Abstract
Objective
To characterize early adoption of a novelmulti-target stool deoxyribonucleic acid (MTsDNA) screening test for colorectal cancer (CRC) and test the hypothesis that adoption differs by demographic characteristics, prior CRC screening behavior, and proceeds predictably over time.
Patients and Methods
We used the Rochester Epidemiology Project infrastructure to assess MTsDNA screening test use among adults aged 50–75 years, and identified 27,147 individuals eligible/due for screening colonoscopy from November 1, 2014 through November 30, 2015, and living in Olmsted County, Minnesota in2014. We used electronic Current Procedure Terminology and Health Care Common Procedure codes to evaluate early adoption of MTsDNA screening test in this population and to test whether early adoption varies by age, sex, race, and prior screening behavior.
Results
Overall, 2,193 (8.1%) and 974 (3.6%) of individuals were screened by colonoscopy and MT-sDNA, respectively. Age, sex, race, and prior screening were significantly and independently associated with MT-sDNA screening use compared to colonoscopy use after adjustment for all other variables. Rates of adoption of MTsDNA screening increased over time and were highest among those aged 50–54 years, females, whites, and had a prior history of screening. MT-sDNA screening use varied predictably by insurance coverage. Rates of colonoscopy decreased over time, while overall CRC screening rates remained steady.
Conclusion
Our results are generally consistent with predictions derived from prior research and Diffusion of Innovation framework, pointing to increasing use of the new screening test over time, and early adoption by younger patients, females, whites and those with prior CRC screening.
Colorectal cancer (CRC) is the second most frequent cause of cancer death in the United States (US).1–3 Colorectal screening can significantly reduce the incidence of CRC and substantially improve CRC survival rates.4,5,6 Several screening tests are available for the early detection of CRC, but nearly one-third of eligible US adults have never been screened.2 Commonly identified barriers to CRC screening include lack of physician recommendation, lack of awareness/knowledge, cost of the test and its sequelae, invasiveness of the test, and fear of the results.7–9 In addition, screening services are inconsistently delivered across practice settings,10,11 and continue to be underutilized overall and in certain ethnic minorities, age groups, and among persons with low socioeconomic status.12 The Healthy People 2020 goal is that 70.5% of adults aged 50–75 years would have CRC screening.13
A recently developed multi-target stool Deoxyribonucleic Acid (MT-sDNA) screening test for CRC (commercialized as Cologuard™), co-developed by Mayo Clinic and Exact Sciences (Madison, WI), holds significant promise for increasing population-adoption of CRC screening. In particular, the MT-sDNA screening test addresses several barriers to colorectal screening. Patient concerns with colonoscopy include the requirement to schedule a separate and lengthy clinic encounter, the need to undergo an arduous bowel preparation regimen, the exposure to sedation or anesthesia, and the discomfort associated with an invasive imaging process. By contrast, the MT-sDNA screening test is a noninvasive, multi-marker, stool-based CRC screening test that detects altered Deoxyribonucleic Acid (DNA), specifically mutant KRAS and methylated BMP3 and NDRG4, as well as a fecal immunochemical test (FIT) for blood released from cancer and precancerous lesions of the colon; the presence of fecal hemoglobin, even in the absence of elevated DNA markers, can lead to a positive result given the weighted nature of the MT-sDNA algorithm.14 Patients may collect and mail stool specimens from their homes with no cathartic bowel preparations and no dietary or medication restrictions.
Results of a multi-center, blinded case-control study demonstrated that the MT-sDNA screening test detects early-stage CRC and large adenomas with high levels of accuracy (92% sensitivity) throughout the colorectum.15 The MT-sDNA screening test outperformed fecal immunochemical tests in detecting cancers (93% versus [vs] 74%), advanced precancerous lesions (42% vs 24%), polyps with high-grade dysplasia (69% vs 46%), and serrated sessile polyps one-centimeter or greater (42% vs 5%). The MT-sDNA screening test was approved by the US Food and Drug Administration in 2014 for CRC screening and the US Preventive Services Task Force (USPSTF) recently issued its final CRC screening recommendations for 2016, assigning an overall A-grade to CRC screening for people ages 50–75 through the use of several screening exams that includes the MT-sDNA screening test.16 Therefore, the MT-sDNA screening test provides clinicians with a highly sensitive and specific screening test option.
