Abstract
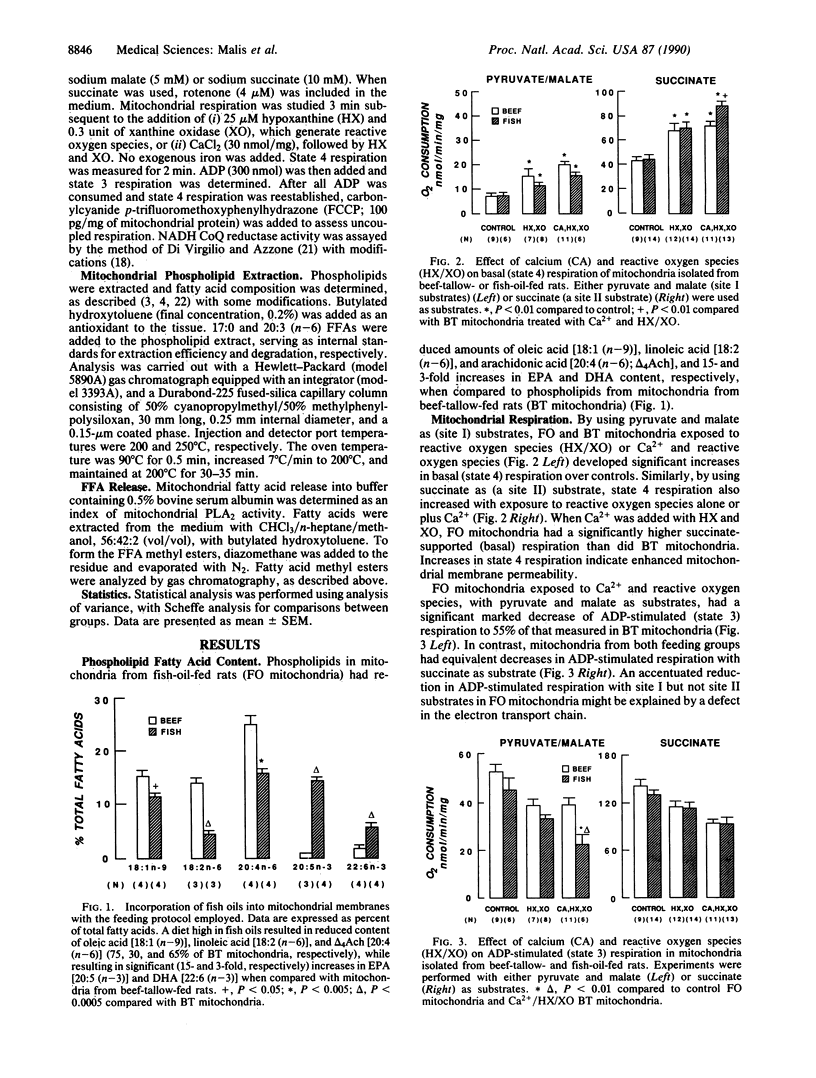
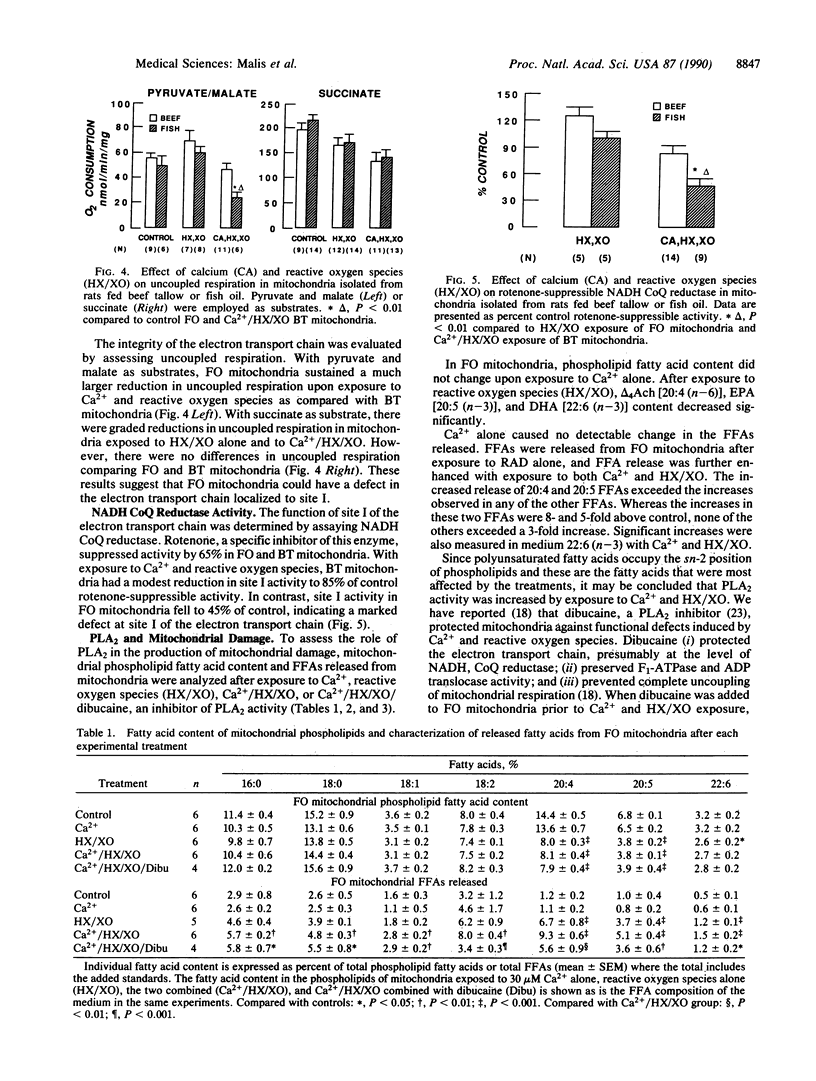
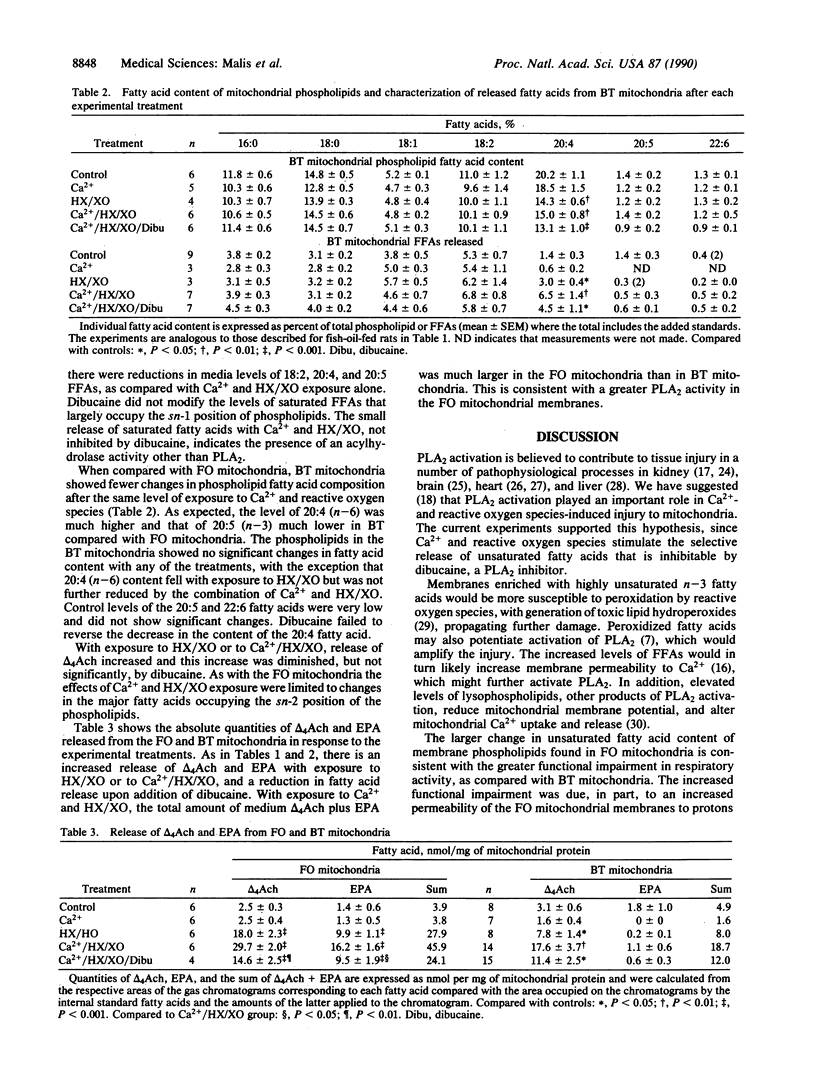
Experiments were designed to evaluate the susceptibility of mitochondrial membranes enriched with n-3 fatty acids to damage by Ca2+ and reactive oxygen species. Fatty acid content and respiratory function were assessed in renal cortical mitochondria isolated from fish-oil- and beef-tallow-fed rats. Dietary fish oils were readily incorporated into mitochondrial membranes. After exposure to Ca2+ and reactive oxygen species, mitochondria enriched in n-3 fatty acids, and using pyruvate and malate as substrates, had significantly greater changes in state 3 and uncoupled respirations, when compared with mitochondria from rats fed beef tallow. Mitochondrial site 1 (NADH coenzyme Q reductase) activity was reduced to 45 and 85% of control values in fish-oil- and beef-tallow-fed groups, respectively. Exposure to Ca2+ and reactive oxygen species enhance the release of polyunsaturated fatty acids enriched at the sn-2 position of phospholipids from mitochondria of fish-oil-fed rats when compared with similarly treated mitochondria of beef-tallow-fed rats. This release of fatty acids was partially inhibited by dibucaine, the phospholipase A2 inhibitor, which we have previously shown to protect mitochondria against damage associated with Ca2+ and reactive oxygen species. The results indicate that phospholipase A2 is activated in mitochondria exposed to Ca2+ and reactive oxygen species and is responsible, at least in part, for the impairment of respiratory function. Phospholipase A2 activity and mitochondrial damage are enhanced when mitochondrial membranes are enriched with n-3 fatty acids.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Au A. M., Chan P. H., Fishman R. A. Stimulation of phospholipase A2 activity by oxygen-derived free radicals in isolated brain capillaries. J Cell Biochem. 1985;27(4):449–453. doi: 10.1002/jcb.240270413. [DOI] [PubMed] [Google Scholar]
