Until recently, advanced stage melanoma was considered an untreatable disease. Hope has now become attached to small molecule inhibitors targeting the melanoma oncogene BRAF(V600E) and approaches to unleash the immune system against these tumors. Still, not every patient will be given respite from these treatments, and the ultimate goal of durable disease remission remains elusive. While it is incompletely understood what the functional barriers to achieving durable responses are, eventual relapse is likely a consequence of a variable degree of cancer heterogeneity that enables selective outgrowth of resistant tumor clones. However, identification of alternative molecular targets that could be used for combinatorial exploit holds promises to extend these initial responses, and may afford sustainable treatment.
In 2011, the BRAF-targeted inhibitor vemurafenib (PLX4032) was found to improve survival for patients with BRAF(V600E)-mutant melanomas [1]. Most, but not all, BRAF(V600E) melanomas will respond to BRAF-targeted drugs, and yet drug resistance will eventually curb patients’ long-term therapeutic benefit. Understanding the cellular mechanisms that preclude initial treatment efficacy (primary resistance) to oncogene-targeted drugs is expected to pave the way for rational combinatorial drug approaches. In addition to reducing the tumor burden, improving these initial responses could also enhance immune-mediated eradication of divergent tumor clones through broadly increasing cancer antigen presentation. Combinatorial approaches that favorably improve BRAF(V600E)-drug responses are consequently highly sought, which is illustrated by co-targeting the downstream target MEK enabling improved survival [2], or alternatively, identification of rational alternative targets unique to cancer cell proliferation.
A recent publication from Herlyn’s laboratory ties two important aspects of cancer cell proliferation together with the potential for combinatorial therapeutic exploit of BRAF(V600E) (Zhang et al., 2016 [3]). It has been known for decades that rapidly growing cancer cells are more responsive to chemotherapeutic agents, and if the tumor is not fully eliminated, the presence of slow-cycling drug-resistant cancer cells will, after some latency, be able to regenerate cancerous growth again [4]. In melanoma, a fraction of those slow cycling cells are positive for the histone methylase JARID1B that is required for tumor nucleation, and which predominantly use mitochondrial oxidative phosphorylation for ATP generation [5]. Seemingly counter-intuitive for cancer cell needs, utilization of mitochondrial oxidative phosphorylation enables highly efficient production of energy (ATP) from sparse nutrients, i.e. glucose. Nonetheless, to fuel rapid cell proliferation, the cellular metabolic demands shift towards enhanced glycolysis to adequately supply cellular building blocks, such as amino acids, lipids, and nucleotides. This glycolytic shift caused by proliferative needs, in turn, exert some degree of cytosolic NAD+ depletion, which can be regenerated from NADH during production of lactate – a fundamental mechanism termed the Warburg effect. It is therefore not surprising that slowly dividing cells have an altered balance of mitochondrial (oxidative phosphorylation) and cytoplasmic (glycolytic) metabolism when compared to rapidly dividing cells. The inherent nature of melanoma cells, however, employs a unique ability to balance metabolic needs through the melanocyte-lineage master transcription factor MITF and the co-activator for mitochondrial biogenesis, PGC1α [6,7]. Generally, mitochondrial biogenesis serves to improve oxidative phosphorylation by replenishing the organelle with new proteins, as well as regulating their numbers by controlling the rates of fusion and fission. A subset of melanoma cells, specifically defined by high expression of MITF and PGC1α, is intrinsically less glycolytic and displays heightened dependence on mitochondrial ATP production and resistance to oxidative stress [6]. Targeted drugs against the oncogenic BRAF-MEK signaling cascade lead to upregulation of the MITF-PGC1α transcription axis, which alleviates metabolic stress that originates from impeded oncogene function [7]. These data suggested that the degree of metabolic reprogramming in melanomas is a consequence of lineage-specific transcription that intersects with oncogenic BRAF function. Building on these observations, Zhang et al. set out to explore how mitochondrial biogenesis and bioenergetics drive resistance to BRAF-targeted agents in melanoma and also examine prospects for combinatorial therapeutic exploit [3].