The MT-sDNA screening test was introduced into Mayo Clinic’s practice in Rochester, Minnesota in October 2014, and the purpose of this study was to characterize early patient adoption of this novel CRC screening test in our local population. Drawing on hypotheses derived from the Diffusion of Innovation (DOI) framework and prior research in cancer screening, we assessed whether early adoption of the MT-sDNA screening test differs by demographic characteristics and by prior screening behavior. The DOI Framework17 is widely used to describe the adoption of health innovations in populations18–23 and has been applied to adoption of cancer screening tests.12,24 We examined adoption of the MT-sDNA screening test over time and assessed its impact on the use of colonoscopy, including second-tier testing. Based on prior research examining the adoption of CRC screening tests, greater use of the MT-sDNA screening test was expected among older age groups, and non-Hispanic whites.25 Although women generally exhibit higher rates of adoption of preventive services, this trend has not consistently been observed for CRC screening;26 therefore, we did not explicate any specific hypothesis for use of the MT-sDNA screening test by sex. Based on prior research demonstrating a general clustering of cancer screening behaviors, we hypothesized greater use of the MT-sDNA screening test among individuals who have routinely engaged in other CRC screening.27,28,29 We also explored trends in CRC screening by MT-sDNA screening, colonoscopy, and overall CRC screening among individuals eligible and due for colorectal screening from November 1, 2014 to November 30, 2015. Based on the tenants of the DOI, we expected to see an increase in the MT-sDNA screening test screening over time.
PATIENTS AND METHODS
Setting and Population
We used the Rochester Epidemiology Project (REP) research infrastructure to assess use of the MT-sDNA screening test among adults, ages 50–75 years, living in Olmsted County, Minnesota in 2014. The REP data linkage infrastructure captures virtually all healthcare in Olmsted County.30–33 Healthcare visit dates are linked to address information, and this information is used to define residency at any given point in time (REP Census). Population coverage for Olmsted County is nearly complete.32 We identified 42,577 individuals ages 50–75 years old residing in Olmsted County from November 1, 2014 to November 30, 2015 with authorization to use their medical records for research (96% of the eligible population) using the REP Census.34 The MT-sDNA screening test, although well-publicized throughout the community, was only available at Mayo Clinic during the course of our study while colonoscopy and other CRC screening tests were available and tracked at all the participating sites. The study procedures were approved by the Mayo Clinic and Olmsted Medical Center Institutional Review Boards.
The diagnostic indexes of the REP were searched electronically to extract International Classification of Diseases, Ninth Revision (ICD-9) current procedure terminology and healthcare common procedure codes. Using these codes, we excluded individuals who were at high risk for CRC, and who were not eligible for MT-sDNA screening. Exclusionary criteria included: previous CRC diagnosis or large polyps; screening before age 45 years or multiple screens before age 50 years; inflammatory bowel disease; familial adenomatous polyposis or Lynch Syndrome. Additionally, we excluded individuals who were already up-to-date with colon screening (colonoscopy screen within 10 years; computed tomography [CT] colonography within five years; sigmoidoscopy within five years with annual fecal occult blood test [FOBT]). Overall, 27,147 individuals were eligible and due for colorectal screening during the study period.
