Abstract
Background
Growing evidence has demonstrated that Ki-67/MIB-1 has an effect on the clinical progression and prognosis in cancers. However, the diagnostic and prognostic values of Ki-67/MIB-1 in thyroid cancer remain unclear.
Materials and methods
The meta-analysis was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Studies were retrieved from PubMed, EBSCO, EMBASE, ISI Web of Science, China National Knowledge Infrastructure, WanFang and Chinese VIP databases. MetaDiSc and STATA12.0 were used to analyze the meta-analysis. Fixed-effect analysis and random-effect analysis were applied to pool the relative ratio based on heterogeneity in this meta-analysis.
Results
In the meta-analysis, 51 eligible studies were included. The pooled sensitivity of Ki-67/MIB-1 was 0.61 (95% confidence interval [CI]: 0.59–0.63) and specificity was 0.75 (95% CI: 0.74–0.77) in thyroid cancer. The pooled positive likelihood ratio was 3.19 (95% CI: 2.30–4.42) and negative likelihood ratio was 0.43 (95% CI: 0.35–0.54). In the diagnosis of thyroid cancer, the pooled diagnostic odds ratio of Ki-67/MIB-1 was 8.54 (95% CI: 5.03–14.49). The area under the symmetric receiver operating characteristic curve was 0.804 (standard error =0.031). Our results showed that there were statistical associations between Ki-67/MIB-1 and age (odds ratio [OR] =1.71, 95% CI: 1.14–2.57, P=0.010), tumor size (OR =1.86, 95% CI: 1.17–2.96, P=0.008), lymph node metastasis (OR =2.49, 95% CI: 1.42–4.39, P=0.002), metastasis status (OR =6.96, 95% CI: 2.46–19.69, P<0.001), tumor node metastasis stage (OR =6.56, 95% CI: 3.80–11.34, P<0.001) and extrathyroid extension (OR =1.91, 95% CI: 1.27–2.87, P=0.002). Furthermore, thyroid cancer patients with a high level of Ki-67/MIB-1 had a worse disease-free survival as compared to patients with a low level of Ki-67/MIB-1 (hazard ratio =5.19, 95% CI: 3.18–8.46, P<0.001). Also, Ki-67/MIB-1 was found to be associated with increased risk of mortality (hazard ratio =3.56, 95% CI: 1.17–10.83, P=0.025).
Conclusion
Our results demonstrated that Ki-67/MIB-1 might act as a potential factor in diagnosing thyroid cancer in Chinese. Also, the meta-analysis indicated that Ki-67/MIB-1 might have an effect on prognosis in non-Chinese thyroid cancer patients.
Keywords: thyroid cancer, Ki-67/MIB-1, diagnosis, progression, prognosis, meta-analysis
Introduction
Thyroid carcinoma, accounting for nearly 1% of all the cancers, is the most common malignancy in the endocrine organs.1 Traditionally, thyroid carcinomas are classified into papillary thyroid cancer (PTC), follicular thyroid cancer, medullary thyroid cancer, poorly differentiated thyroid cancer and anaplastic thyroid cancer, based on histopathology.2 In China, it has been reported that 90 per 100,000 people were diagnosed with thyroid cancer and 6.8 people died among these patients.3 In USA, it has been estimated that 1,980 people died of the disease in 20164 and there will be 64,300 new patients of thyroid cancer. The diagnosis of thyroid cancer is often based on magnetic resonance imaging, ultrasound, computed tomography, fine needle aspiration and radionuclide imaging. Although various methods are well applied in clinics, patients often have poor outcome. Reports have shown that the patients’ age when being diagnosed, sex, tumor size, lymph node, distant metastases and pathologic differentiation of the cancer are the risk factors of prognosis in thyroid cancer.5–8
However, no studies showed the markers had prognostic value in thyroid cancer. Cell proliferative activity is an important factor in cancer biologic behavior. Ki-67, considered as a nuclear antigen, is expressed in all cell nuclei, except those in the G0 phase. Further, MIB-1 acts as a monoclonal antibody which increases against Ki-67. In recent years, Ki-67 has been studied in many cancers, including cervical cancer,9 lung cancer,10 breast cancer11 and thyroid cancer.12 It has been reported that Ki-67 is an independent prognostic factor in thyroid cancer patients.13 A study predicted that patients with Ki-67 labeling index (LI) >3% would show a worse cause-specific survival than those with Ki-67 LI <3%.12 However, Gnemmi et al14 reported that Ki-67 LI (≥4%) is an independent factor and predictor of cause-specific survival.
Though many studies have demonstrated Ki-67/MIB-1 is expressed in thyroid cancer, there is no systematic literature assessing the relationship between Ki-67/MIB-1 expression and clinical factors in thyroid cancer patients. So, the significance of Ki-67/MIB-1 for prognostication of thyroid cancer remains uncertain. Thus, a literature-based meta-analysis study was performed to evaluate the diagnostic and prognostic values of Ki-67/MIB-1 in thyroid cancer.
Materials and methods
Studies selection
Studies were selected to demonstrate the diagnostic and prognostic values of Ki-67/MIB-1 for thyroid cancer. Electronic literatures were searched in PubMed, EBSCO, EMBASE, ISI Web of Science, WanFang, China National Knowledge Infrastructure and Chinese VIP databases from April 1, 1989 to July 31, 2016. The following keywords were used to identify the related publications: “thyroid cancer”, “Ki-67”, “MIB-1”, “proliferative marker”, “proliferative index”, “diagnosis”, “prognostic”, “survival”. The eligible studies were selected in accordance with the following criteria: 1) studies should be published in full assays; 2) the goal of the publication was to illustrate the clinical significance of Ki-67/MIB-1 in primary thyroid cancer; 3) sufficient data were used to determine the connection between Ki-67/MIB-1 and clinicopathologic parameters; 4) when the same patient cohort was reported in different publications, only the most complete and recent study was selected in the meta-analysis.
Also, we screened the references from the reviews and identified articles.
Data extraction and assessment of study quality
Three authors (Deng-hua Pan, Dong-yue Wen and Yi-huan Luo) read the studies carefully and independently. The information of the publications was collected from each study: first author’s name, publication date, the number of patients, patient age, country, follow-up time, antibody of testing Ki-67/MIB-1, the method of detecting Ki-67/MIB-1 expression and threshold used for assessing Ki-67/MIB-1 expression positively. True positive, true negative, false positive and false negative were extracted to construct a diagnostic contingency table. Disease-free survival (DFS) or mortality or distant recurrences-free survival was used to measure the effect of Ki-67/MIB-1 expression on survival in thyroid cancer patients. The following clinical parameters were extracted to evaluate the connection between Ki-67/MIB-1 and thyroid cancer aggressiveness: age, tumor size, lymph node metastasis, metastasis status, extrathyroid extension, tumor node metastasis stage. Minimal size of patients and minimal follow-up time are not defined in this meta-analysis. Studies that met the following criteria were excluded: 1) reviews, conference papers, case reports, expertise public opinion, letters, zoopery were not included; 2) studies without sufficient information to calculate the impact of diagnosis, survival and prognosis of Ki-67/MIB-1 in primary thyroid cancer were excluded; 3) studies with duplicated data from similar or the same population were excluded. QUADAS-2 was used to assess the quality of the studies for diagnosis.15 Newcastle–Ottawa scale (NOS) was used to assess the quality of the studies for prognosis.16 The study with NOS scores ≥6 was identified as a high-quality study and the study with NOS scores <6 was considered as a low-quality study.
Statistical methods
According to the cut-off values, Ki-67/MIB-1 expression was divided into positive and negative groups. The pooled sensitivity and specificity, positive likelihood ratio (LR+), negative likelihood ratio (LR−), diagnostic odds ratio (DOR) and the area under the symmetric receiver operating characteristic curve were used to measure the diagnosis of Ki-67/MIB-1 in thyroid cancer. The odds ratio (OR) and 95% confidence intervals (CIs) were used to estimate the relationship between Ki-67/MIB-1 and clinicopathologic parameters in thyroid cancer patients. When the OR was >1, it indicated that high Ki-67/MIB-1 was a risk factor in thyroid cancer. Hazard ratio (HR) and 95% CI were calculated to measure the effect of Ki-67/MIB-1 on prognosis. Also, when the HR was >1, it indicated that high level of Ki-67/MIB-1 was related to worse survival in thyroid cancer patients.
Further, Cochran’s Q-test was performed to measure heterogeneity. Also, I2 was calculated to assess the inconsistency of the studies. When I2 was over 50% or chi-squared P-value was >0.1, fixed-effect meta-analysis was performed; otherwise, random-effect meta-analysis was used when there was less or no heterogeneity (when I2 was less than 50% or chi-squared P-value was <0.1). MetaDiSc was used to measure the diagnosis of Ki-67/MIB-1 in thyroid cancer. STATA12.0 was used to calculate the progression and prognosis of Ki-67/MIB-1 in thyroid cancer. The potential publication bias was investigated through funnel plot and by computation of Begg’s test. When the P-value was <0.05, it was considered significant.
