Abstract
Thermal stress and prior upper respiratory tract infection are risk factors for the Sudden Infant Death Syndrome. The adverse effects of prior infection are likely mediated by interleukin-1β (IL-1β). Therefore, we examined the single and combined effects of IL-1β and elevated body temperature on the duration of the Laryngeal Chemoreflex (LCR) in decerebrate neonatal piglets ranging in age from post-natal day (P) 3 to P7. We examined the effects of intraperitoneal (I.P.) injections of 0.3 mg/Kg IL-1β with or without I.P. 10 mg/Kg indomethacin pretreatment on the duration of the LCR, and in the same animals we also examined the duration of the LCR when body temperature was elevated approximately 2 °C. We found that IL-1β significantly increased the duration of the LCR even when body temperature was held constant. There was a significant multiplicative effect when elevated body temperature was combined with IL-1β treatment: prolongation of the LCR was significantly greater than the sum of independent thermal and IL-1β-induced prolongations of the LCR. The effects of IL-1β, but not elevated body temperature, were blocked by pretreatment with indomethacin alone. We also tested the interaction between IL-6 given directly into the nucleus of the solitary tract (NTS) bilaterally in 100 ngm microinjections of 50 μL and pre-treatment with indomethacin. Here again, there was a multiplicative effect of IL-6 treatment and elevated body temperature, which significantly prolonged the LCR. The effect of IL-6 on the LCR, but not elevated body temperature, was blocked by pretreatment with indomethacin. We conclude that cytokines interact with elevated body temperature, probably through direct thermal effects on TRPV1 receptors expressed pre-synaptically in the NTS and through cytokine-dependent sensitization of the TRPV1 receptor. This sensitization is likely initiated by cyclo-oxygenase-2 dependent synthesis of prostaglandin E2, which is stimulated by elevated levels of IL-1β or IL-6. Inflammatory sensitization of the LCR coupled with thermal prolongation of the LCR may increase the propensity for apnea and Sudden Infant Death Syndrome.
Keywords: SIDS, Laryngeal chemoreflex, Hyperthermia, Interleukin-1β, Interleukin-6, Nucleus of the solitary tract
1. Introduction
There is widespread recognition that apnea and subsequent hypoxia and bradycardia play an important role in the pathogenesis of SIDS (Hunt and Brouillette, 1987; Kahn et al., 1992; Kinney and Thach, 2009; Leiter and Böhm, 2007; Steinschneider, 1972). It seems likely that babies who die of SIDS experience an asphyxial stress associated with apnea during sleep to which they do not respond appropriately and from which they do not recover (Leiter and Böhm, 2007; Poets et al., 1999; Sridhar et al., 2003). Therefore, events or processes that initiate apnea or enhance apnea duration will promote the occurrence of SIDS, and events or processes that terminate apneas, restore eupnea and enhance arousal from sleep are likely to reduce the occurrence of SIDS. The laryngeal chemoreflex (LCR) is a protective response elicited when fluid enters the larynx, particularly fluid with a low chloride content or low pH (Boggs and Bartlett, 1982; Downing and Lee, 1975; Lee et al., 1977). The LCR is particularly prominent in neonatal animals and is frequently elicited in the normal course of neonatal life (Thach, 1997, 2001). The LCR consists of protective airway responses (laryngeal closure and apnea) that prevent aspiration of fluids into the upper airway, clear the fluids from the airway and preserve oxygen delivery to vital organs. To prevent aspiration, respiration is inhibited (Downing and Lee, 1975), and the glottis is closed (Haraguchi et al., 1983; Sasaki, 1979). To clear the airway, coughing and swallowing are activated (Thach, 2001; van der Velde et al., 2003). To preserve oxygen delivery, bradycardia occurs and blood flow may be redistributed to vital organs (Grogaard et al., 1982). These behaviors are elicited to varying degrees depending on the strength of the reflex response. Weak reflex responses may consist of brief respiratory disruption, coughing and swallowing; whereas the entire range of behaviors may be elicited when the LCR is strongly activated (Thach, 2001; van der Velde et al., 2003). Despite the protective nature of the LCR, many investigators have suggested that the LCR may be an important initiator of apneas that begin a chain of events leading to SIDS (Downing and Lee, 1975; Lanier et al., 1983; Leiter and Böhm, 2007; Page and Jeffery, 2000; Page et al., 1996; Thach, 1997, 2001, 2005). Infants who are susceptible to SIDS may have stronger LCR responses and may have weaker arousal mechanisms and/or difficulty restoring eupnea after reflex apneas—they may fail to autoresuscitate (Cummings et al., 2011; Kinney and Thach, 2009; Leiter, 2009; Leiter and Böhm, 2007; Poets et al., 1999; Sridhar et al., 2003).
A variety of airway receptors contribute to the LCR, and the behavioral diversity of the reflex response may reflect the diversity of receptors activated within the larynx when the LCR is elicited. The LCR can be mediated by ‘water’ receptors within the mucosa of the larynx (Boggs and Bartlett, 1982). More than half of these water sensitive receptors are mechanoreceptive, and the afferent nerves derived from these receptors have rapid conduction velocities, typical of myelinated A-fibers (Anderson et al., 1990; Harding et al., 1978). On the other hand, capsaicin-sensitive laryngeal afferents also elicit apnea, bradycardia, swallowing and other behaviors typical of the LCR. These capsaicin-sensitive fibers are unmyelinated C-fibers and clearly separable from water receptors (Mutoh et al., 2000; Roulier et al., 2003). A-fibers and C-fibers express a different constellation of presynaptic receptors in the NTS (Jin et al., 2004), and transient receptor potential vanilloid 1 (TRPV1) receptors are functionally associated with C-fibers. Both A-fibers and C-fibers run in the internal branch of the superior laryngeal nerve and transmit sensory information from the larynx to the brainstem, where the afferents innervate second order neurons within the caudal NTS (Hayakawa et al., 2001; Patrickson et al., 1991). These second order neurons express non-NMDA glutamate receptors, and when visceral afferents are stimulated, glutamatergic excitatory post-synaptic currents (EPSCs) are elicited in the second order neurons (Doyle et al., 2002; Jin et al., 2004; Peters et al., 2010), and elicit apnea. In previous studies, we have focused on laryngeal stimuli and central regulatory pathways that emphasize the role of C-fiber modulation of the LCR (Xia et al., 2011).
Many risk factors for SIDS seem to enhance the LCR. For example, thermal stress (elevated room temperature, increased covering with bed clothes, etc.) is a risk factor for SIDS, and elevated body temperature enhanced laryngeal adduction induced by superior laryngeal nerve stimulation in dogs (Haraguchi et al., 1983) and prolonged the LCR elicited by injection small volumes of water into the larynx in decerebrate piglets (Curran et al., 2005). The effect of elevated body temperature on the LCR depends on activation of TRPV1 receptors within the nucleus of the tractus solitarius (NTS) (Xia et al., 2011). A recent history of an upper respiratory tract infection (URI) is also a risk factor for SIDS (Steinschneider, 1972), and viral infections, particularly respiratory syncytial virus infections, may prolong reflex apneas in newborn lambs (Lindgren and Grogaard, 1996; Lindgren et al., 1992). The effect of recent URIs depends on the synthesis and release of inflammatory cytokines (Frøen et al., 2000, 2002). In anesthetized piglets, endotoxin administration, which initiates a cytokine cascade through a toll-like receptor, prolonged apneas elicited by insufflation of acidic water into the larynx, and systemic administration of interleukin-1β (IL-1β) prolonged apnea induced by ammonia saturated air insufflated into the larynx (Stoltenberg et al., 1994). Infections that increase IL-1β or tumor necrosis factor-alpha (TNFα) may, therefore, sensitize the LCR. In neither of the foregoing studies was body temperature allowed to rise, but both endotoxin and IL-1β are pyrogenic. Elevated levels of IL-1β and TNFα generate a cascade of cytokines, including IL-6, which in turn stimulate cyclooxygenase-2 (COX-2) activity, and generate prostaglandins, especially prostaglandin E2 (PGE2), which is the ultimate mediator of altered neuronal activity in temperature sensitive cells in the hypothalamus that causes fever (Dinarello, 2004). Therefore, we investigated the interaction between inflammation mediated by IL-1β and the direct effects of temperature on the duration of the LCR. We tested the hypothesis that IL-1β and elevated body temperature would independently increase the duration of the LCR. In addition, we tested the hypothesis that IL-1β and elevated body temperature would demonstrate a significant interaction—that the combined stimuli would have a greater effect than the sum of the two individual stimuli. We also tested the effect of IL-1β in the presence of the mixed cyclooxygenase-1 and -2 inhibitor, indomethacin, to determine whether IL-1β-dependent effects on the LCR are mediated by IL-1β itself or by downstream mediators such as PGE2. Moreover, we tested the hypothesis that the IL-1β-dependent effects on the LCR are mediated centrally within the brainstem by IL-6, the synthesis of which may be increased when IL-1β levels increase.
