Summary
Background:
Tissue engineering is now increasingly focusing on cell-based treatments as promising tools to improve tendon repair. However, many crucial aspects of tendon biology remain to be understood before adopting the best experimental approach for cell-tissue engineering.
Methods:
The role played by Ascorbic Acid (AA) alone and in combination with thyroid hormone T3 in the viability and proliferation of primary human tendon-derived cells was investigated. Human tenocyte viability was detected by Trypan blue exclusion test and cellular proliferation rate was evaluated by CFSE CellTrace™. In addition, the potential role of the AA in the production of Nitric Oxide (NO) was also examined.
Results:
In this in vitro model, an increase in tenocyte proliferation rate was observed as a consequence of progressively increased concentrations of AA (from 10 to 50 µg/ml). The addition of the T3 hormone to the culture further increased tenocyte proliferation rate. In detail, the most evident effect on cellular growth was achieved using the combined supplementation of 50 µg/ml AA and 10−7 M T3.
Conclusion:
We showed that the highest concentration of AA (100 and 500 µg/ml) caused cytotoxicity to human tenocytes. Moreover, it was shown that AA reduces NO synthesis. These results show that AA is a cell proliferation inducer that triggers tenocyte growth, while it reduces NO synthesis.
Keywords: ascorbic acid, T3 thyroid hormone, human tenocytes, nitric oxide, cell proliferation, tendon
Introduction
Tendon healing is a challenging issue in clinical practice and there is a broad consensus in the scientific community that the current methods for surgical repair are insufficient1. As such, cell-mediated engineering therapies are promising alternatives together with orthopedic surgery and traditional scaffold-based therapies. Tendons are specialized tissues consisting of a network of aligned collagen fibrils, which are able to transmit forces between muscles and joints2. Tenocytes are specialized fibroblasts that ensure the homeostasis of the extracellular matrix (ECM) components of tendons through a wide variety of complex mechanisms3. In normal tissues, tenocytes lie among collagen fibers along the tendon’s long axis4. However, tendon overuse together with genetic predisposition factors and dismetabolisms expose humans to tendinopathies. Epidemiological studies report that about 3 to 5 million patients experience tendon injuries each year worldwide4.
Moreover, since tendon healing is a slow process, patients morbidity frequently worsens for the development of severe consequences, such as poor tendon quality, peritendinous adhesions and muscle atrophy. Tissue replacement using auto- or allografted scaffolds is the current surgical reparative approach, despite donor site morbidity, pain and graft failure are recurring issues consequential to this technique. Thus, alternative procedures are strongly needed. In this context, cell-mediated tendon tissue engineering is a promising therapeutic option for regenerative medicine aiming at repairing the tendon lesion by restoring tissue functionality1,5–7. The current cell-based strategy for tendon regeneration involves the expansion and differentiation of tenocyte precursors. Thus, studies finalized at defining standardized protocols to obtain tendon-derived cell culture are necessary. These studies might allow researchers to better understand the proliferation behavior of tenocyte precursors and their differentiation pattern, thus improving the use of cell-based tendon tissue engineering in a clinical setting.
Ascorbic acid (AA) is a well-known organic compound provided with antioxidant properties. AA contributes to prevent oxidative stress through the formation of the ascorbate ion that usually responds to reactive oxygen species (ROS), such as hydroxyl radicals. Since there is clear evidence that AA could help tendons to heal, several studies proposed supplementation of AA during tendinopathy treatments8,9. It has been shown that AA improves the rate and the quality of rat Achilles’ tendon repair. This effect is probably due to an improved angiogenesis and to the protection of hamstring-derived tenocytes from oxidative stress8. Moreover, AA contributes to the synthesis, maturation, secretion and degradation of collagen7,10,11 and it has been shown to increase collagen production in tenocyte culture11,12.
Previous studies from our group showed that tendon expresses thyroid hormones (TH) receptor isoforms and that T3 and T4 hormones enhance tenocyte proliferation in vitro, being 10−7M the most effective concentration for T3. Moreover, T3 and T4 contrast apoptosis in healthy tenocytes in a dose- and time-dependent manner13,14. Our previous data suggested a strong link between TH activation and tenocyte ECM protein expression, in particular collagen I and biglycan secretion, as well as COMP expression15. Furthermore, it has been proven that THs act in association with AA on the ECM protein secretion13,15.
