Abstract
Stem cell therapy (SCT) raises the hope for cardiac regeneration after ischemic heart disease. However, the molecular mechanisms underlying repair of dead myocardium in the ischemic heart is poorly understood. Growing evidences suggest that cardiac matrix stiffness and differential expressions of miRNAs play a crucial role in stem cell survival and differentiation. However, their roles on transplanted stem cells, for the myocardial repair of the ischemic heart, remain unclear. Transplanted stem cells may act in an autocrine and/or paracrine manner to regenerate the dead myocardium. Paracrine mediators such as stem cell-derived exosomes are emerging as a novel therapeutic strategy to overcome some of the limitations of SCT. These exosomes carry microRNAs (miRNAs) that may regulate stem cell differentiation into a specific lineage. MicroRNAs may also contribute to stiffness of surrounding matrix by regulating extracellular matrix (ECM) turnover. The survival of transplanted stem cell depends on its autophagic process that maintains cellular homeostasis. Therefore, exosomes, miRNAs, extracellular matrix turnover, and autophagy may have an integral role in improving the efficacy of SCT. This review elaborates the specific roles of these regulatory components on cardiac regeneration in ischemic hearts during SCT.
Keywords: Exosomes, microRNA, extracellular matrix, trans-differentiation, MMP9, autophagy
Introduction
Ischemic heart disease is a leading cause of mortality in the world. As per the 2012 World Health Organization report 7.4 million people die due to ischemic heart disease (http://www.who.int/mediacentre/factsheets/fs310/en/). Restricted blood supply to the ventricular muscles, due to narrowing of coronary arteries, results in ischemia that compromises oxygen supply to cardiomyocytes and other cells in the myocardium. Severe ischemia leads to acute myocardial infarction (MI) that results into massive loss of cardiomyocytes (1). The adult mammalian heart does not have adequate regenerative capacity to replenish the loss of damaged myocardium after MI. Therefore, MI leads to heart failure (2). Stem cell therapy (SCT) provides a strategy to regenerate new myocardium to replenish dead/damaged myocardium of MI hearts by using exogenous stem cell transplantation (3, 4). However, the survival, proliferation, and differentiation of transplanted stem cells depend on several factors including the stiffness of extracellular matrix (ECM) surrounding the stem cells (5-10). Proteolysis of ECM by matrix metalloproteinase (MMPs) is common in cardiovascular diseases (CVD) (11). MMP9 plays an important role in ECM degradation in pathological hearts that leads to cardiac fibrosis, a stiffer ECM, that may influence cardiac stem cell survival and differentiation (8). MMPs are regulated by microRNAs (miRNAs) (12, 13). MiRNAs are tiny non-coding RNAs that regulate biological functions of a cell by modulating expression of genes (14). MiRNAs have emerged as a novel therapeutic target for CVD (15-17). MiRNAs may play a pivotal role in stem cell survival because they regulate stem cell autophagy (18, 19). Autophagy is a lysosomal degradation process that regulates cellular homeostasis (20). MiRNAs may regulate cardiac stem cell proliferation and differentiation (21) by acting in an autocrine and/or a paracrine fashion (22, 23). MiRNAs encapsulated in an exosome circulate through blood and may have paracrine effects (24). Exosomes are lipid bilayer nanovesicles released by different types of cells when endosomes carrying multivesicles fuse with plasma membrane. Exosomes exert their therapeutic actions by involving in cell-cell interactions and transferring proteins, RNAs (25), and miRNAs (23). Exosomes derived from cardiac stem cells are promising therapeutic candidate because in one hand it may regulate survival, proliferation and differentiation of the transplanted stem cells whereas on the other hand it may overcome the limitations of SCT due to immune rejections, teratoma, or ethical concerns. In this review, we elaborated the roles for exosomes, miRNAs, autophagy, and extracellular matrix turnover in cardiac regeneration during stem cell therapy in ischemic hearts.
Stem cells characteristics and types
Stem cells are pluripotent cells that can differentiate into different lineages to regenerate different types of cells (26). Based on origin, stem cells are classified into embryonic stem cells (ESCs) and adult stem cells (ASCs). ESCs can be maintained in tissue culture, while retaining their pluripotency (27). ESCs in cell culture express the intrinsic transcription factor Oct4 and constitutively receive the extrinsic signal from the leukemia inhibitory factor (LIF) to maintain their pluripotent state (28, 29). Adult stem cells (ASCs) are slow cycling cells that are able to respond to specific environmental signals to either proliferate or differentiate. During differentiation, these ASCs enter into a transient state of rapid proliferation (30), withdraw from cell-cycle, and execute terminal differentiation. ASCs are localized in specific niches, where they utilize many of the extrinsic and intrinsic cues used by their embryonic counterparts in selecting a specific fate. ASCs are roughly categorized into bone marrow stem cells (BM-SCs), circulating pool of progenitor cells such as endothelial progenitor cells (EPCs), and tissue-resident stem cells such as cardiac stem cells (CSCs). BM-SCs are further categorized into mesenchymal stem cells (MSCs) and hematopoietic stem cells (HSCs) (31). According to the expression of surface markers and properties, resident CSCs were classified into different subsets such as c-Kit-positive (c-Kit+) cells, Sca-1-positive (Sca-1POS) cells, side population (SP) cells, cardiosphere cells, and Isl1-positive (Isl1POS) cells (32). CSCs are multipotent cells that can differentiating into multiple lineages; such as cardiomyocytes, smooth muscle cells, and endothelial cells (33, 34).
Regulators of CSCs proliferation and differentiation
The adult heart has small number of CSCs that may have potential for cardiac regeneration (35-37). Stem cells can be differentiated into cardiomyocyte with the treatment of a specific combination of factors (38). CSCs were identified and validated using various markers such as c-kit, MDR-1, and Sca-1 (38, 39). They are heterogeneous and expresses 7%-10% of important cardiogenic transcription factors like Nkx2.5, GATA4, and MEF2 (36). CSCs can divide both symmetrically and asymmetrically, however, asymmetrical division is predominant (40). They regulate myocytes turnover, which is heterogeneous across the heart. Myocytes turnover is faster at the apex and atria and slower at the base-and mid- regions of the ventricle (38, 41). The studies on CSCs differentiation were performed primarily on mice and chick embryos. The formation of cardiomyocytes from mesoderm is regulated by Wnts, BMPs, and Nodal (42, 43). Inhibition of Nodal (a family member of TGF-β), and Wnt promotes formation of cardiomyocytes in xenopus and chick embryos (44-46). Inhibition of Nodal and Wnt is also important for differentiation of mouse ESC into cardiomyocytes (46-48). The transmembrane receptor Notch induces a combination of growth factors that up regulates differentiation of ESC-derived mesoderm subpopulations into cardiac progenitors (49). These growth factors include Wnt5a, BMP6, and secreted frizzled-related protein1 (Sfrp1) (50). The differentiation of committed cardiac progenitors into cardiomyocytes is the last step of differentiation, and is poorly understood. It is believed that Wnt11 plays a crucial role in this last step (48, 51). The transduction of Wnt11 promotes mesenchymal stem cell trans-differentiation into cardiac phenotypes in vitro (52). Several transcription factors regulates differentiation of pluripotent stem cells (PSCs) into cardiac fate. These transcription factors include T Brachyury for primitive streak mesoderm, mesoderm posterior 1(Mesp-1) for cardiogenic mesoderm, and Nkx2.5, T-box (Tbx5/20), GATA4, MEF2C, and Hand1/2 for cardiac mesoderm (53-57). Cardiac development is a complex process that is tightly controlled by the sequential expression of multiple signal transduction proteins and transcription factors working in a synergistic manner. The most studied of these growth factors and signaling pathways include FGFs, BMPs, and Wnts/Nodal (58-61). We have summarized the important regulators of stem cells proliferation and differentiation in Figure 1.
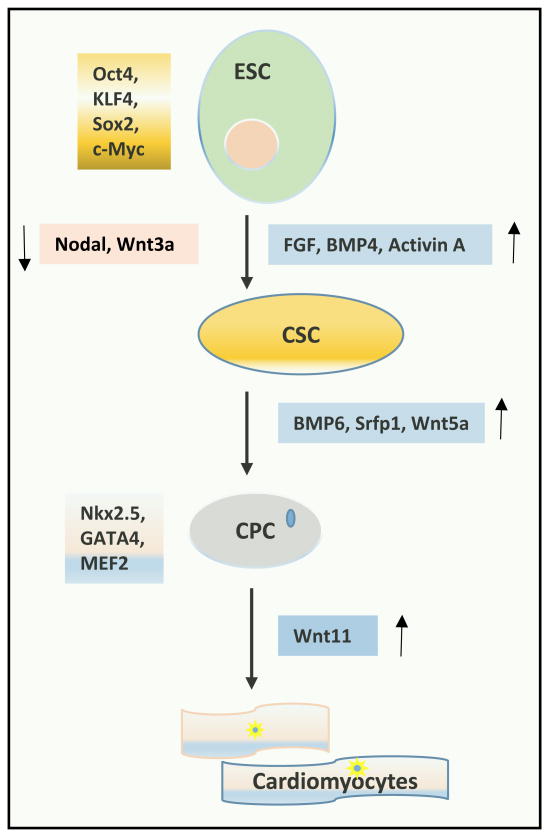
Figure 1.
Regulators for embryonic stem cell (ESC) differentiation into cardiomyocytes. Transcription factors Oct4, KLF4, Sox2 and c-Myc are required for maintaining embryonic stem cell pluripotency. Inhibition of signaling molecules Wnt3a, and nodal while upregulation of FGF, BMP4, and Activin A are required for differentiation of ESC into cardiac stem cell (CSC). Activity of BMP6, Srfp1, and Wnt5a are required for differentiation of CSC into cardiac lineage specific cardiac progenitor cell (CPC). Nkx2.5, GATA4 and MEF2 maintain cardiac lineage specificity. Wnt11 is involved in differentiation of CPC into cardiomyocytes.
Role of autophagy in homeostasis of stem cells
Autophagy is an evolutionary conserved adaptive process required for cellular homeostasis and protecting against various pathological conditions including CVD. During autophagy defective cytoplasmic cargoes are sequestered into double membrane autophagosome which after fusion with lysosome are degraded and recycled (62). Autophagy maintains the quality control of stem and progenitor cells (63). Various properties of the stem cells such as pluripotency, quiescence, differentiation and self-renewal depends on autophagy activation (64, 65). Therefore, autophagy plays an important role in normal functions of stem and progenitor cells (66). Suppression of autophagy through fibroblast growth factor (FGF) signaling inhibits CSC differentiation (67). Autophagy may have different roles in different types of stem cells. It induces apoptosis in BM-MSCs of non-obese diabetic (NOD) mice (68) but promotes MSC-mediated hepatic regeneration in CC14-injured rat liver model (69) and MSC-mediated wound healing in diabetic mellitus patients (70).
