor 1A in the sudden infant death syndrome brainstem medulla and associatens with clinical risk factors. Acta Neuropathol. 2009;117:257–265. doi: 10.1007/s00401-008-0468-x. [DOI] [PubMed] [Google Scholar]
- Maggi CA, Meli A. Suitability of urethane anesthesia for physiopharmacological investigations in various systems. Part 2: Cardiovascular system. Experientia. 1986;42:292–297. doi: 10.1007/BF01942510. [DOI] [PubMed] [Google Scholar]
- Menon AP, Schefft GL, Thach BT. Apnea associated with regurgitation in infants. J Pediatr. 1985;106:625–629. doi: 10.1016/s0022-3476(85)80091-3. [DOI] [PubMed] [Google Scholar]
- Meny RG, Carroll JL, Carbone MT, Kelly DH. Cardiorespiratory recordings from infants dying suddenly and unexpectedly at home. Pediatrics. 1994;93:44–49. [PubMed] [Google Scholar]
- Merahi N, Orer HS, Laporte AM, Gozlan H, Hamon M, Laguzzi R. Baroreceptor reflex inhibition induced by the stimulation of serotonin3 receptors in the nucleus tractus solitarius of the rat. Neuroscience. 1992;46:91–100. doi: 10.1016/0306-4522(92)90011-p. [DOI] [PubMed] [Google Scholar]
- Millhorn DE, Eldridge FL, Waldrop TG. Prolonged stimulation of respiration by endogenous central serotonin. Respir Physiol. 1980;42:171–188. doi: 10.1016/0034-5687(80)90113-9. [DOI] [PubMed] [Google Scholar]
- Mutoh T, Kanamaru A, Kojima K, Nishimura R, Sasaki N, Tsubone H. Effects of perineural capsaicin treatment on cardiopulmonary reflexes elicited by laryngeal instillations of capsaicin and distilled water in sevoflurane-anesthetized dogs. J Vet Med Sci. 2000;62:665–668. doi: 10.1292/jvms.62.665. [DOI] [PubMed] [Google Scholar]
- Netzer F, Mandjee N, Verberne AJ, Bernard JF, Hamon M, Laguzzi R, Sevoz-Couche C. Inhibition of the bradycardic component of the von Bezold-Jarisch reflex and carotid chemoreceptor reflex by periaqueductal gray stimulation: involvement of medullary receptors. Eur J Neurosci. 2009;29:2017–2028. doi: 10.1111/j.1460-9568.2009.06758.x. [DOI] [PubMed] [Google Scholar]
- Ozawa Y, Okado N. Alteration of serotonergic receptors in the brain stems of human patients with respiratory disorders. Neuropediatrics. 2002;33:142–149. doi: 10.1055/s-2002-33678. [DOI] [PubMed] [Google Scholar]
- Page M, Jeffery HE, Post EJ, Wood AKW. Simulated pharyngeal reflux can lead to life-threatening apnea if swallowing and arousal are depressed. J Sudden Infant Death Syndrome and Infant Mortality. 1996;1:281–294. [Google Scholar]
- Palkovits M, Mezey E, Eskay RL, Brownstein MJ. Innervation of the nucleus of the solitary tract and the dorsal vagal nucleus by thyrotropin-releasing hormone-containing raphe neurons. Brain Res. 1986;373:246–251. doi: 10.1016/0006-8993(86)90338-0. [DOI] [PubMed] [Google Scholar]
- Panigrahy A, Filiano JJ, Sleeper LA, Mandell F, Valdes-Dapena M, Krous HF, Rava LA, Foley E, White WF, Kinney HC. Decreased serotonergic receptor binding in rhombic lip-derived regions of the medulla oblongata in the sudden infant death syndrome. J Neuropathol Exp Neurol. 2000;59:377–384. doi: 10.1093/jnen/59.5.377. [DOI] [PubMed] [Google Scholar]
- Panigrahy A, Filiano JJ, Sleeper LA, Mandell F, Valdes-Dapena M, Krous HF, Rava LA, White WF, Kinney HC. Decreased kainate receptor binding in the arcuate nucleus of the Sudden Infant Death Syndrome. J Neuropathol Exp Neurol. 1997;56(11):1253–1261. doi: 10.1097/00005072-199711000-00010. [DOI] [PubMed] [Google Scholar]
- Paterson DS, Trachtenberg FL, Thompson EG, Belliveau RA, Beggs AH, Darnall RA, Chadwick AE, Krous HF, Kinney HC. Multiple serotonergic brainstem abnormalities in the sudden infant death syndrome. JAMA. 2006;296:2124–2132. doi: 10.1001/jama.296.17.2124. [DOI] [PubMed] [Google Scholar]
- Patrickson JW, Smith TE, Zhou S-S. Afferent projections of the superior and recurrent laryngeal nerves. Brain Res. 1991;539:169–174. doi: 10.1016/0006-8993(91)90702-w. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego, CA: 1998. [Google Scholar]
- Pratt GD, Bowery NG. The 5-HT3 receptor ligand, [3H]BRL 43694, binds to presynaptic sites in the nucleus tractus solitarius of the rat. Neuropharmacology. 1989;28:1367–1376. doi: 10.1016/0028-3908(89)90012-9. [DOI] [PubMed] [Google Scholar]
- Pratt GD, Bowery NG, Kilpatrick GJ, Leslie RA, Barnes NM, Naylor RJ, Jones BJ, Nelson DR, Palacids JM, Slater P, et al. Consensus meeting agrees distribution of 5-HT3 receptors in mammalian hindbrain. Trends Pharmacol Sci. 1990;11:135–137. doi: 10.1016/0165-6147(90)90058-g. [DOI] [PubMed] [Google Scholar]