2. Methods
Experiments were performed on 43 piglets (22 male and 21 female) ranging in age from 3 to 7 days (4.9 ± 1.0 days; mean ± SEM) with an average weight of 2.3 ± 0.1 kg. The Institutional Animal Care and Use Committee of Dartmouth College approved all surgery and experimental protocols.
2.1. Surgical preparation
Animals were anesthetized with 2% halothane (2-bromo-2-chloro-1,1,1-trifluoroethane; SigmaAldrich) in O2. A rectal probe was inserted, and rectal temperature (‘body temperature’) was maintained between 38 and 39 °C using a servo-controlled heating pad. Femoral arterial and venous catheters were inserted to measure blood pressure and administer drugs, respectively. Each animal was tracheostomized and artificially ventilated (Harvard Apparatus Dual Phase Respirator, South Natick, MA) to maintain the end-tidal CO2 concentration at approximately 5%, which was also servo-controlled so that end-tidal CO2 did not vary as a function of any of the experimental treatments. After exposing the carotid sinus regions bilaterally, the internal and external carotid arteries were ligated to facilitate decerebration. The vagus nerves were sectioned bilaterally to prevent entrainment of the phrenic rhythm to the mechanical ventilator (Graves et al., 1986; Petrillo et al., 1983). Each animal was placed prone with its head in a stereotaxic apparatus (Kopf Instruments, Tujunga, CA). The skull was opened, the animal was decerebrated at the level of the superior colliculi and all brain tissue rostral to the section was removed by suction. Following decerebration, isoflurane anesthesia was discontinued. Each animal was paralyzed using pancuronium bromide (1 mg/kg, iv; Elkins-Sinn Inc., Cherry Hill, NJ), and supplemental doses of pancuronium were given as required, usually at a rate of 0.5 mg/Kg/h. A phrenic nerve was exposed and sectioned, and the central cut end was placed on a bipolar recording electrode to monitor respiratory output. Phrenic activity was amplified (Gould Universal Amplifier, Cleveland, OH), and the moving time average (“integrated activity”) was calculated electronically (100 ms time constant; CWE, Ardmore, PA). Integrated phrenic nerve activity, body temperature, end-tidal CO2 and blood pressure were recorded on a computer (PowerLab, ADI, Australia) for later analysis.
Intravenous drugs were delivered through the femoral venous catheter. We made microinjections of drugs into the dorsal area of the brainstem using a 0.5 μl syringe (SGE Analytical Sciences, Austin, TX) with a 0.47 mm O.D. The needle of the injection syringe was flexible, and to control the location of the injections more effectively, the needle was passed through a rigid guide tube that ended just above the surface of the dorsal brainstem. The needle was advanced into the dorsal medulla perpendicular to the dorsal surface using visual landmarks—the obex primarily (Niblock et al., 2005). The needle for microinjections was placed in the medulla approximately 30 min before any tests of the LCR were performed. Respiratory activity was stable at the time of each injection. All drugs injected into the medulla were combined with 0.5 μm diameter fluorescent microbeads (Fluoresbrite® YG Micro-spheres, Polysciences, Inc., Warrington, PA). The beads were added to the drugs dissolved in the solvent vehicle (5 μl stock solution of microbeads, which contained 3.64 × 1011 particles/ml, were added to 100 μl vehicle) and distributed within the solvent by shaking before being aspirated into the injection syringe.
We placed a pharyngeal catheter (PE-90) through a nostril and positioned the tip just above the larynx. The catheter was filled with water, and 0.1 ml of water was injected into the larynx using a computer controlled syringe pump each time that we elicited the LCR. Water remained in the catheter between tests, and as a consequence, the temperature of the water injected was near body temperature. The larynx was suctioned periodically as needed. At least 5 min elapsed between tests of the LCR, and the LCR was not tested unless phrenic respiratory activity was stable.
2.2. Neuroanatomy
At the conclusion of each experiment, each piglet was killed with an injection of 500 mg/Kg pentobarbital sodium followed by 5–10 ml of saturated potassium chloride administered I.V. The brainstem was removed from the animal, placed in cryo-embedding medium (Tissue-Tek O.C.T. 458, Sakura Finetek, Torrance, CA) and frozen in isopentane at −70 °C. Brainstems were sectioned (50 μm) in a cryostat at −18 °C, and sections were mounted on gelatinized glass slides, fixed for 15 min in 4% paraformaldehyde in phosphate buffered saline (pH 7.0) and stained with cresyl violet (Bandroft and Cook, 1994; Luna, 1992). The location of the microinjection was identified using a combination of tissue disruption caused by the microinjection syringe tip and the location of fluorescent microbeads. The distribution of fluorescent microbeads was examined under fluorescent light using a TRITC filter (excitation 540 ± 25 nm; dichroic mirror 565 nm) on a Nikon Eclipse E800 microscope (Nikon Instruments Inc., Melville, NY). We recorded the location of the highest concentration of beads as the center of the injection, but we also noted the rostro-caudal extent of spread of the beads.
2.3. Experimental design to study IL-1
We studied three conditions to test the hypothesis that systemic administration of IL-1β would prolong the LCR. In the first study, we treated each animal with 10 mgs/kg indomethacin given IP approximately 60 min before testing the LCR under normothermic, hyperthermic and repeat normothermic conditions. In the second study, we treated each animal with 3 μg/Kg IL-1β given IP approximately 30 min before testing the LCR under the same three conditions. In the final study, we treated each animal with 10 mgs/Kg indomethacin given IP approximately 60 min before testing the LCR, and then we gave 3 μg/Kg IL-1β IP approximately 30 min before testing the LCR again under normothermic, hyperthermic and repeat normothermic conditions. The interval between different temperature conditions was also about 30 min. The body temperature was maintained at approximately 38.5 °C during normothermic conditions and the body temperature was maintained at approximately 41 °C during hyperthermic conditions.
2.4. Experimental design to study IL-6
We studied two drug conditions, IL-6 microinjection into the region of the NTS and IL-6 microinjection after indomethacin treatment, and four thermal conditions. We began the study with a normothermic, untreated, baseline condition before IL-6 treatment during which bilateral 0.5 μL microinjections of vehicle alone were made just caudal to the obex. The LCR was elicited three times to assess the baseline duration of the LCR. After the first set of measurements, IL-6 was administered (100 ngm) in bilateral 0.5 μL microinjections into the NTS just caudal to the obex, and the LCR was tested three times under normothermic conditions approximately 30 min later, followed by assessment of the LCR in a hyperthermic condition and a final normothermic condition after the animal was cooled back its normal body temperature. In the second study, indomethacin 10 mg/Kg was given IP before the start of the study, and the sequence of thermal conditions described above was repeated: a normothermic set of LCR responses was obtained; IL-6, 100 ngm in 0.5 μL was injected bilaterally into the NTS;, and the LCR was studied in the same normothermic, hyperthermic and normothermic sequence of conditions as in the first IL-6 study.
2.5. Data analysis and statistics
We defined the duration of the LCR as the period of respiratory instability (defined as variability of phrenic amplitude and/or respiratory timing) from the beginning of the breath during which the water stimulus was delivered to the onset of at least five regular breaths. These five breaths did not need to have the same frequency or amplitude as the control breaths; we simply required that they be regular (Curran et al., 2005; van der Velde et al., 2003; Xia et al., 2007b). The respiratory disruption measured in this way included both periods of unstable respiratory activity and apneas. We kept the definition of the LCR duration simple and applied it consistently across all animals. We also measured the longest apnea duration of each reflex trial, which is less subject to interpretation. Apnea was defined as a cessation of phrenic activity that was at least 120% greater than the duration of the breath preceding the respiratory cycle during which the water stimulus was delivered. However, apnea did not occur in all tests of the LCR. Measuring both the LCR duration and apnea duration, when present, provided a more complete analysis of the response since apnea duration alone does not capture the behavioral complexity of the LCR, and measuring the LCR duration alone can require subjective judgments. Stimulation of the LCR may induce bradycardia as well as apnea in intact animals. However, we did not analyze the heart rate responses because the animals were vagotomized.
We used a two-way ANOVA to analyze these studies (SYS-TAT 9.0, SPSS, Inc., Chicago, IL). Drug treatments were a between subjects factor, and thermal conditions were a within subjects, repeated factor in the two-way ANOVA. When the ANOVA indicated that significant differences existed among the treatments, specific pre-planned comparisons were made using orthogonal contrasts and P-values adjusted by the Bonferroni method. We made orthogonal contrasts between the two normothermic conditions that bracketed each hyperthermic condition and between the means of these normothermic conditions and the hyperthermic treatment condition. The apnea and LCR durations were not normally distributed, and the variances among treatments were not homogenous; both of which violate the assumptions upon which the ANOVA is based. Therefore, apnea and LCR durations were log-transformed for the statistical analysis, as we have done previously (Curran et al., 2005; Duy et al., 2010), and the ANOVA was performed on the log-transformed data. Data are presented in the text and figures as the mean ± the standard error of the mean of the untransformed values of each variable.
