Abstract
In previous functional magnetic resonance imaging (fMRI) studies of continuous recognition memory it was reported that new items elicit greater hippocampal activity than old (repeated) items (hippocampal ‘novelty’ effects). Rather than reflecting recency differences between new and old items, hippocampal novelty effects may instead reflect the novelty of the association between test items and the experimental context, or a mismatch in the novelty of the test item and the context. The present continuous recognition study assessed these possibilities by manipulating item-context associations on a trial-by-trial basis. Each trial comprised the presentation of an object-word (context-item) pair. Repeated items were paired either with the same context as on their first presentation, a different but previously presented context, or a new context. The task was to judge whether each item was old or new, regardless of the study status of the associated context. We found no evidence that hippocampal novelty effects reflected either item and context recency, or the novelty of the item-context association. Rather, enhanced hippocampal activity was elicited when the novelty of the item and its context mismatched. These findings support the possibility that hippocampal novelty effects reflect, at least in part, the disjunction in novelty between test items and their contexts.
Keywords: continuous recognition, associative novelty, recognition, familiarity, recollection, fMRI
Introduction
It has been reported in numerous studies that neural activity in the hippocampus is enhanced for novel relative to familiar items (for reviews, see Nyberg, 2005; Rugg et al., 2012). For example, in functional magnetic resonance imaging (fMRI) studies of recognition memory, correctly rejected new items are frequently reported to elicit greater hippocampal activity than correctly recognized old items (e.g., Stark and Okado, 2003; Brozinsky et al., 2005; Daselaar et al., 2006; Johnson et al., 2008; Vilberg and Rugg, 2009; Suzuki et al., 2011a, 2011b; Staresina et al., 2012; for reviews, see Nyberg, 2005; Rugg et al., 2012). Using a continuous recognition memory procedure, Johnson et al. (2008) and Suzuki et al. (2011a) identified novelty-sensitive hippocampal regions where such ‘new > old’ effects varied monotonically with the number of times a test item was repeated, suggesting that the effects co-vary with a continuously varying ‘novelty’ or ‘familiarity’ signal. Following previous proposals (e.g., Duzel et al., 2003; Stark and Okado, 2003; Nyberg, 2005), Johnson et al. (2008) and Suzuki et al. (2011a) suggested that these graded effects reflect hippocampally-mediated encoding processes that vary in their level of engagement with the situational novelty of the test item (situational novelty refers to items that, while not necessarily novel in an absolute sense (words, for example), are novel with respect to a given experimental context).
Three variables can potentially contribute to the situational novelty of an item. The first of these is simple recency – the elapsed time since an item was last experienced. The second variable is the novelty of the association between the item and the experimental context. For example, if the MRI scanning environment defines the context, there is not only a difference in the recency of new and old items, but also in the recency and strength of their association with the context. The third variable is the disjunction between the novelty of the item and context. For old items, both the item and the context are familiar. By contrast, for new items, there is a disjunction in familiarity between the item (novel) and the context (familiar). In short, hippocampal novelty effects might depend on the relative novelty of the items, the novelty of an item-context association, or a disjunction between the novelty of the item and the context.
There is evidence to suggest that the hippocampus is sensitive to associative novelty (e.g., Duzel et al., 2003; Kohler et al., 2005; Chen et al., 2011; Howard et al., 2011; Fandakova et al., 2013). For example, in the study of Chen et al. (2011), participants studied face-house associations. At test, participants were cued with a studied face or house and then presented with the original studied associate or an associate from a different studied pair. Correctly rejected novel associates elicited greater hippocampal activity (specifically, in CA1) relative to when familiar associates were correctly recognized.
There is also suggestive evidence that hippocampal activity can be modulated as a function of the disjunction in novelty between items (Pihlajamaki, et al., 2004; Duncan et al., 2009; Turk-Browne et al., 2012). In the Duncan et al. (2009) study, participants studied a pair of items and, after a short delay, judged whether a probe item pair matched or mismatched the studied pair. The probe pair was either the same two items as the sample stimulus, the same items with interchanged spatial locations, or had had one of the objects replaced with a novel item. Hippocampal activity was greater in this last condition than in the two former conditions. As this study did not have a condition where the sample stimulus contained two novel objects, it is unclear whether the reported effect reflects a mismatch in novelty or a simple item novelty effect (see also, Pihlajamaki, et al., 2004). Nevertheless, the finding hints at the possibility that the hippocampus is sensitive to a disjunction in novelty between stimulus elements.
Findings such as those mentioned above lend support to the possibility that previously reported situational novelty effects in the hippocampus during continuous recognition memory (Brozinsky et al., 2005; Johnson et al., 2008; Viskontas et al., 2008; Suzuki et al., 2011a, 2011b) reflect differences between old and new items in their associative novelty, or in the level of disjunction in novelty between an item and its context. In the current study, we assessed these possibilities by manipulating associations between items and their contexts on a trial-by-trial basis. Each trial comprised the presentation of an object-word (context-item) pair. Repeated items were paired either with the same object as on their first presentation (old-Old), a different but previously presented object (old’-Old), or a new object (new-Old). The different pairs are illustrated in Figure 1.
Figure 1.
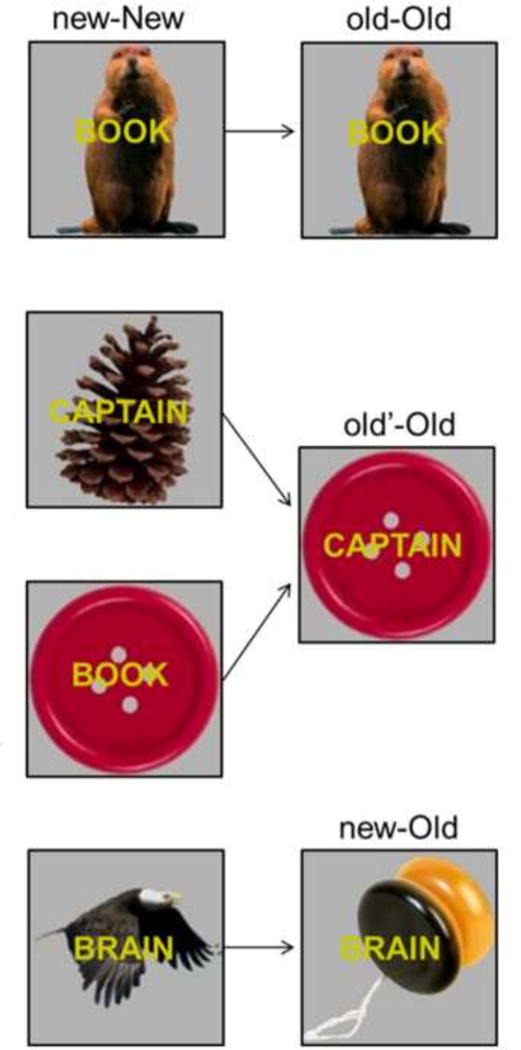
Context-item pairs. Repeated items (words in yellow) were paired with either the same context (object) as on their first presentation (old-Old), a different but previously presented context (old’-Old) or a new context (new-Old). The new-New pairs are the initial presentation of each context-item pair and are shown on the left with the relevant old-Old, old’-Old, and new-Old pairs on the right.
