Abstract
Objectives
In many settings, human papillomavirus (HPV) DNA testing already plays an important role in cervical cancer screening. It is unclear whether hormonal fluctuations associated with menstrual phase or oral contraceptive (OC) use have any effect on HPV detection. We evaluated the effects of OC use and timing of cervical sampling in relation to women’s last menstrual period (LMP) on HPV detection, and viral load in the Brazilian Ludwig-McGill cohort study.
Methods
Women in the cohort were followed every 4–6 months, and at each clinic visit they were asked to complete a questionnaire and to provide a cervical sample for HPV testing. Specimens from 6093 patient visits (n = 2209 women) were categorised according to date of LMP into four distinct phases: follicular (days 5–9), midcycle (days 10–15), luteal (days 16–22), or late luteal (days 23–31).
Results
Compared with follicular phase (referent group), HPV detection did not differ according to reported LMP for midcycle (OR = 1.14, 95% CI 0.95 to 1.37), luteal (OR = 1.03, 95% CI 0.85 to 1.25), or late luteal menstrual phase (OR=1.01, 95% CI 0.83 to 1.24), and was also not influenced by OC use. Analyses restricted to high-risk HPV types (grouped) and HPVs 16 and 18 (separately), produced similar non-significant associations. For HPV-positive samples, we found that the menstrual phase did not influence the total viral load.
Conclusions
These results indicate HPV detection is not associated with menstrual phase. Our findings suggest that standardising the timing of specimen collection for HPV testing is not necessary.
INTRODUCTION
There is now considerable interest in the use of human papillomavirus (HPV) DNA testing for cervical cancer screening. However, there are still uncertainties regarding this test’s accuracy and reliability that need to be addressed to inform evidence-based guidelines. It is not clear whether the phase of a woman’s menstrual period at the time of cervical sampling has an effect on HPV detection, as previous studies have provided conflicting results.1–6 A recent study exploring the effect of oral contraceptive (OC) use on HPV detection revealed a higher detection rate during the follicular phase among non-users, whereas OC users experienced a higher detection rate during the luteal phase.7 Using data collected in the Ludwig-McGill cohort study conducted in Brazil, we evaluated the effect of menstrual phase on HPV detection, and attempted to validate previous findings concerning OC use.
METHODS
Subject selection
Recruitment and follow-up for the Ludwig-McGill cohort study took place between 1993 and 2005 in a population of low-income women in São Paulo, Brazil. Eligible women were: between 18 and 60 years of age, had an intact uterus, not pregnant or planning to become pregnant in the next 12 months, and had not been treated for cervical disease in the last 6 months prior to enrolment.8 Study methods have been described in detail elsewhere.8 The study was approved by review boards and ethical committees of the participating institutions in Brazil and Canada. Informed consent was obtained from all participants prior to enrolment.
Clinical procedures and HPV testing
Participants presented for clinic visits every 4 months (0, 4, 8 and 12 months) during the first year of follow-up, and twice annually in subsequent years. At each visit, subjects were asked to complete a questionnaire regarding risk factors for HPV and cervical cancer, and to provide a cervical sample for Pap cytology and HPV testing. Although subjects were followed for a total of 5 years, information on menstrual phase was only collected during the first year of follow-up. During this first year (visits 1–4), approximately 10% of subjects received an abnormal Pap screening result (ASCUS=4.3%; LSIL=4.1%; HSIL=1.3%).9
An Accelon biosampler (Medscand Inc, Hollywood, Florida, USA) was used to collect a sample of ectocervical and endocervical cells for DNA extraction. Presence of HPV DNA was determined using a PCR assay employing L1 PGMY consensus primers. Typing of the amplified products was performed by hybridisation with individual oligonucleotide probes, and by restriction fragment-length polymorphism analysis to identify 40 different mucosal HPV types. To measure viral load, all HPV-positive specimens were retested using a quantitative low-stringency PCR protocol that detects a broad spectrum of HPVs.10
Statistical analyses
In total, 8504 samples were available from 2458 women during their first year of follow-up. We excluded visits from women with invalid HPV DNA typing information (n=11), or incomplete information on the date of their last menstrual period (LMP) (n=25). In our primary analysis, we excluded visits made by women who were currently menstruating (days 0–4), and by those who had not recently experienced menstruation (postpartum or postmenopausal women). To accomplish that, we excluded visits where women reported their LMP as occurring between <5 (n=907) or >31 days (n=1468) prior to the visit. After applying all these exclusion criteria, information was available for 6093 patient visits from 2209 women with at least one eligible visit. The median duration between clinic visits for these women was 124 days.