Results
Description of studies
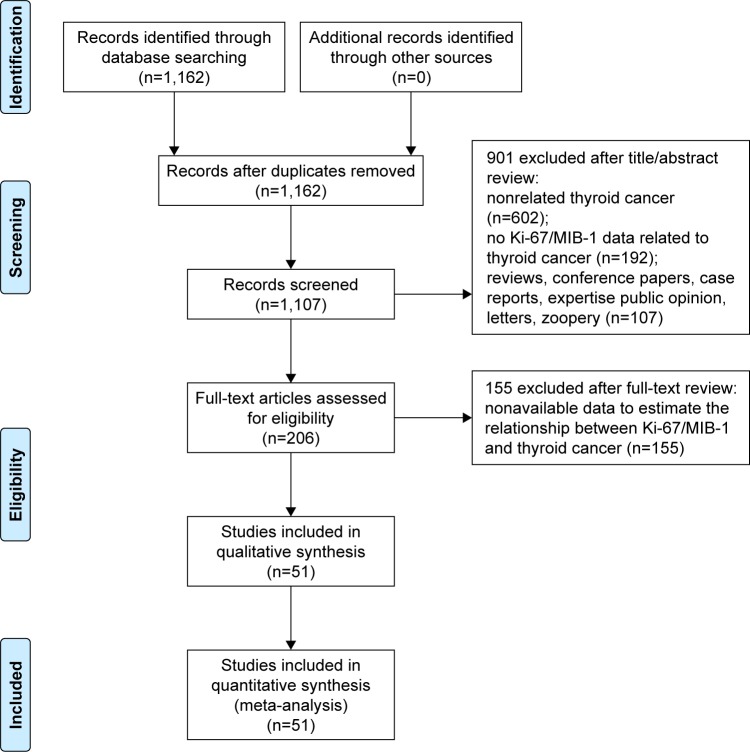
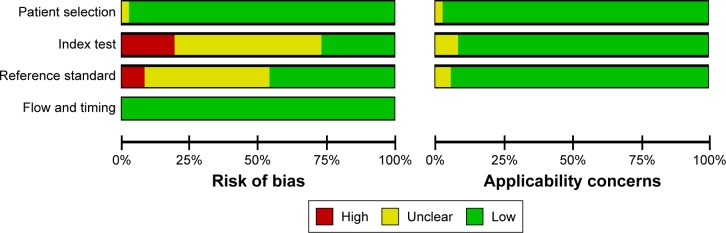
The flow chart of search process is presented in Figure 1. One thousand one hundred and sixty-two relevant studies were identified. After reviewing the abstracts and full text, only 51 studies1,12,14,17–64 were found to be eligible and were included in the meta-analysis. The characteristics of these articles are summarized in Tables 1–3. Table 1 gives the main information of the studies included in diagnosis. Thirty-seven studies detecting the diagnostic value of Ki-67/MIB-1 in thyroid cancer were included in our analysis, including 4,818 samples (2,601 cases and 2,217 controls). The quality assessment of the included studies in diagnosis is shown in Figure 2. The risks of bias in “index test” and “reference standard” were unclear in this meta-analysis. Table 2 shows the correlation between clinicopathologic parameters and Ki-67/MIB-1, including 4,375 samples (1,317 cases and 3,058 controls). As shown in Table 3, a total of 10 studies reporting the relation between Ki-67/MIB-1 and thyroid cancer patient survival were included, of which three studies had data on DFS, seven on mortality and only one on distant recurrences-free survival (DRFS). The studies included in survival analysis had 1,083 samples. The age of patients ranged from 45 to 64.8 years, and the median follow-up time ranged from 68 months to 20 years. Immunohistochemistry (IHC) was used to detect the expression of Ki-67/MIB-1 in all the included studies. The cut-off value ranged from 1% to 10%. The quality assessment of the studies included in prognosis is shown in Table 4.
Figure 1.
Flow diagram of reviewing and selecting studies.
Table 1.
Characteristics of studies included in diagnosis
| First author | Country | Patients | Type of cancer | Type of control | Antibody | Test method | Threshold, % | TP | FP | FN | TN |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Horii et al29 | Japan | 50 | PTC, FTC, ATC | Adenomatous goiter, FA | Ki-67 monoclonal antibody | IHC | 20 | 276 | 2 | 6 | 15 |
| Huang et al34 | China | 160 | PTC | FA, goiter | Mouse monoclonal antibody | IHC | 5 | 103 | 9 | 0 | 128 |
| Chou et al30 | Taiwan | 46 | PTC, FVPTC | NG, normal tissue | Mouse monoclonal antibody anti-Ki-67 | IHC | 10 | 16 | 2 | 13 | 15 |
| Nie et al35 | China | 26 | PTC | TA, NG, toxic goiter | Anti-Ki-67 | IHC | 25 | 9 | 2 | 5 | 10 |
| Liang et al36 | China | 200 | PTC | NG, TA | Ki-67 | IHC | 25 | 93 | 1 | 7 | 99 |
| Gan et al37 | China | 135 | PTC | Adjacent tissue of thyroid carcinoma, normal tissue, NG, FA | Mouse monoclonal antibody | IHC | 10 | 38 | 26 | 7 | 64 |
| Wang et al38 | China | 94 | PTC, FTC, MTC, ATC | NG, TA | Ki-67 | IHC | 10 | 20 | 0 | 45 | 29 |
| Shang et al39 | China | 100 | PTC, FTC, OCTC | PTA, FTA, OCTA | Anti-Ki-67 | IHC | 1.50 | 42 | 29 | 8 | 21 |
| Cui et al63 | China | 87 | PTC, FTC | TA, normal tissue | Mouse monoclonal antibody | IHC | 10 | 35 | 3 | 19 | 20 |
| Feng et al40 | China | 199 | PTC, FTC, MTC | NG, FA, adjacent tissue of thyroid carcinoma | Ki-67 | IHC | 5 | 58 | 16 | 18 | 107 |
| Jin et al64 | China | 79 | PTC, FTC, MTC, ATC | TA, normal thyroid gland tissue beside adenoma | Mouse monoclonal antibody | IHC | 25 | 39 | 3 | 17 | 20 |
| Huang et al42 | China | 70 | PTC | Thyroid hyperplasia, TA | Anti-Ki-67 | IHC | 10 | 20 | 9 | 10 | 31 |
| Li and Du43 | China | 136 | PTC | NG, NG with adenoma hyperplasia, NGWPH | Anti-Ki-67 | IHC | 10 | 55 | 27 | 0 | 54 |
| Hao44 | China | 236 | PTC | NG, TA | Ki-67 | IHC | ND | 17 | 2 | 101 | 116 |
| Song et al27 | China | 592 | PTC | NG, nonmaligant follicular adenoma | Mouse monoclonal antibody | IHC | 10 | 179 | 56 | 262 | 95 |
| Tan et al31 | Turkey | 39 | PTC, FTC | FA | Ki-67 | IHC | 5 | 8 | 10 | 16 | 5 |
| Zeng et al45 | China | 108 | TC | Normal tissue | Mouse monoclonal antibody | IHC | 5 | 46 | 11 | 22 | 29 |
| Qin et al46 | China | 120 | PTC | NG, normal tissue | Anti-Ki-67 | IHC | 10 | 25 | 78 | 15 | 1 |
| Zhao et al47 | China | 129 | PTC | Multinodular goiters, follicular adenomas, Hashimoto’s thyroiditis | Ki-67 | IHC | ND | 34 | 22 | 9 | 64 |
| Jiang et al48 | China | 63 | PTC | TA | Mouse monoclonal antibody | IHC | 10 | 23 | 2 | 12 | 26 |
| Yao et al49 | China | 103 | PTC, FTC, MTC, ATC | Normal tissue, NG, FA | Mouse monoclonal antibody | IHC | 25 | 60 | 10 | 8 | 25 |
| Yang et al50 | China | 55 | FTC | FA | Mouse monoclonal antibody | IHC | 5 | 15 | 6 | 17 | 17 |
| Li51 | China | 246 | PTC | Normal tissue, NG, NGWPH | Mouse monoclonal antibody | IHC | 10 | 68 | 22 | 8 | 68 |
| Shi et al52 | China | 130 | TC | TA, NG, normal thyroid gland tissue | Rabbit monoclonal antibody | IHC | 10 | 42 | 10 | 8 | 70 |
| Zhang et al28 | China | 146 | PTC | Multinodular goiter | Ki-67 | IHC | 1.65 | 57 | 9 | 19 | 61 |
| Maruta et al23 | Japan | 108 | FC | FA | Anti-Ki-67 | IHC | 50 | 47 | 35 | 21 | 5 |
| Guo et al53 | China | 117 | PTC, FTC, ATC | TA, normal thyroid tissues | Anti-Ki-67 | IHC | 10 | 62 | 2 | 87 | 30 |
| Wang et al54 | China | 33 | PTC | Hyalinizing trabecular tumor | MIB-1 | IHC | 10 | 20 | 10 | 0 | 3 |
| Zhou et al55 | China | 120 | PTMC | TA, NG, Hashimoto’s thyroiditis | Anti-Ki-67 | IHC | 25 | 34 | 6 | 46 | 34 |
| Li et al56 | China | 370 | PTC | Benign thyroid nodule | Mouse monoclonal antibody | IHC | 1.00 | 112 | 48 | 68 | 142 |
| Xu et al57 | China | 62 | PTC | Thyroid benign tumor | Ki-67 | IHC | 5 | 32 | 3 | 14 | 13 |
| Wang et al58 | China | 95 | PTC | NGWPH | Ki-67 | IHC | 10 | 26 | 44 | 19 | 6 |
| Zhou et al24 | China | 158 | PTMC | NG, TA, Hashimoto’s thyroiditis | Mouse monoclonal antibody | IHC | 25.00 | 50 | 7 | 58 | 43 |
| Zhu et al59 | China | 96 | PTC | Thyroid hyperplasia | Anti-Ki-67 | IHC | 5 | 40 | 10 | 24 | 22 |
| Li and Pu60 | China | 150 | PTC | Follicular adenoma, normal tissue | Ki-67 | IHC | 5 | 18 | 9 | 32 | 91 |
| Li and Zhang61 | China | 120 | PTC | TA, NG, normal tissue | Mouse monoclonal antibody | IHC | 10 | 25 | 8 | 5 | 82 |
| Feng and Wang62 | China | 60 | MTC | Adjacent tissue of thyroid carcinoma | Mouse monoclonal antibody | IHC | 1 | 36 | 0 | 6 | 18 |
Abbreviations: ATC, anaplastic thyroid cancer; FA, follicular adenoma; FN, false negative; FP, false positive; FTA, follicular thyroid adenoma; FTC, follicular carcinoma; FVPTC, follicular variant of papillary thyroid carcinoma; IHC, immunohistochemistry; MTC, medullary thyroid carcinoma; ND, no data; NG, nodular goiter; NGWPH, nodular goiter with papillary hyperplasia; PTA, papillary thyroid adenoma; OCTA, oxyphilical cell thyroid adenoma; OCTC, oxyphilical cell thyroid carcinoma; PTC, papillary thyroid carcinoma; PTMC, papillary thyroid microcarcinoma; TA, thyroid adenoma; TP, true positive; TN, true negative.