Nitric Oxid (NO) is intracellularly synthesized by a family of isoenzymes that are named nitric oxide synthases (NOSs). So far, three distinct isoforms have been identified: the endothelial NOS (eNOS), the neuronal NOS (nNOS) and the inducible NOS (iNOS). The first two (eNOS and nNOS) are the constitutive forms that are important in blood pressure regulation and memory, respectively16–18. The iNOS iso-form is a highly synthetized enzyme that is involved in the host defense and that can be induced by bacterial cell wall products and pro-inflammatory cytokines, such as interferon-gamma (IFN-γ) and tumor necrosis factor (TNFα). The iNOS isoform has the highest capacity to generate NO. Previous reports showed that all three NOS isoforms are expressed in fibroblasts after tendon injury19. The synthesis of NO is an important factor to increase tissue production during tendon healing and it has been suggested that NO enhances collagen synthesis19.
The main aim of the present study were to clarify whether the supplementation of AA in combination with T3 increases the proliferation rate of human tenocytes and to investigate the role of AA in NO production.
Materials and methods
Patients and methods
All the procedures described in this study were approved by the Ethical Committee of “Tor Vergata” University of Rome. All patients gave written informed consent to be included in this study20. Tendon samples were harvested from healthy supraspinatus tendons biopsy specimens of 5 patients who were arthroscopically operated for shoulder instability (4 men, 1 woman, mean age: 29 ± 5 year). Systemic conditions such as thyroid disorders, diabetes, gynecological condition, neoplasia, rheumatic diseases, epilepsy and any previous or concomitant rotator cuff disease were considered as exclusion criteria.
Tendon cell culture
Primary human tendon-derived cells cultures were established as previously described13. Briefly, cells were isolated from tissue sample by washing several times with Dulbecco’s phosphate-buffered saline (PBS) without Ca2+ and Mg2+ and supplemented with 1% penicillin/streptomycin (Invitrogen, Life Technologies, Carlsbad, CA, USA). Small pieces of fresh isolated tendon were carefully dissected and mechanically disaggregated with the aid of a fine watchmaker forceps to maximize the interface between tissue and medium. Finally, the tendons were immediately placed on Petri dishes of 60 mm of diameter (Greiner CELLSTAR dish, Sigma-Aldrich, Saint Louis, MO, USA), containing 5 mL of α-MEM supplemented with 20% heat-inactivated foetal calf serum (FCS), 1% L-glutamine and 1% penicillin/streptomycin (Gibco, Invitrogen, Life Technologies) at 37°C in 5% CO2 and air with a refresh of medium every 2–3 days. Tenocytes were harvested with StemProAccutase (Life technologies Carlsbad, CA, USA) and centrifuged at 1,500 rpm for 5 min when cells migrated out of tendon pieces and reached 60–80% of confluence (day 19). Collected tenocytes were immediately cultured to avoid phenotype drift with further in vitro passages21. The tenocyte phenotype was confirmed by assessing the mRNA expression of a tenocyte-specific gene (scleraxis, SCX) and of genes coding for collagens α1(I), α2(I) and α1(III) by quantitative real-time PCR (qRT-PCR) with validated primers (Bio-Rad) (data not shown).
Cell viability
Tenocyte-like cells were seeded at 2×104/well in 6-well plates (Greiner CELLSTAR dish, Sigma-Aldrich) in triplicates. Cells were cultured in α-MEM medium supplemented with 10% FCS, 1% L-glutamine and 1% penicillin/streptomycin. Every 3 days, half change of medium was performed. After 24h, cultured cells were exposed to four different concentration of L-ascorbic acid (AA) (Sigma Aldrich, Saint Louis, MO, USA): 10 µg/ml, 50 µg/ml, 100 µg/ml and 500 µg/ml. Thyroid hormone T3 (10−7 M) (Sigma Aldrich) was added when required. Fresh aliquots of T3 were added every 24 h, while AA was refreshed every 3 days. Untreated cells were used as control. After 0, 24, 48 and 72 hours cell viability was assessed by Trypan blue dye exclusion test. Briefly, tendon-derived cells were seeded at 1×104/well in 24-well plates (Greiner CELLSTAR dish, Sigma-Aldrich) in triplicates. Cells were cultured in 1 ml of complete α-MEM. After 0, 24, and 48 h cells were detached, collected and counted in the Burker chamber by means of Trypan Blue (Stem Cells Technologies, Vancouver, Canada) and a light-phase contrast microscope (Nikon Instruments INC., Melville, NY, USA) to evaluate cell viability.