Trans-differentiation of cells
Although differentiation of stem cells into a particular lineage is canonically the strategy for SCT, recent studies revealed that differentiated adult cells can be transdifferentiated into another phenotype by using certain factors. Fibroblasts are present in a large pool in the postnatal heart and they contribute to pathological remodeling via fibrosis. It is observed that by using developmental transcription factors (Gata4, Mef2c, and Tbx5), somatic fibroblast can be reprogrammed into cardiomyocytes in mouse heart (71). In neonatal and adult humans' fibroblasts addition of Gata4, Hand2, Tbx5, myocardin, miR-1 and miR-133 causes trans-differentiation of fibroblast into cardiomyocyte phenotype (72). There are several other factors are involved in this trans-differentiation process (73, 74). However, whether these cardiomyocytes can maintain the cardiomyocytes properties including contractility for prolong time and can maintain synchronous beating with resident cardiomyocytes, is unclear and requires further investigation.
Effect of extracellular matrix turnover on stem cell differentiation
The mechanical force of ECM may influence survival, proliferation, and differentiation of stem cells, and also trans-differentiation of other cells into cardiomyocytes. The mechanical load of the ECM contributes to differentiation of MSCs (75-78). Transforming growth factor- beta (TGF-β) promotes MSC differentiation into a smooth muscle lineage on stiff substrates (79, 80). Soft matrix promotes MSC differentiation into chondrogenic and adipogenic lineages. However, matrix stiffness may not be specific for only one lineage. Biochemical factors such as TGF-β are required to define a unique differentiation pathway (81). ECM stiffness depends on matrix turnover, which is determined by the balance between MMPs and tissue inhibitors of metalloproteinases (TIMPs) (82). MMP-9 and TIMP-4 are predominantly involved in cardiac remodeling. MMP-2 and MMP-9 are collagenases that degrade ECM and contribute to fibrosis (82, 83), where ECM is stiffer (Figure 2). Stiffness of cardiac ECM may play a pivotal role in stem cell therapy (84). MMP9 is also involved in inhibiting EPCs-mediated increase in vessel density in the peri-infarct area in the mouse brain (85). Moreover, it is implicated in migration of c-Kit+ CSCs, which is partially mediated by stem cell factor (SCF) via the activation of PI3K/AKT/MMP-2/-9 signaling pathway (86). These reports indicate the diverse roles of MMPs. Along with MMPs, it was reported that various miRNA family members also regulate ECM. These miRNAs have either pro-, or anti-fibrotic roles in various tissues (87).
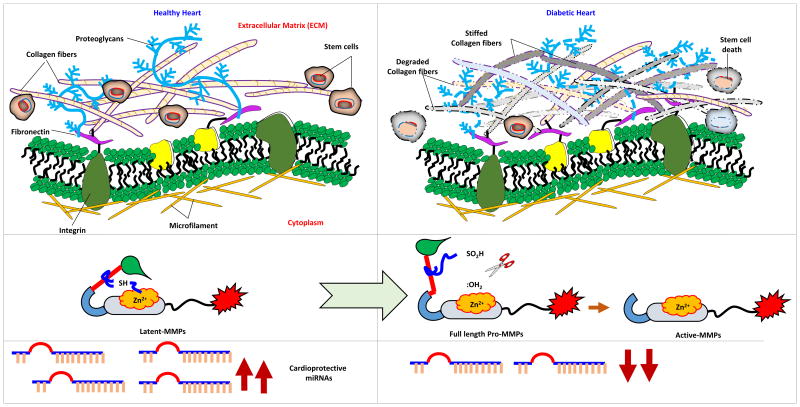
Figure 2.
Extracellular matrix (ECM) remodeling in diabetic heart. In healthy heart collagen and elastin are present in optimal ratio which might help in maintaining the integrity of the ECM and niche of stem cells. In pathological heart such as diabetic heart, activity of MMPs is augmented, expression of cardio-protective miRNAs is attenuated, stiffness of ECM, and apoptosis of stem cells are induced.
MicroRNAs in stem cell proliferation and differentiation
MiRNAs are 22 nucleotide long, non-coding RNAs that modulate gene expression and stem cell proliferation and differentiation (88). They have emerged as a biomarker and a therapeutic target for cardiovascular diseases (15, 89, 90). MiR-29 was found to be an important component of TGF-β signaling, which also regulates collagen synthesis (87). MiR-1, miR-24, miR-29b, miR-101, and miR-200b are anti-fibrotic, whereas miR-15 family, miR-21, miR-34a, miR-192, miR-199b, and miR-208 are pro-fibrotic miRNAs (87). As discussed above, fibrosis changes the ECM tensile properties and ECM related miRNAs can influence stem cell physiology in normal and pathological conditions. In Table 1, we have shown the list of miRNAs targeting important ECM regulators including MMPs, TIMPs, CTGF, and TGF-β (91-137). The information is obtained from online database miRTarbase (http://mirtarbase.mbc.nctu.edu.tw/).
Table 1.
MiRNAs that regulates extracellular matrix turn over in mouse and human hearts.
| Genes involved in ECM turn over | miRNA (Mouse) | Ref. | miRNA (Human) | Ref. |
|---|---|---|---|---|
| MMP9 | miR-204-5p, miR-212-3p, miR-132-3p, miR-320-3p | (91-93) | miR-451a, miR-491-5p, miR-338-3p, miR-21-5p | (91, 94-97) |
| MMP2 | --- | --- | miR-29b-3p, miR-451a, miR-335-5p, miR-338-3p, miR-21-5p, miR-17-5p | (94, 96-100) |
| MMP7 | --- | --- | miR-126-5p, miR-126-3p | (101) |
| MMP12 | --- | --- | miR-145-5p | (102) |
| MMP-14 | --- | --- | miR-335-5p, miR-145-5p | (99, 102) |
| MMP1 | --- | --- | miR-222-3p, miR-203a, miR-145-5p | (102-104) |
| MMP13 | --- | --- | miR-9-5p, miR-100-5p, miR-27b-3p, miR-140-5p, miR-27a-3p, miR-125b-5p, miR-143-3p, miR-132-3p | (105-111) |
| TIMP-1 | --- | --- | miR-519a-3p, miR-26b-5p | (112, 113) |
| TIMP2 | --- | --- | miR-519d-3p, miR-519c-3p, miR-200c-3p | (112, 114) |
| TIMP3 | miR-181b-5p, miR-206-3p, miR-7b-5p, miR-181a-5p, miR-149-5p, miR-124-3p | (115-118) | miR-181b-5p, miR-21-5p, miR-1, miR-222-3p, miR-221-3p, miR-103a-3p, miR-335-5p, miR-124-3p, miR-423-5p, miR-30c-2-3p, miR-18b-5p, miR-16-5p, let-7e-5p | (99, 119-124) |
| TGF-β | --- | ---- | miR-24-3p, miR-29b-3p, miR-144-3p, miR-633, miR-663a, miR-21-5p | (124-129, 145) |
| CTGF | miR-122-5p, miR-425-5p, miR-297a-5p, miR-9-5p | (117, 130) | miR-124-3p, miR-18a-5p, miR-26a-5p, miR-205-5p, miR-145-5p, miR-375, miR-19b-3p, miR-19a-3p | (117, 131-137) |
Abbreviations: MMPs, Matrix metalloproteinases; TIMPs, Tissue inhibitor of metalloproteinases; TGF-β, Transforming growth factor-β; CTGF, Connective tissue growth factor; ECM, Extra cellular matrix.
MicroRNAs (miRNAs) regulate differentiation of stem cells into cardiomyocyte (138). MiR-1 induces differentiation of mESCs and hESCs into cardiac phenotype (51, 139). MiR-1 promotes differentiation of stem cell by targeting HDAC4, which is a negative regulator of MEF2, whereas miR-133 promotes stem cell proliferation by targeting SRF. The differential expression of miRNAs in ESCs and CSCs is nicely reviewed by Kuppusamy et al (140). MiR-1, miR-21, miR -133a, miR -133b, and miR -145 are upregulated both in mouse and human ESC differentiation into cardiac lineage, whereas miR-20b is downregulated during this process in both species (140). Empirical evidences demonstrate that several miRNAs are deregulated during differentiation of embryonic stem cells into cardiac stem cell lineage (140-143). It is also reported that miR-499 along with miR-1 and miR-208 regulates cardiomyocyte differentiation (143). MiR-133-a, -b, miR-125-a, -b, miR-126, miR-23-a, -b, miR-24, miR -30C, miR-132 are differentially expressed during mouse CSC differentiation (144). There are several miRNAs that regulate both ECM turnover (Table 1) and stem cell proliferation and differentiation (Table 2). MiR-1, miR-21-5p, miR-26a-5p, miR-26b-5p, miR-30c-2-3p, miR-126-3p, miR-126-5p, miR-145-5p, miR-30a, miR-30b, miR-99b, miR-125a-5p, miR-129-3p, miR-133a, miR-133b, miR-148a, miR-181b, miR-652 are upregulated whereas miR-17-5p, miR-124-3p, miR-200c-3p, miR-205-5p, miR-20a, miR-20b, miR-106a, miR-106b, miR-182, miR-183, miR-183*, miR-302c, miR-302c* are downregulated during differentiation of SC into cardiomyocytes (140, 143). MiRNAs which are involved in regulating ECM turnover include let-7e-5p (124), miR-100-5p (106), miR-103a-3p (121), miR-125b-5p (109), miR-132-3p (111), miR-140-5p (108), miR-143-3p (110), miR-144-3p (121), miR-16-5p (124), miR-181b-5p (119), miR-18a-5p (132), miR-18b-5p (124), miR-19a-3p (137), miR-19b-3p (132), miR-203a (104), miR-221-3p, miR-222-3p (119), miR-24-3p (125), miR-27a-3p (108), miR-27b-3p (107), miR-29b-3p (145), miR-335-5p (99), miR-338-3p (96), miR-375 (136), miR-423-5p (124), miR-451a (94), miR-491-5p (95), miR-519a-3p, miR-519c-3p, miR-519d-3p (112), miR-633, miR-663a (128), miR-9-5p (105). Therefore, miRNAs play an integral role in SCT (Figure 3).