3. Results
3.1. Effects IL-1β on hyperthermic prolongation of the LCR
Examples of the responses of integrated phrenic nerve activity from a single piglet during four sequential treatment conditions are shown in Fig. 1. When the LCR was elicited first at normal body temperature in the control condition, the apnea elicited lasted 5.8 s and the LCR lasted 9.9 s. Next, the piglet was given IL-1β (3 μg/Kg I.P.), and the LCR was re-evaluated at normal body temperature 20 min after the IL-1β injection when the apnea duration had increased to 19.6 s, and the LCR duration had increased to 23.1 s, even though body temperature had not changed. The combined effects of IL-1β and elevated body temperature were tested by raising the body temperature to 41.1 °C. In this setting, an apnea lasting 23.9 s, was elicited and the LCR lasted 29.3 s. In the final experimental condition, the animal was cooled to its normal body temperature, and when the LCR was elicited again, the apnea duration was reduced to 5.9 s, and the LCR lasted 7.2 s; durations. Thus, Il-1β prolonged the LCR under normothermic conditions, and there was a combined effect of IL-1β and hyperthermia to prolong the LCR even further. The entire sequence of tests took an hour or more, and the effect of IL-1β seemed to have disappeared after approximately 120 min when the final measurements of the LCR were made.
Fig. 1.
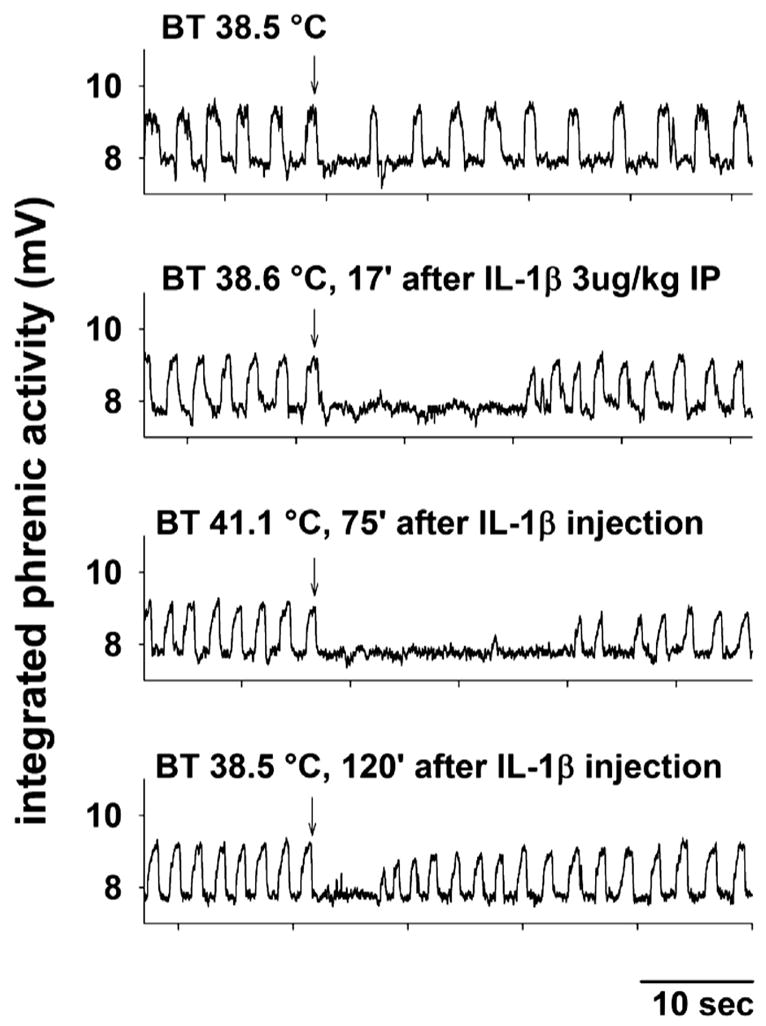
Integrated phrenic activity is shown during four tests of the LCR in a female piglet (post-natal age 4 days and body weight equal 2.3 Kg). The control response to laryngeal injection of 0.1 ml of water (at the downward arrow) at a normal body temperature is shown in the top panel. Seventeen minutes following intraperitoneal injection of 3 μg/Kg IL-1β, the LCR was tested again at the normal body temperature (second panel), and the apnea and LCR durations were prolonged. Elevating the body temperature (third panel) and retesting the LCR 75 min after IL-1β treatment further prolonged the duration of the LCR. In the final condition, normal body temperature was restored, and the LCR was elicited again 120 min after the IL-1β injection (bottom panel). The apnea and LCR durations were not different from the initial, untreated control condition (top panel). ‘BT,’ body temperature; ‘IP,’ intraperitoneal.
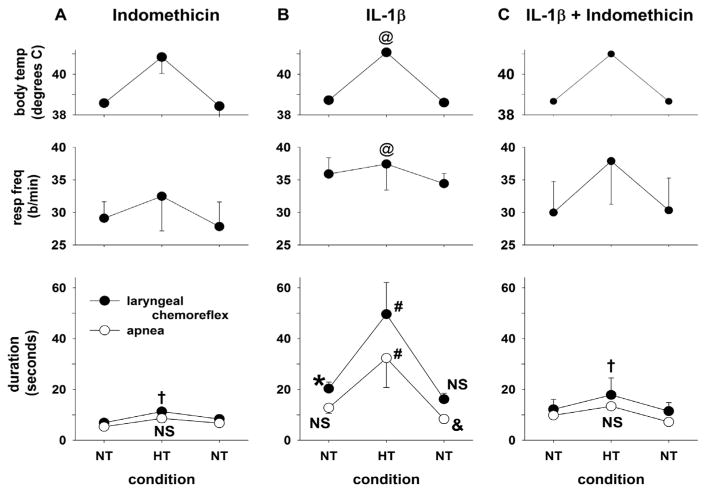
To determine first, whether there was an interaction between hyperthermia and IL-1β treatment and second, whether IL-1β modified the LCR directly (or through activation of cyclooxygenase-1 or -2), we compared the effects of IL-1β treatment at normal and elevated body temperatures to the effect of indomethacin alone or in combination with IL-1β. The average values of the LCR duration, the longest apnea duration, respiratory frequency and body temperature are shown in Fig. 2 during each of these three treatment conditions (indomethacin, n = 6; IL-1β, n = 9 or indomethacin plus IL-1β, n = 7) at each sequential body temperature within each treatment condition. A two-way ANOVA revealed a significant drug treatment by temperature interaction for both apnea duration (P < 0.007) and the LCR duration (P = 0.005), and therefore, we made ten preplanned comparisons among the various treatments and temperatures, we adjusted the significance level to P = 0.005 for each specific comparison in order to keep the probability of making a Type I error at 0.05 for the entire set of comparisons.
Fig. 2.
Mean body temperature, respiratory frequency (resp freq), the duration of the LCR and the duration of apnea have been plotted as functions of experimental conditions. Vertical lines indicate standard errors. One group of piglets received 10 mgs/Kg indomethacin only (Panel A, n = 6); a second group of piglets received 3 μg/Kg IL-1β IP (Panel B, n = 9); and the third group of animals received both indomethacin and IL-1β at these same doses (Panel C, n = 7). In all three conditions, hyperthermia (HT) was associated with a significantly increased respiratory rate compared to normothermic (NT) conditions. This main effect of the hyperthermic condition is indicated by ‘@’ (P < 0.01). Following treatment with indomethacin alone, the LCR was slightly prolonged during the hyperthermic condition compared to normothermic conditions (P < 0.05, ‘†’). The apnea duration was also increased by elevated body temperature, but this did not reach statistical significance (‘NS’). There were no differences in either LCR or apnea duration between the first and second normothermic conditions. After IL-1β treatment, the LCR duration, but not the apnea duration was significantly increased during the normothermic condition compared to the normothermic conditions during indomethacin treatment whether IL-1β was present or not (P < 0.05, ‘*’). Both the LCR and apnea durations were significantly increased during hyperthermia compared to normothermia in the same animals (P < 0.05, ‘#’), and the increases in LCR and apnea durations were significantly greater (P < 0.05) than the changes in these variables after treatment with indomethacin (Panel C). During the second normothermic condition following IL-1β treatment, the LCR duration was not different from the preceding normothermic condition (‘NS’), but the apnea duration was actually significantly less than the first normothermic condition (P < 0.05, ‘&’). When both indomethacin and IL-1β were given together, the response to IL-1β was absent, and the results were identical to indomethacin treatment alone: hyperthermia slightly but significantly prolonged the LCR (P < 0.05, ‘†’), and the mean apnea duration, though also slightly increased, was not significantly different from the response during the normothermic condition (‘NS’).