Cell division assay with CFSE staining
The proliferation rate of human tenocytes was determined by monitoring the decrease of green fluorescence in flow cytometry by means of carboxyfluorescein succinimidyl ester (CFSE) (CellTrace™ Cell Proliferation Kits, Invitrogen, CA, USA). Cells (2×104 tenocytes) were cultured in T-25 flasks (VWR International PBI, MI, Italy) in 5ml α-MEM supplemented with 10% FBS, 1% L-glutamine and 1% penicillin/streptomycin. After 24 h cells were labeled with 2 µM CFSE for 20 min at room temperature. The CFSE readily diffuses into cells and binds covalently to intracellular amines. Five volumes of cold completed α-MEM medium were then added to stop the staining reaction. The cells were then washed three times with PBS to remove the excess of the staining. The stained tenocytes were stimulated as described above to monitor cell division. After 0, 48 and 72 h samples were detached and analyzed by a FACSCalibur flow cytometer (BD Biosciences, San Diego, CA, USA) with a FL1 (FITC) detector in a linear mode. The CFSE monitors the distinct generations of the proliferating cells by dye dilution resulting in a decrease of green fluorescence. Live cells are covalently labeled with a very bright and stable dye, while every generation of cells appears as a different peak of decreasing fluorescent intensity on a flow cytometric histogram. The percentages of apoptotic and dead cells were quantified using Annexin V (BD Pharmingen). The ModFit LT software (Verity Software House, ME, USA) was used to quantify the proliferation indexes and to visualize proliferating cells on statistical histograms.
Estimation of the cell number from cell division number
On the basis of cell division numbers observed in the dilution assay of CFSE fluorescence intensity described above, the cell number following stimulation was calculated using the formula below:
A, B, C, and D indicate percentages of cells that underwent 0, 1, 2, and 3 cycles of cell division, respectively22.
Nitric Oxide
NitrixyteTM probe was used for detecting free NO in tenocytes (Cell Meter™ Fluorimetric Intracellular Nitric Oxide Assay Kit; AAT Bioquest, Inc., Sunnyvale, CA) by flow cytometry. After 24 h of exposure to treatments, cells were harvested and processed according to the manufacturer’s instruction. Hydrogen peroxide (H2O2, 0,5mM) and IFN-γ (5ng/ml) were used as inducers of NO as previously described23,24. Thereafter, the medium was removed and the cultured cells were treated with or without H2O2 and IFN-γ in complete α-MEM with or without two different doses of AA (10 µg/ml, 50 µg/ml) for further 24 h. A negative control was prepared by incubating cells in the absence of inducing agents. This kit uses Nitrixyte orange that can react with NO to generate a bright orange fluorescent product that has spectral properties similar to Cy3 and TRITC. The kit provides to monitor intracellular NO level in live cells. The analysis was performed by using a FACSCalibur flow cytometer (BD Biosciences, San Diego, CA, USA) with a FL2 (PE) detector in a linear mode. Statistical histograms were obtained analyzing samples with FlowJo Software (FlowJo Enterprise, OR, USA) and FacsDiva Software (BD Biosciences, San Diego, CA, USA) and are provided to quantify the fluorescence changes in the phycoerythrin channel.
Statistical Analysis
Data are typical results from a minimum of three independent experiments and are expressed as mean ± SD. One-way Anova was applied for meaning comparison. Comparison of individual treatments was performed using the Student’s t test. Statistical significance in comparison with the corresponding control values was indicated by *p<0.05 versus control.
Results
High concentration of AA affects tenocytes viability
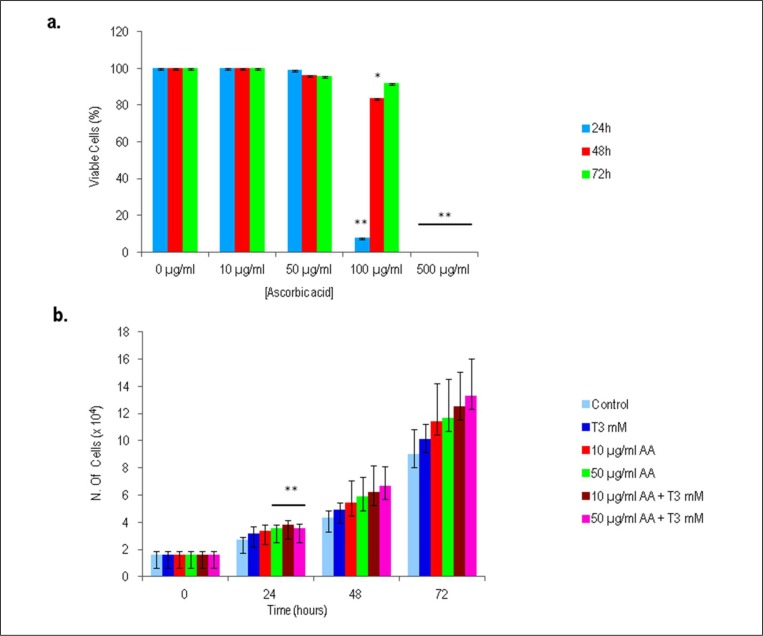
In the first set of experiments, the viability of isolated tenocytes in response to different concentrations of AA were examined by Trypan blue dye exclusion test. A 100% of viability was observed in tenocytes treated with 10 µg/ml of AA or without AA (as control) after 24, 48 and 72 h. Viability of tenocytes exposed at concentration of 50 µg/ml AA only slightly dropped after 24, 48 and 72 h (99, 96 and 96%, respectively), whereas, at concentrations higher than 50 µg/ml AA, tenocytes viability was partially or totally inhibited (Fig. 1a). The addition of 100 µg/ml of AA in cell culture significantly reduced the viability of tenocytes after 24 h of exposure (8%). A supplementation of 500 µg/ml of AA was found to be the most cytotoxic concentration, with a 100% of cells directly stained by Trypan blue dye after 24 h. Thus, concentrations of 10 µg/ml and 50 µg/ml were chosen to evaluate the effect of AA in further investigations (p<00.5).