- Barber A. A., Bernheim F. Lipid peroxidation: its measurement, occurrence, and significance in animal tissues. Adv Gerontol Res. 1967;2:355–403. [PubMed] [Google Scholar]
- Baud L., Nivez M. P., Chansel D., Ardaillou R. Stimulation by oxygen radicals of prostaglandin production by rat renal glomeruli. Kidney Int. 1981 Sep;20(3):332–339. doi: 10.1038/ki.1981.143. [DOI] [PubMed] [Google Scholar]
- Bazán N. G., Jr Effects of ischemia and electroconvulsive shock on free fatty acid pool in the brain. Biochim Biophys Acta. 1970 Oct 6;218(1):1–10. doi: 10.1016/0005-2760(70)90086-x. [DOI] [PubMed] [Google Scholar]
- Bonventre J. V., Swidler M. Calcium dependency of prostaglandin E2 production in rat glomerular mesangial cells. Evidence that protein kinase C modulates the Ca2+-dependent activation of phospholipase A2. J Clin Invest. 1988 Jul;82(1):168–176. doi: 10.1172/JCI113566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S. H., Higgins E., Jr Uncoupling activity of endogenous free fatty acids in rat liver mitochondria. Can J Biochem. 1978 Feb;56(2):111–116. doi: 10.1139/o78-018. [DOI] [PubMed] [Google Scholar]
- Chien K. R., Abrams J., Serroni A., Martin J. T., Farber J. L. Accelerated phospholipid degradation and associated membrane dysfunction in irreversible, ischemic liver cell injury. J Biol Chem. 1978 Jul 10;253(13):4809–4817. [PubMed] [Google Scholar]
- Chien K. R., Han A., Sen A., Buja L. M., Willerson J. T. Accumulation of unesterified arachidonic acid in ischemic canine myocardium. Relationship to a phosphatidylcholine deacylation-reacylation cycle and the depletion of membrane phospholipids. Circ Res. 1984 Mar;54(3):313–322. doi: 10.1161/01.res.54.3.313. [DOI] [PubMed] [Google Scholar]