The task was to judge whether each word was old or new. The task-irrelevant object was assumed to provide the ‘local’ context for the task-relevant item (cf., Tsivilis et al., 2001, 2003). We assumed that our manipulation of local context would overshadow the influence of the relatively invariant experimental context (see also, Turke-Browne et al., 2012; Kim et al., 2014).
In order to assess the above three accounts of hippocampal novelty effects, we conducted three contrasts. To assess the sensitivity of the hippocampus to the relative recency of the test pairs, we contrasted the activity elicited by new-New pairs (initial presentations) with that elicited by old’-Old pairs (new-New > old’-Old). Because both classes of item-context pair comprise novel associations, any difference in activity can be assumed to reflect a difference in when the constituent items and contexts were last experienced. To assess the possibility that the hippocampus is sensitive to associative novelty, we contrasted old’-Old and old-Old pairs (old’-Old > old-Old). In this case, both the context and the item are familiar, and all that varies is the novelty of the item-context association. Lastly, to assess the possibility that hippocampal novelty effects reflect a mismatch between the novelty of an item and its context, we assessed whether new-Old pairs elicited greater activity than any of the other classes of test pair.
Although the primary aim of the study was to identify different classes of novelty-related effects within the hippocampus, we took advantage of our whole-brain fMRI acquisition protocol to investigate novelty and old > new effects throughout the rest of the brain. Only two prior studies have described old > new item effects outside of the medial temporal lobe (MTL) during single-item continuous recognition (Yassa and Stark, 2008; Suzuki et al., 2011a) and, in one of those studies, the extra-MTL effects were restricted to a limited field of view that excluded much of the neocortex (Suzuki, et al., 2011a). Yassa and Stark (2008) reported that, relative to new items, repeated items elicited diminished responses in occipital cortex, extending into the MTL (but, curiously, excluding the hippocampus), along with enhanced activity in bilateral parietal cortex. By contrast, Suzuki et al. (2011a) reported repetition-related activity reductions in retrosplenial and occipito-temporal cortex, accompanied by enhancements in the precuneus and bilateral intraparietal sulcus (IPS). The present experiment afforded the opportunity to extend these findings by examining whether they are modulated by the manipulation of local context.
Results
Behavioral Results
Table 1 shows the mean reaction times (RTs) for correct responses and mean proportions of correct responses for each of the context-item pairs. One-way repeated measure ANOVAs of these variables were conducted across the 4 levels of the factor of pair type (old-Old, old’-Old, new-Old, and new-New). There was a main effect of pair type on RT (F(1.94, 44.53) = 11.03, p < 0.001; degrees of freedom Greenhouse-Geisser corrected for nonsphericity). Follow-up pairwise comparisons revealed that RTs for the new-Old pairs were significantly longer than those for old-Old and old’-Old pairs (both ps < 0.01), which did not differ from one another. Additionally, RTs for the new-New pairs were significantly longer than those for old-Old and old’-Old pairs (both ps < 0.01). RTs did not significantly differ between new-New and new-Old pairs. ANOVA of the accuracy data (computed as hit rate for the old-Old, old’-Old, and new-Old pairs and correct rejection rate for the new-New pair) also revealed a main effect of pair type (F(2.08, 47.80) = 15.84, p < 0.001). Follow-up pairwise comparisons revealed that accuracy for the old-Old pairs was significantly greater than that for either the old’-Old or new-Old pairs (both ps < 0.01), which did not differ from one another. Similarly, accuracy for the new-New pairs was significantly greater than that for either the old’-Old or new-Old pairs (both ps < 0.01).
Table 1.
Mean ± 1 standard deviation proportions of correct responses and associated reaction times (RTs) for each pair type
| Condition | Proportion | RT (ms) |
|---|---|---|
| old-Old | 0.88 ± 0.12 | 837 ± 145 |
| old’-Old | 0.81 ± 0.15 | 851 ± 155 |
| new-Old | 0.80 ± 0.12 | 881 ± 155 |
| new-New | 0.91 ± 0.09 | 908 ± 180 |
fMRI Results
Planned contrasts within the hippocampus
We first tested whether hippocampal novelty effects could be identified by contrasting activity elicited by new-New pairs and that elicited by old’-Old pairs. The contrast failed to identify any significant effects. We went on to test whether hippocampal activity was sensitive to associative novelty by contrasting the activity elicited by old’-Old and old-Old pairs. This contrast also failed to identify significant effects within the hippocampus.
Finally, we tested whether the hippocampus was sensitive to the mismatch between item and context novelty. We searched for a hippocampal mismatch effect by performing a conjunction analysis to identify activity common to the contrasts of new-Old > new-New and new-Old > (old-Old + old’-Old) (contrast weights: +1 −1 and +2 −1 −1, respectively, each contrast thresholded at p < 0.005)1. This procedure identified a 13-voxel cluster in the left anterior hippocampus (peak co−ordinates for the contrast of new-Old > new-New: −33 −13 −14, Z = 3.58, and peak co-ordinates for the contrast of new-Old > (old-Old + old’-Old): −30 −13 −17, Z = 3.16, see Figure 2).
Figure 2.
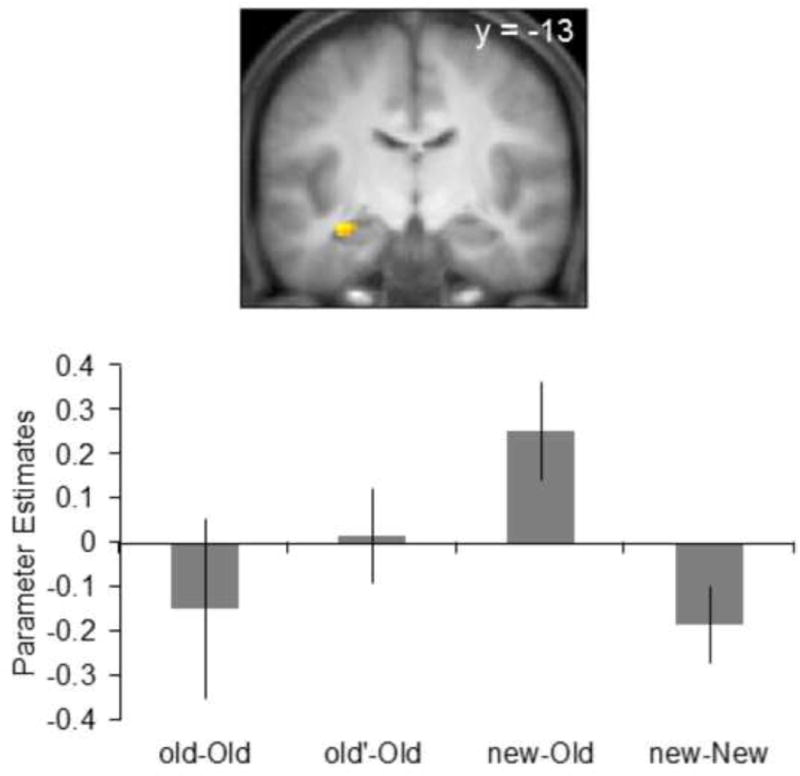
A. Hippocampal effects identified by the conjunction across two contrasts: new-Old > new-New and new-Old > (old-Old +old’-Old). In this and subsequent figures, bar graph shows the mean parameter estimates (± 1 between participant SE) for each pair type extracted from the peak voxels. Parameter estimates are shown for illustrative purposive only.