Figure 1.
Trypan blue dye exclusion test on human tenocytes derived from tendon after 24, 48 and 72 h. a) Effect on cell viability of increasing concentrations of AA. b) Effect on cell viability of 10 µg/ml and 50 µg/ml AA and/or T3 (10−7 M). Untreated tenocytes derived from tendons were used as control. Values results from 6 independent experiments and are expressed as the mean ± SD (n=6) (*p >0.05).
AA combined with T3 does not affect cell viability and increases tenocytes cell growth
Based on our previous study, the effect of 10−7 M T3 in combination with AA was tested. Our data showed that supplementation of AA and T3 increased cell number in a time-and dose-dependent manner, without affecting tenocytes viability. As reported in Figure 1b, after 24 h of cell exposure the increase in cell number was statistically significant when 50 µg/ml AA, 10 µg/ml AA + 10−7 M T3 or 50 µg/ml AA + 10−7 M T3 were added to the cell culture, in comparison to untreated control.
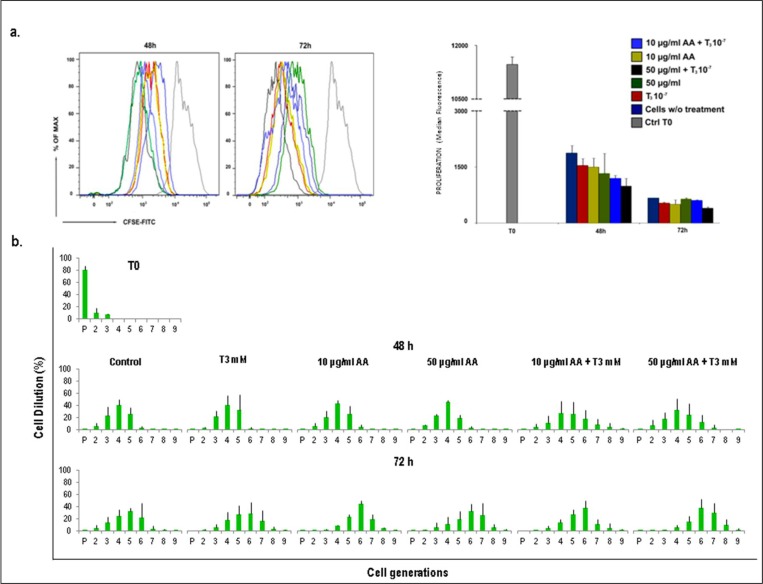
Cell division was enhanced in AA+T3 stimulated tenocytes
To gain more detailed information regarding the effect of AA, T3 and AA+T3 on cellular proliferative ability, isolated human tenocytes were labeled with a fluorescein-based dye (short-named CFSE) and cell proliferation was tracked by flow cytometry. This method not only facilitates the time-dependent quantitative analysis of proliferation rate through the different experimental conditions, but also it enables the acquisition of extensive knowledge regarding each individual cell division occurring within a parental cell population. Thus, isolated human tenocytes were stained with the fluorescent dye and cultured in the presence of: a) 10µu/ml AA; b) 50µu/ml AA; c) 10−7M T3; d) 10µu/ml AA + 10−7M T3 and e) 50µu/ml AA +10−7M T3 Untreated cells were used as controls. Considering CFSE fluorescence dilution as a measure of cell proliferation, an increase of tenocyte proliferation was observed in all the tested conditions (Fig. 2a), with the highest increase when 50µu/ml AA +10−7M T3 were added to the culture.
Figure 2.