To investigate the effect of the phase of menstrual period on HPV positivity and HPV viral load, we stratified the timing of cervical sample collection with reference to the date of LMP into four categories: (1) days 5–9 (follicular phase); (2) days 10–15 (midcycle); (3) days 16–22 (luteal phase) and (4) days 23–31 (late luteal phase). These phases coincide with low serum hormone levels (oestrogen and progesterone), peak serum oestrogen levels and ovulation, peak progesterone levels and reduced serum hormones (both oestrogen and progesterone), respectively. In our primary analyses, the first category (follicular phase) served as the referent group. We included reported LMP information for up to 31 days (rather than 28) to account for those women with late cycles. To investigate whether OC use modifies the effect of menstrual phase on HPV detection, we tested for interaction between these variables and also performed separate stratified analyses according to OC use (never, vs current user). Finally, in an attempt to validate the findings of Schmeink et al7 concerning hormonal contraceptive use, we performed additional sensitivity analyses restricted to women <30 years of age, stratified by OC use (ie, never, vs current user) and using similar exposure categories.
Logistic regression analysis, implementing generalised estimating equations (GEE) was used to estimate ORs and associated 95% CI for the effect of menstrual phase on HPV detection (positivity for any type). GEE was used to account for unknown correlation between outcomes of visits contributed by the same woman. We also present results for separate analyses where the outcome was the detection of single HPV infections (ie, only one HPV type present in specimen), multiple infections (ie, ≥2 HPV types present in specimen), oncogenic HPV types (International Agency for Research on Cancer classification, 2009),11 and HPVs 16 and 18 (separately). We also evaluated the effect of menstrual phase on total viral load on a logarithmic scale (limited to HPV-positive specimens only).
The possibility of confounding by other covariates (eg, age, smoking, parity, OC use and number of sexual partners) was evaluated by including these variables in the model and testing for any significant change in the crude estimates. Except for age, the inclusion of other covariates in the model did not have an important effect on parameter estimates, and so they were left out of the final model.
RESULTS
Accounting for correlation between visits, we did not find appreciable variation in the frequency of HPV-positive results between the four menstrual cycle phases. Among all specimens collected during the follicular, midcycle, luteal and late luteal phases: 15.8% (248/1567), 17.7% (309/1745), 16.3% (256/1570) and 16.5% (200/1211), respectively, tested positive for HPV. Compared with follicular phase (referent group), HPV detection did not differ according to reported LMP for midcycle phase (OR=1.14, 95% CI 0.95 to 1.37), luteal phase (OR=1.03, 95% CI 0.85 to 1.25), or late luteal menstrual phase (OR=1.01, 95% CI 0.83 to 1.24). Similarly, no important differences were observed in the detection of oncogenic HPV types, HPV 16 or HPV 18 (table 1). In comparing the percentage of cervical specimens that tested positive for HPV (any type) according to the number of days since the subject’s LMP (days 5 through 31; figure 1), we observed only minor random variation in HPV detection across the cycle. Stratification by OC use did not result in a statistically significant difference in HPV detection between phases (table 2). In our logistic regression models (fitted using GEE), we also tested whether age or OC use acts as an effect modifier of the relation between menstrual phase and HPV detection. As expected, no interactions were observed between these variables and menstrual phase, that is, estimates remained largely unchanged, and p values for interaction terms were >0.05 (results not shown).
Table 1.