Whole-brain analyses
We conducted whole brain analyses directed towards the identification of generic old > new and new > old item effects. The effects were identified with the contrast between old and new items (old-Old + old’-Old + new-Old) > new-New, and vice-versa; contrast weights: +1 +1 +1 −3 and −1 −1 −1 +3, respectively). No regions demonstrating new > old effects were identified. By contrast, the reverse contrast revealed widespread differences in activity. We elucidated these findings by employing exclusive and inclusive masking to distinguish context-independent and context-dependent effects. Context-independent old > new effects were identified by exclusively masking the above contrast with the bi-directional main effect of context (i.e., the main effect of the ANOVA contrasting the old-Old, old’-Old, and new-Old conditions, thresholded at p < 0.1). Context-independent effects were identified in numerous cortical regions, including the precuneus, inferior parietal lobule, IPS, ventral and dorsal striatum, dorsolateral prefrontal cortex (PFC), and anterior PFC (Figure 3 and Table 2).
Figure 3.
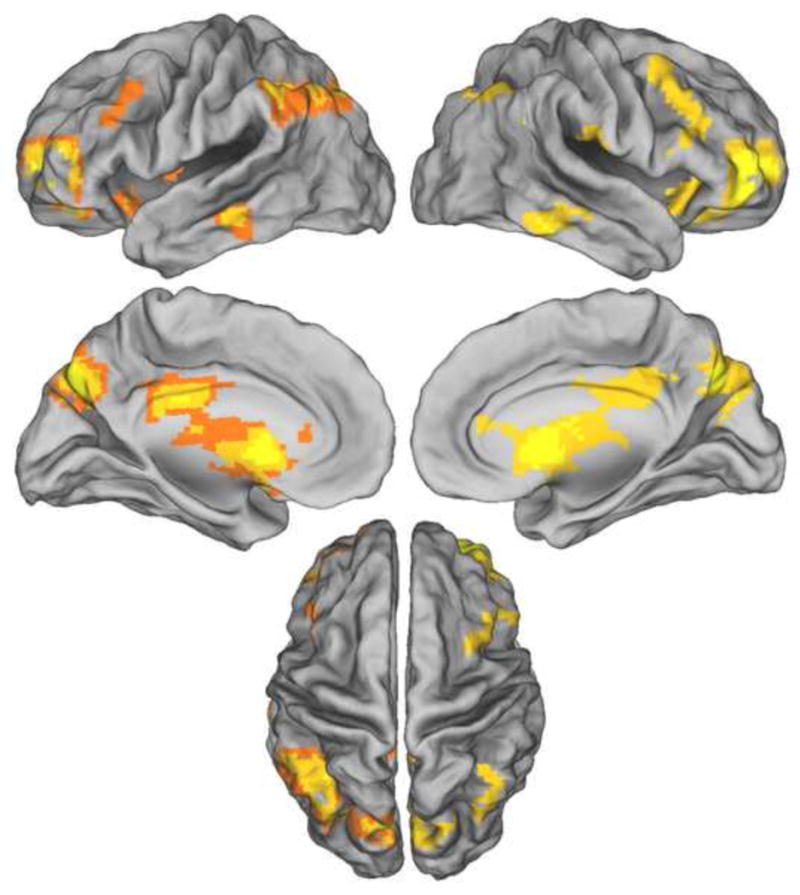
Context-independent old > new effects. Results are overlaid on the standardized brain of the PALS-B12 atlas implemented in Caret5 (VanEssen, 2005). Left hemisphere is on the left.
Table 2.
Loci of context-independent whole-brain effects
| MNI Coordinates
|
Peak Z | Number of above-threshold voxels | Region | ||
|---|---|---|---|---|---|
| x | y | z | |||
| − 9 | − 76 | 40 | 6.91 | 1950 | Left precuneus |
| −35 | −58 | 43 | 5.86 | Left intraparietal sulcus | |
| − 51 | − 49 | 49 | 4.89 | Left inferior parietal lobule | |
| − 9 | 14 | − 5 | 6.65 | 3584 | Left striatum |
| − 3 | − 25 | 28 | 6.05 | Left posterior cingulate cortex | |
| 12 | 17 | 7 | 5.90 | Right striatum | |
| − 33 | 50 | 4 | 6.30 | 454 | Right anterior prefrontal cortex |
| −30 | −46 | −35 | 5.63 | 872 | Left cerebellum |
| 12 | − 82 | − 32 | 4.11 | Right cerebellum | |
| − 66 | − 31 | − 14 | 4.73 | 78 | Left inferior temporal sulcus |
| 63 | − 34 | − 20 | 4.70 | 111 | Right inferior temporal gyrus |
| 66 | − 22 | − 17 | 3.55 | Right middle temporal gyrus | |
| − 9 | 32 | 7 | 4.52 | 43 | Left anterior cingulate cortex |
| 60 | −16 | 19 | 4.30 | 116 | Right postcentral gyrus |
| 33 | − 46 | − 44 | 4.04 | 179 | Right cerebellum |
| 12 | − 31 | − 38 | 3.98 | Right brainstem | |
| −48 | 29 | 28 | 3.86 | 80 | Left middle frontal gyrus |
Coordinates for cluster sub-peaks which lied in distinct cortical regions are listed directly below relevant peak cluster.