Cell tracking assay with CFSE CellTrace™. Tenocytes derived from tendon were stained with CFSE CellTrace™ on day 0. Fluorescence intensity was measured by FACS Calibur flow cytometer in FL1 channel at 48 and 72 h. a) Effect of 10µu/ml AA, 50µu/ml AA, 10−7M T3, 10µu/ml AA + 10−7M T3 and 50µu/ml AA +10−7M T3 on cell proliferation. Fluorescence dilution reflects cell proliferation. A representative histogram shows the highest decrease of CFSE fluorescence adding 50µu/ml AA +10−7M T3 versus control untreated cells. The graph represents the quantification of CFSE median fluorescence index of three independent experiments. b) Histograms show the mean (± SD) of the successive generations of one experiment out of three. Standard deviations are shown.
Subsequently, the rate of cell division of tenocytes stimulated with AA and T3 alone, or in their combination, was examined by FACS analysis. Untreated cells were used as controls. As shown in Figure 2b, cells stained with CFSE and stimulated for 72 h displayed nine rounds of division. Moreover, in all tested conditions, the peak generation phase of cell division were G4 after 48 h and G6 after 72 h. The histograms of CFSE assay in all the described conditions showed different patterns when compared to the controls. In more detail, after 72 h of stimulation with 50µu/ml AA +10−7M T3 cells progressed up to eight divisions, compared to all other conditions in which cells progressed up to seven divisions. This result suggests that the number of cell divisions was higher using a combination of 50µu/ml AA and 10−7M T3, indicating that this type of treatment may accelerate cell proliferation.
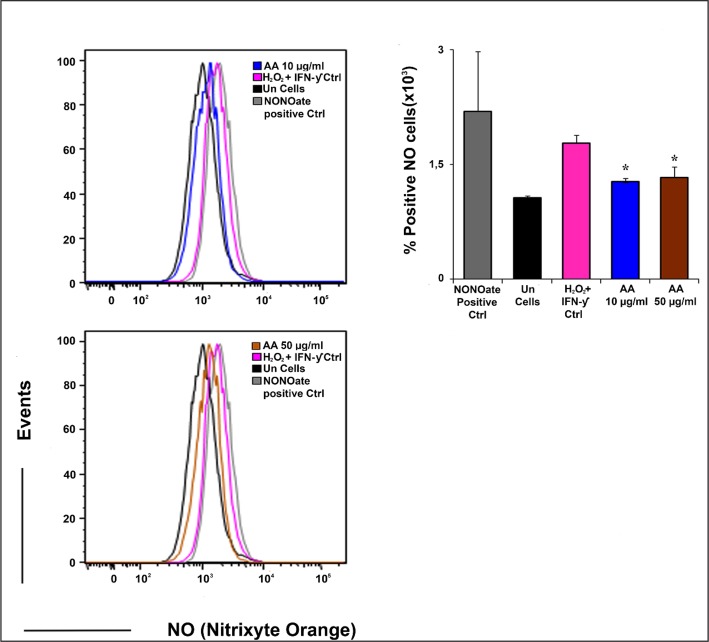
Inhibition of nitric oxide production by AA
The inhibitory activity of AA on NO production induced by H2O2 and INF-γ is shown in Figure 3. Supplementation to the cell culture of 10 µg/ml and 50 µg/ml AA showed a significant inhibitory activity on NO production when compared to positive controls. Moreover, it should be noted that NO synthesis remained higher if compared to the untreated control cells (Fig. 3).
Figure 3.
Detection of NO production. Human tenocytes were incubated with H2O2 (0,5 mM) and IFN-γ (5ng/ml) for 24 h at 37°C to induce NO. Cells were treated with AA (10 µg/ml and 50 µg/ml). NO was detected as described in Material and Methods. The analysis was performed by a FACSCalibur flow cytometer. Representative histograms from flow cytometric analysis illustrate the intensity of fluorescence for the number of cells counted. The values are presented as means ± SEM (*p >0,05).
Discussion
The results observed in vitro suggest a fundamental role of AA in the proliferation behavior of tendon-derived cells and in nitric oxide production. It was found that primary tenocytes exposed to 10 µg/ml and 50 µg/ml of AA were not affected in their cell viability. Conversely, human tenocytes exposed to 100 µg/ml and 500 µg/ml of AA underwent cytotoxicity. Such paradoxal cytotoxic phenomenon was also described by other researchers25,26, who demonstrated toxicity of AA at concentrations higher than 150 µg/ml, due to the high amounts of H2O2 generated as negative feedback. In our experimental model, the toxicity of AA was confirmed starting from lower concentrations than 150 µg/ml, which eventually caused loss of viability and cell death.