ORs for HPV test positivity according to menstrual phase for cumulative patient visits*
| HPV Type | Positive/total samples, n (all phases) | Age-adjusted OR (95% CI)
|
|||
|---|---|---|---|---|---|
| 5–9 days n=1567 (n positive, %) | 10–15 days n=1745 (n positive, %) | 16–22 days n=1570 (n positive, %) | 23–31 days n=1211 (n positive, %) | ||
| Any types | 1013/6093 | 1.0 (ref) (248, 15.8%) | 1.14 (0.95–1.37) (309, 17.7%) | 1.03 (0.85–1.25) (256, 16.3%) | 1.01 (0.83–1.24) (200, 16.5%) |
| Single infection | 224/6093 | 1.0 (ref) (56, 3.6%) | 1.16 (0.81–1.66) (71, 4.1%) | 0.93 (0.63–1.36) (52, 3.3%) | 1.03 (0.69–1.54) (45, 3.7%) |
| Multiple infection | 789/6093 | 1.0 (ref) (192, 12.2%) | 1.14 (0.93–1.40) (238, 13.6%) | 1.07 (0.86–1.32) (204, 13.0%) | 1.00 (0.80–1.26) (155, 12.8%) |
| High-risk types† | 609/6093 | 1.0 (ref) (150, 9.6%) | 1.05 (0.83–1.32) (175, 10.0%) | 1.05 (0.83–1.33) (158, 10.1%) | 1.05 (0.81–1.34) (126, 10.4%) |
| HPV 16 only | 166/6093 | 1.0 (ref) (43, 2.7%) | 0.91 (0.60–1.40) (44, 2.5%) | 1.07 (0.70–1.62) (46, 2.9%) | 0.96 (0.61–1.52) (33, 2.7%) |
| HPV 18 only | 53/6093 | 1.0 (ref) (10, 0.6%) | 1.62 (0.74–3.52) (18, 1.0%) | 1.49 (0.67–3.34) (15, 1.0%) | 1.24 (0.51–3.00) (10, 0.8%) |
All p values were non-significant at 0.05 level of testing.
ORs calculated with logistic regression using generalised estimating equations implementation.
Based on International Agency for Research on Cancer classification 2009.
Figure 1.
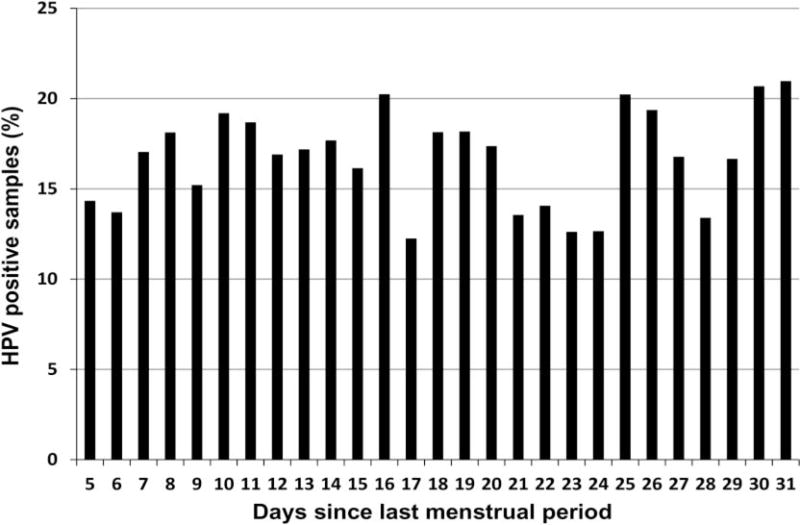
Percentage of cervical samples positive for human papillomavirus DNA according to menstrual phase (number of days since last menstrual period) at time of clinic visit.
Table 2.