- Chien K. R., Sen A., Reynolds R., Chang A., Kim Y., Gunn M. D., Buja L. M., Willerson J. T. Release of arachidonate from membrane phospholipids in cultured neonatal rat myocardial cells during adenosine triphosphate depletion. Correlation with the progression of cell injury. J Clin Invest. 1985 Jun;75(6):1770–1780. doi: 10.1172/JCI111889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua B. H., Shrago E. Reversible inhibition of adenine nucleotide translocation by long chain acyl-CoA esters in bovine heart mitochondria and inverted submitochondrial particles. Comparison with atractylate and bongkrekic acid. J Biol Chem. 1977 Oct 10;252(19):6711–6714. [PubMed] [Google Scholar]
- Corr P. B., Gross R. W., Sobel B. E. Amphipathic metabolites and membrane dysfunction in ischemic myocardium. Circ Res. 1984 Aug;55(2):135–154. doi: 10.1161/01.res.55.2.135. [DOI] [PubMed] [Google Scholar]
- Das D. K., Engelman R. M., Rousou J. A., Breyer R. H., Otani H., Lemeshow S. Role of membrane phospholipids in myocardial injury induced by ischemia and reperfusion. Am J Physiol. 1986 Jul;251(1 Pt 2):H71–H79. doi: 10.1152/ajpheart.1986.251.1.H71. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F., Azzone G. F. Activation of site I redox-driven H+ pump by exogenous quinones in intact mitochondria. J Biol Chem. 1982 Apr 25;257(8):4106–4113. [PubMed] [Google Scholar]
- Fisher M., Upchurch K. S., Levine P. H., Johnson M. H., Vaudreuil C. H., Natale A., Hoogasian J. J. Effects of dietary fish oil supplementation on polymorphonuclear leukocyte inflammatory potential. Inflammation. 1986 Dec;10(4):387–392. doi: 10.1007/BF00915822. [DOI] [PubMed] [Google Scholar]
- Force T., Malis C. D., Guerrero J. L., Varadarajan G. S., Bonventre J. V., Weber P. C., Leaf A. n-3 fatty acids increase postischemic blood flow but do not reduce myocardial necrosis. Am J Physiol. 1989 Oct;257(4 Pt 2):H1204–H1210. doi: 10.1152/ajpheart.1989.257.4.H1204. [DOI] [PubMed] [Google Scholar]
- Kinsella J. E., Lokesh B., Stone R. A. Dietary n-3 polyunsaturated fatty acids and amelioration of cardiovascular disease: possible mechanisms. Am J Clin Nutr. 1990 Jul;52(1):1–28. doi: 10.1093/ajcn/52.1.1. [DOI] [PubMed] [Google Scholar]