Our previous reports demonstrated that T3 and T4 thyroid hormones (THs) increase tenocyte proliferation and synergize with AA inducing the expression of collagen I15. It is plausible to hypothesize that AA in combination with THs might affect tenocyte viability and growth. The reported results not only confirmed our previous observation but also showed that supplementation of AA in combination with T3 increased cell number in a time-and dose-dependent manner, without affecting tenocytes viability.
Isolated human tenocytes showed to respond to all treatments by increasing their proliferation rate. More precisely, the highest cell proliferation rate occurred when AA was used at concentration of 50 µg/ml and in combination with 10−7 M T3, after 72 h of exposure. In addition, cell division analysis showed that 50 µg/ml AA+10−7 M T3 enhanced tenocytes division when compared to all other tested treatments. Considering all these data, it is plausible that the supplementation of AA + T3 may dictate the functional outcome of these cells13–15. In this in vitro system, it may be hypothesized that tenocytes stimulated with AA and T3 (showing higher cell divisions than untreated cells) may induce faster cell differentiation giving rise to an higher number of specialized cells that are able to produce high-quality collagen type I and ECM proteins, as already showed in our previous study15.
It is well known that, during tendon repair, several biological events occur at cellular level such as the release of nitric oxide27. Although several studies suggest a positive role of NO in the tendon repair process28, recently it has been demonstrated that local NO production can hinder tendon tissue recovery and that the inhibition of the NO-releasing system in situ could accelerate tendon recovery27. The present study shows that the NO release significantly decreased when tendon-derived cells were exposed to 10µg/ml and 50µg/ml concentrations of AA. The observed results are consistent with previous studies showing that AA reduces NO production rate28,29. Moreover, our data suggest that strengthening its antioxidant activity, AA could play a potential role in the NO production, probably by modulating iNOS synthesis. According to previous studies30,31, it is reasonable to hypothesize that, in our in vitro model, the major fraction of NO is generated from the inducible isoform of NOS (iNOS) triggered by H2O2 and IFN-γ. As well, it has been reported that iNOS modulates inflammatory cell recruitment and can contribute to the healing process32,33. On the other hand, if synthesized in excessive amount, NO may have many potential toxic effects. Moreover, it should be noticed that AA did not affect the entire NO production; in fact, NO synthesis was still higher if compared to the controls. We hypothesize that AA affects only the overproduction of NO isoforms that are toxic for the cells without affecting the NO physiological mediator role in tendon healing. Further studies are necessary to highlight the link between the reduced NO production by AA and tendon tissue remodeling.
In conclusion, this study shows that the antioxidant AA is a proliferative inducing factor that triggers the proliferation of tendon-derived cells. AA sinergically induces the increase of tenocyte proliferation when combined with T3, at a concentration of 50 µg/ml and 10−7 M, respectively. In the present study, a reduction of NO synthesis by AA was also observed. Translating these considerations in clinical practice, the supplementation of AA + T3 could be effective on human tenocytes proliferation in vivo. However, there are many biological questions that remain unanswered, as well as, many translational factors to solve. Although this study shows that AA has an effect on tenocytes proliferation, there are some intrinsic limitations to this in vitro model. First, the presented data deserve further analyses to fully explain the molecular pathway underlying the synergistic biological effect of AA and T3 on primary tenocytes. Then, it will be fundamental to understand the interplay that occurs between AA and NO production, as well as, to identify the key features underlying this mechanism. Therefore, the potential clinical relevance of the obtained results is highly dependent on the evaluation of the efficacy and toxicity of the combination of AA and T3 in eventual in vivo studies.
Acknowledgments
We thank Dr. Bela Papp for manuscript revision, for discussions and helpful suggestions. We thank Fondazione PCFF ONLUS for buying Cell Meter™ Fluorimetric Intracellular Nitric Oxide Assay Kit.
Footnotes
Conflict of interest
The Author has no financial or personal relationships with other people or organizations that could inappropriately influence their work.