In the comparisons among indomethacin, IL-1β or indomethacin plus IL-1β (Fig. 2), it is apparent that when indomethacin was present with or without IL-1β (Fig. 2A and C), there was thermal prolongation of the LCR (P < 0.05, ‘†’) and though the apnea duration increased, this change did not reach statistical significance (‘NS’). In contrast, IL-1β alone dramatically altered the LCR responses (Fig. 2B). First, there was a significant initial normothermic effect of IL-1β as seen from the significant prolongation of the LCR comparing the normothermic effects after IL-1β treatment to the normothermic treatments after indomethacin and indomethacin plus IL-1β (P < 0.05, ‘*’). Apnea duration was longer under this first normothermic condition after IL-1β treatment compared to the combined normothermic indomethacin containing treatments, but this change was not statistically significant. During the second normothermic condition following IL-1β treatment, the LCR duration was not different from the preceding normothermic condition (‘NS’), but the apnea duration was actually significantly less than the first normothermic condition (‘&’, P < 0.05). Thus, IL-1β treatment increased the LCR duration even under normothermic conditions, a response that indomethacin treatment blocked.
The most interesting aspect of the results of IL-1β treatment are that riding on top of a relatively small normothermic IL-1β-related prolongation of the LCR, there is a large and statistically significant prolongation of both apnea and LCR duration during combined hyperthermia plus IL-1β treatment (P < 0.05, ‘#’; Fig. 2B), and this amplification of the hyperthermic prolongation of the LCR was absent in animals treated with indomethacin alone or in combination with IL-1β (Panels A and C). Temperature and IL-1β have an interactive effective - prolongation of the LCR after IL-1β treatment was much greater than the sum of the effects of hyperthermia plus either indomethacin or IL-1β treatment alone. We cannot draw this conclusion with certainty however: there are no results for hyperthermia alone, and it is possible that the apparent interaction is due to IL-1β having not yet reached its time of greatest effectiveness until the hyperthermic test within each treatment group. Finally, it seems unlikely that the IL-1β treatment effect is due to IL-1β itself, since indomethacin, which blocks synthetic processes downstream from IL-1β in the cascade of inflammatory mediators and cytokines, blocked the IL-1β-mediated effects on the LCR.
In previous studies of the thermal modulation of the LCR in decerebrate piglets, we have seen variable effects on respiratory frequency; the effects were often attributed to elevated body temperature, but sometimes attributable to drug treatments (Duy et al., 2010). In the current study, IL-1β treatment did not affect respiratory frequency (there was no drug treatment effect or drug treatment by temperature effect). Elevated body temperature on the other hand significantly increased the respiratory frequency in all three drug treatment conditions (P = 0.01, ‘@’). This thermal effect on respiratory frequency was not altered by indomethacin treatment, which is further evidence that IL-1β was not involved in this change in respiratory frequency.
We measured other variables to make sure that the piglets were physiologically similar across treatment conditions. First, baseline body temperature was not different among treatment groups, and the increase in body temperature during the hyperthermic conditions was similar in all three treatment groups and statistically significant (P < 0.001, ‘@’). Blood pressure was not different among treatment groups nor among the temperature conditions or drug treatments. The end-tidal CO2 was also similar across drug treatment groups, although it was approximately 1 mm Hg higher during the hyperthermic conditions compared to the normothermic condition in all three treatment groups (P = 0.01). This may reflect an increase in metabolic rate at the higher temperature, and it also reflects our imperfect ability as experimenters to match mechanical ventilation to metabolism with 1 mm Hg end-tidal PCO2 accuracy.
3.2. Effects of IL-6 on the LCR
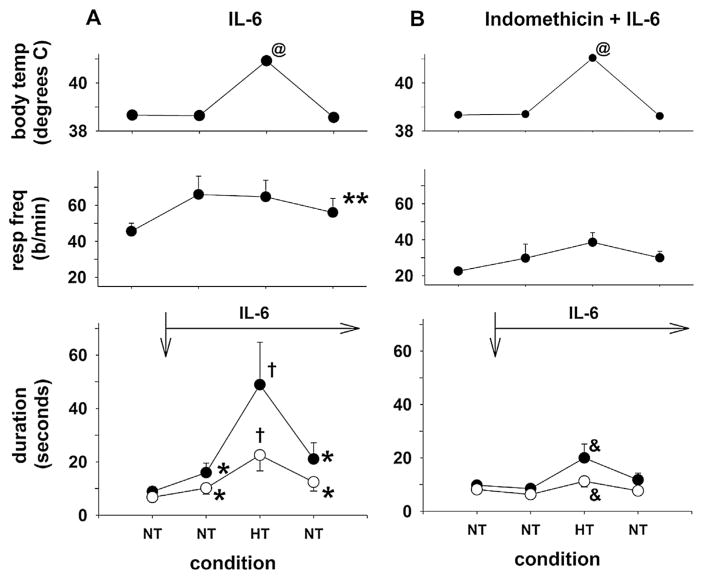
Elevated levels of IL-1β initiate a cascade of pro-inflammatory cytokines, and IL-6 levels rise sharply after treatment with IL-1β. Moreover, IL-6 is a cytokine pyrogen in its own right (Dinarello, 2004; Tsakiri et al., 2008). Therefore, we tested the hypotheses that IL-6 treatment would prolong the LCR, and that any IL-6-mediated prolongation of the LCR would be amplified by increased body temperature. We also determined whether IL-6-induced changes in the LCR were dependent on cyclo-oxygenase activity by combining IL-6 plus indomethacin in one treatment group. We modified the experimental design by including an initial untreated, control condition of the LCR at normal body temperature to make it easier to detect any effect of IL-6 under normothermic conditions without a prior bout of hyperthermia. This modification of the experimental design allowed us to compare only two drug treatment groups (IL-6 (n = 6) versus combined IL-6 and indomethacin (n = 6)) as shown in Fig. 3. In addition, we gave the IL-6 centrally, bilateral 100 ngm of IL = 6 in 0.5 μL injections into the NTS, to determine whether IL-6 could act within the brainstem to prolong the LCR. As before, there was a significant drug treatment by the thermal treatment interaction, which permitted us to make a series of specific, orthogonal contrasts.
Fig. 3.
Mean body temperature, respiratory frequency (resp freq), the duration of the LCR and the duration of apnea have been plotted as functions of experimental conditions. One group of piglets received 100 ngs IL-6 in 0.5 μl directly injected bilaterally into the NTS after the first normothermic condition (Panel A, the time of injection is indicated by the downward arrow), and a second group of piglets received 10 mgs/Kg indomethacin IP at the start of the study before 100 ngs IL-6 in 0.5 μl were directly injected bilaterally into the NTS at the time indicated by the downward arrow (Panel B). Body temperature increased significantly during the hyperthermic condition, by design, (P < 0.001, ‘@’). IL-6 treatment significantly increased the respiratory rate (P < 0.05, ‘**’), but this increase was present and unchanged in both normothermic and hyperthermic conditions. Following treatment with IL-6, both the LCR and apnea durations were significantly increased during normothermia (P < 0.05, ‘*’), and the LCR and apnea durations were significantly increased when hyperthermia and IL-6 treatment were combined (P < 0.05, ‘†’). During the second normothermic condition after IL-6 treatment, the LCR and apnea durations were not significantly different from the preceding normothermic, IL-6 treated condition, but still longer than the original normothermic condition preceding IL-6 treatment (P < 0.05, ‘**’). Thus, the IL-6 and hyperthermia caused a significant increase in the durations of the LCR and apnea, which were significantly greater than in the normothermic condition (P < 0.05) and significantly greater than the effect of IL-6 when combined with indomethacin (Panel B). Administration of IL-6 after treatment with indomethacin did not increase the LCR and apnea durations above the simple effect of hyperthermia alone. During hyperthermia and combined IL-6 and indomethacin treatment, the LCR and apnea durations were both increased significantly compared to the preceding normothermic conditions, and the increase in LCR and apnea durations was significantly less than the comparable increase in LCR and apnea durations when IL-6 alone was administered (P < 0.05, ‘&’).
IL-6 treatment prolonged the LCR. The normothermic conditions after IL-6 treatment (the second and fourth within animal treatments; Fig. 3A) were not different from each other, but IL-6 treatment increased the durations of apnea and the LCR at normal body temperature compared to the normothermic pre-IL-6 condition (P < 0.05, ‘*’). Moreover, the apnea and LCR durations during the hyperthermic condition were significantly greater than the apnea and LCR durations during the normothermic conditions after IL-6 treatment (P < 0.05, ‘†’). When IL-6 was given in combination with indomethacin, there were no differences among any of the three normothermic conditions, though hyperthermia still increased the apnea and LCR durations compared to the normothermic conditions (P < 0.05, ‘&’). Moreover, the hyperthermic apnea duration was significantly greater in the IL-6 treatment group compared to the apnea and LCR durations in the combined IL-6 and indomethacin treatment (P = 0.016 for apnea duration and P = 0.007 for the LCR duration). Thus, IL-6 treatment prolonged the LCR under normothermic conditions; IL-6-dependent prolongation was significantly amplified by elevating body temperature; and treatment with indomethacin completely blocked these effects of IL-6 treatment and revealed the isolated hyperthermic prolongation of the LCR.
In this set off studies, the respiratory frequency was elevated in the IL-6 treatment group across all body temperature conditions compared to the combined IL-6 and indomethacin treatment (P = 0.036, ‘**’). Thus, IL-6 may have some effect on respiratory frequency independent of cyclo-oxygenase activation. Body temperature was similar among drug treatment groups and treatment conditions and significantly elevated during the hyperthermic treatment condition (P < 0.001, ‘@’). Blood pressure and end-tidal CO2 levels were similar across all treatment conditions in all treatment groups.