In this recent paper, Zhang and colleagues present compelling data to indicate that melanoma cell lines defined by intrinsic low expression of a mitochondrial biogenesis signature exhibit greater apoptotic resistance to BRAF-targeted drugs [3]. Although resistant cells were found to have a lower mitochondrial potential, indicative of less utilization of oxidative phosphorylation; in response to BRAF-targeted inhibitors, however, the capacity to increase mitochondrial biogenesis blunted the therapeutic effects. Through assessing growth, cell death, respiration, and mitochondrial markers in cell lines, Zhang et al. designated treatment resistance with selection for a slow growing phenotype and increased mitochondrial biogenesis. Consistent with mitochondrial biogenesis as a means to circumvent BRAF-targeted inhibitors, analyses in patient biopsies revealed a general on-treatment increase in the expression of the nuclear encoded genes; mitochondrial transcription factor (TFAM), mitochondrial superoxide dismutase (SOD2), as well as an accumulation of mitochondrial DNA (mtDNA) content. Based on these findings, they went further to demonstrate that suppression of TFAM, or the mitochondrial protein chaperone TRAP1, augmented the functional effects of inhibiting BRAF(V600E) in melanoma cells. As proof-of-concept for rational combinatorial treatment with BRAF-inhibitors, administration of a mitochondria-targeted molecular chaperone inhibitor, gamitrinib, was found to effectively prevent metabolic shift towards mitochondrial oxidative phosphorylation in vitro, and also blunt tumor growth in vivo.
These new data on inhibiting some capacities of mitochondrial bioenergetics to prevent targeted BRAF-treatment resistance are certainly mechanistically sound. It remains, however, to be elucidated specifically how mitochondrial biogenesis confers resistance. To this end, is it improving bioenergetic functions, increasing detoxification through SOD2 seen also in patient tumors, or generally protecting mitochondria from organelle specific autophagy? Specifically, it is unclear if the mechanism of gamitrinib action is through inhibition of mitochondrial energetics, or targeting survival itself, such as provoking cell death through disabling the integrity of the mitochondrial transition pore. The use of specific electron transfer chain inhibitors and genetic approaches to target protein components would therefore be necessary to gain further mechanistic insights. Moreover, it is unclear to which extent acquired resistance (secondary) to targeted BRAF-inhibition, such as NRAS- and selection of MEK-mutations seen in patients affect mitochondrial energetics and utilization of alternative metabolic routes.
Finally, although the pharmacodynamics of the mitochondrial chaperone inhibitor - gamitrinib, may be favorable in tumor cells compared to normal tissue cells. It remains still to ascertain that reducing mitochondrial oxidative phosphorylation does not affect other tissues that would be detrimental to clinical implementation and long term efficacy, such as certain immune cells and neurons, associated with heightened reliance on mitochondrial oxidative phosphorylation. With this in mind and the burgeoning recognition that the effects of BRAF(V600E)-targeted drugs may involve priming of the immune system, hence, extrapolating these combinatorial treatment studies obtained using immune-compromised animals may not necessarily deliver sought clinical efficacy. While these data need verification in additional systems, this study suggests that mitochondrial biogenesis and/or energy production may prove useful for therapeutic exploit. This study consequently provides an important step towards understanding the principal mechanisms governing melanoma cells’ resistance to BRAF-targeted drugs, and does deliver a strike against their capacity to evade these treatments.
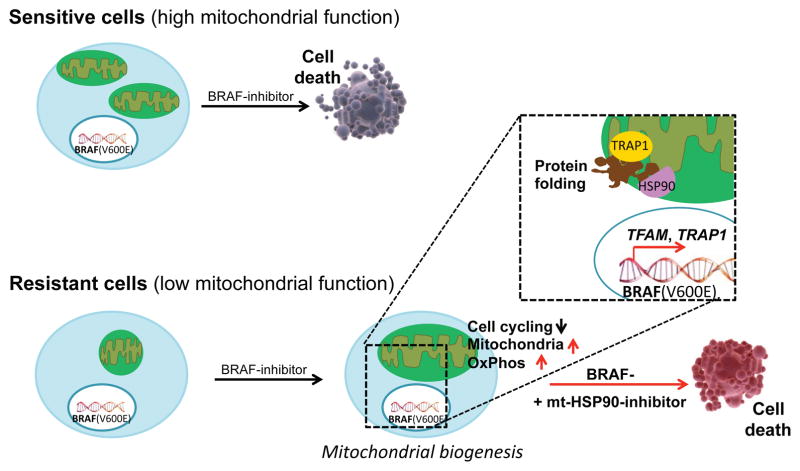
Figure 1. Suppression of mitochondrial biogenesis sensitizes melanoma cells to MAPK inhibition.