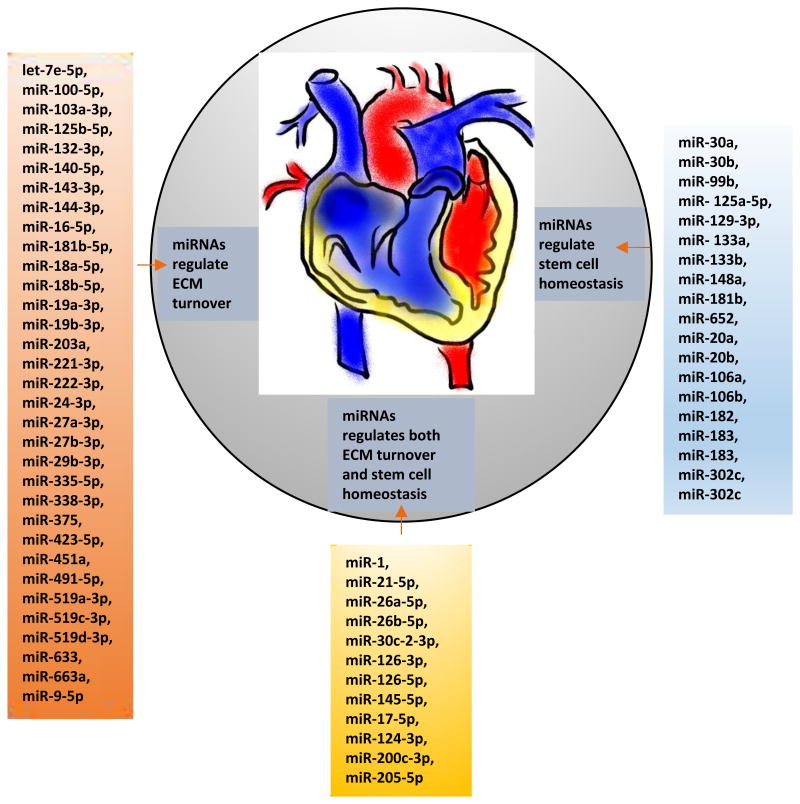
Figure 3.
MiRNAs involve in ECM turn over and stem cell differentiation. Left panel shows 33 miRNAs that regulate ECM turnover, right panel shows 19 miRNAs that regulate stem cell homeostasis, and middle panel represents miRNAs that regulate both stem cell homeostasis and ECM turnover.
MicroRNAs in trans-differentiation
Cardiac fibroblasts can be reprogrammed to cardiomyocytes using combination of different miRNAs (miR-1, miR- 133, miR- 208 and miR- 499) (146). Administration of these miRNAs into ischemic boarder zone of MI hearts induces trans-differentiation of cardiac fibroblasts into cardiomyocytes. Although miR-1 may be sufficient to induce cardiomyocyte trans-differentiation, the combination of miR-133, -208, and -499 is much more effective in the trans-differentiation process. Fibroblast-turned cardiomyocytes have all the properties of functional cardiomyocytes including contractility and spontaneous calcium oscillations (146). Therefore, trans-differentiation of fibroblast to cardiomyocytes by miRNAs provides a novel opportunity in SCT.
Stem cell therapy for cardiac regeneration
Stem cell therapy (SCT) is one of the propitious approaches to promote cardiac regeneration or repair myocardium after MI (147, 148). In vitro and in vivo studies have shown the transformation of various types of stem cells such as ESC (149), iPSCs (150), BM-SCs(151, 152), and adult tissue derived MSCs(14, 152, 153), HSCs (154), CSCs (155), adipose stem cells (156), and EPCs (157, 158) into cardiomyocyte lineage. Growing evidence suggest that cardiac regeneration by SCT is influenced by several paracrine factors (159, 160). Moreover, the homing of transplanted stem cells is dictated by the cytokines released from the damaged tissue (161). Broad range of cytokines, chemokines, growth factors such as vascular endothelial growth factors (VEGF), fibroblast growth factors (FGF), insulin-like growth factor-1 (IGF-1), and hepatocyte growth factor (HGF) have been shown to stimulate regeneration. Exosomes are one of the various paracrine mediators, which play an important role as regulators in cell autonomous repair mechanisms (162).
Role of miRNA containing exosomes in cardiac regeneration
Exosomes originate from inward folding of cell membranes which results in the formation of multiple intraluminal vesicles in the endosome called multivascular bodies (MVBs). These MVBs fuse with the plasma membrane releasing intraluminal vesicles into the extracellular matrix in the form of exosomes (163-165). They are present as extracellular space as vesicles (163). The diameter of exosome range from 30-120 nm. Exosome was first reported in sheep reticulocytes in early 1950s (165, 166). Exosomes are secreted from various types of cells including stem cells (167), cardiomyocytes (168), B cells (169), T cells (170), dendritic cells (171), platelets (172), Schwann cells (173), endothelial cells (174), and tumor cells (175). They are present in various body fluids such as blood, urine, plasma, semen, and broncho-alveolar lavage, and play an important role in intercellular communication (176, 177). They also play a pivotal role in modulation of immune responses and cell signaling pathways (178-180).
Different types of exosomes behave differently based on their origin. Stem cell exosomes are released by different types of stem cells such as pluripotent stem cell (embryonic stem cell and induced pluripotent derived exosomes) and adult stem cell (mesenchymal, endothelial progenitor and cardiac progenitor cell derived exosomes). The role of stem cell exosomes on cardiac repair along with their roles in normal and infarcted heart is reviewed by others (165, 181). Exosomes released during stress or pathological conditions behave differently compared to healthy conditions (182). ESCs serve as a promising source of exosomes due to their unique microRNA and protein content to augment endogenous CPCs proliferation and differentiation. Mir-290 family is highly expressed in ESC-derived exosomes in the mouse cardiomyocytes, which is evident from the elevated levels of miR-291, miR-294 and miR-295. These exosomes might have an important role in ESC exosome-mediated cardiac repair. Therefore, these exosome are implicated in stem cell survival, proliferation and differentiation into cardiomyocyte lineage (183). Few studies have reported cardioprotective effects of CPC-derived exosomes in myocardial ischemia/reperfusion (I/R) injury and MI model. CPC exosomes with miR-451/144 might exert beneficial effects (184). They also enhanced endothelial cell migration through extracellular matrix metalloproteinase inducer (EMMPRIN) (185). Exosomes with various miRNAs derived from CPCs in hypoxic conditions improve cardiac function in the injured heart (186). Cardiosphere derived cell (CDC) exosomes with miR-146a have elicited signature beneficial effects in MI model by improving global function and decreasing scar mass. Though therapeutic regeneration was observed with miR-146a-treated hearts but CDC exosomes excel in having more promising effects (187). CDC exosomes carrying miR-22 and miR-24, and play a prominent role in cardiac regeneration (188, 189). Exosomes are also released from mature cells present in the heart like cardiomyocytes and fibroblasts (190, 191). MiR-320 enriched exosomes in diabetic cardiomyocytes transfer miR-320 into endothelial cells and inhibit endothelial cell proliferation, migration, and myocardial angiogenesis in diabetics (191).
Stem cell therapy and stem cell-derived exosomes in clinical trial
The first stem cell based clinical trial with intracoronary infusion was “transplantation of progenitor cells and regenerative enhancement in acute myocardial infarction (TOPCARE)”, where bone marrow-derived mononuclear cells (BMMNCs) were used (192). Although, there was initial success with this population of cells for acute myocardial infarction (AMI) and chronic heart failure (CHF) but later in larger trials, no significant improvement in heart conditions was observed (193). Subsequent clinical trials were based on purified cell population. In Act34-CMI trial, CD34+ EPCs were used for chronic myocardial infarction (CMI) and reduction in frequency of angina was reported. However, in another trial (POSEIDON) using purified BM derived human MSCs, no improvement in ejection fraction was observed in patients (193). Cardiac specific stem cells were used in recently concluded SCIPIO trial. It is reported that c-kit-positive, lineage negative CSCs improve post- infarction left ventricle function (194). However, another group found that c-kit+ cells have minimal contribution to cardiomyocytes in the adult heart (195). There is controversy on whether c-kit positive cells are the marker of cardiac stem cells (196). CADUCEUS clinical trial used CSC cardiosphere and observed no cardiac benefits in AMI patients whereas C-CURE trial used cardiopoietic hMSCs and reported positive results in ischemic cardiomyopathy patients (193). Apart from various phase-I and II clinical trials, there are few ongoing phase-III clinical trials – BMI, CHART-1, CHART-2. The BMI trial used BMMNCs whereas CHART-1 and 2 used MSCs isolated from patient's bone marrow (197). Clinical trials on human ESCs and iPSCs in various ailments is reviewed by others (198). Transcoronary infusion of CPCs in patients with hypoplastic left heart syndrome, the (HLHS)- TICAP trial showed improvement in right ventricular ejection fraction that persisted during 36-month follow up (199). A list of existing and ongoing stem cell clinical trials are summarized in a recent review article by Poulin et al (200).
Limitations and Future perspective of stem cell therapy
Although several types of stem cells were used in clinical trials, they were successful only at different phases of clinical trials but mostly failed in larger trials, may be due to inappropriate choice of endpoints and/or less considerations for regulatory pathways involved in myocardial regeneration (201). Careful analyses of results from clinical trials will help us to understand the challenges to get success in stem cell therapy for heart failure (202, 203). To understand the cause of failure of larger clinical trial, it is imperative to evaluate the gene expression profiles of the transplanted stem cells after engraftment and to develop strategies that can facilitate the engraftment and differentiation of transplanted stem cells. The success of stem cell therapy may depend on homing and differentiation of transplanted stem cells to cardiac lineages that contribute to myocardial regeneration, the effect of paracrine factors that stimulate endogenous resident stem cell's differentiation to contribute to myocardial regeneration, and the microenvironment surrounding the niche of the stem cells that facilitate survival and differentiation of stem cells (88, 204). Recent studies demonstrated that stem cell exosomes could be a promising target for myocardial regeneration, and several preclinical trials reported improvement in myocardial regeneration by stem cell exosomes (205-208). Therefore, exosomes could be a novel approach for cardiac regeneration (209), and are given in pre-clinical studies for evaluating its safety and efficacy. MiRNAs from these exosomes can be also used as a biomarker for clinical outcome of the patients (210). Although miRNAs are now in clinical trials (211), stem cell-derived exosomes need further investigations to translate its role in SCT. One of the limitations of exosome-mediated cardiac regeneration is specificity and yield of exosomes (212). Developing techniques to isolate cardiac specific exosomes, and to deliver them to the border zone of the ischemic heart, understanding the mechanism of action of exosomes delivered to the ischemic heart, are some of the strategies for successful use of exosomes in regenerating damaged myocardium. An alternative strategy for replenishing the dead myocardium could be trans-differentiation of fibroblast into functional cardiomyocytes or inducing cardiomyocyte to reenter into cell cycle (213). Considering of ECM stiffness and its impact on stem cells, regulation of MMPs especially inhibition of MMP9 can be an important approach. Similarly, regulating of autophagy of stem cells is crucial for their survival and differentiation.
In summary, we can harness the basic science knowledge and clinical outcomes from the previous clinical trials to understand the factors that regulate survival of transplanted stem cells, differentiation of engrafted stem cells into a specific lineage such as cardiomyocytes, maintenance of cardiomyocyte's properties for prolong time. At the same time, we need to use systematic approach to improve cardiac regeneration in MI hearts and it may include autophagy, exosome, miRNAs, ECM stiffness, and trans-differentiation (Figure 4).