ORs for human papillomavirus detection (any type) according to menstrual phase and oral contraceptive pill use for cumulative patient visits*
| OC Use | Positive/total samples, n (all phases) | Age-adjusted OR (95% CI)
|
|||
|---|---|---|---|---|---|
| 5–9 days (46/315, 14.6%) | 10–15 days (65/344, 18.9%) | 16–22 days (63/328, 19.2%) | 23–31 days (46/264, 17.4%) | ||
| Never user | 148/863 | 1.0 (ref) (31/210, 14.8%) | 1.19 (0.71–2.01) (39/236, 16.5%) | 1.40 (0.85–2.31) (47/239, 19.7%) | 1.07 (0.62–1.86) (31/183, 16.9%) |
| Current user | 72/383 | 1.0 (ref) (15/105, 14.3%) | 1.86 (0.93–3.77) (26/108, 24.0%) | 1.32 (0.61–2.85) (16/89, 18.0%) | 1.32 (0.60–2.91) (15/81, 18.5%) |
| <6 years | 51/236 | 1.0 (ref) (10/61, 16.4%) | 1.82 (0.76–4.39) (17/64, 26.6%) | 1.48 (0.58–3.78) (12/54, 22.2%) | 1.42 (0.56–3.60) (12/57, 21.0%) |
| ≥6 years | 21/147 | 1.0 (ref) (5/44, 11.4%) | 2.03 (0.61–6.70) (9/44, 20.4%) | 1.00 (0.25–4.04) (4/35, 11.4%) | 1.11 (0.24–5.17) (3/24, 12.5%) |
All p values were non-significant at 0.05 level of testing.
ORs calculated with logistic regression using generalised estimating equations implementation.
OC, oral contraceptive.
The geometric mean viral load among HPV-positive samples was 4.26 viral copies/cell (median=0.50, interquartile range=0.25–27). Using follicular phase as the referent group, viral load detection (dichotomised at the upper tertile) did not differ according to reported menstrual phase for midcycle (OR=1.17, 95% CI 0.91 to 1.51), luteal (OR=1.11, 95% CI 0.85 to 1.44), or late luteal phase (OR=0.97, 95% CI 0.72 to 1.29). As shown in figure 2, there was some minor variation between viral load detection and number of days since LMP, particularly for days 29–31.
Figure 2.
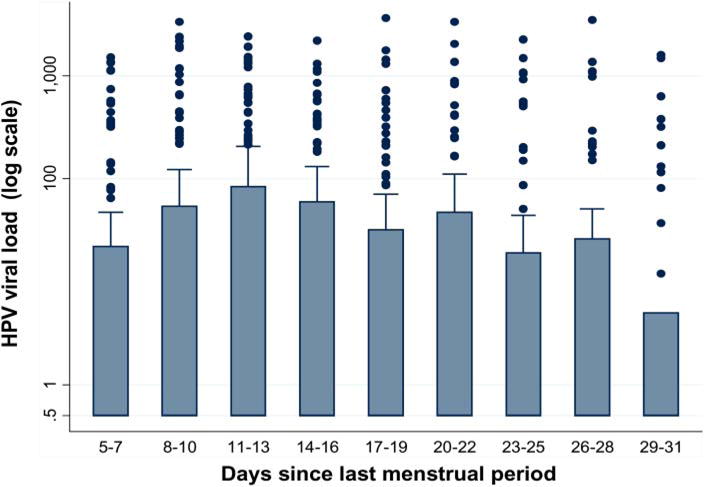
Human papillomavirus load (number of copies per cell among HPV-positive samples) according to menstrual phase (number of days since last menstrual period) at time of clinic visit, measured using low-stringency PCR. This figure is only reproduced in colour in the online version.
DISCUSSION
Our findings suggest that levels of HPV positivity do not vary during the menstrual cycle. Previous studies that examined this topic have produced conflicting results. Four studies,1–4 including a study based on PCR testing of tampon specimens,1 did not find any difference. Other studies based on repeated testing have found higher HPV detection during the follicular5 and luteal phases6 of the menstrual cycle. Only one other study evaluated HPV viral load, and investigators found a modest increase during midcycle.4 It is unclear why viral load detection would be higher for specimens collected during this time, but it has been suggested that peak oestrogen levels at midcycle could promote this effect by enhancing HPV viral replication, or by reducing cellular adhesion.4 In this study, we found no indication that viral load varies with menstrual phase. It is unlikely that the decrease in viral load for days 29–31 was due to any true biological effect. This category had fewer data in relation to the others and, therefore, variation found here may simply be a reflection of sparse data. We were also unable to confirm previous findings suggesting that OC use acts as an effect-modifier of the relation between menstrual phase and HPV detection. Additional analyses designed to directly compare our results with Schmeink and colleagues,7 (ie, restricted to women <30 years of age and with similar menstrual phase grouping) also did not reveal any statistically significant differences in HPV detection according to OC use.