- Lamers J. M., Hülsmann W. C. Inhibition of (Na+ + K+)-stimulated ATPase of heart by fatty acids. J Mol Cell Cardiol. 1977 Apr;9(4):343–346. doi: 10.1016/s0022-2828(77)80039-4. [DOI] [PubMed] [Google Scholar]
- Leaf A., Weber P. C. Cardiovascular effects of n-3 fatty acids. N Engl J Med. 1988 Mar 3;318(9):549–557. doi: 10.1056/NEJM198803033180905. [DOI] [PubMed] [Google Scholar]
- Lee T. H., Hoover R. L., Williams J. D., Sperling R. I., Ravalese J., 3rd, Spur B. W., Robinson D. R., Corey E. J., Lewis R. A., Austen K. F. Effect of dietary enrichment with eicosapentaenoic and docosahexaenoic acids on in vitro neutrophil and monocyte leukotriene generation and neutrophil function. N Engl J Med. 1985 May 9;312(19):1217–1224. doi: 10.1056/NEJM198505093121903. [DOI] [PubMed] [Google Scholar]
- Lenzen S., Görlich J. K., Rustenbeck I. Regulation of transmembrane ion transport by reaction products of phospholipase A2. I. Effects of lysophospholipids on mitochondrial Ca2+ transport. Biochim Biophys Acta. 1989 Jun 26;982(1):140–146. doi: 10.1016/0005-2736(89)90184-3. [DOI] [PubMed] [Google Scholar]
- Lorenz R., Spengler U., Fischer S., Duhm J., Weber P. C. Platelet function, thromboxane formation and blood pressure control during supplementation of the Western diet with cod liver oil. Circulation. 1983 Mar;67(3):504–511. doi: 10.1161/01.cir.67.3.504. [DOI] [PubMed] [Google Scholar]
- Malis C. D., Bonventre J. V. Mechanism of calcium potentiation of oxygen free radical injury to renal mitochondria. A model for post-ischemic and toxic mitochondrial damage. J Biol Chem. 1986 Oct 25;261(30):14201–14208. [PubMed] [Google Scholar]
- Nishida T., Shibata H., Koseki M., Nakao K., Kawashima Y., Yoshida Y., Tagawa K. Peroxidative injury of the mitochondrial respiratory chain during reperfusion of hypothermic rat liver. Biochim Biophys Acta. 1987 Jan 16;890(1):82–88. doi: 10.1016/0005-2728(87)90071-5. [DOI] [PubMed] [Google Scholar]