References
- 1.Gaspar D, Spanoudes K, Holladay C, Pandit A, Zeugolis D. Progress in cell-based therapies for tendon repair. Adv Drug Deliv Rev. 2015;84:240–256. doi: 10.1016/j.addr.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 2.Josza L, Kannus P, Balint JB, Reffy A. Three-dimensional ultrastructure of human tendon. Acta Anat. 1991;142:306–312. doi: 10.1159/000147207. [DOI] [PubMed] [Google Scholar]
- 3.Moore MJ, De Beaux A. A quantitative ultrastructural study of rat tendon from birth to maturity. J Anat. 1987;153:163–169. [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma P, Maffulli N. Biology of tendon injury: healing, modeling and remodeling. J Musculoskelet Neuronal Interact. 2006;6(2):181–190. [PubMed] [Google Scholar]
- 5.Maffulli N, Wong J, Almekinders LC. Types and epidemiology of tendinopathy. Clin Sports Med. 2003:675–692. doi: 10.1016/s0278-5919(03)00004-8. [DOI] [PubMed] [Google Scholar]
- 6.Woo SL, Hildebrand K, Watanabe N, Fenwick JA, Papageorgiou CD, Wang JH. Tissue engineering of ligament and tendon healing. Clin Orthop Relat Res. 1999:S312–23. doi: 10.1097/00003086-199910001-00030. [DOI] [PubMed] [Google Scholar]
- 7.Goh JC, Ouyang HW, Teoh SH, Chan CK, Lee EH. Tissue-engineering approach to the repair and regeneration of tendons and ligaments. Tissue Eng. 2003;9(Suppl 1):S31–44. doi: 10.1089/10763270360696969. [DOI] [PubMed] [Google Scholar]
- 8.Omeroğlu S, Peker T, Türközkan N, Omeroğlu H. High-dose vitamin C supplementation accelerates the Achilles tendon healing in healthy rats. Arch Orthop Trauma Surg. 2009;129(2):281–286. doi: 10.1007/s00402-008-0603-0. [DOI] [PubMed] [Google Scholar]
- 9.Fusini F, Bisicchia S, Bottegoni C, Gigante A, Zanchini F, Busilacchi A. Nutraceutical supplement in the management of tendinopathies: a systematic review. Muscles Ligaments Tendons J. 2016;6(1):48–57. doi: 10.11138/mltj/2016.6.1.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poulsen ET, Larsen A, Zollo A, Jørgensen AL, Sanggaard KW, Enghild JJ, Matrone C. New Insights to Clathrin and Adaptor Protein 2 for the Design and Development of Therapeutic Strategies. Int J Mol Sci. 2015(12):29446–29453. doi: 10.3390/ijms161226181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alcantra-Martos T, Delgado-Martinez AD, Vega MV, Carrascal MT, Munuera-Martinez L. Effect of vitamin C on fracture healing in elderly Osteogenic Disorder Shionogi rats. J Bone Joint Surg Br. 2007;89:402–407. doi: 10.1302/0301-620X.89B3.18007. [DOI] [PubMed] [Google Scholar]
- 12.Russell JE, Manske PR. Ascorbic acid requirement for optimal flexor tendon repair in vitro. J Orthop Res. 1991;9:714–719. doi: 10.1002/jor.1100090511. [DOI] [PubMed] [Google Scholar]
- 13.Oliva F, Berardi AC, Misiti S, Verga Falzacappa C, Iacone A, Maffulli N. Thyroid hormones enhance growth and counteract apoptosis in human tenocytes isolated from rotator cuff tendons. Cell Death Dis. 2013;4:e705. doi: 10.1038/cddis.2013.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oliva F, Piccirilli E, Berardi AC, Frizziero A, Tarantino U, Maffulli N. Hormones and tendinopathies: the current evidence. Br Med Bull. 2016;117(1):39–58. doi: 10.1093/bmb/ldv054. [DOI] [PubMed] [Google Scholar]
- 15.Berardi AC, Oliva F, Berardocco M, la Rovere M, Accorsi P, Maffulli N. Thyroid hormones increase collagen I and cartilage oligomeric matrix protein (COMP) expression in vitro human tenocytes. Muscles Ligaments Tendons J. 2014;4(3):285–291. [PMC free article] [PubMed] [Google Scholar]
- 16.Bredt DS, Glatt CE, Hwang PM, Fotuhi M, Dawson TM, Snyder SH. Nitric oxide synthase protein and mRNA are discretely localized in neuronal populations of the mammalian CNS together with NADPH diaphorase. Neuron. 1991;7(4):615–624. doi: 10.1016/0896-6273(91)90374-9. [DOI] [PubMed] [Google Scholar]