Identification of Colorectal Cancer Screening
Diagnostic indices of the REP were searched electronically to identify receipt of MT-sDNA, colonoscopy, CT colonography and sigmoidoscopy with annual FOBT. MT-sDNA testing and results were identified using laboratory codes (58030-ROCLIS-Cologuard). Screening colonoscopy, CT colonography and sigmoidoscopy was identified using current procedure terminology codes for screening or diagnostic tests with a modifier indicating an initial screen (Supplemental Table). Only 27 patients screened with CT colonography or flexible sigmoidoscopy with an annual FOBT/FIT during this timeframe, so use of these tests was not examined. Colonoscopy was the most frequently used screening test for colon cancer in our population and MT-sDNA screening was introduced into our clinical practice as an alternative to colonoscopy screening for average risk patients; therefore, we compared use of MT-sDNA to colonoscopy.
Analysis
The proportion of eligible individuals who were initially screened by MT-sDNA and colonoscopy during the study period were described and compared separately by age, sex, race and prior screening using chi-square tests. Prior cancer screening was defined as colonoscopy testing more than 10 years prior, CT colonography more than five years prior or sigmoidoscopy with an annual FOBT more than five years prior. Multivariable logistic regression models were used to assess factors that might be associated with choosing the MT-sDNA screening test vs colonoscopy, including age, sex, race and history of prior screening. Results are presented as odds ratios and 95% confidence intervals. Among patients who had MT-sDNA screening, we calculated the percentage with Mayo Clinic employee and dependent insurance, other private insurance, government insurance and no insurance.
The rate of MT-sDNA screening and colonoscopy screening per month, defined as the number screened each month divided by the eligible and due population, was plotted from November 1, 2014 through November 30, 2015. Generalized estimating equations with a Poisson distribution were used to test for temporal trends in the MT-sDNA and colonoscopy screening.
RESULTS
Table 1 summarizes CRC screening by colonoscopy and MT-sDNA screening by the sociodemographic characteristics of the eligible population who were due for CRC screening (n=27,147). The counts and percentages shown in Table 1 only include the first screening test within this timeframe. The overall percentage of the eligible and due population who were screened by colonoscopy was higher than the percentage screened by MT-sDNA (8.08% vs 3.59%, P=<.001).
Table 1.
Population characteristics and rate and odds of MT-sDNA screening (compared to colonoscopy) by age, sex, race, and prior CRC screening
| N, (%) | Colonoscopy N (%) |
MT-sDNA N (%) |
Odds of MT-sDNA OR (95% CI)2 |
|
|---|---|---|---|---|
| Total | N=27,147 | 2,193 (8.08) | 974 (3.59) | |
| Age | <.0011 | <.0011 | .0022 | |
| 50–54 | 7,294 (26.9) | 739 (10.13) | 346 (4.74) | Ref |
| 55–59 | 7,238 (26.7) | 387 (5.35) | 118 (1.63) | 0.60 (0.47, 0.79) |
| 60–64 | 5,514 (20.3) | 489 (8.87) | 225 (4.08) | 0.95 (0.77, 1.16) |
| 65–69 | 4,002 (14.7) | 331 (8.27) | 164 (4.1) | 0.99 (0.78, 1.24) |
| 70–75 | 3,099 (11.4) | 247 (7.97) | 121 (3.9) | 0.94 (0.73, 1.22) |
| Sex | .421 | <.0011 | <.0012 | |
| Males | 12,662 (46.6) | 1,041 (8.22) | 357 (2.82) | Ref |
| Females | 14,485 (53.4) | 1,152 (7.95) | 617 (4.26) | 1.56 (1.33, 1.82) |