- Okayasu T., Curtis M. T., Farber J. L. Structural alterations of the inner mitochondrial membrane in ischemic liver cell injury. Arch Biochem Biophys. 1985 Feb 1;236(2):638–645. doi: 10.1016/0003-9861(85)90668-x. [DOI] [PubMed] [Google Scholar]
- Philipson K. D., Ward R. Effects of fatty acids on Na+-Ca2+ exchange and Ca2+ permeability of cardiac sarcolemmal vesicles. J Biol Chem. 1985 Aug 15;260(17):9666–9671. [PubMed] [Google Scholar]
- Reinhart P. H., van de Pol E., Taylor W. M., Bygrave F. L. An assessment of the calcium content of rat liver mitochondria in vivo. Biochem J. 1984 Mar 1;218(2):415–420. doi: 10.1042/bj2180415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouslin W., Millard R. W. Mitochondrial inner membrane enzyme defects in porcine myocardial ischemia. Am J Physiol. 1981 Feb;240(2):H308–H313. doi: 10.1152/ajpheart.1981.240.2.H308. [DOI] [PubMed] [Google Scholar]
- Sevanian A., Kim E. Phospholipase A2 dependent release of fatty acids from peroxidized membranes. J Free Radic Biol Med. 1985;1(4):263–271. doi: 10.1016/0748-5514(85)90130-8. [DOI] [PubMed] [Google Scholar]
- Smith M. W., Collan Y., Kahng M. W., Trump B. F. Changes in mitochondrial lipids of rat kidney during ischemia. Biochim Biophys Acta. 1980 May 28;618(2):192–201. doi: 10.1016/0005-2760(80)90025-9. [DOI] [PubMed] [Google Scholar]
- von Schacky C., Fischer S., Weber P. C. Long-term effects of dietary marine omega-3 fatty acids upon plasma and cellular lipids, platelet function, and eicosanoid formation in humans. J Clin Invest. 1985 Oct;76(4):1626–1631. doi: 10.1172/JCI112147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Schacky C., Siess W., Fischer S., Weber P. C. A comparative study of eicosapentaenoic acid metabolism by human platelets in vivo and in vitro. J Lipid Res. 1985 Apr;26(4):457–464. [PubMed] [Google Scholar]