- 17.Lamas S, Marsden PA, Li GK, Tempst P, Michel T. Endothelial nitric oxide synthase: molecular cloning and characterization of a distinct constitutive enzyme isoform. Proc Natl Acad Sci U S A. 1992;89(14):6348–6352. doi: 10.1073/pnas.89.14.6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cho HJ, Xie QW, Calaycay J, Mumford RA, Swiderek KM, Lee TD, Nathan C. Calmodulin is a subunit of nitric oxide synthase from macrophages. J Exp Med. 1999;176(2):599–604. doi: 10.1084/jem.176.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murrell GA. Using nitric oxide to treat tendinopathy. Br J Sports Med. 2007;41:227–231. doi: 10.1136/bjsm.2006.034447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Padulo J, Oliva F, Frizziero A, Maffulli N. Muscles, Ligaments and Tendons Journal - Basic principles and recommendations in clinical and field science research: 2016 Update. MLTJ. 2016;6(1):1–5. doi: 10.11138/mltj/2016.6.1.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yao L, Bestwick CS, Bestwick LA, Maffulli N, Aspden RM. Phenotypic drift in human tenocyte culture. Tissue Eng. 2006;12(7):1843–1849. doi: 10.1089/ten.2006.12.1843. [DOI] [PubMed] [Google Scholar]
- 22.Roederer M. Interpretation of cellular proliferation data: avoid the Panglossian. Cytometry Part A. 2011;79A:95–101. doi: 10.1002/cyto.a.21010. [DOI] [PubMed] [Google Scholar]
- 23.Wei T, Chen C, Hou J, Xin W, Mori A. Nitric Oxide induces oxidative stress and apoptptosis in neuronal cells. Biochim Biophys Acta. 2000;1498:72–79. doi: 10.1016/s0167-4889(00)00078-1. [DOI] [PubMed] [Google Scholar]
- 24.Sherman PA, Laubach VE, Reep BR, Wood ER. Purification and cDNA sequence of an inducibile nitric oxide synthase from a human tumor cell line. Biochemistry. 1993;32:11600–11605. doi: 10.1021/bi00094a017. [DOI] [PubMed] [Google Scholar]
- 25.Hakimi O, Poulson R, Thakkar D, Yapp C, Carr A. Ascorbic acid is essential for significant collagen deposition by human tenocytes in vitro. Oxid Antioxid Med Sci. 2014;3(2):119–127. [Google Scholar]
- 26.Clément MV, Ramalingam J, Long LH, Halliwell B. The in vitro cytotoxicity of ascorbate depends on the culture medium used to perform the assay and involves hydrogen peroxide. Antioxid Redox Signal. 2001;3(1):157–163. doi: 10.1089/152308601750100687. [DOI] [PubMed] [Google Scholar]
- 27.Moraes SA, Oliveira KR, Crespo-López ME, Picanço-Diniz DL, Herculano AM. Local NO synthase inhibition produces histological and functional recovery in Achilles tendon of rats after tenotomy: tendon repair and local NOS inhibition. Cell Tissue Res. 2013;353(3):457–463. doi: 10.1007/s00441-013-1662-2. [DOI] [PubMed] [Google Scholar]
- 28.Heinrich UR, Fischer I, Brieger J, Rümelin A, Schmidtmann I, Li H, Mann WJ, Helling K. Ascorbic acid reduces noise-induced nitric oxide production in the guinea pig ear. Laryngoscope. 2008;118(5):837–842. doi: 10.1097/MLG.0b013e31816381ae. [DOI] [PubMed] [Google Scholar]
- 29.Ribeiro DA, Buttros JB, Oshima CT, Bergamaschi CT, Campos RR. Ascorbic acid prevents acute myocardial infarction induced by isoproterenol in rats: role of inducible nitric oxide synthase production. J Mol Histol. 2009;40(2):99–105. doi: 10.1007/s10735-009-9218-1. [DOI] [PubMed] [Google Scholar]
- 30.Tate DJ, Jr, Patterson JR, Velasco-Gonzalez C, et al. Interferon-gamma-induced nitric oxide inhibits the proliferation of murine renal cell carcinoma cells. Int J Biol Sci. 2012;8(8):1109–1120. doi: 10.7150/ijbs.4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heller R, Unbehaun A, Schellenberg B, Mayer B, Werner-Felmayer G, Werner ER. L-ascorbic acid potentiates endothelial nitric oxide synthesis via a chemical stabilization of tetrahydrobiopterin. J Biol Chem. 2001;276(1):40–47. doi: 10.1074/jbc.M004392200. [DOI] [PubMed] [Google Scholar]
- 32.Lin J, Wang MX, Wei A, Zhu W, Murrell GA. The cell specific temporal expression of nitric oxide synthase isoforms during achilles tendon healing. Inflamm Res. 2001;50(10):515–522. doi: 10.1007/PL00000228. [DOI] [PubMed] [Google Scholar]
- 33.Torricelli P, Veronesi F, Pagani S, Maffulli N, Masiero S, Frizziero A, Fini M. In vitro tenocyte metabolism in aging and oestrogen deficiency. Age (Dordr) 2013;35(6):2125–2136. doi: 10.1007/s11357-012-9500-0. [DOI] [PMC free article] [PubMed] [Google Scholar]