| Race/Ethnicity | <.0011 | <.0011 | .032 | |
| White | 23,028 (84.8) | 1,929 (8.38) | 892 (3.87) | Ref |
| Black | 957 (3.5) | 60 (6.27) | 13 (1.47) | 0.43 (0.23, 0.82) |
| Asian | 1,063 (3.9) | 70 (6.59) | 27 (2.54) | 0.85 (0.54, 1.35) |
| Hispanic | 1,212 (4.5) | 82 (6.77) | 30 (2.48) | 0.80 (0.52, 1.23) |
| Other/Unknown | 887 (1.0) | 52 (5.86) | 12 (1.25) | 0.58 (0.31, 1.08) |
| Prior CRC Screening | .551 | <.0011 | <.0012 | |
| Yes | 321 (1.2) | 23 (7.17) | 53 (16.51) | Ref |
| No | 26,826 (98.8) | 2,170 (8.09) | 921 (3.43) | 0.17 (0.10, 0.28) |
Chi-square P-value comparing screening rates across categories of the given characteristic separately for colonoscopy and MT-sDNA
Odds ratio and 95% confidence interval from multiple logistic regression analysis. The dependent variable for this analysis was screening method and all characteristics listed in the table were included as explanatory variables. Odds ratios>1 indicate an increased likelihood of screening with MT- sDNA. P-value represents Type 3 analysis of the effect across categories of the given characteristic
Abbreviations: CI, Confidence Interval; CRC, Colorectal Cancer; MT-sDNA, Multi-Target Stool Deoxyribonucleic Acid; OR, Odds Ratio
Adoption of MT-sDNA screening varied significantly by age, sex, race/ethnicity and prior screening (Table 1). The highest rate of adoption of MT-sDNA screening was among those ages 50–54 years (4.74%) with somewhat lower rates of adoption observed among those ages 60–75 years and a significantly lower rate among those ages 55–59 (1.63%), all P<.001. Among males, 2.82% had a MT-sDNA screening test. The rate among females was significantly higher at 4.26%. The highest adoption rate for MT-sDNA screening by race/ethnicity was observed among whites (3.87%) and the lowest rate was observed among blacks (1.47%).
Prior screening status was not significantly associated with colonoscopy (P=.55). However, compared to those without prior CRC screening (3.43%), a significantly higher percentage of those who had prior CRC screening adopted the MT-sDNA screening test (16.51%; P<.001). Among patients who had MT-sDNA screening, 54.21% had Mayo Clinic insurance, 15.40% had other private insurance, 29.26% had government insurance, and 1.13% did not have insurance.
Table 1 also summarizes the results of a multivariable logistic regression analysis exploring whether any of the demographic characteristics or prior screening were independently associated with use of MT-sDNA screening among patients who were screened within our designated timeframe. Younger age, female sex, white race, and prior CRC screening remained significantly associated with the use of MT-sDNA screening compared to the use of colonoscopy after adjustment for all other variables. Specifically, those aged 55–59 years were significantly less likely (adjusted odds ratio [OR]=0.6; 95% confidence interval [CI], 0.5, 0.8) to use MT-sDNA screening than those ages 50–54 years. Females were more likely (adjusted OR=1.6; 95% CI, 1.3, 1.8) than males to use MT-sDNA screening compared to colonoscopy. Compared to whites, blacks were less likely (adjusted OR=0.4; 95% CI, 0.2, 0.8) to use the MT-sDNA screening compared to colonoscopy. Finally, compared to those who had a prior CRC screening test, those without a prior CRC screening were less likely (adjusted OR=0.2; 95% CI, 0.1, 0.3) to use the MT-sDNA compared to colonoscopy.