Table 2.
The relationships between Ki-67/MIB-1 expression and clinicopathologic parameters
| Clinicopathologic parameters | Studies (n) | Test group
|
Control group
|
Heterogeneity
|
Meta-analysis model | OR (95% CI) | P-value | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Events | Total | Events | Total | I2, % | P-value | |||||
| Age (45 years) | 10 | 124 | 303 | 91 | 239 | 0.00 | 0.806 | Fixed-effect analysis | 1.71 (1.14–2.57) | 0.01 |
| Tumor size (4 cm) | 5 | 35 | 165 | 76 | 730 | 2.00 | 0.395 | Fixed-effect analysis | 1.86 (1.17–2.96) | 0.008 |
| Lymph node metastasis | 15 | 233 | 345 | 226 | 509 | 50.20 | 0.014 | Random-effect analysis | 2.49 (1.42–4.39) | 0.002 |
| Metastasis status | 7 | 75 | 133 | 121 | 920 | 59.80 | 0.021 | Random-effect analysis | 6.96 (2.46–19.69) | <0.001 |
| TNM stage | 7 | 113 | 146 | 103 | 247 | 0.00 | 0.96 | Fixed-effect analysis | 6.56 (3.80–11.34) | <0.001 |
| Extrathyroid extension | 4 | 64 | 225 | 79 | 413 | 31.30 | 0.224 | Fixed-effect analysis | 1.91 (1.27–2.87) | 0.002 |
Abbreviations: CI, confidence interval; OR, odds ratio; TNM, tumor node metastasis.
Table 3.
The features of the studies relating Ki-67/MIB-1 to patients’ prognosis
| Type of survival | First author | Country | Year | Patients | Age | Median FU | Test method | Antibody | Threshold, % | HR (95% CI) | P-value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| DFS | Kjellman et al1 | Sweden | 2003 | 30 | 62 | 146 months | IHC | MIB-1 | 1.85 | 4.93 (1.91–12.77) | 0.001 |
| Ito et al12 | Japan | 2010 | 371 | 49 | 125 months | IHC | Anti Ki-67 antibody | 1 | 4.13 (2.19–7.75) | <0.001 | |
| Miyauchi et al22 | Japan | 2013 | 390 | 50.8 | 88 months | IHC | Anti Ki-67 antibody (clone MIB-1) | >10 | 15.33 (4.13–56.96) | <0.001 | |
| Mortality | Wang et al33 | Sweden | 1996 | 21 | 53 | 10.5 years | IHC | Ki-67 | 5 | 0.514 (0.086–3.089) | 0.467 |
| Tisell et al26 | Sweden | 2003 | 36 | 45 | 20 years | IHC | Anti-Ki67 | 1 | 2.12 (0.862–5.233) | 0.102 | |
| Ito et al12 | Japan | 2010 | 371 | 49 | 125 months | IHC | Anti Ki-67 antibody | 3 | 25.64 (2.49–250) | 0.006 | |
| Chen et al20 | USA | 2011 | 9 | 64.8 | 32.5 months | IHC | MIB-1 | 5 | 0.088 (0.008–1.026) | 0.052 | |
| Miyauchi et al22 | Japan | 2013 | 390 | 50.8 | 88 months | IHC | Anti Ki-67 antibody (clone MIB-1) | >10 | 34.08 (3.81–305.16) | 0.002 | |
| Gnemmi et al14 | France | 2014 | 82 | ND | 68 months | IHC | MIB1 | 4 | 6.126 (1.662–22.574) | 0.007 | |
| Jovanovic et al32 | Macedonia | 2015 | 20 | 51.2 | 73 months | IHC | MIB-1 | 6.50 | 24.25 (3.63–161.99) | 0.001 | |
| Feng and Wang62 | China | 2014 | 42 | 53.2 | 7.8 years | IHC | MIB-1 | 1 | 2.28 (0.61–8.55) | 0.228 | |
| DRFS | Gnemmi et al14 | France | 2014 | 82 | ND | 68 months | IHC | MIB-1 | 4 | 7.322 (3.141–17.07) | <0.001 |
Abbreviations: CI, confidence interval; DFS, disease-free survival; DRFS, distant recurrences-free survival; FU, follow-up; HR, hazard ratio; IHC, immunohistochemistry; ND, no data.
Figure 2.
Risk of bias of studies included in diagnosis with QUADAS-2 tool.
Table 4.
Quality assessment of included studies according to NOS
| Studies
|
Selection
|
Comparability
|
Outcome
|
Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| First author | Year | A | B | C | D | E | F | G | H | score |
| Kjellman et al1 | 2003 | * | * | * | * | * | * | 6 | ||
| Feng and Wang62 | 2015 | * | * | * | * | * | * | 6 | ||
| Ito et al12 | 2010 | * | * | * | * | * | * | 6 | ||
| Jovanovic et al32 | 2015 | * | * | * | * | * | * | 6 | ||
| Gnemmi et al14 | 2014 | * | * | * | * | * | * | * | 7 | |
| Miyauchi et al22 | 2013 | * | * | * | * | * | 5 | |||
| Chen et al20 | 2011 | * | * | * | * | * | * | 6 | ||
| Wang et al33 | 1996 | * | * | * | * | * | * | 6 | ||
| Tisell et al26 | 2003 | * | * | * | * | * | * | 6 | ||
Notes: A: representative of the exposed cohort; B: selection of the nonexposed cohort; C: ascertainment of exposure; D: demonstration that outcome of interest was not present at the start of study; E: comparability of cohorts on the basis of the design or analysis; F: assessment of outcome; G: adequacy of follow-up for outcomes to occur (≥2 years or outcomes occurred in all patients); H: adequacy of follow-up of cohorts (follow-up rate ≥75%).
Abbreviation: NOS, Newcastle–Ottawa scale.
Effect of Ki-67/MIB-1 on diagnosis
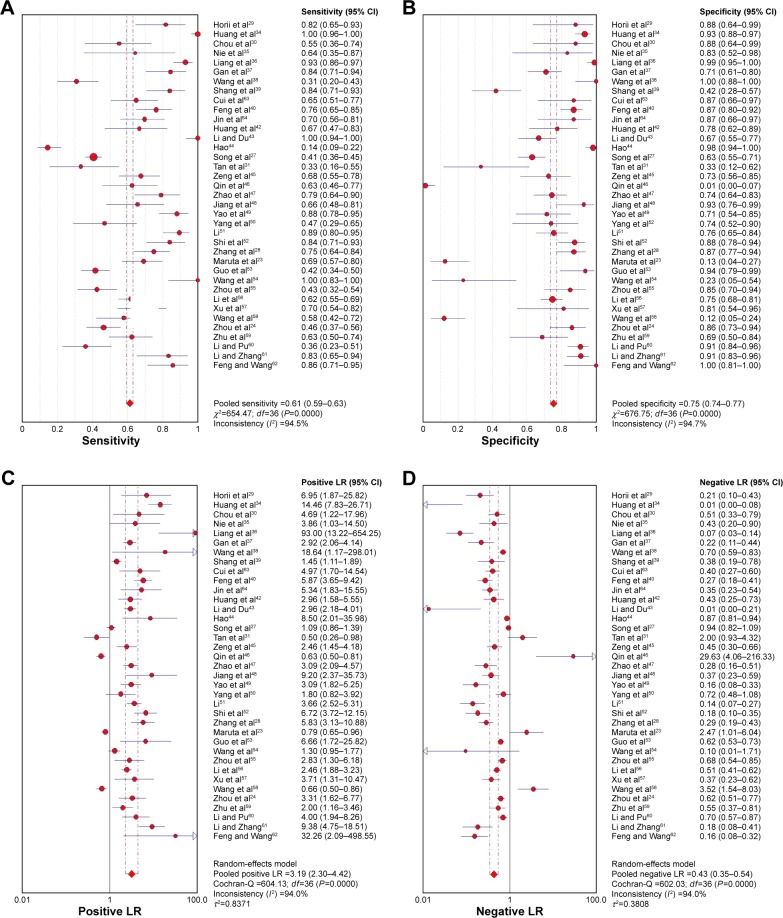
Due to heterogeneity, random-effect model was used to calculate the overall performance of Ki-67/MIB-1 in thyroid tissues in diagnosing thyroid cancer. Ki-67/MIB-1 was found to be a valuable diagnostic factor for thyroid cancer. The pooled sensitivity was 0.61 (95% CI: 0.59–0.63; Figure 3A) and specificity was 0.75 (95% CI: 0.74–0.77; Figure 3B). The pooled LR+ was 3.19 (95% CI: 2.30–4.42; Figure 3C) and the pooled LR− was 0.43 (95% CI: 0.35–0.54; Figure 3D). The pooled DOR of Ki-67/MIB-1 was 8.54 (95% CI: 5.03–14.49; Figure 4). The area under the symmetric receiver operating characteristic curve was 0.804 (standard error =0.031; Figure 5). For Ki-67/MIB-1, the summary indexes of 37 studies are displayed in forest plots. High heterogeneity was found in sensitivity (I2=94.5%, P<0.001) and specificity (I2=94.7%, P<0.001). Also, subgroup analysis was performed to identify the possible reasons for heterogeneity. There were 12 studies with Ki-67/MIB-1 cut-off value ≤5%, which revealed a pooled sensitivity of 0.70 (95% CI: 0.67–0.73), specificity of 0.80 (95% CI: 0.77–0.83) and DOR of 7.49 (95% CI: 3.61–15.52). Fifteen studies had Ki-67/MIB-1 cut-off value of 10%, a pooled sensitivity of 0.56 (95% CI: 0.53–0.59), specificity of 0.67 (95% CI: 0.63–0.70) and DOR of 7.73 (95% CI: 2.68–22.30). There were eight studies with a cut-off value >10%, and the pooled sensitivity, specificity and DOR were 0.78 (95% CI: 0.75–0.81), 0.79 (95% CI: 0.74–0.84) and 15.40 (95% CI: 3.41–69.62), respectively. In the subgroup analysis of PTC, the results showed that the pooled sensitivity, specificity and DOR were 0.63 (95% CI: 0.60–0.65), 0.74 (95% CI: 0.72–0.76) and 8.22 (95% CI: 4.08–16.56), respectively. In the subgroup analysis of papillary thyroid microcarcinoma (PTMC), the results showed that the pooled sensitivity, specificity and DOR were 0.45 (95% CI: 0.37–0.52), 0.86 (95% CI: 0.77–0.92) and 4.76 (95% CI: 2.48–9.17), respectively (Table S1).