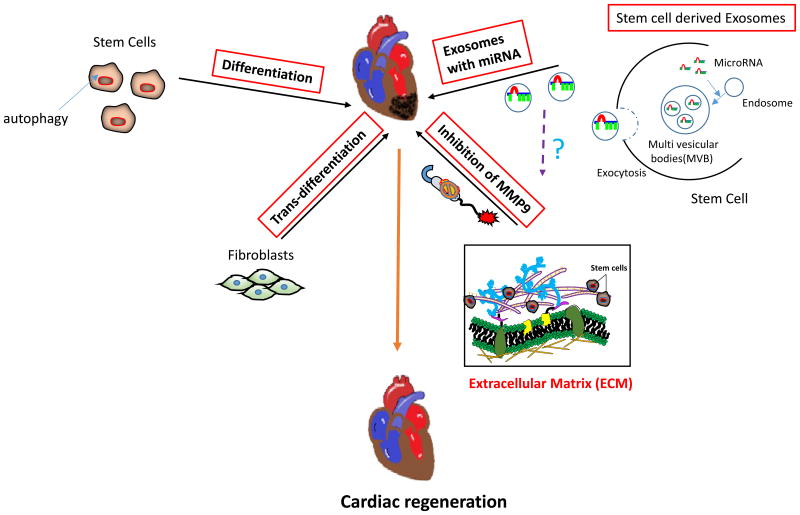
Figure 4.
Schematics for systemic approach for stem cell therapy. Cardiac regeneration can be achieved by using different approaches such as regulating autophagy in stem cells, using stem cell-derived exosomes, inhibiting matrix metalloproteinase-9 (MMP9) that may reduce stiffness of extracellular matrix to promote stem cell proliferation and differentiation, inducing trans-differentiation of fibroblasts into cardiomyocytes.
Supplementary Material
Acknowledgments
This work was supported in part by the National Institutes of Health grants: HL-113281 and HL-116205 to Paras Kumar Mishra.
Footnotes
Conflict of interest: Authors confirm that there are no conflicts of interest.
References
- 1.Li M, Izpisua Belmonte JC. Mending a Faltering Heart. CIRC RES. 2016;118(2):344–351. doi: 10.1161/CIRCRESAHA.115.306820. [DOI] [PubMed] [Google Scholar]
- 2.Narula J, Haider N, Virmani R, et al. Apoptosis in myocytes in end-stage heart failure. N ENGL J MED. 1996;335(16):1182–1189. doi: 10.1056/NEJM199610173351603. [DOI] [PubMed] [Google Scholar]
- 3.Sanganalmath SK, Bolli R. Cell therapy for heart failure: a comprehensive overview of experimental and clinical studies, current challenges, and future directions. CIRC RES. 2013;113(6):810–834. doi: 10.1161/CIRCRESAHA.113.300219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anversa P, Leri A, Kajstura J, et al. Myocyte growth and cardiac repair. J MOL CELL CARDIOL. 2002;34(2):91–105. doi: 10.1006/jmcc.2001.1506. [DOI] [PubMed] [Google Scholar]
- 5.Gilbert PM, Havenstrite KL, Magnusson KE, et al. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. SCIENCE. 2010;329(5995):1078–1081. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holst J, Watson S, Lord MS, et al. Substrate elasticity provides mechanical signals for the expansion of hemopoietic stem and progenitor cells. NAT BIOTECHNOL. 2010;28(10):1123–1128. doi: 10.1038/nbt.1687. [DOI] [PubMed] [Google Scholar]
- 7.Lei Y, Gojgini S, Lam J, et al. The spreading, migration and proliferation of mouse mesenchymal stem cells cultured inside hyaluronic acid hydrogels. BIOMATERIALS. 2011;32(1):39–47. doi: 10.1016/j.biomaterials.2010.08.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mishra PK, Chavali V, Metreveli N, et al. Ablation of MMP9 induces survival and differentiation of cardiac stem cells into cardiomyocytes in the heart of diabetics: a role of extracellular matrix. CAN J PHYSIOL PHARMACOL. 2012;90(3):353–360. doi: 10.1139/y11-131. [DOI] [PubMed] [Google Scholar]
- 9.Reilly GC, Engler AJ. Intrinsic extracellular matrix properties regulate stem cell differentiation. J BIOMECH. 2010;43(1):55–62. doi: 10.1016/j.jbiomech.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 10.Shav D, Einav S. The effect of mechanical loads in the differentiation of precursor cells into mature cells. ANN N Y ACAD SCI. 2010;1188:25–31. doi: 10.1111/j.1749-6632.2009.05079.x. [DOI] [PubMed] [Google Scholar]
- 11.Fingleton B. Matrix metalloproteinases as valid clinical targets. CURR PHARM DES. 2007;13(3):333–346. doi: 10.2174/138161207779313551. [DOI] [PubMed] [Google Scholar]
- 12.Wang J, Gao Y, Ma M, et al. Effect of miR-21 on renal fibrosis by regulating MMP-9 and TIMP1 in kk-ay diabetic nephropathy mice. CELL BIOCHEM BIOPHYS. 2013;67(2):537–546. doi: 10.1007/s12013-013-9539-2. [DOI] [PubMed] [Google Scholar]
- 13.Chaturvedi P, Kalani A, Medina I, et al. Cardiosome mediated regulation of MMP9 in diabetic heart: role of mir29b and mir455 in exercise. J CELL MOL MED. 2015;19(9):2153–2161. doi: 10.1111/jcmm.12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartel DP. MicroRNAs: target recognition and regulatory functions. CELL. 2009;136(2):215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mishra PK, Tyagi N, Kumar M, et al. MicroRNAs as a therapeutic target for cardiovascular diseases. J CELL MOL MED. 2009;13(4):778–789. doi: 10.1111/j.1582-4934.2009.00744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nouraee N, Mowla SJ. miRNA therapeutics in cardiovascular diseases: promises and problems. FRONT GENET. 2015;6:232. doi: 10.3389/fgene.2015.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wronska A, Kurkowska-Jastrzebska I, Santulli G. Application of microRNAs in diagnosis and treatment of cardiovascular disease. ACTA PHYSIOL (OXF) 2015;213(1):60–83. doi: 10.1111/apha.12416. [DOI] [PubMed] [Google Scholar]
- 18.Maiese K. MicroRNAs and SIRT1: A Strategy for Stem Cell Renewal and Clinical Development? J TRANSL SCI. 2015;1(3):55–57. [PMC free article] [PubMed] [Google Scholar]
- 19.Morgado AL, Xavier JM, Dionisio PA, et al. MicroRNA-34a Modulates Neural Stem Cell Differentiation by Regulating Expression of Synaptic and Autophagic Proteins. MOL NEUROBIOL. 2015;51(3):1168–1183. doi: 10.1007/s12035-014-8794-6. [DOI] [PubMed] [Google Scholar]
- 20.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. CELL. 2008;132(1):27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Purvis N, Bahn A, Katare R. The Role of MicroRNAs in Cardiac Stem Cells. STEM CELLS INT. 2015;2015:194894. doi: 10.1155/2015/194894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ratajczak MZ, Kucia M, Jadczyk T, et al. Pivotal role of paracrine effects in stem cell therapies in regenerative medicine: can we translate stem cell-secreted paracrine factors and microvesicles into better therapeutic strategies? LEUKEMIA. 2012;26(6):1166–1173. doi: 10.1038/leu.2011.389. [DOI] [PubMed] [Google Scholar]
- 23.Zhu H, Fan GC. Extracellular/circulating microRNAs and their potential role in cardiovascular disease. AM J CARDIOVASC DIS. 2011;1(2):138–149. [PMC free article] [PubMed] [Google Scholar]
- 24.Johnstone RM. Revisiting the road to the discovery of exosomes. BLOOD CELLS MOL DIS. 2005;34(3):214–219. doi: 10.1016/j.bcmd.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Lugini L, Cecchetti S, Huber V, et al. Immune surveillance properties of human NK cell-derived exosomes. J IMMUNOL. 2012;189(6):2833–2842. doi: 10.4049/jimmunol.1101988. [DOI] [PubMed] [Google Scholar]
- 26.Mishra PK, Singh SR, Joshua IG, et al. Stem cells as a therapeutic target for diabetes. FRONT BIOSCI. 2010;15:461–477. doi: 10.2741/3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. SCIENCE. 1998;282(5391):1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 28.Nichols J, Zevnik B, Anastassiadis K, et al. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. CELL. 1998;95(3):379–391. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- 29.Niwa H, Burdon T, Chambers I, et al. Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. GENES DEV. 1998;12(13):2048–2060. doi: 10.1101/gad.12.13.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Potten CS, Schofield R, Lajtha LG. A comparison of cell replacement in bone marrow, testis and three regions of surface epithelium. BIOCHIM BIOPHYS ACTA. 1979;560(2):281–299. doi: 10.1016/0304-419x(79)90022-2. [DOI] [PubMed] [Google Scholar]
- 31.Bernardi S, Severini GM, Zauli G, et al. Cell-based therapies for diabetic complications. EXP DIABETES RES. 2012;2012:872504. doi: 10.1155/2012/872504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leonardini A, Avogaro A. Abnormalities of the cardiac stem and progenitor cell compartment in experimental and human diabetes. ARCH PHYSIOL BIOCHEM. 2013;119(4):179–187. doi: 10.3109/13813455.2013.798334. [DOI] [PubMed] [Google Scholar]
- 33.Shen YH, Hu X, Zou S, et al. Stem cells in thoracic aortic aneurysms and dissections: potential contributors to aortic repair. ANN THORAC SURG. 2012;93(5):1524–1533. doi: 10.1016/j.athoracsur.2012.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams AR, Hare JM. Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. CIRC RES. 2011;109(8):923–940. doi: 10.1161/CIRCRESAHA.111.243147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anversa P, Kajstura J, Leri A, et al. Life and death of cardiac stem cells: a paradigm shift in cardiac biology. CIRCULATION. 2006;113(11):1451–1463. doi: 10.1161/CIRCULATIONAHA.105.595181. [DOI] [PubMed] [Google Scholar]
- 36.Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. CELL. 2003;114(6):763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 37.Hierlihy AM, Seale P, Lobe CG, et al. The post-natal heart contains a myocardial stem cell population. FEBS LETT. 2002;530(1-3):239–243. doi: 10.1016/s0014-5793(02)03477-4. [DOI] [PubMed] [Google Scholar]
- 38.Barile L, Messina E, Giacomello A, et al. Endogenous cardiac stem cells. PROG CARDIOVASC DIS. 2007;50(1):31–48. doi: 10.1016/j.pcad.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 39.Bearzi C, Rota M, Hosoda T, et al. Human cardiac stem cells. PROC NATL ACAD SCI U S A. 2007;104(35):14068–14073. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perez-Moreno M, Jamora C, Fuchs E. Sticky business: orchestrating cellular signals at adherens junctions. CELL. 2003;112(4):535–548. doi: 10.1016/s0092-8674(03)00108-9. [DOI] [PubMed] [Google Scholar]