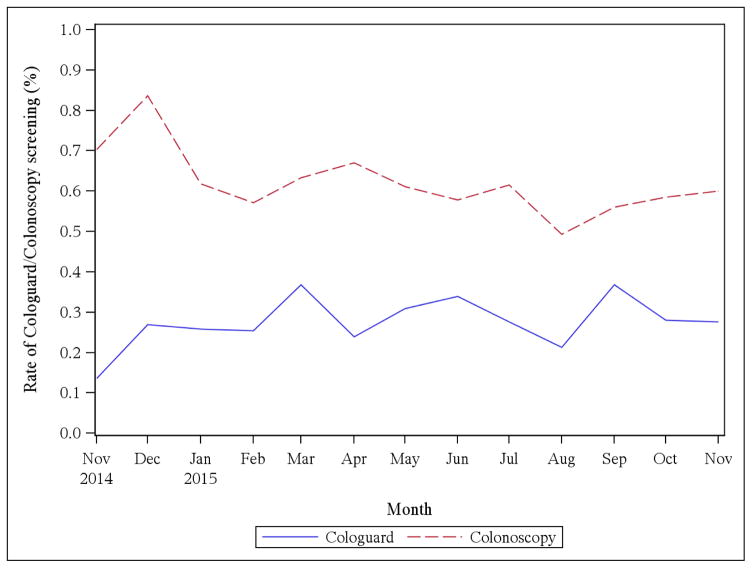
Figure 1 summarizes rates of MT-sDNA screening and colonoscopy screening per month during the study period revealing a significant increase in the rate of MT-sDNA screening (P=.01) and a significant decrease in colonoscopy (P<.001). Overall CRC screening rates observed over this 12-month period were flat with no significant increase or decrease observed. We also evaluated the rates of second-tier screening by colonoscopy following a positive MT-sDNA screening test and found that 80.8% of those who had a positive MT-sDNA screening test followed up within three months and 89.7% followed up with a colonoscopy by the end of February 2016.
Figure 1. Rate of MT-sDNA screening and Colonoscopy Screening Per Month.
There is a significant increase in the rate of MT-sDNA screening (P=.01) and a significant decrease in the rate of colonoscopy (P<.001).
Abbreviation: MT-sDNA, Multi-Target Stool Deoxyribonucleic Acid
DISCUSSION
We assessed whether early adoption of MT-sDNA screening differs by demographic characteristics and prior screening, and examined the adoption of MT-sDNA screening over time and assessed its impact on the use of colonoscopy, including second-tier testing. Our hypothesis that greater use of MT-sDNA screening would be observed among older age groups was not supported; rates of MT-sDNA screening adoption were highest among those ages 50–54 years. Given mixed results in prior research around sex differences in CRC screening, we did not make any specific hypothesis about differences in MT-sDNA screening adoption by sex. However, we did observe higher rates of adoption among females compared to males and this is consistent with prior research on use of other preventive services. Consistent with our prediction, non-Hispanic whites demonstrated the highest level of adoption of MT-sDNA screening compared to other racial and ethnic groups.
We hypothesized greater use of MT-sDNA screening among individuals who have routinely engaged in other CRC screening and found that rates of MT-sDNA screening use were higher among those who had prior CRC screening, which is consistent with previous research.27,28,29 Rates of CRC screening in Olmsted County are relatively high, with 81% of the population reporting a prior colonoscopy or sigmoidoscopy.35 The comparable rate for Minnesota is 68.5% and for the US is 61.3%.35 This may, in part, explain why the adoption of MT-sDNA screening most frequently occurred among those with prior screening. The population of Olmsted County, MN has socioeconomic characteristics similar to the upper Midwest.36 However, the population is more highly educated than the general US population (39% vs 28% with a bachelor’s degree or higher), and has a higher median household income ($64,000 vs $52,000 per year).37 The proportion of the population with health insurance is high, and is similar to the upper Midwest and the east coast of the US.38 These factors may explain the higher rates of screening this population. In addition, uptake rates of this new test may be higher and more rapid than in other parts of the country given the local media attention to the development of the test. However, studies such as these are necessary to understand how populations vary throughout the country and can serve as useful referent populations for understanding variability in health and health care, and can highlight important differences that can be targeted for interventions.39,40
Diffusion of Innovation Framework
The DOI process proceeds along an S-curve in a population with an initial small group adopting an innovation (innovators/early adopters), followed by greater adoption in a population, and then later in the process a small percentage of the population may adopt an innovation (late adopters/laggards).20,22 As cancer screening tests have been introduced into clinical practice, they have generally followed the signature DOI process.12