Figure 3.
Forest plots for the accuracy of Ki-67/MIB-1 for the diagnosis of thyroid cancer.
Notes: (A) Sensitivity; (B) specificity; (C) positive LR (LR+); (D) negative LR (LR−).
Abbreviations: CI, confidence interval; df, degrees of freedom; LR, likelihood ratio.
Figure 4.
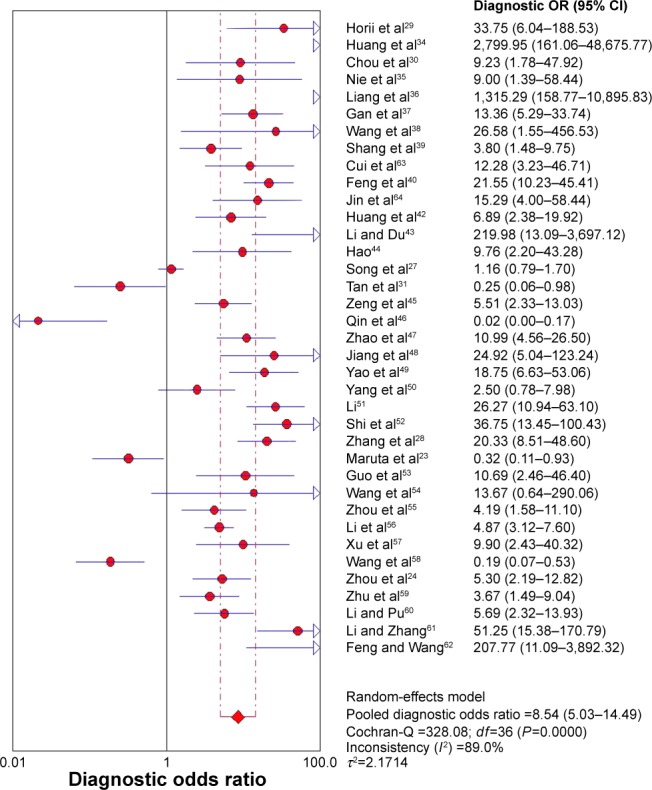
Forest plot of DOR of Ki-67/MIB-1 for the diagnosis of thyroid cancer.
Note: The pooled DOR of Ki-67/MIB-1 for the diagnosis of thyroid cancer was 8.54 (95% CI: 5.03–14.49).
Abbreviations: CI, confidence interval; df, degrees of freedom; DOR, diagnostic odds ratio; OR, odds ratio.
Figure 5.
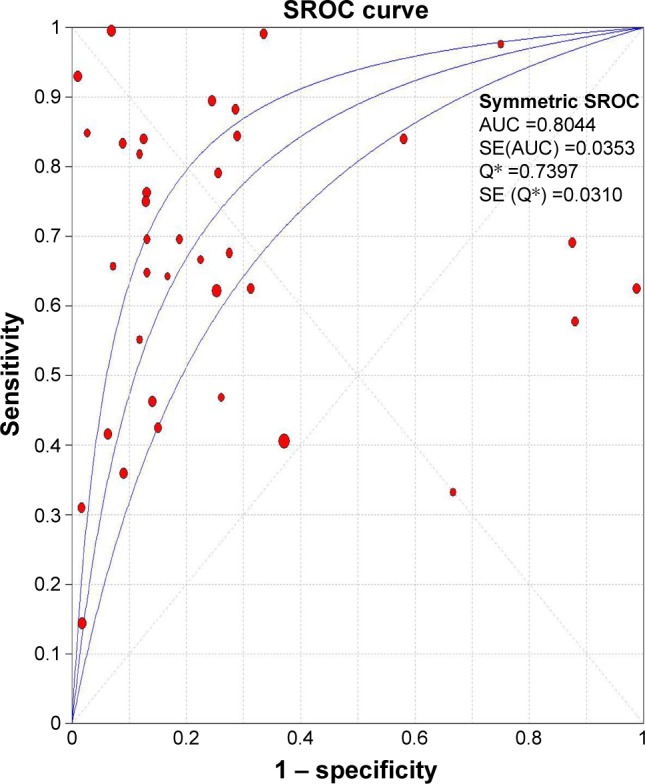
SROC curve for the accuracy of Ki-67/MIB-1 in the diagnosis of thyroid cancer.
Notes: The area under the SROC curve was 0.804 (SE =0.031). Q* represents the index to judge the accuracy of the diagnostic experiment.
Abbreviations: AUC, area under the curve; SE, standard error; SROC, symmetric receiver operating characteristic.
Associations of Ki-67/MIB-1 with clinicopathologic parameters
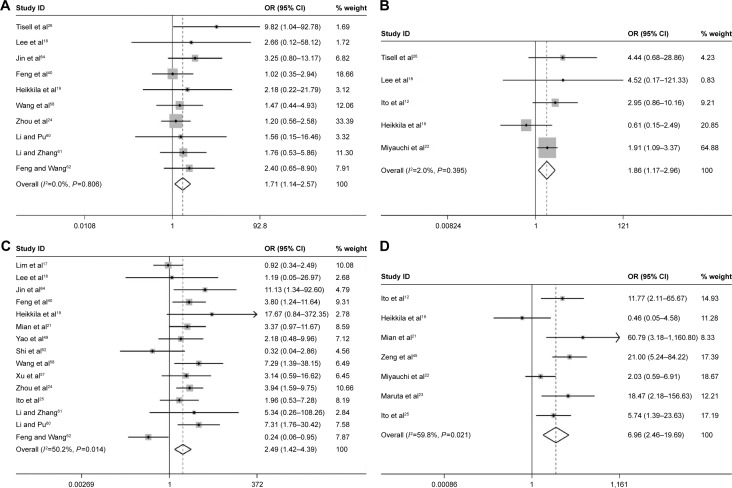
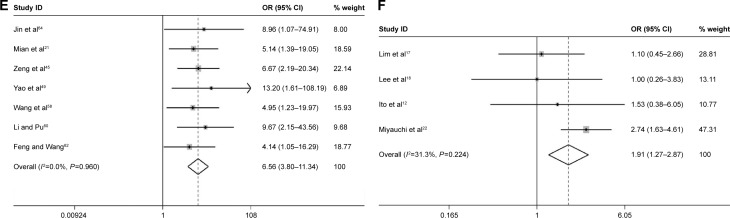
Table 2 shows the main results of the effects value of Ki-67/MIB-1 on clinicopathologic parameters in thyroid cancer patients. The results suggested that older patients (>45 years old) had high level of Ki-67/MIB-1 with a pooled OR of 1.71 (95% CI: 1.14–2.57, P=0.010; I2=0.00%, P=0.806; Figure 6A). The level of Ki-67/MIB-1 was higher in large tumor size (>4 cm) than in small-sized tumor (<4 cm; pooled OR =1.86, 95% CI: 1.17–2.96, P=0.008; I2=2.00%, P=0.395; Figure 6B). Overall, 15 studies had data to estimate the relationship between Ki-67/MIB-1 and lymph node metastasis. The pooled OR estimated from 15 studies indicated that Ki-67/MIB-1 was lower in negative lymph node metastasis than in positive lymph node metastasis (pooled OR =2.49, 95% CI: 1.42–4.39, P=0.002; I2=50.20%, P=0.014; Figure 6C). Also, the association between Ki-67/MIB-1 and metastasis status was calculated and the combined OR was 6.96 (95% CI: 2.46–19.69, P<0.001; Figure 6D). The combined OR for tumor node metastasis stage was 6.56 (95% CI: 3.80–11.33, P<0.001; I2=0.00%, P=0.960), suggesting that overexpression of Ki-67/MIB-1 was significantly correlated with advanced stage (Figure 6E). In addition, four studies provided insufficient information to estimate the effect of extrathyroid extension. The pooled OR was 1.91 (95% CI: 1.27–2.87, P=0.002; Figure 6F).