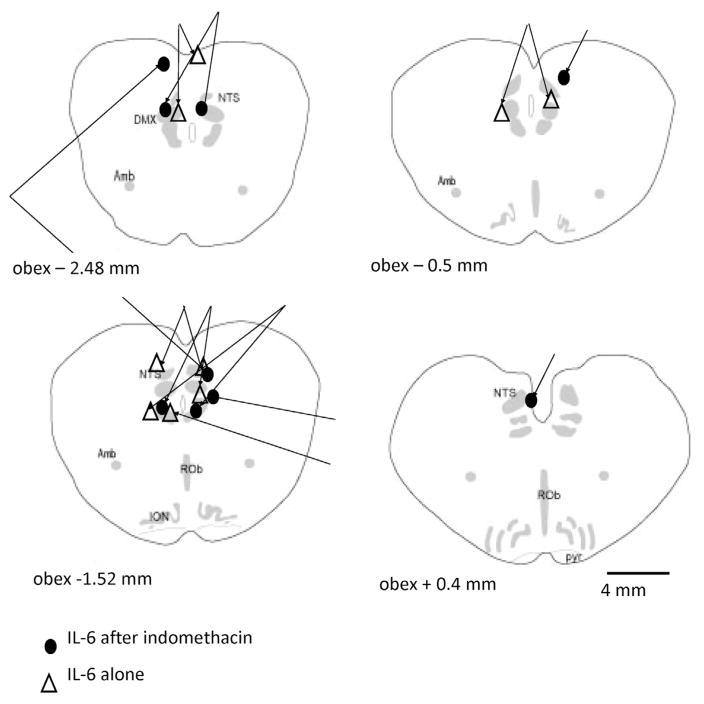
The locations of the microinjections of IL-6 are shown in Fig. 4. The IL-6 microinjections are intermixed throughout the NTS. The sites of injections do not differ among the animals that received indomethacin or control injections. One set of microinjections was caudal to the NTS (not shown in Fig. 4). Nevertheless, the respiratory data for this animal, which received IL-6 and no indomethacin, were incorporated into the statistical analysis.
Fig. 4.
The sites of injection of IL-6 are noted in the schematic cross-sections of the piglet brainstem, which start caudal to the obex of the neonatal piglet medulla. Microinjections in the same animal are ‘yoked’ together by lines. The sites of IL-6 injection were not different between the indomethacin treated and non-treated animals. In 4 animals, we found only a single injection site, and both microinjection sites were found caudal to the NTS in one animal treated with IL-6, but not indomethacin. Anatomical abbreviations: ROb, raphé obscurus; DMX, dorsal vagal motor nucleus; Amb, nucleus ambiguus; ION, inferior olivary nucleus; NTS, nucleus tractus solitaries; pyr, pyramids.
4. Discussion
The main findings in this study are that IL-1β and IL-6, pro-inflammatory cytokines, prolong the LCR during normothermic conditions and amplify the prolongation of the LCR that occurred when body temperature was elevated approximately 2.5 °C. The lengthening of the LCR caused by IL-1β and IL-6 was prevented by co-administration of indomethacin, an inhibitor of cyclo-oxygenase-1 and -2. The results reveal separate, but interacting effects of elevated body temperature and IL-1β or IL-6 since the LCR was most effectively prolonged when both elevated body temperature and IL-1β or IL-6 were present. Indomethacin suppressed the response to IL-1β and IL-6, but some degree of thermally mediated prolongation of the LCR remained even after treatment with indomethacin.
4.1. Mechanisms of IL-1β activation of the LCR
The effect of IL-1β on apnea length has been investigated previously. IL-1β pretreatment increased the duration of apnea elicited either by inhalation of ammonia or laryngeal instillation of hydrochloric acid in anesthetized piglets even when body temperature was held constant (Frøen et al., 2000; Stoltenberg et al., 1994). These authors speculated about the possible mechanism of this effect, but since the role of TRPV1 in the regulation of the LCR was described (Xia et al., 2011), we can propose a mechanistically plausible, molecular chain of events between IL-1β and control of the LCR. TRPV1 channels are temperature sensitive, and particularly interesting, for that reason, in respect to the thermal prolongation of the LCR. TRPV1 channels, which are non-specific cation channels, are present in the laryngeal mucosa (Hamamoto et al., 2009), and in the nodose ganglion (Helliwell et al., 1998) and also expressed within the NTS pre-synaptically on visceral C-fiber afferents (Mezey et al., 2000; Patterson et al., 2003; Sun et al., 2009). Activation of TRPV1 channels within the pre-synaptic membrane on unmyelinated C-fibers appears to amplify glutamate release onto second order neurons in the NTS. TRPV1 channels are usually thought to have a thermal threshold of greater than 42 °C (Julius and Basbaum, 2001). However, TRPV1 channel activation in the NTS, which enhances asynchronous release of glutamate following visceral afferent stimulation, was thermally modulated by temperatures between 25 and 38 °C (Peters et al., 2010). Consistent with this more physiological TRPV1 thermal threshold of activation, elevating body temperature in neonatal piglets by 1–2 °C was sufficient to elicit a TRPV1-dependent thermal prolongation of the LCR (Xia et al., 2011). Thus, the activity of TRPV1 channels in the NTS was modulated at temperatures well within the physiological range in neonatal animals.
TRPV1 channels are promiscuous and multiple, non-thermal stimuli may modify their activity, irrespective of temperature (Jia and Lee, 2007; Premkumar and Abooj, 2013). Many of the nonthermal activators and modulators of TRPV1 activity are mediators of inflammation. TRPV1 activation is elicited or potentiated by capsaicin, low pH, anandamide (Smart et al., 2000), bradykinin and a variety of cytokines, including TNF-a, IL-1β and IL-6 (Schaible, 2014), some of which in turn stimulate the synthesis and release of eicosanoids, such as prostaglandin E2 (PGE2) (Hwang et al., 2000). PGE2 is produced by cyclo-oxygenase-2 (COX-2), and expression of COX-2 is increased when IL-1β levels increase (Samad et al., 2001). PGE2 binds to the EP prostaglandin receptors, increases intracellular levels of calcium and activates adenyl cyclase and increases cAMP, which leads to activation of protein kinase A (Kawabata, 2011; Premkumar and Abooj, 2013). Protein kinase A, in turn, phos-phorylates TRPV1, and it is the phosphorylation of TRPV1 that is thought to enhance its conductance and enhance calcium entry into the cell after exposure to PGE2 (Jeske et al., 2008). PGE2-dependent sensitization of TRPV1 leads to greater presynaptic calcium entry and greater release of glutamate (Sekiyama et al., 1995). Thermal hyperalgesia in peripheral nerves is attributed to PGE2-dependent sensitization of TRPV1 so that thermal stimuli that previously caused no discomfort, now elicit pain (Latremoliere and Woolf, 2009; Moriyama et al., 2005; Samad et al., 2001). We believe that these same processes described in neurons in the dorsal horn are also present in C-fiber vagal sensory neurons that terminate in the NTS. Thus, IL-1β and IL-6 may increase PGE2 in the NTS and sensitize TRPV1 channels, and thereby amplify the potency of thermal information. The enhanced thermal prolongation of the LCR can be seen as a form of enhanced reflex thermal hyperalgesia or reflex allodynia analogous to enhanced painful thermal hyperalgesia in peripheral nerves following central sensitization, in which TRPV1 channels play a prominent role (Grace et al., 2014; Kawabata, 2011; Matta and Ahern, 2011; Premkumar and Abooj, 2013). Thus, the multiplicative effects of IL-1β and elevated body temperature arise from the interactions of these factors at the presynaptic TRPV1 receptors on laryngeal C-fibers.
It is our hypothesis that thermal activation of pre-synaptic TRPV1 channels within the NTS amplifies glutamate release at second order neurons when the body temperature is increased. In this model, the duration of the LCR is proportional to the intensity of glutamatergic excitations of the second order neurons within the NTS, and TRPV1-dependent enhanced glutamatergic excitation of second order neurons is responsible for thermal prolongation of apnea and respiratory disruption associated with the LCR. That the apneic response should be amplified by heating may reflect an inverse correlation between metabolic rate and temperature, as shown for the inhibitory duration of the Hering-Breuer reflex, which was also longer at higher temperatures, but lower metabolic rates (Merazzi and Mortola, 1999). Furthermore, we believe that IL-1β and IL-6 prolong the LCR as a result of increased binding of PGE2 to its receptor and the resulting phosphorylation and sensitization of TPRV1 receptors. Thus, TRPV1 and those modulators of TRPV1 that increase its activity or sensitivity appear to provide excitatory amplification of the activation of second order neurons in the NTS, which is transmitted to the respiratory control element(s) in the ventral medulla that ultimately generate apnea and prolong the LCR.