- 41.Urbanek K, Cesselli D, Rota M, et al. Stem cell niches in the adult mouse heart. PROC NATL ACAD SCI U S A. 2006;103(24):9226–9231. doi: 10.1073/pnas.0600635103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gadue P, Huber TL, Paddison PJ, et al. Wnt and TGF-beta signaling are required for the induction of an in vitro model of primitive streak formation using embryonic stem cells. PROC NATL ACAD SCI U S A. 2006;103(45):16806–16811. doi: 10.1073/pnas.0603916103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lindsley RC, Gill JG, Murphy TL, et al. Mesp1 coordinately regulates cardiovascular fate restriction and epithelial-mesenchymal transition in differentiating ESCs. CELL STEM CELL. 2008;3(1):55–68. doi: 10.1016/j.stem.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Foley AC, Mercola M. Heart induction by Wnt antagonists depends on the homeodomain transcription factor Hex. GENES DEV. 2005;19(3):387–396. doi: 10.1101/gad.1279405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Foley AC, Korol O, Timmer AM, et al. Multiple functions of Cerberus cooperate to induce heart downstream of Nodal. DEV BIOL. 2007;303(1):57–65. doi: 10.1016/j.ydbio.2006.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schneider VA, Mercola M. Wnt antagonism initiates cardiogenesis in Xenopus laevis. GENES DEV. 2001;15(3):304–315. doi: 10.1101/gad.855601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Naito AT, Shiojima I, Akazawa H, et al. Developmental stage-specific biphasic roles of Wnt/beta-catenin signaling in cardiomyogenesis and hematopoiesis. PROC NATL ACAD SCI U S A. 2006;103(52):19812–19817. doi: 10.1073/pnas.0605768103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ueno S, Weidinger G, Osugi T, et al. Biphasic role for Wnt/beta-catenin signaling in cardiac specification in zebrafish and embryonic stem cells. PROC NATL ACAD SCI U S A. 2007;104(23):9685–9690. doi: 10.1073/pnas.0702859104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qin G, Ii M, Silver M, et al. Functional disruption of alpha4 integrin mobilizes bone marrow-derived endothelial progenitors and augments ischemic neovascularization. J EXP MED. 2006;203(1):153–163. doi: 10.1084/jem.20050459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen VC, Stull R, Joo D, et al. Notch signaling respecifies the hemangioblast to a cardiac fate. NAT BIOTECHNOL. 2008;26(10):1169–1178. doi: 10.1038/nbt.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rajala K, Pekkanen-Mattila M, Aalto-Setala K. Cardiac differentiation of pluripotent stem cells. STEM CELLS INT. 2011;2011:383709. doi: 10.4061/2011/383709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.He Z, Li H, Zuo S, et al. Transduction of Wnt11 promotes mesenchymal stem cell transdifferentiation into cardiac phenotypes. STEM CELLS DEV. 2011;20(10):1771–1778. doi: 10.1089/scd.2010.0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hiroi Y, Kudoh S, Monzen K, et al. Tbx5 associates with Nkx2-5 and synergistically promotes cardiomyocyte differentiation. NAT GENET. 2001;28(3):276–280. doi: 10.1038/90123. [DOI] [PubMed] [Google Scholar]
- 54.Peterkin T, Gibson A, Patient R. GATA-6 maintains BMP-4 and Nkx2 expression during cardiomyocyte precursor maturation. EMBO J. 2003;22(16):4260–4273. doi: 10.1093/emboj/cdg400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Plageman TF, Jr, Yutzey KE. Differential expression and function of Tbx5 and Tbx20 in cardiac development. J BIOL CHEM. 2004;279(18):19026–19034. doi: 10.1074/jbc.M314041200. [DOI] [PubMed] [Google Scholar]
- 56.Riley P, Anson-Cartwright L, Cross JC. The Hand1 bHLH transcription factor is essential for placentation and cardiac morphogenesis. NAT GENET. 1998;18(3):271–275. doi: 10.1038/ng0398-271. [DOI] [PubMed] [Google Scholar]
- 57.Watt AJ, Battle MA, Li J, et al. GATA4 is essential for formation of the proepicardium and regulates cardiogenesis. PROC NATL ACAD SCI U S A. 2004;101(34):12573–12578. doi: 10.1073/pnas.0400752101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marvin MJ, Di RG, Gardiner A, et al. Inhibition of Wnt activity induces heart formation from posterior mesoderm. GENES DEV. 2001;15(3):316–327. doi: 10.1101/gad.855501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mima T, Ueno H, Fischman DA, et al. Fibroblast growth factor receptor is required for in vivo cardiac myocyte proliferation at early embryonic stages of heart development. PROC NATL ACAD SCI U S A. 1995;92(2):467–471. doi: 10.1073/pnas.92.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Winnier G, Blessing M, Labosky PA, et al. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. GENES DEV. 1995;9(17):2105–2116. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- 61.Zhang H, Bradley A. Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. DEVELOPMENT. 1996;122(10):2977–2986. doi: 10.1242/dev.122.10.2977. [DOI] [PubMed] [Google Scholar]
- 62.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. CELL. 2008;132(1):27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rodolfo C, Di BS, Cecconi F. Autophagy in stem and progenitor cells. CELL MOL LIFE SCI. 2016;73(3):475–496. doi: 10.1007/s00018-015-2071-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vessoni AT, Muotri AR, Okamoto OK. Autophagy in stem cell maintenance and differentiation. STEM CELLS DEV. 2012;21(4):513–520. doi: 10.1089/scd.2011.0526. [DOI] [PubMed] [Google Scholar]
- 65.Phadwal K, Watson AS, Simon AK. Tightrope act: autophagy in stem cell renewal, differentiation, proliferation, and aging. CELL MOL LIFE SCI. 2013;70(1):89–103. doi: 10.1007/s00018-012-1032-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guan JL, Simon AK, Prescott M, et al. Autophagy in stem cells. AUTOPHAGY. 2013;9(6):830–849. doi: 10.4161/auto.24132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guan JL, Simon AK, Prescott M, et al. Autophagy in stem cells. AUTOPHAGY. 2013;9(6):830–849. doi: 10.4161/auto.24132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meng Y, Ji J, Tan W, et al. Involvement of autophagy in the procedure of endoplasmic reticulum stress introduced apoptosis in bone marrow mesenchymal stem cells from nonobese diabetic mice. CELL BIOCHEM FUNCT. 2016;34(1):25–33. doi: 10.1002/cbf.3161. [DOI] [PubMed] [Google Scholar]
- 69.Jung J, Choi JH, Lee Y, et al. Human placenta-derived mesenchymal stem cells promote hepatic regeneration in CCl4 -injured rat liver model via increased autophagic mechanism. STEM CELLS. 2013;31(8):1584–1596. doi: 10.1002/stem.1396. [DOI] [PubMed] [Google Scholar]
- 70.Han YF, Sun TJ, Han YQ, et al. Clinical perspectives on mesenchymal stem cells promoting wound healing in diabetes mellitus patients by inducing autophagy. EUR REV MED PHARMACOL SCI. 2015;19(14):2666–2670. [PubMed] [Google Scholar]
- 71.Ieda M, Fu JD, Delgado-Olguin P, et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. CELL. 2010;142(3):375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nam YJ, Song K, Luo X, et al. Reprogramming of human fibroblasts toward a cardiac fate. PROC NATL ACAD SCI U S A. 2013;110(14):5588–5593. doi: 10.1073/pnas.1301019110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wada R, Muraoka N, Inagawa K, et al. Induction of human cardiomyocyte-like cells from fibroblasts by defined factors. PROC NATL ACAD SCI U S A. 2013;110(31):12667–12672. doi: 10.1073/pnas.1304053110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fu JD, Stone NR, Liu L, et al. Direct reprogramming of human fibroblasts toward a cardiomyocyte-like state. STEM CELL REPORTS. 2013;1(3):235–247. doi: 10.1016/j.stemcr.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kaunas R, Nguyen P, Usami S, et al. Cooperative effects of Rho and mechanical stretch on stress fiber organization. PROC NATL ACAD SCI U S A. 2005;102(44):15895–15900. doi: 10.1073/pnas.0506041102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kurpinski K, Chu J, Hashi C, et al. Anisotropic mechanosensing by mesenchymal stem cells. PROC NATL ACAD SCI U S A. 2006;103(44):16095–16100. doi: 10.1073/pnas.0604182103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kurpinski K, Park J, Thakar RG, et al. Regulation of vascular smooth muscle cells and mesenchymal stem cells by mechanical strain. MOL CELL BIOMECH. 2006;3(1):21–34. [PubMed] [Google Scholar]
- 78.Park JS, Chu JS, Cheng C, et al. Differential effects of equiaxial and uniaxial strain on mesenchymal stem cells. BIOTECHNOL BIOENG. 2004;88(3):359–368. doi: 10.1002/bit.20250. [DOI] [PubMed] [Google Scholar]
- 79.Kurpinski K, Lam H, Chu J, et al. Transforming growth factor-beta and notch signaling mediate stem cell differentiation into smooth muscle cells. STEM CELLS. 2010;28(4):734–742. doi: 10.1002/stem.319. [DOI] [PubMed] [Google Scholar]
- 80.Wang D, Park JS, Chu JS, et al. Proteomic profiling of bone marrow mesenchymal stem cells upon transforming growth factor beta1 stimulation. J BIOL CHEM. 2004;279(42):43725–43734. doi: 10.1074/jbc.M407368200. [DOI] [PubMed] [Google Scholar]
- 81.Park JS, Chu JS, Tsou AD, et al. The effect of matrix stiffness on the differentiation of mesenchymal stem cells in response to TGF-beta. BIOMATERIALS. 2011;32(16):3921–3930. doi: 10.1016/j.biomaterials.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tyagi SC, Hoit BD. Metalloproteinase in myocardial adaptation and maladaptation. J CARDIOVASC PHARMACOL THER. 2002;7(4):241–246. doi: 10.1177/107424840200700407. [DOI] [PubMed] [Google Scholar]
- 83.Ali MA, Schulz R. Activation of MMP-2 as a key event in oxidative stress injury to the heart. FRONT BIOSCI. 2009;14:699–716. doi: 10.2741/3274. [DOI] [PubMed] [Google Scholar]
- 84.Mishra PK, Givvimani S, Chavali V, et al. Cardiac matrix: a clue for future therapy. BIOCHIM BIOPHYS ACTA. 2013;1832(12):2271–2276. doi: 10.1016/j.bbadis.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Morancho A, Ma F, Barcelo V, et al. Impaired vascular remodeling after endothelial progenitor cell transplantation in MMP9-deficient mice suffering cortical cerebral ischemia. J CEREB BLOOD FLOW METAB. 2015;35(10):1547–1551. doi: 10.1038/jcbfm.2015.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Guo J, Jie W, Shen Z, et al. SCF increases cardiac stem cell migration through PI3K/AKT and MMP2/9 signaling. INT J MOL MED. 2014;34(1):112–118. doi: 10.3892/ijmm.2014.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pottier N, Cauffiez C, Perrais M, et al. FibromiRs: translating molecular discoveries into new anti-fibrotic drugs. TRENDS PHARMACOL SCI. 2014;35(3):119–126. doi: 10.1016/j.tips.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 88.Tyagi AC, Sen U, Mishra PK. Synergy of microRNA and stem cell: a novel therapeutic approach for diabetes mellitus and cardiovascular diseases. CURR DIABETES REV. 2011;7(6):367–376. doi: 10.2174/157339911797579179. [DOI] [PubMed] [Google Scholar]