Figure 6.
Forest plots of Ki-67/MIB-1 and clinicopathologic parameters in thyroid cancer patients.
Notes: (A) Age; (B) tumor size; (C) lymph node metastasis; (D) metastasis status; (E) TNM stage; (F) extrathyroid extension. Weights are from random-effects analysis.
Abbreviations: CI, confidence interval; OR, odds ratio; TNM, tumor node metastasis.
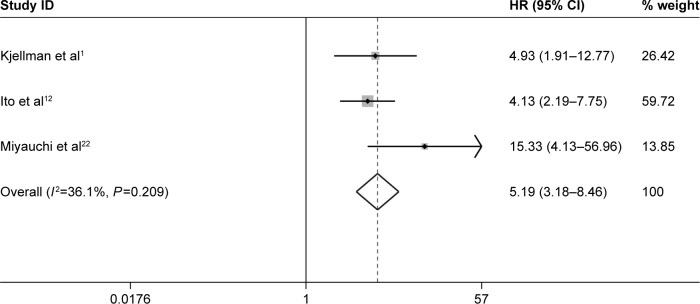
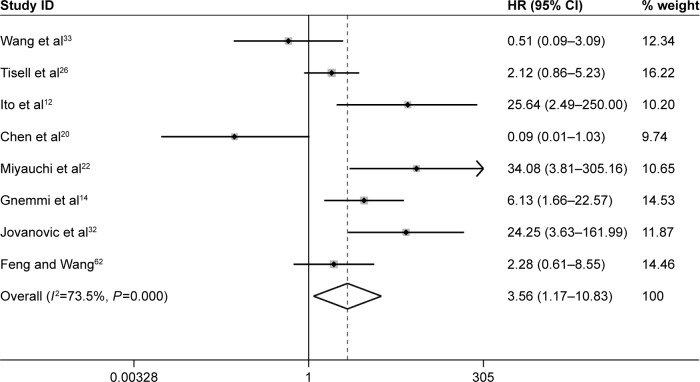
Impact of Ki-67/MIB-1 expression on survival in thyroid cancer
Furthermore, three studies assessing the association of Ki-67/MIB-1 expression on DFS were identified in this meta-analysis. The combined HR was 5.19. It was demonstrated that overexpression of Ki-67/MIB-1 was associated with worse DFS in thyroid cancer by fixed-effect model (95% CI: 3.18–8.46, P<0.001; I2=36.1%, P=0.209; Figure 7). The meta-analysis showed overexpression of Ki-67/MIB-1 had effect on mortality (HR =3.56, 95% CI: 1.17–10.83, P=0.025) in eight studies, with heterogeneity (I2=73.5%, P<0.001; Figure 8). Worsened mortality was found among patients with Ki-67/MIB-1 cut-off value >10% (HR =34.08, 95% CI: 3.81–305.00, P=0.002) by subgroup analysis. Nevertheless, when Ki-67/MIB-1 was more than 5%, the patients were afflicted with poor mortality (HR =28.06, 95% CI: 6.68–117.87, P<0.001) in thyroid cancer. No connection was found between Ki-67/MIB-1 and thyroid cancer when Ki-67/MIB-1 was less than 5% (HR =1.98, 95% CI: 0.65–6.06, P=0.230). In subgroup analysis, no significant association was found between medullary thyroid cancer and mortality (HR =0.96, 95% CI: 0.24–2.16). In addition, no significant relationship was observed between PTC and mortality (HR =1.47, 95% CI: 2.23–5.18; Table S2).
Figure 7.
Meta-analysis evaluating the association between Ki-67/MIB-1 and DFS (fixed-effect analysis).
Note: The combined fixed-effect HR was 5.19 (95% CI: 3.18–8.46, P<0.001), indicating that high level of Ki-67/MIB-1 was associated with worse DFS.
Abbreviations: CI, confidence interval; DFS, disease-free survival; HR, hazard ratio.
Figure 8.
Meta-analysis evaluating the association between Ki-67/MIB-1 and mortality (random-effect analysis).
Notes: The pooled fixed-effect HR was 3.56 (95% CI: 1.17–10.83, P=0.025), indicating that high level of Ki-67/MIB-1 was associated with increased of risk of mortality. Weights are from random-effect analysis.
Abbreviations: CI, confidence interval; HR, hazard ratio.
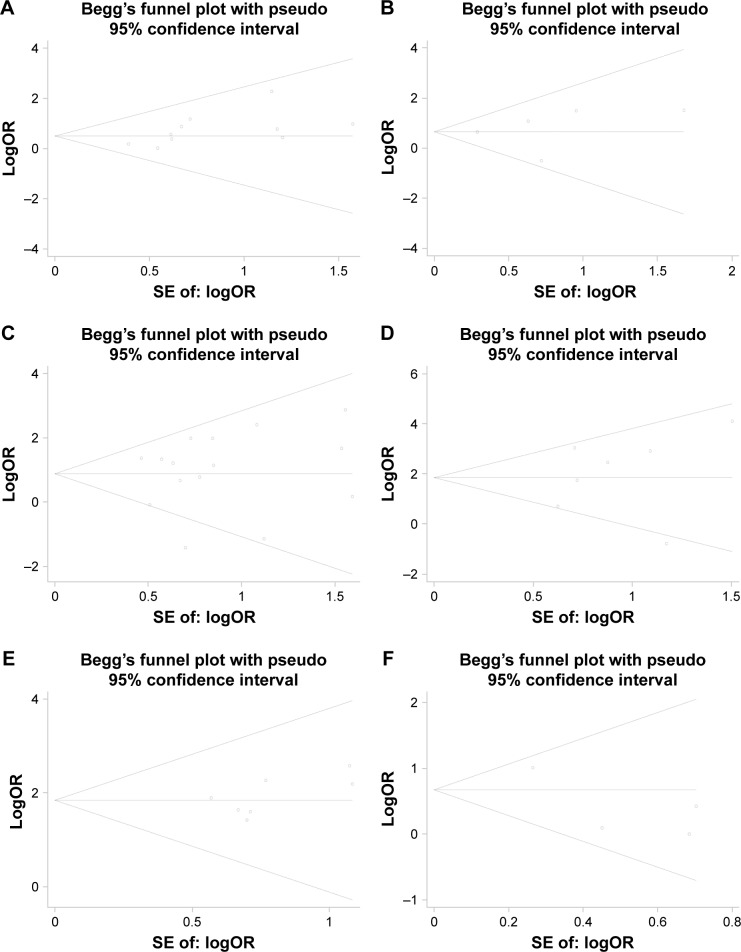
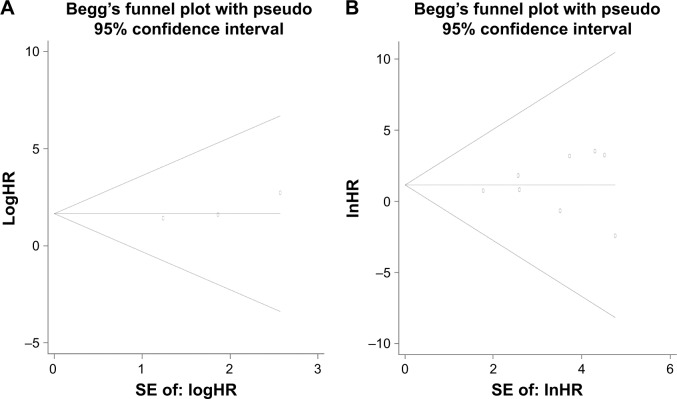
Publication bias
The Begg’s test and funnel plot showed that there was no evidence of publication bias present among all the analyses in our meta-analysis (all P>0.05; Figures 9 and 10).
Figure 9.
Assessment of publication bias for clinicopathologic parameters.
Notes: (A) Age; (B) tumor size; (C) lymph node metastasis; (D) metastasis status; (E) TNM stage; (F) extrathyroid extension.
Abbreviations: OR, odds ratio; SE, standard error; TNM, tumor node metastasis.
Figure 10.
Assessment of publication bias for DFS and mortality.
Notes: (A) DFS; (B) mortality.
Abbreviations: DFS, disease-free survival; HR, hazard ratio; SE, standard error.
Discussion
Cell proliferative activity is regarded as an important factor for evaluating the biologic behavior of cancer cells. It is a key process in the development of tumors when the balance between cell death and proliferation is destroyed. We used IHC to detect cell cycle-specific antigens and this method can assess the proliferative activity of cells. Ki-67/MIB-1 is the most widely used marker for assessing the proliferative capacity of tumor cells. Increased expression of Ki-67/MIB-1 has been linked to increased invasiveness in many cancers.10,65–67 Many meta-analyses have shown that high expression of Ki-67/MIB-1 contributed to poor survival in many tumors.68–72 However, there is no consensus on the association between high level of Ki-67/MIB-1 and thyroid cancer at present. Meta-analysis is a systematical method applied widely to evaluate the prognostic indicators in different trials. Thus, in this meta-analysis, we explored the diagnosis and prognosis of Ki-67/MIB-1 expression in thyroid cancer.