4.2. SIDS, inflammation and thermal stress
SIDS is often associated with heat stress (Blair et al., 2008; Guntheroth and Spiers, 2001; Kleemann et al., 1998; Williams et al., 1996). Although there is not universal agreement (Sheers-Masters et al., 2004), many reports indicate that death frequently occurs in overheated rooms or when the infant is excessively bundled or covered by blankets (Fleming et al., 1990; Ponsonby et al., 1998; Williams et al., 1996). As emphasized by Guntheroth and Spiers (2001), the danger of the prone sleeping position may relate more to heat stress than to asphyxia. Prone infants – particularly those who are covered by a blanket and/or have their faces in the bedclothes – lose heat more slowly than supine infants do, both from the body surface and via the respiratory tract (Bolton et al., 1996). We hypothesize, therefore, that thermal stress, by enhancing the LCR, may increase the likelihood of prolonged apneas that result ultimately in sudden death in neonates in whom the LCR and the thermal effects on the LCR are particularly strong. The demonstration that TRPV1 channels provide a thermal sensor that enhances the LCR provides a molecular mechanism through which heat stress and other epidemiologically identified risk factors for SIDS may operate to increase the risk of prolonged apnea and sudden infant death.
Recent upper respiratory tract infections are also a risk factor for SIDS (Dalveit et al., 2003; Raza and Blackwell, 1999), and recent viral infection, particularly with Respiratory Syncytial Virus, increases the potency of the LCR (Lindgren et al., 1992). The infection-related increase in potency of the LCR seems to be mediated by inflammatory mediators (TNF-α, IL-1β and IL-6). IL-1β does not cross the blood brain barrier well, but production of IL-1β and IL-6 can be induced in neurons and glia in the brainstem (Jafri et al., 2013), or IL-1β or activators of Il-1β may be transmitted retrograde from the larynx into the central nervous system through the superior laryngeal nerves (Lindgren and Grogaard, 1996; Lindgren et al., 1992; Park et al., 2005; Thrane et al., 1995). Thus, a recent respiratory tract infection may increase the concentration of inflammatory mediators and sensitize TRPV1 channels (Julius and Basbaum, 2001; Venkatachalam and Montell, 2007). Increased activity of the TRP channels, even at normal body temperatures, may amplify the LCR and increase the likelihood of prolonged apneas and the risk of SIDS following infections. In the presence of fever plus cytokine-mediated TRPV1 sensitization, the LCR would be even further prolonged, as shown in Figs. 2 and 3 where there was a strong interaction between elevated body temperature and IL-1β or IL-6 pretreatment. Thus, TRPV1 channel activity may provide a common molecular mechanism for the epidemiological association of SIDS with thermal stress and recent infections.
4.3. Limitations of the methods
We have discussed the limitations of studying the LCR in decerebrate neonatal piglets in previous studies (Duy et al., 2010; Xia et al., 2008). We pointed out in these discussions that thermal stress has been identified as a risk factor for SIDS, but this does not mean necessarily that infants who died of SIDS had an elevated body temperature. In this respect, our studies of decerebrate piglets may not accurately mimic the thermal risk factors for SIDS. Another aspect of our experimental LCR maneuvers that distinguish them from naturally occurring apneas is that the animals are continuously ventilated and so do not become hypoxic or hypercapnic during the periods of phrenic “apnea”. We found in this study and have found in previous studies somewhat variable effects of drug or changes in body temperature on respiratory frequency (Curran et al., 2005; Xia et al., 2007a, b). It is worth noting that, to the extent an increased respiratory frequency represents an increased respiratory drive, such an increase in frequency would tend to decrease, rather than increase the duration of apnea and the LCR. Hence, the changes in LCR and apnea durations that we observed are not likely to be attributable to the changes in respiratory frequency that we observed. In addition, small differences in the level of decerebration can alter respiratory frequency among different animals.
4.4. Summary
IL-1β and IL-6 prolonged the duration of the LCR in decerebrate piglets, an effect that was blocked by inhibition of cyclo-oxygenase. Therefore, the cytokine-mediated prolongation of the LCR probably depends on the synthesis of PGE2, which, through a cascade of intracellular messengers, phosphorylates and sensitizes TRPV1 channels. Sensitization of TRPV1 channels may create laryngeal chemoreflex allodynia in which moderate laryngeal stimulation, which may occur commonly even in normal infants, may cause profound apnea, especially in the setting of recent inflammation, which may accompany and follow recent upper respiratory tract infections—a well-known risk for SIDS. The impact of cytokine-mediated sensitization of the LCR may be compounded if body temperature is also elevated since TRPV1 channels mediate thermal prolongation of the LCR, and there appears to be a multiplicative interaction between cytokine-mediated sensitization of the LCR and thermal prolongation of the LCR. Thus, a recent upper respiratory tract infection associated with increased IL-1β production and a fever may provide a particularly potent combination of factors that enhance the propensity for prolonged and severe apneas, which may begin the process that leads to sudden death in susceptible infants.
Acknowledgments
This work was supported by grants 36379 and 42707 from the NICHD.
References
- Anderson JW, Sant’Ambrosio FB, Mathew OP, Sant’Ambrogio G. Water-responsive laryngeal receptors in the dog are not specialized endings. Respir Physiol. 1990;79:33–43. doi: 10.1016/0034-5687(90)90058-7. [DOI] [PubMed] [Google Scholar]
- Bandroft JD, Cook HC. The central and peripheral nervous system. In: Bandroft JD, editor. Manual of Histological Techniques and Their Diagnostic Application. Churchill Livingstone; New York: 1994. pp. 350–351. [Google Scholar]
- Blair PS, Mitchell EA, Heckstall-Smith EMA, Fleming PJ. Head covering—a major modifiable risk factor for Sudden Infant Death Syndrome: a systematic review. Arch Dis Child. 2008;93:778–783. doi: 10.1136/adc.2007.136366. [DOI] [PubMed] [Google Scholar]
- Boggs DF, Bartlett D., Jr Chemical specificity of a laryngeal apneic reflex in puppies. J Appl Physiol. 1982;53:455–462. doi: 10.1152/jappl.1982.53.2.455. [DOI] [PubMed] [Google Scholar]
- Bolton DPG, Nelson EAS, Taylor BJ, Weatherall IL. Thermal balance in infants. J Appl Physiol. 1996;80:2234–2242. doi: 10.1152/jappl.1996.80.6.2234. [DOI] [PubMed] [Google Scholar]
- Cummings KJ, Hewitt JC, Li A, Daubenspeck JA, Nattie EE. Postnatal loss of brainstem serotonin neurones compromises the ability of neonatal rats to survive episodic severe hypoxia. J Physiol. 2011;589:5247–5256. doi: 10.1113/jphysiol.2011.214445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran AK, Xia L, Leiter JC, Bartlett D., Jr Elevated body temperature enhances the laryngeal chemoreflex in decerebrate piglets. J Appl Physiol. 2005;98:780–786. doi: 10.1152/japplphysiol.00906.2004. [DOI] [PubMed] [Google Scholar]
- Dalveit AK, Irgens LM, Øyen N, Skjaerven R, Markstad T, Wennergren G. Circadian variations in sudden infant death syndrome: associations with maternal smoking, sleeping position and infections. The Nordic Epidemiological SIDS Study. Acta Paediatr. 2003;92:1007–1013. [PubMed] [Google Scholar]
- Dinarello CA. Infection, fever, and exogenous and endogenous pyrogens: some concepts have changed. J Endotoxin Res. 2004;10:201–222. doi: 10.1179/096805104225006129. [DOI] [PubMed] [Google Scholar]
- Downing SE, Lee JC. Laryngeal chemosensitivity: a possible mechanism of sudden infant death. Pediatrics. 1975;55:640–649. [PubMed] [Google Scholar]