Context-dependent old > new effects were identified by inclusively masking the old > new item contrast with the main effect of the ANOVA contrasting the old-Old, old’-Old, and new-Old pairs (thresholded at p < 0.01, giving an estimated conjoint significance of p < 0.0001; Fisher, 1950; Lazar et al., 2002). Context-dependent old > new effects were uniquely identified a 37-voxel cluster in the left anterior PFC (peak coordinates of −39 59 4, Z = 3.36, Figure 4). Parameter estimates were then extracted from the peak voxel within this cluster and follow-up comparisons were conducted to investigate the pattern of activity among the three conditions containing old items. Note that these comparisons are unbiased with respect to the statistical procedure employed to identify the peak voxels. As depicted in Figure 4, follow-up comparisons on peak parameter estimates revealed that activity associated with the old-Old pairs was significantly greater than that elicited by either the old’-Old or new-Old pairs (both ps < 0.01). Activity did not significantly differ between the old’-Old and new-Old pairs.
Figure 4.
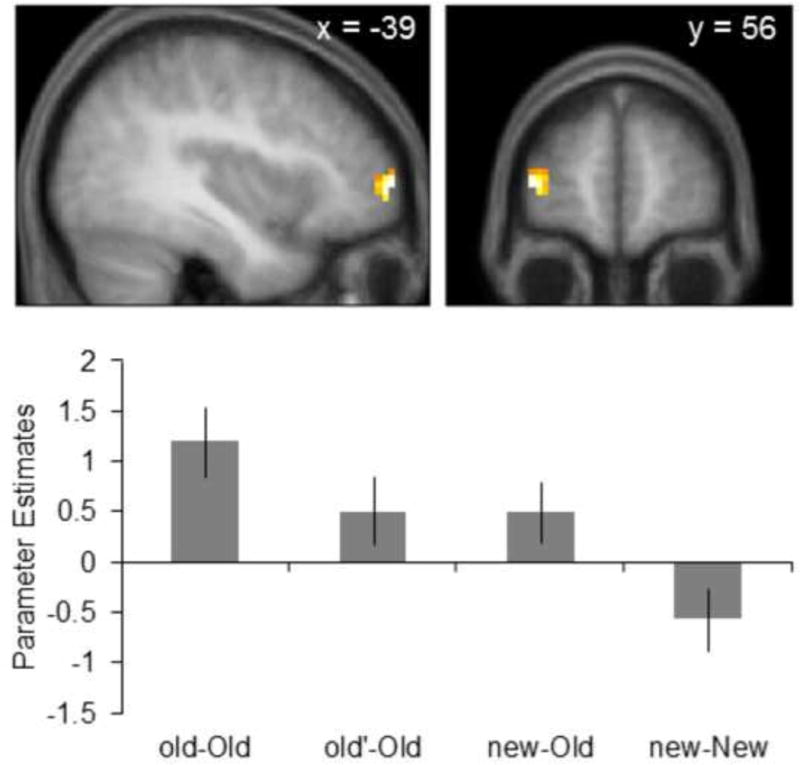
Context-dependent old > new effects within the left anterior prefrontal cortex. Parameter estimates for the new-New pairs are shown for illustrative purposes only as follow-up comparisons were conducted excluding this pair.
Potential RT confounds
As described in the behavioral results, RT differences were observed across the four context-item pairs. These findings raise the possibility that differences in the fMRI activity elicited by the different pair types were influenced by these differences in RT. To assess this possibility, we repeated all the fMRI analyses reported above where participant-specific mean RTs for each pair type were entered as covariates in the second-level repeated measures ANOVA (see, ‘Image Acquisition and Analysis’). These analyses gave rise to results essentially identical to those identified in the original analyses. Thus, there was no evidence that RT influenced the current fMRI findings.
Discussion
In previous continuous recognition studies, hippocampal novelty effects were identified by contrasting the first and second presentations of single items (new > old effects). Here we assessed whether these effects reflect a difference in recency between test items or whether instead they reflect either the relative novelty of item-context associations, or the mismatch between the novelty of the item and its context. We failed to identify any region sensitive to differences in the novelty of item-context pairs, as would have been expected if hippocampal novelty effects merely reflected differences in when the items and their contexts were last experienced. We also failed to identify associative novelty effects in the hippocampus. We did however identify a hippocampal novelty mismatch effect. Additionally, a whole-brain analysis revealed old > new effects in several regions. With one exception, these effects were insensitive to variations in local context.
Behavioral findings
The contrast of the recognition accuracy scores across the four pair types gave rise to two findings. First, accuracy for the old-Old pairs was significantly greater than that for the old’-Old and new-Old pairs. This effect might be a reflection of the benefit to item memory accruing from the combination of the familiarity of the item and its association with the context (cf. Speer and Curran, 2007). An alternative interpretation is that the benefit to item memory when repeating an identical stimulus configuration may reflect something akin to the unitization of the item and the context (cf. Yonelinas et al., 2010). Finally, it is possible that participants consciously recollected item-context pairs when they were repeated, and that the recollected information enhanced recognition accuracy (cf. Donaldson and Rugg, 1998, 1999; Yonelinas, 2002). However, as is discussed below (see, ‘Whole brain analyses’), we think it is unlikely that recollection played much of a role in recognition decisions in the present task.
The second result from the analyses of the behavioral data was that recognition accuracy for the old’-Old and the new-Old pairs did not significantly differ. Thus, there was no evidence that the study status of the context biased recognition judgments on the accompanying item. It seems unlikely therefore that the participants integrated information about the study status of contexts and items when making recognition judgments.
Hippocampal fMRI findings
The hippocampus was insensitive to the combined novelty of an item and its context, as this was assessed with the contrast between new-New and old’-Old pairs. The absence of a new > old effect is striking in light of the frequency with which such effects have been reported in prior studies (see Introduction). This null finding is consistent with the possibility that the hippocampal novelty effects previously reported in studies of continuous recognition (Brozinsky et al., 2005; Johnson et al., 2008; Viskontas et al., 2008; Suzuki et al., 2011a; but see, Yassa and Stark, 2008) reflect something other than simple recency. Additionally, we found no evidence for a hippocampal associative novelty effect (as operationalized by the contrast between old’-Old > old-Old pairs). Thus there was no support for the hypothesis that previously reported hippocampal new > old effects observed during continuous recognition reflect the novelty of the association between an item and its context.
The hippocampus was however sensitive to the mismatch between the novelty of an item and its context. This finding provides support for the possibility that prior new > old hippocampal effects (see above) reflected a mismatch in item-context novelty. The current finding raises the further possibility that previously reported hippocampal novelty effects in experiments that employed a study-test procedure (for reviews, see Nyberg, 2005; Rugg et al., 2012) also reflected a mismatch between item and contextual novelty.