- Raul L. Serotonin2 receptors in the nucleus tractus solitarius: characterization and role in the baroreceptor reflex arc. Cell Mol Neurobiol. 2003;23:709–726. doi: 10.1023/A:1025096718559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remmers JE, Richter DW, Ballantyne D, Bainton CR, Klein JP. Reflex prolongation of stage I of expiration. Pflügers Arch. 1986;407:190–198. doi: 10.1007/BF00580675. [DOI] [PubMed] [Google Scholar]
- Roulier S, Arsenault J, Reix P, Dorion D, Praud JP. Effects of C fiber blockade on cardiorespiratory responses to laryngeal stimulation in concious lambs. Respir Physiol Neurobiol. 2003;136:13–23. doi: 10.1016/s1569-9048(03)00108-3. [DOI] [PubMed] [Google Scholar]
- Saetta M, Mortola JP. Interaction of hypoxic and hypercapnic stimuli on breathing pattern in the newborn rat. J Appl Physiol (1985) 1987;62:506–512. doi: 10.1152/jappl.1987.62.2.506. [DOI] [PubMed] [Google Scholar]
- Say M, Machaalani R, Waters KA. Changes in serotonergic receptors 1A and 2A in the piglet brainstem after intermittent hypercapnic hypoxia (IHH) and nicotine. Brain Res. 2007;1152:17–26. doi: 10.1016/j.brainres.2007.03.037. [DOI] [PubMed] [Google Scholar]
- Sevoz C, Callera JC, Machado BH, Hamon M, Laguzzi R. Role of serotonin3 receptors in the nucleus tractus solitarii on the carotid chemoreflex. Am J Physiol. 1997;272:H1250–H1259. doi: 10.1152/ajpheart.1997.272.3.H1250. [DOI] [PubMed] [Google Scholar]
- Thach BT. Sudden infant death syndrome: Can gastroesophageal reflux cause sudden infant death? Am J Med. 2000;108:144S–148S. doi: 10.1016/s0002-9343(99)00354-x. [DOI] [PubMed] [Google Scholar]
- Thach BT. Maturation and transformation of reflexes that protect the laryngeal airway from liquid aspiration from fetal to adult life. Am J Med. 2001;111:69S–77S. doi: 10.1016/s0002-9343(01)00860-9. [DOI] [PubMed] [Google Scholar]
- Trulson ME, Jacobs BL. Raphe unit activity in freely moving cats: correlation with level of behavioral arousal. Brain Res. 1979;163:135–150. doi: 10.1016/0006-8993(79)90157-4. [DOI] [PubMed] [Google Scholar]
- van der Velde L, Curran A, Filiano JJ, Darnall RA, Bartlett D, Jr, Leiter JC. Prolongation of the laryngeal chemoreflex after inhibition of the rostroventral medulla in piglets: A role in SIDS? J Appl Physiol. 2003;94:1883–1895. doi: 10.1152/japplphysiol.01103.2002. [DOI] [PubMed] [Google Scholar]
- Varnas K, Halldin C, Hall H. Autoradiographic distribution of serotonin transporters and receptor subtypes in human brain. Hum Brain Mapp. 2004;22:246–260. doi: 10.1002/hbm.20035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veasey SC, Fornal CA, Metzler CW, Jacobs BL. Response of serotonergic caudal raphe neurons in relation to specific motor activities in freely moving cats. J Neurosci. 1995;15:5346–5359. doi: 10.1523/JNEUROSCI.15-07-05346.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waeber C, Hoyer D, Palacios JM. 5-hydroxytryptamine3 receptors in the human brain: autoradiographic visualization using [3H]ICS 205–930. Neuroscience. 1989;31:393–400. doi: 10.1016/0306-4522(89)90382-5. [DOI] [PubMed] [Google Scholar]
- Xia L, Bartlett D, Jr, Leiter JC. TRPV1 channels in the nucleus of the solitary tract mediate thermal prolongation of the LCR in decerebrate piglets. Respir Physiol Neurobiol. 2011;176:21–31. doi: 10.1016/j.resp.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia L, Bartlett D, Jr, Leiter JC. Interleukin-1beta and interleukin-6 enhance thermal prolongation fo the LCR in decerebrate piglets. Resp Physiol Neurobiol. 2016 doi: 10.1016/j.resp.2016.05.006. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia L, Damon TA, Leiter JC, Bartlett D., Jr Focal warming of the nucleus of the solitary tract prolongs the laryngeal chemoreflex in decerebrate piglets. J Appl Physiol. 2007;102:54–62. doi: 10.1152/japplphysiol.00720.2006. [DOI] [PubMed] [Google Scholar]
- Xia L, Leiter JC, Bartlett D., Jr Laryngeal apnea in rat pups: effects of age and body temperature. J Appl Physiol. 2008;104:269–274. doi: 10.1152/japplphysiol.00721.2007. [DOI] [PubMed] [Google Scholar]
- Xia L, Leiter JC, Bartlett D., Jr Gestational nicotine exposure exaggerates hyperthermic enhancement of laryngeal chemoreflex in rat pups. Respir Physiol Neurobiol. 2010;171:17–21. doi: 10.1016/j.resp.2010.01.011. [DOI] [PubMed] [Google Scholar]