- 89.Callis TE, Deng Z, Chen JF, et al. Muscling through the microRNA world. EXP BIOL MED (MAYWOOD) 2008;233(2):131–138. doi: 10.3181/0709-MR-237. [DOI] [PubMed] [Google Scholar]
- 90.van RE, Sutherland LB, Thatcher JE, et al. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. PROC NATL ACAD SCI U S A. 2008;105(35):13027–13032. doi: 10.1073/pnas.0805038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee Y, Yang X, Huang Y, et al. Network modeling identifies molecular functions targeted by miR-204 to suppress head and neck tumor metastasis. PLOS COMPUT BIOL. 2010;6(4):e1000730. doi: 10.1371/journal.pcbi.1000730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ucar A, Vafaizadeh V, Jarry H, et al. miR-212 and miR-132 are required for epithelial stromal interactions necessary for mouse mammary gland development. NAT GENET. 2010;42(12):1101–1108. doi: 10.1038/ng.709. [DOI] [PubMed] [Google Scholar]
- 93.Bronisz A, Godlewski J, Wallace JA, et al. Reprogramming of the tumour microenvironment by stromal PTEN-regulated miR-320. NAT CELL BIOL. 2012;14(2):159–167. doi: 10.1038/ncb2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nan Y, Han L, Zhang A, et al. MiRNA-451 plays a role as tumor suppressor in human glioma cells. BRAIN RES. 2010;1359:14–21. doi: 10.1016/j.brainres.2010.08.074. [DOI] [PubMed] [Google Scholar]
- 95.Yan W, Zhang W, Sun L, et al. Identification of MMP-9 specific microRNA expression profile as potential targets of anti-invasion therapy in glioblastoma multiforme. BRAIN RES. 2011;1411:108–115. doi: 10.1016/j.brainres.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 96.Huang XH, Chen JS, Wang Q, et al. miR-338-3p suppresses invasion of liver cancer cell by targeting smoothened. J PATHOL. 2011;225(3):463–472. doi: 10.1002/path.2877. [DOI] [PubMed] [Google Scholar]
- 97.Moriyama T, Ohuchida K, Mizumoto K, et al. MicroRNA-21 modulates biological functions of pancreatic cancer cells including their proliferation, invasion, and chemoresistance. MOL CANCER THER. 2009;8(5):1067–1074. doi: 10.1158/1535-7163.MCT-08-0592. [DOI] [PubMed] [Google Scholar]
- 98.Rossi M, Pitari MR, Amodio N, et al. miR-29b negatively regulates human osteoclastic cell differentiation and function: implications for the treatment of multiple myeloma-related bone disease. J CELL PHYSIOL. 2013;228(7):1506–1515. doi: 10.1002/jcp.24306. [DOI] [PubMed] [Google Scholar]
- 99.Tavazoie SF, Alarcon C, Oskarsson T, et al. Endogenous human microRNAs that suppress breast cancer metastasis. NATURE. 2008;451(7175):147–152. doi: 10.1038/nature06487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yang F, Yin Y, Wang F, et al. miR-17-5p Promotes migration of human hepatocellular carcinoma cells through the p38 mitogen-activated protein kinase-heat shock protein 27 pathway. HEPATOLOGY. 2010;51(5):1614–1623. doi: 10.1002/hep.23566. [DOI] [PubMed] [Google Scholar]
- 101.Felli N, Felicetti F, Lustri AM, et al. miR-126&126* restored expressions play a tumor suppressor role by directly regulating ADAM9 and MMP7 in melanoma. PLOS ONE. 2013;8(2):e56824. doi: 10.1371/journal.pone.0056824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kano M, Seki N, Kikkawa N, et al. miR-145, miR-133a and miR-133b: Tumor-suppressive miRNAs target FSCN1 in esophageal squamous cell carcinoma. INT J CANCER. 2010;127(12):2804–2814. doi: 10.1002/ijc.25284. [DOI] [PubMed] [Google Scholar]
- 103.Liu X, Yu J, Jiang L, et al. MicroRNA-222 regulates cell invasion by targeting matrix metalloproteinase 1 (MMP1) and manganese superoxide dismutase 2 (SOD2) in tongue squamous cell carcinoma cell lines. CANCER GENOMICS PROTEOMICS. 2009;6(3):131–139. [PMC free article] [PubMed] [Google Scholar]
- 104.Stanczyk J, Ospelt C, Karouzakis E, et al. Altered expression of microRNA-203 in rheumatoid arthritis synovial fibroblasts and its role in fibroblast activation. ARTHRITIS RHEUM. 2011;63(2):373–381. doi: 10.1002/art.30115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jones SW, Watkins G, Le GN, et al. The identification of differentially expressed microRNA in osteoarthritic tissue that modulate the production of TNF-alpha and MMP13. OSTEOARTHRITIS CARTILAGE. 2009;17(4):464–472. doi: 10.1016/j.joca.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 106.Henson BJ, Bhattacharjee S, O'Dee DM, et al. Decreased expression of miR-125b and miR-100 in oral cancer cells contributes to malignancy. GENES CHROMOSOMES CANCER. 2009;48(7):569–582. doi: 10.1002/gcc.20666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Akhtar N, Rasheed Z, Ramamurthy S, et al. MicroRNA-27b regulates the expression of matrix metalloproteinase 13 in human osteoarthritis chondrocytes. ARTHRITIS RHEUM. 2010;62(5):1361–1371. doi: 10.1002/art.27329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tardif G, Hum D, Pelletier JP, et al. Regulation of the IGFBP-5 and MMP-13 genes by the microRNAs miR-140 and miR-27a in human osteoarthritic chondrocytes. BMC MUSCULOSKELET DISORD. 2009;10:148. doi: 10.1186/1471-2474-10-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xu N, Zhang L, Meisgen F, et al. MicroRNA-125b down-regulates matrix metallopeptidase 13 and inhibits cutaneous squamous cell carcinoma cell proliferation, migration, and invasion. J BIOL CHEM. 2012;287(35):29899–29908. doi: 10.1074/jbc.M112.391243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Osaki M, Takeshita F, Sugimoto Y, et al. MicroRNA-143 regulates human osteosarcoma metastasis by regulating matrix metalloprotease-13 expression. MOL THER. 2011;19(6):1123–1130. doi: 10.1038/mt.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Grimson A, Farh KK, Johnston WK, et al. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. MOL CELL. 2007;27(1):91–105. doi: 10.1016/j.molcel.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fornari F, Milazzo M, Chieco P, et al. In hepatocellular carcinoma miR-519d is up-regulated by p53 and DNA hypomethylation and targets CDKN1A/p21, PTEN, AKT3 and TIMP2. J PATHOL. 2012;227(3):275–285. doi: 10.1002/path.3995. [DOI] [PubMed] [Google Scholar]
- 113.Gennarino VA, Sardiello M, Avellino R, et al. MicroRNA target prediction by expression analysis of host genes. GENOME RES. 2009;19(3):481–490. doi: 10.1101/gr.084129.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chuang TD, Panda H, Luo X, et al. miR-200c is aberrantly expressed in leiomyomas in an ethnic-dependent manner and targets ZEBs, VEGFA, TIMP2, and FBLN5. ENDOCR RELAT CANCER. 2012;19(4):541–556. doi: 10.1530/ERC-12-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang B, Hsu SH, Majumder S, et al. TGFbeta-mediated upregulation of hepatic miR-181b promotes hepatocarcinogenesis by targeting TIMP3. ONCOGENE. 2010;29(12):1787–1797. doi: 10.1038/onc.2009.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Limana F, Esposito G, D'Arcangelo D, et al. HMGB1 attenuates cardiac remodelling in the failing heart via enhanced cardiac regeneration and miR-206-mediated inhibition of TIMP-3. PLOS ONE. 2011;6(6):e19845. doi: 10.1371/journal.pone.0019845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chi SW, Zang JB, Mele A, et al. Argonaute HITS-CLIP decodes microRNA-mRNA interaction maps. NATURE. 2009;460(7254):479–486. doi: 10.1038/nature08170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yu JY, Chung KH, Deo M, et al. MicroRNA miR-124 regulates neurite outgrowth during neuronal differentiation. EXP CELL RES. 2008;314(14):2618–2633. doi: 10.1016/j.yexcr.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lu Y, Roy S, Nuovo G, et al. Anti-microRNA-222 (anti-miR-222) and -181B suppress growth of tamoxifen-resistant xenografts in mouse by targeting TIMP3 protein and modulating mitogenic signal. J BIOL CHEM. 2011;286(49):42292–42302. doi: 10.1074/jbc.M111.270926. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 120.Zhang A, Liu Y, Shen Y, et al. miR-21 modulates cell apoptosis by targeting multiple genes in renal cell carcinoma. UROLOGY. 2011;78(2):474–479. doi: 10.1016/j.urology.2011.03.030. [DOI] [PubMed] [Google Scholar]
- 121.Baek D, Villen J, Shin C, et al. The impact of microRNAs on protein output. NATURE. 2008;455(7209):64–71. doi: 10.1038/nature07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang C, Zhang J, Hao J, et al. High level of miR-221/222 confers increased cell invasion and poor prognosis in glioma. J TRANSL MED. 2012;10:119. doi: 10.1186/1479-5876-10-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yu D, Zhou H, Xun Q, et al. microRNA-103 regulates the growth and invasion of endometrial cancer cells through the downregulation of tissue inhibitor of metalloproteinase 3. ONCOL LETT. 2012;3(6):1221–1226. doi: 10.3892/ol.2012.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Helwak A, Kudla G, Dudnakova T, et al. Mapping the human miRNA interactome by CLASH reveals frequent noncanonical binding. CELL. 2013;153(3):654–665. doi: 10.1016/j.cell.2013.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Luna C, Li G, Qiu J, et al. MicroRNA-24 regulates the processing of latent TGFbeta1 during cyclic mechanical stress in human trabecular meshwork cells through direct targeting of FURIN. J CELL PHYSIOL. 2011;226(5):1407–1414. doi: 10.1002/jcp.22476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Martin J, Jenkins RH, Bennagi R, et al. Post-transcriptional regulation of Transforming Growth Factor Beta-1 by microRNA-744. PLOS ONE. 2011;6(10):e25044. doi: 10.1371/journal.pone.0025044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tili E, Michaille JJ, Alder H, et al. Resveratrol modulates the levels of microRNAs targeting genes encoding tumor-suppressors and effectors of TGFbeta signaling pathway in SW480 cells. BIOCHEM PHARMACOL. 2010;80(12):2057–2065. doi: 10.1016/j.bcp.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Liu ZY, Zhang GL, Wang MM, et al. MicroRNA-663 targets TGFB1 and regulates lung cancer proliferation. ASIAN PAC J CANCER PREV. 2011;12(11):2819–2823. [PubMed] [Google Scholar]