A seemingly analogous hippocampal novelty mismatch effect was identified by Turk-Browne et al. (2012). In that study, participants viewed a continuous stream of scenes and made an indoor/outdoor judgment to each scene. The scenes were organized into triplets. The first two scenes of each triplet were assumed to serve as the ‘temporal’ context for the third scene, the item. Contexts were repeated either with the same item as on their initial presentation or with a novel item. Additionally, some repeated items were preceded by novel contexts. Greater hippocampal activity was elicited in this latter condition relative to when both contexts and items were novel. Turk-Browne and colleagues (2012) interpreted this elevated hippocampal activity as evidence for a hippocampally-mediated retrieval process. They proposed that when a familiar item was paired with a novel context, the resulting mismatch elicited retrieval of the context originally associated with the item. A similar interpretation can be applied in the present case for the hippocampal effect elicited by new-Old pairs (where, as in Turk-Browne et al. (2012), a novel context preceded a familiar item). An alternative possibility however is that the enhanced hippocampal activity elicited by new-Old pairs reflected a hippocampally-mediated encoding process that ‘overwrote’ the original item-context association. It will be important for future studies to disambiguate these two possibilities.
According to both of the above accounts, enhanced hippocampal activity for old’-Old relative to old-Old pairs should also have been evident, a finding we failed to observe. A possible explanation for this null result is that the inter-trial interval between presentations of new-New pairs that were interchanged to form old’-Old pairs was too short on average to permit the formation of unique item-context associations. This possibility arises from the finding that context-item associations can seemingly be formed when the context and the item are separated in time (Turk-Browne et al., 2012; see also, Howard and Kahana, 2002; Sederberg et al., 2008). By this argument, the context belonging to a given new-New pair might also have formed part of the context associated with the word belonging to a succeeding new-New pair, if the inter-pair lag was sufficiently short. We assessed this possibility in a follow-up analysis in which we segregated old’-Old pairs according to whether the new-New pairs from which they were created were separated by 5 or fewer (old’-Old-close) or more than 5 intervening pairs (old’-Old-far). The contrast between old’-Old-far > old’-Old-close pairs failed to identify any effects within the hippocampus or at the whole-brain level. Thus, there was no evidence that the activity elicited by old’-Old pairs differed according to the temporal separation of the original and re-paired context. These findings therefore provide no support for the possibility that the enhanced hippocampal activity elicited by new-Old pairs reflects the novelty of the item-context association, rather than a mismatch in the novelty of the context and its associated item.
It is also worth noting that our failure to identify an associative novelty effect is difficult to reconcile with the idea that associative novelty effects are driven by expectancy violations (Kumaran and Maguire, 2006, 2007a; Chen et al., 2013; for a review, see Kumaran and Maguire, 2007b). According to this idea, a repeated context would be predicted to generate an ‘expectation’ for the item it was originally paired with. In the case of old’-Old pairs, the context should have generated an expectancy for the originally associated item, an expectation that was violated with the presentation of a novel associate. It is currently uncertain whether such expectancy violations depend on the explicit, intentional retrieval of items associated with a repeated context, and thus this possibility cannot be ruled out at present. In the present experiment, contexts were both task-irrelevant (participants were instructed to treat them merely as a warning cue for the upcoming word) and, in addition, preceded the critical items (words) by only 300 ms. Together, these factors may have led to participants failing to use repeated contexts to explicitly generate expectations about the associated word. In short, additional research is required to elucidate conditions under which associative novelty effects are observed.
Whole-brain fMRI findings
In contrast to prior continuous recognition studies (Yassa and Stark, 2008; Suzuki et al., 2011a), we failed to identify any cortical regions demonstrating enhanced activity for new relative to old items. Whereas this null finding might indicate that, like their hippocampal counterparts, extra-hippocampal novelty effects depend upon a mismatch in item and contextual novelty, it should be noted that both of the aforementioned prior studies employed multiple repetitions of old items. In neither case was it demonstrated that new > old effects were reliably present for the contrast between new items and items that had been repeated once only, the situation analogous to the present study. Thus, it remains to be determined whether cortical novelty effects are context-dependent.
Unlike new > old effects, old > new effects were robust and widespread throughout the cortex. Context-independent effects were observed in, among other regions, posterior parietal cortex in the vicinity of the IPS, along with the anterior PFC, the striatum, and precuneus. It is notable that old > new effects were not identified in cortical regions typically associated with the successful recollection of qualitative information about a study episode (e.g., the angular gyrus, medial PFC, and posterior cingulate/retrosplenial cortex; for reviews, see Kim, 2010; Rugg and Vilberg, 2013). Rather, the present effects include regions – such as the IPS and anterior PFC - that correspond to those previously reported to accompany familiarity-based recognition judgments (for reviews, see Vilberg and Rugg, 2008; Kim, 2010). Thus the current findings support the assumption that the recognition of test items during continuous recognition is typically supported primarily by an acontextual familiarity signal (Johnson et al., 2008; Suzuki et al., 2011a,b; Rugg et al., 2012, but see, Huijbers et al., 2010).
Only one cortical region – the left anterior PFC – demonstrated a context-dependent old > new effect. This took the form of greater activity for old-Old than for either old’-Old and new-Old pairs. The left anterior PFC has been previously implicated in familiarity- rather than recollection-based recognition judgments (e.g. Yonelinas et al., 2005; Vilberg and Rugg, 2007; for a review, see Kim, 2010). Thus the present results raise the possibility that the region identified here as context-sensitive is especially sensitive to the strength of the familiarity signal supporting recognition judgments, reflecting the enhancement of familiarity arising from the repetition of the same item-context configuration (see, ‘Behavioral findings’). As was noted previously, along with the anterior PFC, the IPS has also been implicated in familiarity-based recognition judgments (for reviews, see Vilberg and Rugg, 2008; Kim, 2010). Indeed, it has been reported that activity in this region co-varies with familiarity strength (Yonelinas et al., 2005, Suzuki et al., 2011a, Johnson et al., 2013). In light of these findings, one might have expected this region to parallel the anterior PFC in demonstrating greater activity for old-Old than either old’-Old or new-Old pairs. Although evidence for such an effect was not forthcoming from the analyses described above, such an effect was evident in the left IPS for the directed contrast between old-Old and the other two classes of test pairs containing old items (peak co-ordinates of −39 −64 55, Z = 2.54, 101 voxels at a threshold of p < 0.05). Thus, there is little or no evidence from the present study to suggest that the left anterior PFC and the left IPS are differentially sensitive to the strength of the familiarity signal supporting recognition memory judgments.
Conclusion
Remarkably, we were unable to identify any regions, either within or outside the hippocampus, where activity was enhanced for novel relative to familiar item-context pairs. Hence, we found no evidence to suggest that previously reported hippocampal novelty effects reflect differences in the time since an item was last experienced. We did however identify a hippocampal effect that reflected the mismatch in novelty between an item and its local context. This finding supports the possibility that hippocampal novelty effects reflect a disparity in novelty between test items and their contexts.