Major strengths of our study were its size and longitudinal design; however, we recognise that there are some limitations. We had to depend on patient recall for information on the date of LMP, and so there was likely some misclassification of this variable, which could have biased our results towards the null. However, when we restricted our analysis to only women who completed high school, or who reported a regular monthly period, there was no meaningful change in our results.
In this study, subjects were asked not to attend the clinic for specimen collection during days of active menstruation. This was based on evidence that cervical samples collected on these days normally lead to poor quality smears12 that are more likely to result in false negative diagnoses.13,14 In our primary analysis, we therefore excluded samples collected from subjects on these days (ie, reported LMP between 0 and 4 days). When we later included these samples as part of our sensitivity analyses, we found it led to almost no change in our results, and did not affect our overall interpretation.
Women in this study with normal Pap cytology results had lower HPV viral load in comparison with those with abnormal cytology. Because these women may be more susceptible to epithelial fluctuations during the menstrual cycle which could affect HPV detection, we also performed analyses restricted to these cytologically normal women. Again, we observed no important change in our results. Since we did not find much variability in HPV viral load during the menstrual cycle, we did not expect there to be much variation in detection of cytologic abnormalities. Nevertheless, for a complete assessment of this issue, we also compared cytology results (stratified by lesion grade) according to reported LMP As expected, we found no significant difference in lesion grade frequency according to menstrual phase.
In conclusion, our findings are consistent with the bulk of the literature in suggesting that menstrual phase does not have an effect on HPV detection It is expected that HPV testing will eventually become the main primary screening tool in prevention of cervical cancer, and concerns about the accuracy of HPV testing during different phases of the menstrual cycles may compel clinicians to consider standardising the timing of specimen collection. Our findings suggest that this will not be necessary.
Key messages.
-
▸
HPV DNA testing now plays an important role in cervical cancer screening.
-
▸
It is still not clear whether menstrual phase affects the accuracy of this test, or if oral contraceptive use somehow modifies this effect.
-
▸
Results from this study strongly suggest that cervical HPV detection is not affected by menstrual phase.
Acknowledgments
Ludwig—McGill cohort study team members: affiliated with the Ludwig Institute for Cancer Research in Sao Paulo, Brazil: Maria Luiza Baggio, Lenice Galan, João Simão Sobrinho, José Carlos Mann Prado, Silvaneide Ferreira, Lara Termini, Maria Cecília Costa, Romulo Miyamura, Andrea Trevisan, Patricia Thomann, João Candeias, Laura Sichero, Paula Rahal, Antonio Ruiz, Jane Kaiano, Monica Santos, Patricia Savio, Paulo Maciag, Tatiana Rabachini, Luisa Villa (Co-Principal Investigator). Affiliated with McGill University in Montreal, Canada: Marie-Claude Rousseau, Salaheddin Mahmud, Nicolas Schlecht, Helen Trottier, Alex Ferenczy, Thomas Rohan, Myriam Chevarie-Davis, Joseph Tota, Agnihotram Ramanakumar, Eliane Duarte, Sophie Kulaga, Juliette Robitaille, Robert Platt, Eduardo Franco (Principal Investigator).
Funding Financial support was provided by the Ludwig Institute for Cancer Research (intramural grant to LLV and ELF), the US National Cancer Institute (grant CA70269 to ELF), and the Canadian Institutes of Health Research (operating grant 49 396 and team grant 83 320 to ELF).
Footnotes
Contributors ELF and LLV designed the parent cohort study. ELF conceived the research question. JET conducted the analysis and drafted the manuscript. AVR and SMM were involved with data management and the analysis. AT evaluated cervical specimens and generated viral load information. All authors contributed to interpretation of the data and were responsible for critical revisions to the paper. All authors read and approved the final manuscript.
Competing interests ELF has served as occasional consultant or advisory board member to companies that produce HPV vaccines (Merck and GSK) or cervical screening diagnostic assays (Roche, Qiagen, Gen-Probe, BD). Through his institution he has also received funding from Merck for investigator initiated research. SMM is affiliated with an organisation that received unrestricted grant funding from GSK. LLV is consultant of Merck, Sharp & Dohme for the Quadrivalent HPV vaccine, Roche and Qiagen for HPV DNA testing assays.