- 129.Gabriely G, Wurdinger T, Kesari S, et al. MicroRNA 21 promotes glioma invasion by targeting matrix metalloproteinase regulators. MOL CELL BIOL. 2008;28(17):5369–5380. doi: 10.1128/MCB.00479-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tsai WC, Hsu SD, Hsu CS, et al. MicroRNA-122 plays a critical role in liver homeostasis and hepatocarcinogenesis. J CLIN INVEST. 2012;122(8):2884–2897. doi: 10.1172/JCI63455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lv XB, Jiao Y, Qing Y, et al. miR-124 suppresses multiple steps of breast cancer metastasis by targeting a cohort of pro-metastatic genes in vitro. CHIN J CANCER. 2011;30(12):821–830. doi: 10.5732/cjc.011.10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ernst A, Campos B, Meier J, et al. De-repression of CTGF via the miR-17-92 cluster upon differentiation of human glioblastoma spheroid cultures. ONCOGENE. 2010;29(23):3411–3422. doi: 10.1038/onc.2010.83. [DOI] [PubMed] [Google Scholar]
- 133.Sander S, Bullinger L, Klapproth K, et al. MYC stimulates EZH2 expression by repression of its negative regulator miR-26a. BLOOD. 2008;112(10):4202–4212. doi: 10.1182/blood-2008-03-147645. [DOI] [PubMed] [Google Scholar]
- 134.Xie H, Zhao Y, Caramuta S, et al. miR-205 expression promotes cell proliferation and migration of human cervical cancer cells. PLOS ONE. 2012;7(10):e46990. doi: 10.1371/journal.pone.0046990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lee HK, Bier A, Cazacu S, et al. MicroRNA-145 is downregulated in glial tumors and regulates glioma cell migration by targeting connective tissue growth factor. PLOS ONE. 2013;8(2):e54652. doi: 10.1371/journal.pone.0054652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tsukamoto Y, Nakada C, Noguchi T, et al. MicroRNA-375 is downregulated in gastric carcinomas and regulates cell survival by targeting PDK1 and 14-3-3zeta. CANCER RES. 2010;70(6):2339–2349. doi: 10.1158/0008-5472.CAN-09-2777. [DOI] [PubMed] [Google Scholar]
- 137.Pichiorri F, Suh SS, Ladetto M, et al. MicroRNAs regulate critical genes associated with multiple myeloma pathogenesis. PROC NATL ACAD SCI U S A. 2008;105(35):12885–12890. doi: 10.1073/pnas.0806202105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Takaya T, Ono K, Kawamura T, et al. MicroRNA-1 and MicroRNA-133 in spontaneous myocardial differentiation of mouse embryonic stem cells. CIRC J. 2009;73(8):1492–1497. doi: 10.1253/circj.cj-08-1032. [DOI] [PubMed] [Google Scholar]
- 139.Ivey KN, Muth A, Arnold J, et al. MicroRNA regulation of cell lineages in mouse and human embryonic stem cells. CELL STEM CELL. 2008;2(3):219–229. doi: 10.1016/j.stem.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kuppusamy KT, Sperber H, Ruohola-Baker H. MicroRNA regulation and role in stem cell maintenance, cardiac differentiation and hypertrophy. CURR MOL MED. 2013;13(5):757–764. doi: 10.2174/1566524011313050007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Seeger FH, Zeiher AM, Dimmeler S. MicroRNAs in stem cell function and regenerative therapy of the heart. ARTERIOSCLER THROMB VASC BIOL. 2013;33(8):1739–1746. doi: 10.1161/ATVBAHA.113.300138. [DOI] [PubMed] [Google Scholar]
- 142.Kane NM, Thrasher AJ, Angelini GD, et al. Concise review: MicroRNAs as modulators of stem cells and angiogenesis. STEM CELLS. 2014;32(5):1059–1066. doi: 10.1002/stem.1629. [DOI] [PubMed] [Google Scholar]
- 143.Wilson KD, Hu S, Venkatasubrahmanyam S, et al. Dynamic microRNA expression programs during cardiac differentiation of human embryonic stem cells: role for miR-499. CIRC CARDIOVASC GENET. 2010;3(5):426–435. doi: 10.1161/CIRCGENETICS.109.934281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bras-Rosario L, Matsuda A, Pinheiro AI, et al. Expression profile of microRNAs regulating proliferation and differentiation in mouse adult cardiac stem cells. PLOS ONE. 2013;8(5):e63041. doi: 10.1371/journal.pone.0063041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Luna C, Li G, Qiu J, et al. Cross-talk between miR-29 and transforming growth factor-betas in trabecular meshwork cells. INVEST OPHTHALMOL VIS SCI. 2011;52(6):3567–3572. doi: 10.1167/iovs.10-6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jayawardena TM, Egemnazarov B, Finch EA, et al. MicroRNA-mediated in vitro and in vivo direct reprogramming of cardiac fibroblasts to cardiomyocytes. CIRC RES. 2012;110(11):1465–1473. doi: 10.1161/CIRCRESAHA.112.269035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Abdelwahid E, Siminiak T, Guarita-Souza LC, et al. Stem cell therapy in heart diseases: a review of selected new perspectives, practical considerations and clinical applications. CURR CARDIOL REV. 2011;7(3):201–212. doi: 10.2174/157340311798220502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Passier R, van Laake LW, Mummery CL. Stem-cell-based therapy and lessons from the heart. NATURE. 2008;453(7193):322–329. doi: 10.1038/nature07040. [DOI] [PubMed] [Google Scholar]
- 149.Wu X, Ding S, Ding Q, et al. Small molecules that induce cardiomyogenesis in embryonic stem cells. J AM CHEM SOC. 2004;126(6):1590–1591. doi: 10.1021/ja038950i. [DOI] [PubMed] [Google Scholar]
- 150.Zhang J, Wilson GF, Soerens AG, et al. Functional cardiomyocytes derived from human induced pluripotent stem cells. CIRC RES. 2009;104(4):e30–e41. doi: 10.1161/CIRCRESAHA.108.192237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Strauer BE, Brehm M, Zeus T, et al. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. CIRCULATION. 2002;106(15):1913–1918. doi: 10.1161/01.cir.0000034046.87607.1c. [DOI] [PubMed] [Google Scholar]
- 152.Orlic D, Kajstura J, Chimenti S, et al. Transplanted adult bone marrow cells repair myocardial infarcts in mice. ANN N Y ACAD SCI. 2001;938:221–229. doi: 10.1111/j.1749-6632.2001.tb03592.x. [DOI] [PubMed] [Google Scholar]
- 153.Schuleri KH, Feigenbaum GS, Centola M, et al. Autologous mesenchymal stem cells produce reverse remodelling in chronic ischaemic cardiomyopathy. EUR HEART J. 2009;30(22):2722–2732. doi: 10.1093/eurheartj/ehp265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Jackson KA, Majka SM, Wang H, et al. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J CLIN INVEST. 2001;107(11):1395–1402. doi: 10.1172/JCI12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. CELL. 2003;114(6):763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 156.Gomez-Mauricio RG, Acarregui A, Sanchez-Margallo FM, et al. A preliminary approach to the repair of myocardial infarction using adipose tissue-derived stem cells encapsulated in magnetic resonance-labelled alginate microspheres in a porcine model. EUR J PHARM BIOPHARM. 2013;84(1):29–39. doi: 10.1016/j.ejpb.2012.11.028. [DOI] [PubMed] [Google Scholar]
- 157.Badorff C, Brandes RP, Popp R, et al. Transdifferentiation of blood-derived human adult endothelial progenitor cells into functionally active cardiomyocytes. CIRCULATION. 2003;107(7):1024–1032. doi: 10.1161/01.cir.0000051460.85800.bb. [DOI] [PubMed] [Google Scholar]
- 158.Rupp S, Badorff C, Koyanagi M, et al. Statin therapy in patients with coronary artery disease improves the impaired endothelial progenitor cell differentiation into cardiomyogenic cells. BASIC RES CARDIOL. 2004;99(1):61–68. doi: 10.1007/s00395-003-0441-3. [DOI] [PubMed] [Google Scholar]
- 159.Tang XL, Rokosh G, Sanganalmath SK, et al. Intracoronary administration of cardiac progenitor cells alleviates left ventricular dysfunction in rats with a 30-day-old infarction. CIRCULATION. 2010;121(2):293–305. doi: 10.1161/CIRCULATIONAHA.109.871905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gnecchi M, He H, Noiseux N, et al. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006;20(6):661–669. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 161.Haider HK, Jiang S, Idris NM, et al. IGF-1-overexpressing mesenchymal stem cells accelerate bone marrow stem cell mobilization via paracrine activation of SDF-1alpha/CXCR4 signaling to promote myocardial repair. CIRC RES. 2008;103(11):1300–1308. doi: 10.1161/CIRCRESAHA.108.186742. [DOI] [PubMed] [Google Scholar]
- 162.Sahoo S, Klychko E, Thorne T, et al. Exosomes from human CD34(+) stem cells mediate their proangiogenic paracrine activity. CIRC RES. 2011;109(7):724–728. doi: 10.1161/CIRCRESAHA.111.253286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Han C, Sun X, Liu L, et al. Exosomes and Their Therapeutic Potentials of Stem Cells. STEM CELLS INT. 2016;2016:7653489. doi: 10.1155/2016/7653489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Stoorvogel W, Strous GJ, Geuze HJ, et al. Late endosomes derive from early endosomes by maturation. CELL. 1991;65(3):417–427. doi: 10.1016/0092-8674(91)90459-c. [DOI] [PubMed] [Google Scholar]
- 165.Kishore R, Khan M. More Than Tiny Sacks: Stem Cell Exosomes as Cell-Free Modality for Cardiac Repair. CIRC RES. 2016;118(2):330–343. doi: 10.1161/CIRCRESAHA.115.307654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. CELL. 1983;33(3):967–978. doi: 10.1016/0092-8674(83)90040-5. [DOI] [PubMed] [Google Scholar]
- 167.Lai RC, Arslan F, Lee MM, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. STEM CELL RES. 2010;4(3):214–222. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 168.Vrijsen KR, Sluijter JP, Schuchardt MW, et al. Cardiomyocyte progenitor cell-derived exosomes stimulate migration of endothelial cells. J CELL MOL MED. 2010;14(5):1064–1070. doi: 10.1111/j.1582-4934.2010.01081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Raposo G, Nijman HW, Stoorvogel W, et al. B lymphocytes secrete antigen-presenting vesicles. J EXP MED. 1996;183(3):1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Peters PJ, Geuze HJ, van der Donk HA, et al. Molecules relevant for T cell-target cell interaction are present in cytolytic granules of human T lymphocytes. EUR J IMMUNOL. 1989;19(8):1469–1475. doi: 10.1002/eji.1830190819. [DOI] [PubMed] [Google Scholar]