Whereas BRAF(V600E) melanoma cells with high basal level of mitochondrial biogenesis show an increased sensitivity to MAPK inhibition, those that harbor lower level of mitochondria are intrinsically resistant to MAPK blockage. Upon treatment with BRAF inhibitors, the resistant cells become slow-cycling. Concomitantly, slow-cycling resistant cells elevate their mitochondrial biogenesis as mediated by the nuclear-encoded TFAM and TRAP1, leading to the expansion of mitochondrial genome and enhanced mitochondrial protein folding. Consequently, suppression of mitochondrial biogenesis such as by abrogating the mitochondrial protein folding with the HSP90 inhibitor gamitrinib significantly reduces tumor bioenergetics, and also enhances the efficacy of BRAF-targeted inhibitors.
References
- 1.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, Hogg D, Lorigan P, Lebbe C, Jouary T, Schadendorf D, Ribas A, O’Day SJ, Sosman JA, Kirkwood JM, Eggermont AM, Dreno B, Nolop K, Li J, Nelson B, Hou J, Lee RJ, Flaherty KT, McArthur GA BRIM-3 Study Group. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Long GV, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, Larkin J, Garbe C, Jouary T, Hauschild A, Grob JJ, Chiarion Sileni V, Lebbe C, Mandalà M, Millward M, Arance A, Bondarenko I, Haanen JB, Hansson J, Utikal J, Ferraresi V, Kovalenko N, Mohr P, Probachai V, Schadendorf D, Nathan P, Robert C, Ribas A, DeMarini DJ, Irani JG, Casey M, Ouellet D, Martin AM, Le N, Patel K, Flaherty K. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med. 2014;371:1877–1888. doi: 10.1056/NEJMoa1406037. [DOI] [PubMed] [Google Scholar]
- 3.Zhang G, Frederick DT, Wu L, et al. Targeting mitochondrial biogenesis to overcome drug resistance to MAPK inhibitors. J Clin Invest. 2016 doi: 10.1172/JCI82661. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skipper HE. Improvement of the model systems. Cancer Res. 1969;29:2329–2333. [PubMed] [Google Scholar]
- 5.Roesch A, Vultur A, Bogeski I, Wang H, Zimmermann KM, Speicher D, Körbel C, Laschke MW, Gimotty PA, Philipp SE, Krause E, Pätzold S, Villanueva J, Krepler C, Fukunaga-Kalabis M, Hoth M, Bastian BC, Vogt T, Herlyn M. Overcoming intrinsic multidrug resistance in melanoma by blocking the mitochondrial respiratory chain of slow-cycling JARID1B(high) cells. Cancer Cell. 2013;23:811–825. doi: 10.1016/j.ccr.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vazquez F, Lim JH, Chim H, Bhalla K, Girnun G, Pierce K, Clish CB, Granter SR, Widlund HR, Spiegelman BM, Puigserver P. PGC1α expression defines a subset of human melanoma tumors with increased mitochondrial capacity and resistance to oxidative stress. Cancer Cell. 2013;23:287–301. doi: 10.1016/j.ccr.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haq R, Shoag J, Andreu-Perez P, Yokoyama S, Edelman H, Rowe GC, Frederick DT, Hurley AD, Nellore A, Kung AL, Wargo JA, Song JS, Fisher DE, Arany Z, Widlund HR. Oncogenic BRAF regulates oxidative metabolism via PGC1α and MITF. Cancer Cell. 2013;23:302–315. doi: 10.1016/j.ccr.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]