According to the DOI framework, adoption is driven by: the relative advantage of the innovation; compatibility with the values, experiences, and needs of potential adopters; perceived complexity of adopting the innovation; trialability, or ability to try or test an innovation; and the visibility or observability of the innovation within the population of potential adopters.20,22 The MT-sDNA screening enjoys several advantages over colonoscopy across these domains from a patient-experience perspective. Colonoscopy requires unpleasant bowel preparation, is time consuming, often requires patients to take time off of work, and is more costly than MT-sDNA screening. Thus, MT-sDNA screening is likely to be perceived as more compatible with patient preferences and less complex than colonoscopy. However, at this very early stage of diffusion, potential adopters and referring clinicians are likely to be more aware of colonoscopy as an option for CRC screening than MT-sDNA screening. In applications relevant to cancer screening, lack of awareness of recommendations for screening practice among patients and providers (resulting in lack of referral or recommendation) have been cited consistently as barriers to screening adoption, particularly during the early stages of diffusion.12
We observed an overall increase in the adoption of MT-sDNA screening over time, which is consistent with the hypotheses derived from the DOI framework.17 As the DOI framework would predict, initial adoption rates of MT-sDNA screening are low and lag behind that of previously diffused CRC screening technologies, in particular colonoscopy. Our results also suggest that certain groups (including men and blacks) may benefit from targeted campaigns to improve awareness of the screening test. The recent USPSTF A-grade recommendation for CRC screening by DNA-based stool sample tests coupled with increasing insurance coverage will likely boost overall awareness of the test availability.
Over this same time period, we observed a decrease in use of colonoscopy, and no change in overall CRC screening. This is consistent with our finding that the adoption of the MT-sDNA screening was much higher among those who had previously had a CRC screening. This suggests that the introduction of MT-sDNA screening into this patient population led to the use of MT-sDNA screening among persons who may have had a colonoscopy if the MT-sDNA screening had not been made available, rather than use of MT-sDNA screening among persons who would have otherwise chosen not to have CRC screening. Prior research has provided evidence that nearly all patients (97%) who refused colonoscopy accepted alternative, non-invasive stool-based or blood-based CRC screening options. Thus, the introduction of MT-sDNA screening as an alternative screening tool for CRC has potential to improve the overall CRC screening rates in the population if adopted by those previously resistant to the use of other screening modalities, such as colonoscopy. During the timeframe of our investigation, the MT-sDNA screening test was not covered by all insurance payers; however, it was covered for Medicare and Medicaid patients and for patients who were employees or dependents of employees at Mayo Clinic. Indeed, patterns of use reflected insurance coverage wherein those patients with insurance coverage engaged in MT-sDNA screening significantly more often than those who did not have coverage. With the recent USPSTF A recommendation coverage rates will very likely increase thereby increasing access to and adoption of the test.
Limitations
Use of existing clinical and laboratory data for our analysis limits the data available for evaluation of our hypotheses. In particular, we did not have access to patients’ income-level, education-level, and employment status – all known to predict early adoption of screening tests. Patient insurance data are only available for the Mayo Clinic practice sites so we were not able to include the insurance status in all of our analyses; however, we were able to examine the insurance status among those who had a MT-sDNA screening. Another limitation of our study, and an area of inquiry that merits further investigation, is that with the available data, we were unable to evaluate associations between healthcare provider credentials and practice site with ordering of the MT-sDNA screening.
CONCLUSIONS
Our results are generally consistent with predictions derived from prior research and the DOI framework. Looking forward, this framework predicts that we will see a significant rise in population adoption of this screening test followed by an eventual leveling out. The recent USPSTF endorsement, and increasing coverage by health insurance companies, will likely accelerate this process. For average-risk patients, the MT-sDNA screening offers an alternative screening test for CRC screening with potentially fewer barriers to use. Future research is encouraged to track continued dissemination of this test in the population, to assess its impact on use of traditional CRC screening, and to ascertain patients’ experiences relevant to this test.