Ethics approval McGill University (MU); Ludwig Institute for Cancer Research (LICR).
Provenance and peer review Not commissioned; externally peer reviewed.
References
- 1.Fairley CK, Robinson PM, Chen S, et al. The detection of HPV DNA, the size of tampon specimens and the menstrual cycle. Genitourin Med. 1994;70:171–4. doi: 10.1136/sti.70.3.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wheeler CM, Greer CE, Becker TM, et al. Short-term fluctuations in the detection of cervical human papillomavirus DNA. Obstet Gynecol. 1996;88:261–8. doi: 10.1016/0029-7844(96)00120-2. [DOI] [PubMed] [Google Scholar]
- 3.Harper DM, Longacre MR, Noll WW, et al. Factors affecting the detection rate of human papillomavirus. Ann Fam Med. 2003;1:221–7. doi: 10.1370/afm.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sherman ME, Carreon JD, Schiffman M. Performance of cytology and human papillomavirus testing in relation to the menstrual cycle. Br J Cancer. 2006;94:1690–6. doi: 10.1038/sj.bjc.6603151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Ham MA, Melchers WJ, Hanselaar AJ, et al. Fluctuations in prevalence of cervical human papillomavirus in women frequently sampled during a single menstrual cycle. Br J Cancer. 2002;87:373–6. doi: 10.1038/sj.bjc.6600485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schneider A, Kirchhoff T, Meinhardt G, et al. Repeated evaluation of human papillomavirus 16 status in cervical swabs of young women with a history of normal Papanicolaou smears. Obstet Gynecol. 1992;79(5 Pt 1):683–8. [PubMed] [Google Scholar]
- 7.Schmeink CE, Massuger LF, Lenselink CH, et al. Effect of the menstrual cycle and hormonal contraceptives on human papillomavirus detection in young, unscreened women. Obstet Gynecol. 2010;116:67–75. doi: 10.1097/AOG.0b013e3181e238f0. [DOI] [PubMed] [Google Scholar]
- 8.Franco E, Villa L, Rohan T, et al. Design and methods of the Ludwig-McGill longitudinal study of the natural history of human papillomavirus infection and cervical neoplasia in Brazil. Ludwig-McGill Study Group. Revista panamericana de salud publica=Pan American J Public Health. 1999;6:223–33. doi: 10.1590/s1020-49891999000900001. [DOI] [PubMed] [Google Scholar]
- 9.Trottier H, Mahmud S, Costa MC, et al. Human papillomavirus infections with multiple types and risk of cervical neoplasia. Cancer Epidemiol Biomarkers Prev. 2006;15:1274–80. doi: 10.1158/1055-9965.EPI-06-0129. [DOI] [PubMed] [Google Scholar]
- 10.Schlecht NF, Trevisan A, Duarte-Franco E, et al. Viral load as a predictor of the risk of cervical intraepithelial neoplasia. Int J Cancer. 2003;103:519–24. doi: 10.1002/ijc.10846. [DOI] [PubMed] [Google Scholar]
- 11.Bouvard V, Baan R, Straif K, et al. A review of human carcinogens-Part B: biological agents. Lancet Oncol. 2009;10:321–2. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- 12.Vooijs GP, van der Graaf Y, Elias AG. Cellular composition of cervical smears in relation to the day of the menstrual cycle and the method of contraception. Acta Cytol. 1987;31:417–26. [PubMed] [Google Scholar]
- 13.Ransdell JS, Davey DD, Zaleski S. Clinicopathologic correlation of the unsatisfactory Papanicolaou smear. Cancer. 1997;81:139–43. [PubMed] [Google Scholar]
- 14.Nygard JF, Sauer T, Nygard M, et al. CIN 2/3 and cervical cancer in an organised screening programme after an unsatisfactory or a normal Pap smear: a seven-year prospective study of the Norwegian population-based screening programme. J Med Screen. 2004;11:70–6. doi: 10.1258/096914104774061047. [DOI] [PubMed] [Google Scholar]