Methods and materials
Participants
Twenty-four participants (mean age of 23 yrs, range 18–30; 11 females) with normal or corrected-to-normal vision completed the experiment; each participant contributed data to the analyses described below. Participants had no contraindications for MRI and reported themselves to be right-handed and free of neurological and psychiatric disease. Informed consent was obtained prior to participation. The experimental protocol was approved by The University of Texas, Southwestern Medical Center at Dallas Institutional Review Board.
Stimulus Materials
Experimental items consisted of 272 color pictures of objects obtained from Hemera Photo Objects 50,000 Volume II (2001). Each object was paired with a semantically unrelated concrete word. The words were selected from the word norms compiled by Nelson et al. (2004) and ranged in length from 3 to 9 letters and in frequency from 1 to 600 counts/million. For each participant, the object-word (context-item) pairs were randomly sorted and selected to create 4 lists, one for each of the pair types. The first list provided the initial presentations of context-item pairs (new-New pairs), the second list comprised repeated items paired with the same context as the initial presentation (old-Old pairs), the third list comprised repeated items paired with a different repeated context (old’-Old pairs), and the fourth list comprised repeated items paired with a new context (new-Old pairs).
The aforementioned 4 lists were used to create 4 continuous recognition runs (no context-item pairs were repeated across runs). Each run consisted of 96 context-item trials with an additional 22 null trials (3500 ms of fixation). Context-item pairs were presented at the center of a grey screen. On each trial, the context (object) was first presented in isolation for 300 ms. The item (word), presented in 25-point Arial font with yellow characters, was then superimposed across the center of the context for 500 ms, after which both the context and item were removed. A central fixation cross was then displayed for 2700 ms followed immediately by the presentation of the next context or the onset of a null trial. Contexts subtended maximum horizontal and vertical visual angles of 4.61°. The maximum horizontal angle subtended by the items was also 4.61°.
Trials in each run were organized into a series of 6 blocks (participants were unaware of this organization and, from their perspective, viewed only a continuous stream of context-item pairs). The initial block consisted of 8 new-New pairs. Each subsequent block consisted of a 1:1 ratio of new and old items (i.e., 8 new-New, 2 old-Old, 2 old’-Old, and 4 new-Old pairs). Critical fMRI contrasts were conducted on the last 5 blocks. Items were repeated after between 1 and 30 intervening items (with a mean lag of 14 items)2. The mean lag for context repetitions for old-Old and old’-Old pairs was 15 contexts. Trial presentation was pseudorandom, and no more than 3 context-item pairs of any given type were presented successively (following the 8 initial new-New pairs). No more than two null trials were successively presented. Each run terminated with 20 s of fixation.
Experimental Procedures
The task was to judge whether each item was being viewed for the first or the second time, and to respond ‘new’ or ‘old’, respectively. Participants were instructed to treat the associated context as a warning cue to prepare for the upcoming item. It was stressed that the identity and study status of the context were irrelevant and that decisions should be based solely upon the study status of the following item. Instructions also stressed the need to respond as quickly as possible without sacrificing accuracy. Responses were made on a button-box with the middle (old response) and ring (new responses) fingers of the right hand. Prior to entering the scanner participants completed a short practice run consisting of 7 context-item pairs (with at least 1 trial of each condition) that were presented on a laptop computer.
Image Acquisition and Analysis
MR images were acquired on a 3 T Philips Achieva MRI scanner (Philips Medical Systems) using a 32-channel head coil. Functional images were acquired using an echo-planar imaging (EPI) sequence (SENSE factor of 1.5, flip angle 70°, 80 × 80 matrix, FOV = 24 cm, TR = 2000 ms, TE = 30 ms, 34 slices, 3 mm slice thickness, 1 mm gap, 3 mm isotropic voxels). The images were acquired oriented parallel to the anterior/posterior commissure plane in ascending order. For each continuous recognition run, 205 volumes were acquired (yielding an effective sampling rate of 2 Hz, given the 3.5 s inter-trial interval). Anatomic images were acquired using a magnetization rapidly acquired gradient echo sequence (MPRAGE; 240 × 240 matrix, 1 mm isotropic voxels).
fMRI analysis was conducted with Statistical Parametric Mapping (SPM8, Wellcome Department of Cognitive Neurology, London, UK). Functional image preprocessing included realignment to the mean image, slice-time correction (using the middle slice as reference), reorientation, spatial smoothing with a 8 mm full-width-half-maximum (FWHM) Gaussian kernel, and spatial normalization to Montreal Neurological Institute (MNI) space using a standard EPI template (Cocosco et al.,1997). Anatomic images were normalized to MNI space for each individual participant (using the T1 template of SPM8) and then averaged across participants to create a mean image across all participants. The timeseries in each voxel was temporally smoothed using a high-pass filter of 128 s and scaled to a constant mean within a session. Sessions were not concatenated prior to first-level analysis (see below).
fMRI data were analyzed in two stages. In the first stage, events of interest were defined as the new-New, old-Old, old’-Old, and new-Old pairs attracting an accurate response (old pairs derived from the new-New pairs presented in the lead-in block of each run were treated as events of no-interest). Pairs associated with inaccurate responses, false-alarms, and failures to respond, along with new-New pairs from the lead-in blocks, were modeled as a single event of no-interest. Delta functions were used to model neural activity at the onset of the context for each pair. The associated BOLD response was then modeled by convolving the delta functions with the canonical hemodynamic response function and its temporal and dispersion derivatives (Friston et al., 1998). In all, the first-level GLM for each participant contained 4 events of interest (new-New, old-Old, old’-Old, and new-Old), a single event of no-interest, 6 regressors corresponding to the motion parameters (three for rotation and three for rigid-body translation), and a constant for each run which modeled mean image intensity. An AR(1) model was implemented to correct for serial correlations in the error covariance (Friston et al., 2002).
Individual participant parameter estimates for the four events of interest (old-Old, old’-Old, new-Old, and new-New) were derived from the preceding single-subject GLMs and entered into a second-level one-way repeated measures ANOVA in which participants were modeled as a random-effect. To evaluate effects within the hippocampus, a mask was created by manually tracing the hippocampus using the across-participant mean normalized 3D anatomical image (based on standard anatomical landmarks; Insausti et al., 1998; Pruessner et al., 2000). The mask image was smoothed with an 8-mm FWHM Gaussian smoothing kernel using MRIcron software (Rorden et al., 2007). A voxel-wise threshold of p < 0.005 was used for targeted a priori contrasts (see Introduction) aimed at identifying effects within the hippocampus (see also, Johnson et al., 2008; Suzuki et al., 2011a, 2011b). Correction for multiple comparisons (p < 0.05) was effected by imposition of a cluster extent threshold of 10 voxels within the hippocampal mask. The threshold was estimated using a Monte Carlo simulation of 10,000 iterations with a FWHM of 8 mm implemented using the AlphaSIM tool in Analysis of Functional NeuroImages (AFNI) software (http://afni.nimh.nih.gov/afni/AFNI_Help/AlphaSim.html). For whole-brain analyses an individual voxel threshold of p < 0.001 was used, corrected to p < 0.05 by imposition of a cluster extent threshold of 21 voxels (estimated using the same procedure as just described). All pairwise contrasts (see Results) were conducted using the error term derived from the parent ANOVA. The peak voxels of significant effects are reported in MNI coordinates.