Supplementary Material
Acknowledgments
Financial Support
This study was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676, and by the Mayo Clinic Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery, Rochester, MN. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- CRC
Colorectal Cancer
- CT
Computed Tomography
- DNA
Deoxyribonucleic Acid
- DOI
Diffusion of Innovation
- FOBT
Fecal Occult Blood Test
- FIT
Fecal Immunochemical Test
- MT-sDNA
Multi-Target Stool DNA
- REP
Rochester Epidemiology Project
- US
United States
- USPSTF
United States Preventive Services Task Force
Footnotes
Conflict of Interest Disclosure:
Dr. Kisiel reports other support (that could result in the sharing of potential future royalties) from Exact Sciences, outside the submitted work. Dr. Kisiel has a patent (61/784,429) pending. Dr. St. Sauver reports grants from the National Institute of Aging, during the conduct of the study. Dr. Tulledge-Scheitel reports other support in the form of shares of stock from Exact Science, outside the submitted work. All other author report no conflicts.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.U.S. Cancer Statistics Working Group. United States cancer statistics: 1999–2010 incidence and mortality web-based report. US Department of Health and Human Services, National Cancer Institute, Centers for Disease Control and Prevention; [Accessed January 15, 2016]. http://www.cdc.gov/uscs. [Google Scholar]
- 2.Klabunde CN, Joseph DA, King JB, White A, Plescia M. Vital Signs: Colorectal Cancer Screening Test Use - United States, 2012. Mmwr-Morbid Mortal W. 2013;62(44):881–888. [PMC free article] [PubMed] [Google Scholar]
- 3.American Cancer Society. Cancer Facts and Figures, 2016. Atlanta GA: American Cancer Society; 2016. [Google Scholar]
- 4.Agency for Healthcare Research and Quality. Guide to Clinical Preventive Services, 2010–2011: Recommendations of the U.S. Preventive Services Task Force. Rockville, MD: US Department of Health and Human Services; 2010. [PubMed] [Google Scholar]
- 5.American Cancer Society. Cancer Facts and Figures 2014. Atlanta, GA: American Cancer Society; 2014. [Google Scholar]
- 6.Zauber AG, Lansdorp-Vogelaar I, Knudsen AB, Wilschut J, van Ballegooijen M, Kuntz KM. Evaluating test strategies for colorectal cancer screening: a decision analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149(9):659–669. doi: 10.7326/0003-4819-149-9-200811040-00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vernon SW. Participation in colorectal cancer screening: a review. J Natl Cancer Inst. 1997;89(19):1406–1422. doi: 10.1093/jnci/89.19.1406. [DOI] [PubMed] [Google Scholar]
- 8.Cokkinides VE, Chao A, Smith RA, Vernon SW, Thun MJ. Correlates of underutilization of colorectal cancer screening among U.S. adults, age 50 years and older. Prev Med. 2003;36(1):85–91. doi: 10.1006/pmed.2002.1127. [DOI] [PubMed] [Google Scholar]
- 9.Zapka JG, Puleo E, Vickers-Lahti M, Luckmann R. Healthcare system factors and colorectal cancer screening. Am J Prev Med. 2002;23(1):28–35. doi: 10.1016/s0749-3797(02)00444-0. [DOI] [PubMed] [Google Scholar]
- 10.Grol R, Grimshaw J. From best evidence to best practice: effective implementation of change in patients’ care. Lancet. 2003;362(9391):1225–1230. doi: 10.1016/S0140-6736(03)14546-1. [DOI] [PubMed] [Google Scholar]
- 11.McIlfatrick S, Keeney S, McKenna H, McCarley N, McElwee G. Investigating the role of the general practitioner in cancer prevention: a mixed methods study. BMC Fam Pract. 2013:14. doi: 10.1186/1471-2296-14-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finney Rutten LJ, Nelson DE, Meissner HI. Examination of population-wide trends in barriers to cancer screening from a diffusion of innovation perspective (1987–2000) Prev Med. 2004;38(3):258–268. doi: 10.1016/j.ypmed.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 13.Office of Disease Prevention and Health Promotion. Healthy People 2020. U.S. Department of Health and HUman Services; [Accessed July 6, 2016]. https://www.healthypeople.gov/2020/data-search/Search-the-Data?nid=4054. [Google Scholar]