In our study, we found the valuable predicting effect of Ki-67/MIB-1 for the diagnosis of thyroid cancer with a high accuracy for Chinese. In clinical practice, detecting Ki-67/MIB-1 expression may contribute to diagnosing thyroid cancer when combined with clinical symptoms, laboratory examinations and other radiologic imaging. Our analysis demonstrated that patients with overexpression of Ki-67/MIB-1 seemed to have a poor survival in thyroid cancer. Also, Ki-67/MIB-1 was found to be associated with tumor size, lymph node metastasis, metastasis status, extrathyroid extension and other clinicopathologic parameters. However, the mechanism of Ki-67/MIB-1 in thyroid cancer is still unclear. Ki-67/MIB-1 as a cellular marker has a positive effect on cell proliferation. Ki-67/MIB-1 expression detected by IHC can evaluate tumor cell proliferation. A previous study confirmed that Sp1 plays an important role in regulation of Ki-67/MIB-1 gene expression.73 Another study pointed out that E2F2 transcription factor was positively correlated with Ki-67/MIB-1 expression in human glioblastoma74 and E2F1–3 factors are the transcriptional activator in tumor progression and the retinoblastoma tumor suppressor pathway regulates E2F1–3 factors which could control cellular proliferation.75
In this meta-analysis, heterogeneity existed among the studies. Heterogeneity was still a potential problem which affected the meta-analysis results, though random-effect models were used to analyze the data. Meanwhile, in order to reduce heterogeneity, only the studies with the method of IHC to detect Ki-67/MIB-1 were included in this meta-analysis. However, evaluation standards, study location, number of patients, sex and age of patients were different, which contributed to the heterogeneity. Also, various cut-off values were used to define thyroid cancer with Ki-67/MIB-1 positive expression by different investigators, which potentially contributed to the heterogeneity. So, it is difficult to apply a standard critical value in clinical practice. Spyratos et al76 found that few tumors with low proliferation rate were under misclassification when the cut-off value of Ki-67/MIB-1 was 10%, and it is acceptable to identify a highly proliferative tumor with a Ki-67/MIB-1 cut-off value of 25%. In this meta-analysis, the cut-off values of Ki-67/MIB-1 ranged from 0.5% to 25%. Therefore, different Ki-67/MIB-1 cut-offs may cause heterogeneity. Higher sensitivity, specificity and DOR were found in patients with cut-off value >10% by diagnostic subgroup analysis. Furthermore, those patients may have worse mortality. Given the small size of studies included in mortality analysis, further research with larger sample size would be needed to explore the impact of KI-67/MIB-1 on mortality. Besides, HRs were extracted from survival curves or calculated from data which might be less than another variance. Most of the studies included in diagnosis were from China; however, most of the studies related to DFS and mortality were from outside of China. We found that Ki-67/MIB-1 had a diagnostic value in Chinese. However, we did not have enough data to calculate the relationship between Ki-67/MIB-1 and mortality for Chinese. So, regional variation may become a score of heterogeneity. Besides, due to several types of thyroid cancer dealt with in the same study, we conducted subgroup analysis in this meta-analysis. Ki-67/MIB-1 had diagnostic effect on different thyroid cancer types. We did not find significant association between Ki-67/MIB-1 and different thyroid cancer types.
Despite the above limitations, the current meta-analysis proves the associations between high Ki-67/MIB-1 and tumor deterioration, poor DFS and increased mortality in patients with thyroid cancer. In conclusion, we showed that high expression of Ki-67/MIB-1 was significantly connected with tumor size, lymph metastasis, metastasis status, extrathyroid extension and poor prognosis of thyroid cancer in this study.
Conclusion
Our meta-analysis shows that Ki-67/MIB-1 may be a biomarker for clinical deterioration in Chinese and has an effect on prognosis in thyroid cancer among non-Chinese. Therefore, detection of Ki-67/MIB-1 in the clinic will be beneficial to the treatment and prognostic assessment for thyroid cancer patients. However, well-designed prospective studies are necessary to further confirm our results.
Supplementary materials
Table S1.
Subgroup analysis evaluating the diagnostic value of Ki-67/MIB-1 in thyroid cancer
| Groups | No of studies | Pooled sensitivity (95% CI) | Pooled specificity (95% CI) | DOR (95% CI) |
|---|---|---|---|---|
| Cut-off, % | ||||
| ≤5 | 12 | 0.70 (0.67–0.73) | 0.80 (0.77–0.83) | 7.49 (3.61–15.52) |
| 10 | 15 | 0.56 (0.53–0.59) | 0.67 (0.63–0.70) | 7.73 (2.68–22.30) |
| >10 | 8 | 0.78 (0.75–0.81) | 0.79 (0.74–0.84) | 15.40 (3.41–69.62) |
| Types | ||||
| PTC | 21 | 0.63 (0.60–0.65) | 0.74 (0.72–0.76) | 8.22 (4.08–16.56) |
| PTMC | 2 | 0.45 (0.37–0.52) | 0.86 (0.77–0.92) | 4.76 (2.48–9.17) |
Abbreviations: CI, confidence interval; DOR, diagnostic odds ratio; PTC, papillary thyroid carcinoma; PTMC, papillary thyroid microcarcinoma.
Table S2.
Subgroup analysis evaluating the prognostic value of Ki-67/MIB-1 for mortality in thyroid cancer
| Groups | Pooled HR | 95% CI | Heterogeneity test
|
Statistical method | |
|---|---|---|---|---|---|
| I2, % | P-value | ||||
| Univariate cut-off, % | |||||
| ≤5 | 1.98 | 0.65–6.06 | 68.80 | 0.007 | Random |
| >5 | 28.06 | 6.68–117.87 | 0.00 | 0.818 | Random |
| <10 | 2.73 | 0.89–8.38 | 72.20 | 0.001 | Random |
| Types | |||||
| PTC | 1.47 | 2.23–5.18 | 87.10 | <0.001 | Random |
| MTC | 0.96 | 0.24–2.16 | 65.50 | 0.035 | Random |
Abbreviations: CI, confidence interval; HR, hazard ratio; MTC, medullary thyroid carcinoma; PTC, papillary thyroid carcinoma.
Acknowledgments
The study was supported by funds from the Guangxi Scientific Research and Technology Development Plan (1598011-4). The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the paper.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Kjellman P, Wallin G, Hoog A, Auer G, Larsson C, Zedenius J. MIB-1 index in thyroid tumors: a predictor of the clinical course in papillary thyroid carcinoma. Thyroid. 2003;13(4):371–380. doi: 10.1089/105072503321669866. [DOI] [PubMed] [Google Scholar]
- 2.Sofiadis A, Tani E, Foukakis T, et al. Diagnostic and prognostic potential of MIB-1 proliferation index in thyroid fine needle aspiration biopsy. Int J Oncol. 2009;35(2):369–374. [PubMed] [Google Scholar]
- 3.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 4.Torre LA, Sauer AM, Chen MS, Jr, Kagawa-Singer M, Jemal A, Siegel RL. Cancer statistics for Asian Americans, Native Hawaiians, and Pacific Islanders, 2016: Converging incidence in males and females. CA Cancer J Clin. 2016;66(3):182–202. doi: 10.3322/caac.21335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ito Y, Miyauchi A, Ito M, et al. Prognosis and prognostic factors of differentiated thyroid carcinoma after the appearance of metastasis refractory to radioactive iodine therapy. Endocr J. 2014;61(8):821–824. doi: 10.1507/endocrj.ej14-0181. [DOI] [PubMed] [Google Scholar]
- 6.Ito Y, Tomoda C, Uruno T, et al. Ultrasonographically and anatomo-pathologically detectable node metastases in the lateral compartment as indicators of worse relapse-free survival in patients with papillary thyroid carcinoma. World J Surg. 2005;29(7):917–920. doi: 10.1007/s00268-005-7789-x. [DOI] [PubMed] [Google Scholar]
- 7.Ito Y, Tomoda C, Uruno T, et al. Prognostic significance of extrathyroid extension of papillary thyroid carcinoma: massive but not minimal extension affects the relapse-free survival. World J Surg. 2006;30(5):780–786. doi: 10.1007/s00268-005-0270-z. [DOI] [PubMed] [Google Scholar]
- 8.Ito Y, Kakudo K, Hirokawa M, et al. Biological behavior and prognosis of familial papillary thyroid carcinoma. Surgery. 2009;145(1):100–105. doi: 10.1016/j.surg.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Yu JQ, Zhou Q, Zheng YF, Bao Y. Expression of vimentin and Ki-67 proteins in cervical squamous cell carcinoma and their relationships with clinicopathological features. Asian Pac J Cancer Prev. 2015;16(10):4271–4275. doi: 10.7314/apjcp.2015.16.10.4271. [DOI] [PubMed] [Google Scholar]