Highlights.
-
►
New items elicit greater hippocampal activity than old items (‘novelty’ effects)
-
►
These effects may reflect a novelty mismatch between test items and the context
-
►
Enhanced hippocampal activity was elicited when item-context novelty mismatched
-
►
Prior hippocampal novelty effects may thus reflect mismatch in item-context novelty
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The conjunction isolates only what is common (i.e., a mismatch in novelty) across both contrasts of new-Old > new-New and new-Old > (old-Old + old’-Old). The purpose of using this conjunction was to avoid the identification of differences that would be exclusive to one of the two contrasts.
When items repeated at lags of less than or equal to 5 intervening items were excluded from these analyses an identical pattern of results was observed. Thus, the present effects are unlikely to reflect the influence of items maintained in short-term or working memory between successive presentations.
References
- Brozinsky CJ, Yonelinas AP, Kroll NEA, Ranganath C. Lag-sensitive repetition suppression effects in the anterior parahippocampal gyrus. Hippocampus. 2005;15:557–561. doi: 10.1002/hipo.20087. [DOI] [PubMed] [Google Scholar]
- Chen J, Olsen RK, Preston AR, Glover GH, Wagner AD. Associative retrieval processes in the human medial temporal lobe: hippocampal retrieval success and CA1 mismatch detection. Learning & Memory. 2011;18:523–8. doi: 10.1101/lm.2135211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Dastjerdi M, Foster BL, LaRocque KF, Rauschecker AM, Parvizi J, Wagner AD. Human hippocampal increases in low-frequency power during associative prediction violations. Neuropsychologia. 2013;51:2344–2351. doi: 10.1016/j.neuropsychologia.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocosco CA, Kollokian V, Kwan RS, Evans AC. BrainWeb: Online interface to a 3D MRI simulated brain database; Proceedings of the third international conference on function mapping of the human brain, Copenhagen; Denmark. 1997. p. S235. [Google Scholar]
- Daselaar SM, Fleck MS, Cabeza R. Triple dissociation in the medial temporal lobes: recollection, familiarity, and novelty. Journal of Neurophysiology. 2006;96:1902–11. doi: 10.1152/jn.01029.2005. [DOI] [PubMed] [Google Scholar]
- Donaldson DI, Rugg MD. Recognition memory for new associations: electrophysiological evidence for the role of recollection. Neuropsychologia. 1998;36:377–95. doi: 10.1016/s0028-3932(97)00143-7. [DOI] [PubMed] [Google Scholar]
- Donaldson DI, Rugg MD. Event-related potential studies of associative recognition and recall: electrophysiological evidence for context dependent retrieval processes. Brain Research Cognitive Brain Research. 1999;8:1–16. doi: 10.1016/s0926-6410(98)00051-2. [DOI] [PubMed] [Google Scholar]
- Duncan K, Curtis C, Davachi L. Distinct memory signatures in the hippocampus: intentional States distinguish match and mismatch enhancement signals. The Journal of Neuroscience. 2009;29:131–139. doi: 10.1523/JNEUROSCI.2998-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duzel E, Habib R, Rotte M, Guderian S, Tulving E, Heinze HJ. Human hippocampal and parahippocampal activity during visual associative recognition memory for spatial and nonspatial stimulus configurations. The Journal of Neuroscience. 2003;23:9439–9444. doi: 10.1523/JNEUROSCI.23-28-09439.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fandakova Y, Lindenberger U, Shing YL. Deficits in process-specific prefrontal and hippocampal activations contribute to adult age differences in episodic memory interference. Cerebral Cortex. 2014;24:1832–1844. doi: 10.1093/cercor/bht034. [DOI] [PubMed] [Google Scholar]
- Fisher RA. Statistical methods for research workers. London: Oliver and Boyd; 1950. [Google Scholar]
- Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R. Event-related fMRI: Characterizing differential responses. Neuroimage. 1998;7:30–40. doi: 10.1006/nimg.1997.0306. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Penny W, Phillips C, Kiebel S, Hinton G, Ashburner J. Classical and Bayesian inference in neuroimaging: Theory. Neuroimage. 2002;16:465–483. doi: 10.1006/nimg.2002.1090. [DOI] [PubMed] [Google Scholar]
- Insausti R, Juottonen K, Soininen H, Insausti AM, Partanen K, Vainio P, et al. MR volumetric analysis of the human entorhinal, perirhinal, and temporopolar cortices. American Journal of Neuroradiology. 1998;19:659–671. [PMC free article] [PubMed] [Google Scholar]
- Johnson JD, Muftuler LT, Rugg MD. Multiple repetitions reveal functionally and anatomically distinct patterns of hippocampal activity during continuous recognition memory. Hippocampus. 2008;18:975–980. doi: 10.1002/hipo.20456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JD, Suzuki M, Rugg MD. Recollection, familiarity, and content-sensitivity in lateral parietal cortex: a high-resolution fMRI study. Frontiers in Human Neuroscience. 2013;7:219. doi: 10.3389/fnhum.2013.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard LR, Kumaran D, Olafsdottir HF, Spiers HJ. Double dissociation between hippocampal and parahippocampal responses to object-background context and scene novelty. The Journal of Neuroscience. 2011;31:5253–5261. doi: 10.1523/JNEUROSCI.6055-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard MW, Kahana MJ. A Distributed Representation of Temporal Context. Journal of Mathematical Psychology. 2002;46:269–299. [Google Scholar]
- Huijbers W, Pennartz CMA, Daselaar SM. Dissociating the “retrieval success” regions of the brain: effects of retrieval delay. Neuropsychologia. 2010;48:491–7. doi: 10.1016/j.neuropsychologia.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Kim H. Dissociating the roles of the default-mode, dorsal, and ventral networks in episodic memory retrieval. NeuroImage. 2010;50:1648–1657. doi: 10.1016/j.neuroimage.2010.01.051. [DOI] [PubMed] [Google Scholar]
- Kim G, Lewis-Peacock JA, Norman KA, Turk-Browne NB. Pruning of memories by context-based prediction error. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:8997–9002. doi: 10.1073/pnas.1319438111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler S, Danckert S, Gati JS, Menon RS. Novelty responses to relational and non-relational information in the hippocampus and the parahippocampal region: A comparison based on event-related fMRI. Hippocampus. 2005;15:763–774. doi: 10.1002/hipo.20098. [DOI] [PubMed] [Google Scholar]