- Doyle MW, Bailey TW, Jin YH, Andresen MC. Vanilloid receptors presynaptically modulate cranial visceral afferent synaptic transmission in nucleus tractus solitarius. J Neurosci. 2002;22:8222–8229. doi: 10.1523/JNEUROSCI.22-18-08222.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duy MP, Xia L, Bartlett D, Jr, Leiter JC. An adenosine A2A agonist injected in the NTS prolongs the LCR by a GABAergic mechanism in decerebrate piglets. Exp Physiol. 2010;95:774–787. doi: 10.1113/expphysiol.2010.052647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming PJ, Gilbert R, Azaz Y, Berry PJ, Rudd PT, Stewart A, Hall E. Interaction between bedding and sleeping position in the SIDS: a population based case-control study. BMJ. 1990;301:85–89. doi: 10.1136/bmj.301.6743.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frøen JF, Akre H, Stray-Pedersen B, Saugstad OD. Adverse effects of nicotine and interleukin-1beta on autoresuscitation after apnea in piglets: implications for Sudden Infant Death Syndrome. Pediatrics. 2000;105:E52. doi: 10.1542/peds.105.4.e52. [DOI] [PubMed] [Google Scholar]
- Frøen JF, Akre H, Stray-Pedersen B, Saugstad OD. Prolonged apneas and hypoxia mediated by nicotine and endotoxin in piglets. Biol Neonat. 2002;81:119–125. doi: 10.1159/000047196. [DOI] [PubMed] [Google Scholar]
- Grace PM, Hutchinson MR, Maier SF, Watkins LR. Pathological pain and the neuroimmune interface. Nat Rev Immunol. 2014;14:217–231. doi: 10.1038/nri3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves C, Glass L, Laporta D, Meloche R, Grassino A. Respiratory phase locking during mechanical ventilation in anesthetized subjects. Am J Physiol. 1986;250:R902–R909. doi: 10.1152/ajpregu.1986.250.5.R902. [DOI] [PubMed] [Google Scholar]
- Grogaard J, Lindstrom DP, Stahlman MT, Marchal F, Sundell H. The cardiovascular response to laryngeal water administration in young lambs. J Dev Physiol. 1982;4:353–370. [PubMed] [Google Scholar]
- Guntheroth WG, Spiers PS. Thermal stress in Sudden Infant Death: is there an ambiguity with the rebreathing hypothesis? Pediatrics. 2001;107:693–698. doi: 10.1542/peds.107.4.693. [DOI] [PubMed] [Google Scholar]
- Hamamoto T, Takumida M, Kirakawa K, Tatsukawa T, Ishibashi T. Localization of transient receptor potential vanilloid (TRPV) in the human larynx. Acta Otolaryngol. 2009;129:560–568. doi: 10.1080/00016480802273108. [DOI] [PubMed] [Google Scholar]
- Haraguchi S, Fung RQ, Sasaki R. Effect of hyperthermia on the laryngeal closure reflex. Implications in the sudden infant death syndrome. Ann Otol Rhinol Laryngol. 1983;92:24–28. doi: 10.1177/000348948309200106. [DOI] [PubMed] [Google Scholar]
- Harding R, Johnson P, McClelland ME. Liquid-sensitive laryngeal receptors in the developing sheep cat and monkey. J Physiol (London) 1978;277:409–422. doi: 10.1113/jphysiol.1978.sp012281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa T, Takanaga A, Maeda S, Seki M, Yajima Y. Subnuclear distribution of afferents from the oral, pharyngeal and laryngeal regions in the nucleus tractus solitarii of the rat: a study using transganglionic transport of cholera toxin. Neurosci Res. 2001;39:221–232. doi: 10.1016/s0168-0102(00)00218-2. [DOI] [PubMed] [Google Scholar]
- Helliwell RJA, McLatchie LM, Clarke MJ, Winter JB, Bevan S, McIntyre P. Capsaicin sensitivity is associated with the expression of vanilloid (capsaicin) receptor (VR1) mRNA in adult rat sensory ganglia. Neurosci Lett. 1998;250:177–180. doi: 10.1016/s0304-3940(98)00475-3. [DOI] [PubMed] [Google Scholar]
- Hunt CE, Brouillette RT. Sudden infant death syndrome: 1987 perspective. J Pediatr. 1987;110:669–678. doi: 10.1016/s0022-3476(87)80001-x. [DOI] [PubMed] [Google Scholar]
- Hwang SW, Cho H, Lee SY, Kang CJ, Jung JY, Cho S, Min KH, Suh YG, Kim DH, Oh U. Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc Natl Acad Sci. 2000;97:6155–6160. doi: 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafri A, Belkadi A, Zaidi SI, Getsy P, Wilson CG, Martin RJ. Lung inflammation induces IL-1beta expression in hypoglossal neurons in rat brainstem. Respir Physiol Neurobiol. 2013;188:21–28. doi: 10.1016/j.resp.2013.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeske NA, Diogenes A, Ruparel NB, Fehrenbacher JC, Henry M, Akopian AN, Hargreaves KM. A-kinase anchoring protein mediates TRPV1 thermal hyperalgesia through PKA phosphorylation of TRPV1. Pain. 2008;138:604–616. doi: 10.1016/j.pain.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Lee LY. Role of TRPV receptors in respiratory diseases. Biochim Biophys Acta. 2007;1772:915–927. doi: 10.1016/j.bbadis.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Jin YH, Bailey TW, Li B, Schild JH, Andresen MC. Puringergic and vanilloid receptor activation releases glutamate from separate cranial afferent terminals in nucleus tractus solitarius. J Neurosci. 2004;24:4709–4717. doi: 10.1523/JNEUROSCI.0753-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- Kahn A, Groswasser J, Rebuffat E, Scottiaux M, Blum D, Foerster M, Franco P, Bochner A, Alexander M, Bachy A, Richard P, Verghote M, Le Polain D, Wayenberg JL. Sleep and cardiorespiratory characteristics of infant victims of sudden death: a prospective case-control study. Sleep. 1992;15:287–292. doi: 10.1093/sleep/15.4.287. [DOI] [PubMed] [Google Scholar]
- Kawabata A. Prostaglandin E2 and pain—an update. Biol Pharm Bull. 2011;34:1170–1173. doi: 10.1248/bpb.34.1170. [DOI] [PubMed] [Google Scholar]
- Kinney HC, Thach BT. The sudden infant death syndrome. N Engl J Med. 2009;361:795–805. doi: 10.1056/NEJMra0803836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleemann WJ, Schlaud M, Fieguth A, Hiller AS, Rothämel T, Tröger HD. Body and head position, covering of the head by bedding and risk of sudden infant death (SID) Int J Legal Med. 1998;112:22–26. doi: 10.1007/s004140050192. [DOI] [PubMed] [Google Scholar]
- Lanier B, Richardson MA, Cummings C. Effect of hypoxia on laryngeal reflex apnea—implications for sudden infant death. Otolaryngol Head Neck Surg. 1983;91:597. doi: 10.1177/019459988309100602. [DOI] [PubMed] [Google Scholar]
- Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JC, Stoll BJ, Downing SE. Properties of the laryngeal chemoreflex in neonatal piglets. Am J Physiol. 1977;233:R30–R36. doi: 10.1152/ajpregu.1977.233.1.R30. [DOI] [PubMed] [Google Scholar]
- Leiter JC, Böhm I. Mechanisms of pathogenesis in the Sudden Infant Death Syndrome (SIDS) Respir Physiol Neurobiol. 2007;159:127–138. doi: 10.1016/j.resp.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Leiter JC. Serotonin, gasping, autoresuscitation and SIDS—a contrarian view. J Appl Physiol. 2009:106. doi: 10.1152/japplphysiol.00329.2009. [DOI] [PubMed] [Google Scholar]
- Lindgren C, Grogaard J. Reflex apnoea response and inflammatory mediators in infants with respiratory tract infection. Acta Paediatr. 1996;85:798–803. doi: 10.1111/j.1651-2227.1996.tb14154.x. [DOI] [PubMed] [Google Scholar]
- Lindgren C, Jing L, Graham B, Grogaard J, Sundell H. Respiratory syncytial virus infection reinforces reflex apnea in young lambs. Pediatr Res. 1992;31:381–385. doi: 10.1203/00006450-199204000-00015. [DOI] [PubMed] [Google Scholar]
- Luna LG. Histopathologic Methods and Color Atlas of Special Stains and Tissue Artifacts. American Histolabs, Inc; Gaithersburg: 1992. [Google Scholar]
- Matta JA, Ahern GP. TRPV1 and synaptic transmission. Curr Pharm Biotechnol. 2011;12:95–101. doi: 10.2174/138920111793937925. [DOI] [PubMed] [Google Scholar]
- Merazzi D, Mortola JP. Effects of changes in ambient temperature on the Hering-Breuer reflex of the conscious newborn rat. Pediatr Res. 1999;45:370–376. doi: 10.1203/00006450-199903000-00014. [DOI] [PubMed] [Google Scholar]
- Mezey A, Toth ZE, Cortright DN, Arzubi MK, Krause JE, Elde R, Guo A, Blumberg PM, Szallasi A. Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like immunoreactivity, in the central nervous system of the rat and human. Proc Natl Acad Sci. 2000;97:3655–3660. doi: 10.1073/pnas.060496197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama T, Higashi T, Togashi K, Iida T, Segi E, Sugimoto Y, Tominaga T, Narumiya S, Tominaga M. Sensitization of TRPV1 by EP1 and IP reveals peripheral nociceptive mechanism of prostaglandins. Mol Pain. 2005;1:3. doi: 10.1186/1744-8069-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutoh T, Kanamaru A, Kojima K, Nishimura R, Sasaki N, Tsubone H. Effects of perineural capsaicin treatment on cardiopulmonary reflexes elicited by laryngeal instillations of capsaicin and distilled water in sevoflurane-anesthetized dogs. J Vet Med Sci. 2000;62:665–668. doi: 10.1292/jvms.62.665. [DOI] [PubMed] [Google Scholar]