- 10.Ghanim B, Klikovits T, Hoda MA, et al. Ki67 index is an independent prognostic factor in epithelioid but not in non-epithelioid malignant pleural mesothelioma: a multicenter study. Br J Cancer. 2015;112(5):783–792. doi: 10.1038/bjc.2015.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hao S, He ZX, Yu KD, Yang WT, Shao ZM. New insights into the prognostic value of Ki-67 labeling index in patients with triple-negative breast cancer. Oncotarget. 2016;7(17):24824–24831. doi: 10.18632/oncotarget.8531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito Y, Miyauchi A, Kakudo K, Hirokawa M, Kobayashi K, Miya A. Prognostic significance of ki-67 labeling index in papillary thyroid carcinoma. World J Surg. 2010;34(12):3015–3021. doi: 10.1007/s00268-010-0746-3. [DOI] [PubMed] [Google Scholar]
- 13.Pascual J, Berciano J. An open trial of buspirone in migraine prophylaxis. Preliminary report. Clin Neuropharmacol. 1991;14(3):245–250. doi: 10.1097/00002826-199106000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Gnemmi V, Renaud F, Do Cao C, et al. Poorly differentiated thyroid carcinomas: application of the Turin proposal provides prognostic results similar to those from the assessment of high-grade features. Histopathology. 2014;64(2):263–273. doi: 10.1111/his.12246. [DOI] [PubMed] [Google Scholar]
- 15.Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 16.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 17.Lim DJ, Baek KH, Lee YS, et al. Clinical, histopathological, and molecular characteristics of papillary thyroid microcarcinoma. Thyroid. 2007;17(9):883–888. doi: 10.1089/thy.2007.0001. [DOI] [PubMed] [Google Scholar]
- 18.Lee YS, Ha SA, Kim HJ, et al. Minichromosome maintenance protein 3 is a candidate proliferation marker in papillary thyroid carcinoma. Exp Mol Pathol. 2010;88(1):138–142. doi: 10.1016/j.yexmp.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 19.Heikkila A, Siironen P, Hagstrom J, et al. Follicular thyroid neoplasm: clinicopathologic features suggesting malignancy. APMIS. 2010;118(11):846–854. doi: 10.1111/j.1600-0463.2010.02668.x. [DOI] [PubMed] [Google Scholar]
- 20.Chen JH, Faquin WC, Lloyd RV, Nose V. Clinicopathological and molecular characterization of nine cases of columnar cell variant of papillary thyroid carcinoma. Mod Pathol. 2011;24(5):739–749. doi: 10.1038/modpathol.2011.2. [DOI] [PubMed] [Google Scholar]
- 21.Mian C, Pennelli G, Barollo S, et al. Combined RET and Ki-67 assessment in sporadic medullary thyroid carcinoma: a useful tool for patient risk stratification. Eur J Endocrinol. 2011;164(6):971–976. doi: 10.1530/EJE-11-0079. [DOI] [PubMed] [Google Scholar]
- 22.Miyauchi A, Kudo T, Hirokawa M, et al. Ki-67 labeling index is a predictor of postoperative persistent disease and cancer growth and a prognostic indicator in papillary thyroid carcinoma. Eur Thyroid J. 2013;2(1):57–64. doi: 10.1159/000347148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maruta J, Hashimoto H, Yamashita H, et al. Value of thyroid specific peroxidase and Ki-67 stains in preoperative cytology for thyroid follicular tumors. Diagn Cytopathol. 2015;43(3):202–209. doi: 10.1002/dc.23204. [DOI] [PubMed] [Google Scholar]
- 24.Zhou Y, Jiang HG, Lu N, Lu BH, Chen ZH. Expression of ki67 in papillary thyroid microcarcinoma and its clinical significance. Asian Pac J Cancer Prev. 2015;16(4):1605–1608. doi: 10.7314/apjcp.2015.16.4.1605. [DOI] [PubMed] [Google Scholar]
- 25.Ito Y, Uruno T, Takamura Y, et al. Papillary microcarcinomas of the thyroid with preoperatively detectable lymph node metastasis show significantly higher aggressive characteristics on immunohistochemical examination. Oncology. 2005;68(2–3):87–96. doi: 10.1159/000085701. [DOI] [PubMed] [Google Scholar]
- 26.Tisell LE, Oden A, Muth A, et al. The Ki67 index a prognostic marker in medullary thyroid carcinoma. Br J Cancer. 2003;89(11):2093–2097. doi: 10.1038/sj.bjc.6601453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song Q, Wang D, Lou Y, et al. Diagnostic significance of CK19, TG, Ki67 and galectin-3 expression for papillary thyroid carcinoma in the northeastern region of China. Diagn Pathol. 2011;6:126. doi: 10.1186/1746-1596-6-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Meng Z, Zhang M, et al. Immunohistochemical evaluation of midkine and nuclear factor-kappa B as diagnostic biomarkers for papillary thyroid cancer and synchronous metastasis. Life Sci. 2014;118(1):39–45. doi: 10.1016/j.lfs.2014.09.025. [DOI] [PubMed] [Google Scholar]
- 29.Horii A, Yoshida J, Sakai M, et al. Ki-67 positive fractions in benign and malignant thyroid tumours: application of flow cytometry. Acta Otolaryngol. 1999;119(5):617–620. doi: 10.1080/00016489950180883. [DOI] [PubMed] [Google Scholar]
- 30.Chou SJ, Chen CM, Harn HJ, Chen CJ, Liu YC. In situ detection of hTERT mRNA relates to Ki-67 labeling index in papillary thyroid carcinoma. J Surg Res. 2001;99(1):75–83. doi: 10.1006/jsre.2001.6124. [DOI] [PubMed] [Google Scholar]
- 31.Tan A, Etit D, Bayol U, Altinel D, Tan S. Comparison of proliferating cell nuclear antigen, thyroid transcription factor-1, Ki-67, p63, p53 and high-molecular weight cytokeratin expressions in papillary thyroid carcinoma, follicular carcinoma, and follicular adenoma. Ann Diagn Pathol. 2011;15(2):108–116. doi: 10.1016/j.anndiagpath.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 32.Jovanovic R, Kostadinova-Kunovska S, Janevska V, et al. Novel RET mutations in macedonian patients with medullary thyroid carcinoma: genotype-phenotype correlations. Pril (Makedon Akad Nauk Umet Odd Med Nauki) 2015;36(1):93–107. [PubMed] [Google Scholar]
- 33.Wang W, Johansson H, Bergholm U, Wilander E, Grimelius L. Apoptosis and expression of the proto-oncogenes bcl-2 and p53 and the proliferation factor Ki-67 in human medullary thyroid carcinoma. Endocr Pathol. 1996;7(1):37–45. doi: 10.1007/BF02739913. [DOI] [PubMed] [Google Scholar]
- 34.Huang X, Zhang P, Chen J, Gao l, Ling Y. Expression of p21ras protein, treanforming growth factorβ1 and Ki-67 in papillary thyroid carcinoma. J Fujian Med University. 2000;2:129–131. [Google Scholar]
- 35.Nie M, Du M, Li X. Expression of cytokeratin19 and Ki67 in papillary thyroid carcinoma. Heilongjiang Med J. 2005;29(3):168–169. [Google Scholar]
- 36.Liang F, Fu Q, Dai C, Wang G, Li J, Zhao M. Pathological study of papillary thyroid carcinoma and papillary thyroid typerplasia. Cancer Res Clin. 2006;18(11):755–756. [Google Scholar]
- 37.Gan X, Zhu W, Zhang X, Wan L. The expression and significance of Ki-67 and CK-19 in papillary thyroid carcinoma. Zhejiang Practical Med. 2007;12(6):391–392. [Google Scholar]
- 38.Wang C, Sun J, Teng M, Huan D. Expression of RET, HBME-1 and Ki67 in thyroid benign and malignant tumors. Practical Oncol J. 2007;21(5):427–429. [Google Scholar]
- 39.Shang L, Yang Y, Li D, Wei D, Chen X. Expressions of CD26, Ki67 and EGFR proteins in primany thyroid neoplasms and their value in differential diagnosis. Chinese J Endocrinol Meta. 2008;24(2):174–177. [Google Scholar]
- 40.Feng W, Cao Y, Zhang J. Expression of Ki-67 and survivin in thyroid cacner and their significance. J Practical Med. 2009;25(19):3215–3218. [Google Scholar]
- 41.Wang L, Xi F, Yang Y, et al. Expression of survivin and Ki-67 and their significance in thyroid tumors. Shandong Med J. 2009;49(28):9–11. [Google Scholar]
- 42.Huang H, Mei J, Xu L, Chen R. Expression of CK19, Ki67 and VEGF and their clinical significances in papillary thyroid carcinoma. J Nanchang University (Medical Sciences) 2010;50(11):24–27. [Google Scholar]
- 43.Li N, Du J. Expression of HBME-1, CK19, Gal-3 and Ki-67 in papillary thyroid lesions. J Harbin Med University. 2010;44(6):575–578. [Google Scholar]
- 44.Hao G. The expresion p53, p21 and Ki-67 in papillary thyroid carcinoma. China Modern Med. 2010;(2):38–38. [Google Scholar]
- 45.Zeng X, Yang X, Wang T. Expression and significance of tumor-related factors COX-2 and Ki-67 in thyroid neoplasm tissue. Practical Preventive Med. 2011;18(4):612–614. [Google Scholar]