- 171.Zitvogel L, Regnault A, Lozier A, et al. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. NAT MED. 1998;4(5):594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]
- 172.Heijnen HF, Schiel AE, Fijnheer R, et al. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. BLOOD. 1999;94(11):3791–3799. [PubMed] [Google Scholar]
- 173.Fevrier B, Vilette D, Archer F, et al. Cells release prions in association with exosomes. PROC NATL ACAD SCI U S A. 2004;101(26):9683–9688. doi: 10.1073/pnas.0308413101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Dignat-George F, Boulanger CM. The many faces of endothelial microparticles. ARTERIOSCLER THROMB VASC BIOL. 2011;31(1):27–33. doi: 10.1161/ATVBAHA.110.218123. [DOI] [PubMed] [Google Scholar]
- 175.Wolfers J, Lozier A, Raposo G, et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. NAT MED. 2001;7(3):297–303. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- 176.Simons M, Raposo G. Exosomes--vesicular carriers for intercellular communication. CURR OPIN CELL BIOL. 2009;21(4):575–581. doi: 10.1016/j.ceb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 177.Mittelbrunn M, Gutierrez-Vazquez C, Villarroya-Beltri C, et al. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. NAT COMMUN. 2011;2:282. doi: 10.1038/ncomms1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Dai S, Wan T, Wang B, et al. More efficient induction of HLA-A*0201-restricted and carcinoembryonic antigen (CEA)-specific CTL response by immunization with exosomes prepared from heat-stressed CEA-positive tumor cells. CLIN CANCER RES. 2005;11(20):7554–7563. doi: 10.1158/1078-0432.CCR-05-0810. [DOI] [PubMed] [Google Scholar]
- 179.Korkut C, Ataman B, Ramachandran P, et al. Trans-synaptic transmission of vesicular Wnt signals through Evi/Wntless. CELL. 2009;139(2):393–404. doi: 10.1016/j.cell.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Soderberg A, Barral AM, Soderstrom M, et al. Redox-signaling transmitted in trans to neighboring cells by melanoma-derived TNF-containing exosomes. FREE RADIC BIOL MED. 2007;43(1):90–99. doi: 10.1016/j.freeradbiomed.2007.03.026. [DOI] [PubMed] [Google Scholar]
- 181.Chistiakov DA, Orekhov AN, Bobryshev YV. Cardiac Extracellular Vesicles in Normal and Infarcted Heart. INT J MOL SCI. 2016;17(1) doi: 10.3390/ijms17010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Garcia NA, Moncayo-Arlandi J, Sepulveda P, et al. Cardiomyocyte exosomes regulate glycolytic flux in endothelium by direct transfer of GLUT transporters and glycolytic enzymes. CARDIOVASC RES. 2015 doi: 10.1093/cvr/cvv260. [DOI] [PubMed] [Google Scholar]
- 183.Khan M, Nickoloff E, Abramova T, et al. Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. CIRC RES. 2015;117(1):52–64. doi: 10.1161/CIRCRESAHA.117.305990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Chen L, Wang Y, Pan Y, et al. Cardiac progenitor-derived exosomes protect ischemic myocardium from acute ischemia/reperfusion injury. BIOCHEM BIOPHYS RES COMMUN. 2013;431(3):566–571. doi: 10.1016/j.bbrc.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Vrijsen KR, Sluijter JP, Schuchardt MW, et al. Cardiomyocyte progenitor cell-derived exosomes stimulate migration of endothelial cells. J CELL MOL MED. 2010;14(5):1064–1070. doi: 10.1111/j.1582-4934.2010.01081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Gray WD, French KM, Ghosh-Choudhary S, et al. Identification of therapeutic covariant microRNA clusters in hypoxia-treated cardiac progenitor cell exosomes using systems biology. CIRC RES. 2015;116(2):255–263. doi: 10.1161/CIRCRESAHA.116.304360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ibrahim AG, Cheng K, Marban E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. STEM CELL REPORTS. 2014;2(5):606–619. doi: 10.1016/j.stemcr.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wang J, Huang W, Xu R, et al. MicroRNA-24 regulates cardiac fibrosis after myocardial infarction. J CELL MOL MED. 2012;16(9):2150–2160. doi: 10.1111/j.1582-4934.2012.01523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Gurha P, Abreu-Goodger C, Wang T, et al. Targeted deletion of microRNA-22 promotes stress-induced cardiac dilation and contractile dysfunction. CIRCULATION. 2012;125(22):2751–2761. doi: 10.1161/CIRCULATIONAHA.111.044354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lyu L, Wang H, Li B, et al. A critical role of cardiac fibroblast-derived exosomes in activating renin angiotensin system in cardiomyocytes. J MOL CELL CARDIOL. 2015;89(Pt B):268–279. doi: 10.1016/j.yjmcc.2015.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wang X, Huang W, Liu G, et al. Cardiomyocytes mediate anti-angiogenesis in type 2 diabetic rats through the exosomal transfer of miR-320 into endothelial cells. J MOL CELL CARDIOL. 2014;74:139–150. doi: 10.1016/j.yjmcc.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Behfar A, Crespo-Diaz R, Terzic A, et al. Cell therapy for cardiac repair--lessons from clinical trials. NAT REV CARDIOL. 2014;11(4):232–246. doi: 10.1038/nrcardio.2014.9. [DOI] [PubMed] [Google Scholar]
- 193.Behfar A, Crespo-Diaz R, Terzic A, et al. Cell therapy for cardiac repair--lessons from clinical trials 1. NAT REV CARDIOL. 2014;11(4):232–246. doi: 10.1038/nrcardio.2014.9. [DOI] [PubMed] [Google Scholar]
- 194.Tang XL, Rokosh G, Sanganalmath SK, et al. Intracoronary administration of cardiac progenitor cells alleviates left ventricular dysfunction in rats with a 30-day-old infarction. CIRCULATION. 2010;121(2):293–305. doi: 10.1161/CIRCULATIONAHA.109.871905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.van Berlo JH, Kanisicak O, Maillet M, et al. c-kit+ cells minimally contribute cardiomyocytes to the heart. NATURE. 2014;509(7500):337–341. doi: 10.1038/nature13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Sultana N, Zhang L, Yan J, et al. Resident c-kit(+) cells in the heart are not cardiac stem cells. NAT COMMUN. 2015;6:8701. doi: 10.1038/ncomms9701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Behfar A, Crespo-Diaz R, Terzic A, et al. Cell therapy for cardiac repair--lessons from clinical trials. NAT REV CARDIOL. 2014;11(4):232–246. doi: 10.1038/nrcardio.2014.9. [DOI] [PubMed] [Google Scholar]
- 198.Ilic D, D L, M C, C S. Human embryonic and induced pluripotent stem cells in clinical trials. BRITISH MEDICAL BULLETIN. 2015:19–27. doi: 10.1093/bmb/ldv045. [DOI] [PubMed] [Google Scholar]
- 199.Tarui S, Ishigami S, Ousaka D, et al. Transcoronary infusion of cardiac progenitor cells in hypoplastic left heart syndrome: Three-year follow-up of the Transcoronary Infusion of Cardiac Progenitor Cells in Patients With Single-Ventricle Physiology (TICAP) trial. J THORAC CARDIOVASC SURG. 2015;150(5):1198–1207. 1208. doi: 10.1016/j.jtcvs.2015.06.076. [DOI] [PubMed] [Google Scholar]
- 200.Poulin MF, Deka A, Mohamedali B, et al. Clinical Benefits of Stem Cells for Chronic Symptomatic Systolic Heart Failure A Systematic Review of the Existing Data and Ongoing Trials. CELL TRANSPLANT. 2016 doi: 10.3727/096368916X692087. [DOI] [PubMed] [Google Scholar]
- 201.Banovic M, Loncar Z, Behfar A, et al. Endpoints in stem cell trials in ischemic heart failure. STEM CELL RES THER. 2015;6:159. doi: 10.1186/s13287-015-0143-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Oh H, Ito H, Sano S. Challenges to success in heart failure: Cardiac cell therapies in patients with heart diseases. J CARDIOL. 2016 doi: 10.1016/j.jjcc.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 203.Micheu MM, Scafa-Udriste A, DorobanTu M. Bringing cardiac stem cell therapy from bench to bedside: lessons from the past and future perspectives. ROM J MORPHOL EMBRYOL. 2016;57(2):367–372. [PubMed] [Google Scholar]
- 204.Bruyneel AA, Sehgal A, Malandraki-Miller S, et al. Stem Cell Therapy for the Heart: Blind Alley or Magic Bullet? J CARDIOVASC TRANSL RES. 2016 doi: 10.1007/s12265-016-9708-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ong SG, Lee WH, Huang M, et al. Cross talk of combined gene and cell therapy in ischemic heart disease: role of exosomal microRNA transfer. CIRCULATION. 2014;130(11 Suppl 1):S60–S69. doi: 10.1161/CIRCULATIONAHA.113.007917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Mackie AR, Klyachko E, Thorne T, et al. Sonic hedgehog-modified human CD34+ cells preserve cardiac function after acute myocardial infarction. CIRC RES. 2012;111(3):312–321. doi: 10.1161/CIRCRESAHA.112.266015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Lai RC, Arslan F, Lee MM, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. STEM CELL RES. 2010;4(3):214–222. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 208.Akyurekli C, Le Y, Richardson RB, et al. A systematic review of preclinical studies on the therapeutic potential of mesenchymal stromal cell-derived microvesicles. STEM CELL REV. 2015;11(1):150–160. doi: 10.1007/s12015-014-9545-9. [DOI] [PubMed] [Google Scholar]
- 209.Singla DK. Stem cells and exosomes in cardiac repair. CURR OPIN PHARMACOL. 2016;27:19–23. doi: 10.1016/j.coph.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 210.Spinetti G, Fortunato O, Caporali A, et al. MicroRNA-15a and microRNA-16 impair human circulating proangiogenic cell functions and are increased in the proangiogenic cells and serum of patients with critical limb ischemia. CIRC RES. 2013;112(2):335–346. doi: 10.1161/CIRCRESAHA.111.300418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Janssen HL, Reesink HW, Lawitz EJ, et al. Treatment of HCV infection by targeting microRNA. N ENGL J MED. 2013;368(18):1685–1694. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- 212.Das S, Halushka MK. Extracellular vesicle microRNA transfer in cardiovascular disease. CARDIOVASC PATHOL. 2015;24(4):199–206. doi: 10.1016/j.carpath.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 213.Lin Z, Pu WT. Strategies for cardiac regeneration and repair. SCI TRANSL MED. 2014;6(239):239rv1. doi: 10.1126/scitranslmed.3006681. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.