- Niblock MM, Luce CJ, Belliveau RA, Paterson DS, Kelley ML, Sleeper LA, Filiano J, Kinney HC. Comparative anatomical assessment of the piglet as a model for the developing human medullary serotonergic system. Brain Res Rev. 2005;50:169–183. doi: 10.1016/j.brainresrev.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Page M, Jeffery HE. The role of gastro-oesophageal reflux in the aetiology of SIDS. Early Hum Dev. 2000;59:127–149. doi: 10.1016/s0378-3782(00)00093-1. [DOI] [PubMed] [Google Scholar]
- Page M, Jeffery HE, Post EJ, Wood AKW. Simulated pharyngeal reflux can lead to life-threatening apnea if swallowing and arousal are depressed. J Sudd Infant Death Syndr Infant Mortal. 1996;1:281–294. [Google Scholar]
- Park KH, Cho SH, Song CE, Kim DH, Kim HT. Neuroimmunological activation of the afferent laryngeal neural circuit in experimentally induced laryngeal inflammation. Acta Otolaryngol. 2005;125:184–190. doi: 10.1080/00016480410017170. [DOI] [PubMed] [Google Scholar]
- Patrickson JW, Smith TE, Zhou SS. Afferent projections of the superior and recurrent laryngeal nerves. Brain Res. 1991;539:169–174. doi: 10.1016/0006-8993(91)90702-w. [DOI] [PubMed] [Google Scholar]
- Patterson LM, Zheng H, Ward SM, Berthoud HR. Vanilloid receptor (VR1) expression in vagal afferent neurons innervating the gastrointestinal tract. Cell Tissue Res. 2003;311:277–287. doi: 10.1007/s00441-002-0682-0. [DOI] [PubMed] [Google Scholar]
- Peters JH, McDougall SJ, Fawley JA, Smith SM, Andresen MC. Primary afferent activation of thermosensitive TRPV1 triggers asynchronous glutamate release at central neurons. Neuron. 2010;65:657–669. doi: 10.1016/j.neuron.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrillo GA, Glass L, Trippenbach T. Phase locking of the respiratory rhythm in cats to a mechanical ventilator. Can J Physiol Pharmacol. 1983;61:599–607. doi: 10.1139/y83-092. [DOI] [PubMed] [Google Scholar]
- Poets CF, Meny RG, Chobanian MR, Bonofiglo RE. Gasping and other cardiorespiratory patterns during sudden infant death. Pediatr Res. 1999;45:350–354. doi: 10.1203/00006450-199903000-00010. [DOI] [PubMed] [Google Scholar]
- Ponsonby AL, Dwyer T, Couper D, Cochrane JA. Association between use of a quilt and sudden infant death syndrome: case-control study. BMJ. 1998;316:195–196. doi: 10.1136/bmj.316.7126.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premkumar LS, Abooj M. TRP channels and analgesia. Life Sci. 2013;92:415–424. doi: 10.1016/j.lfs.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raza MW, Blackwell CC. Sudden infant death syndrome, virus infections and cytokines. FEMS Immunol Med Microbiol. 1999;25:85–96. doi: 10.1111/j.1574-695X.1999.tb01330.x. [DOI] [PubMed] [Google Scholar]
- Roulier S, Arsenault J, Reix P, Dorion D, Praud JP. Effects of C fiber blockade on cardiorespiratory responses to laryngeal stimulation in concious lambs. Respir Physiol Neurobiol. 2003;136:13–23. doi: 10.1016/s1569-9048(03)00108-3. [DOI] [PubMed] [Google Scholar]
- Samad TA, Moore KA, Sapirstein A, Billet S, Allchorne A, Poole S, Bonventre JV, Woolf CJ. Interleukin-1beta-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature. 2001;410:471–475. doi: 10.1038/35068566. [DOI] [PubMed] [Google Scholar]
- Sasaki CT. Development of laryngeal function: etiologic significance in the sudden infant death syndrome. Laryngoscope. 1979;89:1964–1982. doi: 10.1288/00005537-197912000-00010. [DOI] [PubMed] [Google Scholar]
- Schaible HG. Nociceptive neurons detect cytokines in arthritis. Arthr Res Ther. 2014;16:470. doi: 10.1186/s13075-014-0470-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiyama N, Mizuta S, Hori A, Kobayashi S. Prostaglandin E2 facilitates excitatory synaptic transmission in the nucleus tractus solitarii of rats. Neurosci Lett. 1995;188:101–104. doi: 10.1016/0304-3940(95)11407-n. [DOI] [PubMed] [Google Scholar]
- Sheers-Masters JR, Schootman M, Thach BT. Heat stress and Sudden Infant Death Syndrome incidence: a United States population epidemiologic study. Pediatrics. 2004;113:e586–e592. doi: 10.1542/peds.113.6.e586. [DOI] [PubMed] [Google Scholar]
- Smart D, Gunthorpe MJ, Jerman JC, Nasir S, Gray JE, Muir AI, Chambers JK, Randall AD, Davis JB. The endogenous lipid anadamide is a full agonist at the human vanilloid receptor (hVR1) Br J Pharm. 2000;129:227–230. doi: 10.1038/sj.bjp.0703050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridhar R, Thach BT, Kelly DH, Henslee JA. Characterization of successful and failed autoresuscitation in human infants. Pediatr Pulmonol. 2003;36:113–122. doi: 10.1002/ppul.10287. [DOI] [PubMed] [Google Scholar]
- Steinschneider A. Prolonged apnea and the sudden infant death syndrome: clinical and laboratory observations. Pediatrics. 1972;50:646–654. [PubMed] [Google Scholar]
- Stoltenberg L, Sundar T, Almaas R, Storm H, Rognumm TO, Saugstad OD. Changes in apnea and autoresuscitation in piglets after intravenous and intrathecal interleukin-1 beta injection. J Perinat Med. 1994;22:421–432. doi: 10.1515/jpme.1994.22.5.421. [DOI] [PubMed] [Google Scholar]
- Sun H, Li DP, Chen SR, Hittelman WN, Pan HL. Sensing of blood pressure increase by transient receptor potential vanilloid 1 receptors on baroreceptors. J Pharmacol Exp Ther. 2009;331:851–859. doi: 10.1124/jpet.109.160473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thach BT. Reflux associated apnea in infants: evidence for a laryngeal chemoreflex. Am J Med. 1997;103(5A):120S–124S. doi: 10.1016/s0002-9343(97)00336-7. [DOI] [PubMed] [Google Scholar]
- Thach BT. Maturation and transformation of reflexes that protect the laryngeal airway from liquid aspiration from fetal to adult life. Am J Med. 2001;111:69S–77S. doi: 10.1016/s0002-9343(01)00860-9. [DOI] [PubMed] [Google Scholar]
- Thach BT. The role of respiratory control disorders in SIDS. Respir Physiol Neurobiol. 2005;149:343–353. doi: 10.1016/j.resp.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Thrane PS, Maehlen J, Stoltenberg L, Brandtzaeg P. Retrograde axonal cytokine transport: a pathway for immunostimulation in the brain inducing hypoxia and sudden infant death? Med Hypotheses. 1995;44:81–84. doi: 10.1016/0306-9877(95)90074-8. [DOI] [PubMed] [Google Scholar]
- Tsakiri N, Kimber I, Rothwell NJ, Pinteaux E. Interleukin-1-induced interleukin-6 synthesis is mediated by the neutral sphingomyelinase/Src kinase pathway in neurones. Br J Pharmacol. 2008;153:775–783. doi: 10.1038/sj.bjp.0707610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SM, Taylor BJ, Mitchell EA. Sudden infant death syndrome: insulation from bedding and clothing and its effect modifiers. Int J Epidemiol. 1996;25:366–375. doi: 10.1093/ije/25.2.366. [DOI] [PubMed] [Google Scholar]
- Xia L, Damon T, Niblock MM, Bartlett D, Jr, Leiter JC. Unilateral microdialysis of gabazine in the dorsal medulla reverses thermal prolongation of the laryngeal chemoreflex in decerebrate piglets. J Appl Physiol. 2007a;103:1864–1872. doi: 10.1152/japplphysiol.00524.2007. [DOI] [PubMed] [Google Scholar]
- Xia L, Bartlett D, Jr, Leiter JC. An adenosine A2A antagonist injected in the NTS reverses thermal prolongation of the LCR in decerebrate piglets. Respir Physiol Neurobiol. 2008;164:358–365. doi: 10.1016/j.resp.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia L, Bartlett D, Jr, Leiter JC. TRPV1 channels in the nucleus of the solitary tract mediate thermal prolongation of the LCR in decerebrate piglets. Respir Physiol Neurobiol. 2011;176:21–31. doi: 10.1016/j.resp.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Velde L, Curran A, Filiano JJ, Darnall RA, Bartlett D, Jr, Leiter JC. Prolongation of the laryngeal chemoreflex after inhibition of the rostroventral medulla in piglets: a role in SIDS? J Appl Physiol. 2003;94:1883–1895. doi: 10.1152/japplphysiol.01103.2002. [DOI] [PubMed] [Google Scholar]
- Xia L, Damon TA, Leiter JC, Bartlett D., Jr Focal warming of the nucleus of the solitary tract prolongs the laryngeal chemoreflex in decerebrate piglets. J Appl Physiol. 2007b;102:54–62. doi: 10.1152/japplphysiol.00720.2006. [DOI] [PubMed] [Google Scholar]