- 46.Qin L, Niu J, Liu S, He J, Xu Y. Significance and Expression of MCM3 and Ki-67 in Normal Thyroid, Nodular Goiter and Carcinoma. Journal of Shihezi University (Natural Science) 2012;30(3):356–360. [Google Scholar]
- 47.Zhao L, Lin J, Shi B, Lin Q, Huai Y, Wang K. Expression and clinical pathology significance of P53, ki-67, galectin-3, HBME-1, 34βE12 and CK19 in papillary thyroid carcinomas. Basic Amp Clin Med. 2012;32(10):1202–1206. [Google Scholar]
- 48.Jiang W, Wang Y, Chen M, Zhu C. Expression and relationship of XIAP, P53 and Ki67 in papillary thyroid carcinoma. BMJ. 2012;34(11):971–974. [Google Scholar]
- 49.Yao H, Wei Z, Liang X, et al. The expressin and prognosis of tumor metastasis gene KISS-1 in the tissues of thyroid cancer. Chinese Remedies Clinics. 2012;12(11):1408–1411. [Google Scholar]
- 50.Yang R, Teng X, Ding W, Sun K. Expressions of MCM2, Ki67, CyclinD1 in Follicular Carcinoma and Follicular Adenoma of the Thyroid and the Comparison with Galectin-3 and CK19. J Basic Clin Oncol. 2013;(6):464–468. [Google Scholar]
- 51.Li X. Expression and clinial significance of Receptor for Activated Protein C Kinase1 and Ki67 in papillary thyroid carcinoma. J Dalian Med University. 2013 [Google Scholar]
- 52.Shi L, Zhang A, Luo Y, Zhao S, Tian H, Yang Y. Abnormal expressions of positive cell cycle control factors and thyroid carcinoma occurrence and progression. J Southern Med University. 2013;33(7):1031–1035. [PubMed] [Google Scholar]
- 53.Guo M, Xing Y, Chen W, Zhang J, Meng D. Bcl-1, P53 and ki-67 expression in the thyroid carcinoma and its clinical significance. J Modern Oncol. 2014;22(6):1294–1297. [Google Scholar]
- 54.Wang Y, Wang H, Zhu H. Expression and diagnosistic value of CK19, HBME-1 and MIB-1 between hyalinizing trabecular tumor and thyroid papillary carcinoma. Practical Oncol J. 2014;(4):326–330. [Google Scholar]
- 55.Zhou Y, Jiang H, Lu B, Chen Z, LuN Y, Li J. Expression and Clinical Significance of Ki67, CK19, Galectin-3 and HBME-1 in Papillary Thyroid Carcinoma Zhejiang. Practical Med. 2014;19(6):395–397. [Google Scholar]
- 56.Li T, Wu G, Cai D. Clinical significance of four immunohistochemical markers in the diagnosis of the papillary thyroid carcinoma. Shanghai Med Pharm J. 2014;35(10):24–27. [Google Scholar]
- 57.Xu Y, Li X, Yu J. The relationship between BRAF gene mutation and Ki67 protein expression in papillary thyroid carcinoma. J Nanchang University (Medical Sciences) 2014;54(10):12–14. [Google Scholar]
- 58.Wang L, Sun J, Hou H, Peng J. The expression and significance of C-erB-2 and Ki-67 in thyroid papillary carcinomas. J Chinese Physician. 2014;16(1):76–78. [Google Scholar]
- 59.Zhu C, Shuai J, Shao Q, Hu G. Discuss application of antibody marker MC combining with CK19, CK19, TPO, Galectin-3, KI-67 in thyroid papillary lesions. World Latest Med Info. 2015;(53):11–12. [Google Scholar]
- 60.Li G, Pu Y. Expression of CD147 and Ki-67 in papillary thyroid cacner and significance. J Modern Oncol. 2015;23(13):1818–1820. [Google Scholar]
- 61.Li Y, Zhang J. Clinical Significance of the ProtientExpression of MCM7,CDK2 and Ki-67 in Thyroid Cancer. Practical J Cancer. 2015;0(3):359–361. [Google Scholar]
- 62.Feng J, Wang J. Expression and clinical significance of Ki67 and calcitonin in medullary thyroid carcinoma. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2014;28(24):1921–1924. Chinese. [PubMed] [Google Scholar]
- 63.Cui W, Chen X, Liu C, Zhang W. Expressions of Gal-3, PTTG and Ki-67 in primany thyroid neoplasms and their value in differential diagnosis. Shaanxi Medical J. 2009;38(6):670–672. [Google Scholar]
- 64.Jin D, Li L, Liu X, Liang S, Xi F, Gao J. Expression of survivin and Ki-67 and their significance in thyroid tumors. Chinese J of Clin and Experimental Pathol. 2009;25:154–157. [Google Scholar]
- 65.Zhang T, Zhao C, Luo L, Zhao H, Cheng J, Xu F. The expression of Mcl-1 in human cervical cancer and its clinical significance. Med Oncol. 2012;29(3):1985–1991. doi: 10.1007/s12032-011-0005-y. [DOI] [PubMed] [Google Scholar]
- 66.Joseph MG, Shibani A, Panjwani N, et al. Usefulness of Ki-67, mitoses, and tumor size for predicting metastasis in carcinoid tumors of the lung: a study of 48 cases at a tertiary care centre in Canada. Lung Cancer Int. 2015;2015:545601. doi: 10.1155/2015/545601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ahmed S, Rashed H, Hegazy A, Mohamed AM, Elmesallamy W. Prognostic value of ALDH1, EZH2 and Ki-67 in astrocytic gliomas. Turk Patoloji Derg. 2016;32(2):70–81. doi: 10.5146/tjpath.2015.01344. [DOI] [PubMed] [Google Scholar]
- 68.Tian Y, Ma Z, Chen Z, et al. Clinicopathological and prognostic value of Ki-67 expression in bladder cancer: a systematic review and meta-analysis. PLoS One. 2016;11(7):e0158891. doi: 10.1371/journal.pone.0158891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Petrelli F, Viale G, Cabiddu M, Barni S. Prognostic value of different cut-off levels of Ki-67 in breast cancer: a systematic review and meta-analysis of 64,196 patients. Breast Cancer Res Treat. 2015;153(3):477–491. doi: 10.1007/s10549-015-3559-0. [DOI] [PubMed] [Google Scholar]
- 70.Luo Y, Zhang X, Mo M, et al. High Ki-67 immunohistochemical reactivity correlates with poor prognosis in bladder carcinoma: a comprehensive meta-analysis with 13,053 patients involved. Medicine (Baltimore) 2016;95(15):e3337. doi: 10.1097/MD.0000000000003337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Luo Y, Ren F, Liu Y, et al. Clinicopathological and prognostic significance of high Ki-67 labeling index in hepatocellular carcinoma patients: a meta-analysis. Int J Clin Exp Med. 2015;8(7):10235–10247. [PMC free article] [PubMed] [Google Scholar]
- 72.Pan D, Wei K, Ling Y, Su S, Zhu M, Chen G. The prognostic role of Ki-67/MIB-1 in cervical cancer: a systematic review with meta-analysis. Med Sci Monit. 2015;21:882–889. doi: 10.12659/MSM.892807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tian H, Qian GW, Li W, et al. A critical role of Sp1 transcription factor in regulating the human Ki-67 gene expression. Tumour Biol. 2011;32(2):273–283. doi: 10.1007/s13277-010-0119-4. [DOI] [PubMed] [Google Scholar]
- 74.Jin Q, Zhang W, Qiu XG, et al. Gene expression profiling reveals Ki-67 associated proliferation signature in human glioblastoma. Chin Med J (Engl) 2011;124(17):2584–2588. [PubMed] [Google Scholar]
- 75.Wu L, Timmers C, Maiti B, et al. The E2F1–3 transcription factors are essential for cellular proliferation. Nature. 2001;414(6862):457–462. doi: 10.1038/35106593. [DOI] [PubMed] [Google Scholar]
- 76.Spyratos F, Ferrero-Pous M, Trassard M, et al. Correlation between MIB-1 and other proliferation markers: clinical implications of the MIB-1 cutoff value. Cancer. 2002;94(8):2151–2159. doi: 10.1002/cncr.10458. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Subgroup analysis evaluating the diagnostic value of Ki-67/MIB-1 in thyroid cancer
| Groups | No of studies | Pooled sensitivity (95% CI) | Pooled specificity (95% CI) | DOR (95% CI) |
|---|---|---|---|---|
| Cut-off, % | ||||
| ≤5 | 12 | 0.70 (0.67–0.73) | 0.80 (0.77–0.83) | 7.49 (3.61–15.52) |
| 10 | 15 | 0.56 (0.53–0.59) | 0.67 (0.63–0.70) | 7.73 (2.68–22.30) |
| >10 | 8 | 0.78 (0.75–0.81) | 0.79 (0.74–0.84) | 15.40 (3.41–69.62) |
| Types | ||||
| PTC | 21 | 0.63 (0.60–0.65) | 0.74 (0.72–0.76) | 8.22 (4.08–16.56) |
| PTMC | 2 | 0.45 (0.37–0.52) | 0.86 (0.77–0.92) | 4.76 (2.48–9.17) |
Abbreviations: CI, confidence interval; DOR, diagnostic odds ratio; PTC, papillary thyroid carcinoma; PTMC, papillary thyroid microcarcinoma.
Table S2.
Subgroup analysis evaluating the prognostic value of Ki-67/MIB-1 for mortality in thyroid cancer
| Groups | Pooled HR | 95% CI | Heterogeneity test
|
Statistical method | |
|---|---|---|---|---|---|
| I2, % | P-value | ||||
| Univariate cut-off, % | |||||
| ≤5 | 1.98 | 0.65–6.06 | 68.80 | 0.007 | Random |
| >5 | 28.06 | 6.68–117.87 | 0.00 | 0.818 | Random |
| <10 | 2.73 | 0.89–8.38 | 72.20 | 0.001 | Random |
| Types | |||||
| PTC | 1.47 | 2.23–5.18 | 87.10 | <0.001 | Random |
| MTC | 0.96 | 0.24–2.16 | 65.50 | 0.035 | Random |
Abbreviations: CI, confidence interval; HR, hazard ratio; MTC, medullary thyroid carcinoma; PTC, papillary thyroid carcinoma.