- Kumaran D, Maguire EA. An unexpected sequence of events: Mismatch detection in the human hippocampus. PLoS Biology. 2006;4:2372–2382. doi: 10.1371/journal.pbio.0040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran D, Maguire EA. Match mismatch processes underlie human hippocampal responses to associative novelty. The Journal of Neuroscience. 2007a;27:8517–8524. doi: 10.1523/JNEUROSCI.1677-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran D, Maguire EA. Which computational mechanisms operate in the hippocampus during novelty detection? Hippocampus. 2007b;17:735–748. doi: 10.1002/hipo.20326. [DOI] [PubMed] [Google Scholar]
- Lazar NA, Luna B, Sweeney JA, Eddy WF. Combining brains: A survey of methods for statistical pooling of information. Neuroimage. 2002;16:538–550. doi: 10.1006/nimg.2002.1107. [DOI] [PubMed] [Google Scholar]
- Lisman J, Grace AA, Duzel E. A neoHebbian framework for episodic memory; role of dopamine-dependent late LTP. Trends in Neurosciences. 2011;34:536–547. doi: 10.1016/j.tins.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DL, McEvoy CL, Schreiber TA. The University of South Florida free association, rhyme, and word fragment norms. Behavior Research Methods, Instruments, & Computers. 2004;36:402–407. doi: 10.3758/bf03195588. [DOI] [PubMed] [Google Scholar]
- Nyberg L. Any novelty in hippocampal formation and memory? Current Opinion in Neurology. 2005;18:424–428. doi: 10.1097/01.wco.0000168080.99730.1c. [DOI] [PubMed] [Google Scholar]
- Pihlajamaki M, Tanila H, Könönen M, Hänninen T, Hämäläinen A, Soininen H, Aronen HJ. Visual presentation of novel objects and new spatial arrangements of objects differentially activates the medial temporal lobe subareas in humans. The European Journal of Neuroscience. 2004;19:1939–49. doi: 10.1111/j.1460-9568.2004.03282.x. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Köhler S, Crane J, Pruessner M, Lord C, Byrne A, et al. Volumetry of temporopolar, perirhinal, entorhinal and parahippocampal cortex from high-resolution MR images: considering the variability of the collateral sulcus. Cerebral Cortex. 2002;12:1342–1353. doi: 10.1093/cercor/12.12.1342. [DOI] [PubMed] [Google Scholar]
- Rorden C, Karnath H, Bonilha L. Improving Lesion- Symptom Mapping. Journal of Cognitive Neuroscience. 2007;19:1081–1088. doi: 10.1162/jocn.2007.19.7.1081. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Vilberg KL. Brain networks underlying episodic memory retrieval. Current Opinion in Neurobiology. 2013;23:255–260. doi: 10.1016/j.conb.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugg MD, Vilberg KL, Mattson JT, Yu SS, Johnson JD, Suzuki M. Item memory, context memory and the hippocampus: FMRI evidence. Neuropsychologia. 2012;50:3070–3079. doi: 10.1016/j.neuropsychologia.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sederberg PB, Howard MW, Kahana MJ. A context-based theory of recency and contiguity in free recall. Psychological Review. 2008;115:893–912. doi: 10.1037/a0013396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speer NK, Curran T. ERP correlates of familiarity and recollection processes in visual associative recognition. Brain Research. 2007;1174:97–109. doi: 10.1016/j.brainres.2007.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, Fell J, Do Lam ATA, Axmacher N, Henson RN. Memory signals are temporally dissociated in and across human hippocampus and perirhinal cortex. Nature Neuroscience. 2012;15:1167–1173. doi: 10.1038/nn.3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark CEL, Okado Y. Making memories without trying: medial temporal lobe activity associated with incidental memory formation during recognition. The Journal of Neuroscience. 2003;23:6748–6753. doi: 10.1523/JNEUROSCI.23-17-06748.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Johnson JD, Rugg MD. Decrements in hippocampal activity with item repetition during continuous recognition: an fMRI study. Journal of Cognitive Neuroscience. 2011a;23:1522–1532. doi: 10.1162/jocn.2010.21535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Johnson JD, Rugg MD. Recollection-related hippocampal activity during continuous recognition: A high-resolution fMRI study. Hippocampus. 2011b;21:575–583. doi: 10.1002/hipo.20781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsivilis D, Otten LJ, Rugg MD. Context Effects on the Neural Correlates of Recognition Memory : An Electrophysiological Study. 2001;31:497–505. doi: 10.1016/s0896-6273(01)00376-2. [DOI] [PubMed] [Google Scholar]
- Tsivilis D, Otten LJ, Rugg MD. Repetition effects elicited by objects and their contexts: An fMRI study. Human Brain Mapping. 2003;19:145–154. doi: 10.1002/hbm.10116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk-Browne NB, Simon MG, Sederberg PB. Scene representations in parahippocampal cortex depend on temporal context. The Journal of Neuroscience. 2012;32:7202–7. doi: 10.1523/JNEUROSCI.0942-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC. A Population-Average, Landmark- and Surface-based (PALS) atlas of human cerebral cortex. NeuroImage. 2005;28:635–62. doi: 10.1016/j.neuroimage.2005.06.058. [DOI] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD. Dissociation of the neural correlates of recognition memory according to familiarity, recollection, and amount of recollected information. Neuropsychologia. 2007;45:2216–2225. doi: 10.1016/j.neuropsychologia.2007.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD. Memory retrieval and the parietal cortex: A review of evidence from a dual-process perspective. Neuropsychologia. 2008;46:1787–1799. doi: 10.1016/j.neuropsychologia.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viskontas IV, Knowlton BJ, Steinmetz PN, Fried I. Differences in mnemonic processing by neurons in the human hippocampus and parahippocampal regions. Journal of Cognitive Neuroscience. 2006;18:1654–1662. doi: 10.1162/jocn.2006.18.10.1654. [DOI] [PubMed] [Google Scholar]
- Yassa MA, Stark CEL. Multiple signals of recognition memory in the medial temporal lobe. Hippocampus. 2008;18:945–954. doi: 10.1002/hipo.20452. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. The nature of recollection and familiarity: A review of 30 years of research. Journal of Memory and Language. 2002;46:441–517. [Google Scholar]
- Yonelinas AP, Aly M, Wang WC, Koen JD. Recollection and familiarity: Examining controversial assumptions and new directions. Hippocampus. 2010;20:1178–1194. doi: 10.1002/hipo.20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP, Otten LJ, Shaw KN, Rugg MD. Separating the brain regions involved in recollection and familiarity in recognition memory. The Journal of Neuroscience. 2005;25:3002–3008. doi: 10.1523/JNEUROSCI.5295-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