- 14.Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med. 2014;370(14):1287–1297. doi: 10.1056/NEJMoa1311194. [DOI] [PubMed] [Google Scholar]
- 15.Ahlquist DA, Zou H, Domanico M, et al. Next-generation stool DNA test accurately detects colorectal cancer and large adenomas. Gastroenterology. 2012;142(2):248–256. doi: 10.1053/j.gastro.2011.10.031. quiz e225–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin JS, Piper MA, Perdue LA, et al. Screening for Colorectal Cancer: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2016;315(23):2576–2594. doi: 10.1001/jama.2016.3332. [DOI] [PubMed] [Google Scholar]
- 17.Rogers EM. Diffusion of innovations. 4. New York: Free Press; 1995. [Google Scholar]
- 18.Carboneau C. Using diffusion of innovations and academic detailing to spread evidence-based practices. J Healthc Qual. 2005;27(2):48–52. doi: 10.1111/j.1945-1474.2005.tb01117.x. [DOI] [PubMed] [Google Scholar]
- 19.Greenhalgh T, Robert G, Macfarlane F, Bate P, Kyriakidou O. Diffusion of innovations in service organizations: systematic review and recommendations. Milbank Q. 2004;82(4):581–629. doi: 10.1111/j.0887-378X.2004.00325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rogers EM. Diffusion of preventive innovations. Addict Behav. 2002;27(6):989–993. doi: 10.1016/s0306-4603(02)00300-3. [DOI] [PubMed] [Google Scholar]
- 21.Denis JL, Hebert Y, Langley A, Lozeau D, Trottier LH. Explaining diffusion patterns for complex health care innovations. Health Care Manage Rev. 2002;27(3):60–73. doi: 10.1097/00004010-200207000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Rogers EM. Lessons for guidelines from the diffusion of innovations. Jt Comm J Qual Improv. 1995;21(7):324–328. doi: 10.1016/s1070-3241(16)30155-9. [DOI] [PubMed] [Google Scholar]
- 23.Sondik EJ. Discussion of diffusion of medical innovations. Prog Clin Biol Res. 1989:29335–39. [PubMed] [Google Scholar]
- 24.Hahm MI, Park EC, Choi KS, Lee HY, Park JH, Park S. Inequalities in adoption of cancer screening from a diffusion of innovation perspective: identification of late adopters. Cancer Epidemiol. 2011;35(1):90–96. doi: 10.1016/j.canep.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 25.Courtney RJ, Paul CL, Sanson-Fisher RW, et al. Individual- and provider-level factors associated with colorectal cancer screening in accordance with guideline recommendation: a community-level perspective across varying levels of risk. BMC Public Health. 2013:13. doi: 10.1186/1471-2458-13-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McQueen A, Vernon SW, Meissner HI, Klabunde CN, Rakowski W. Are there gender differences in colorectal cancer test use prevalence and correlates? Cancer Epidem Biomar. 2006;15(4):782–791. doi: 10.1158/1055-9965.EPI-05-0629. [DOI] [PubMed] [Google Scholar]
- 27.Carlos RC, Fendrick AM, Patterson SK, Bernstein SJ. Associations in breast and colon cancer screening behavior in women. Acad Radiol. 2005;12(4):451–458. doi: 10.1016/j.acra.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 28.Guerrero-Preston R, Chan C, Vlahov D, Mitchell MK, Johnson SB, Freeman H. Previous cancer screening behavior as predictor of endoscopic colon cancer screening among women aged 50 and over, in NYC 2002. J Community Health. 2008;33(1):10–21. doi: 10.1007/s10900-007-9067-3. [DOI] [PubMed] [Google Scholar]
- 29.Shapiro JA, Seeff LC, Nadel MR. Colorectal cancer-screening tests and associated health behaviors. Am J Prev Med. 2001;21(2):132–137. doi: 10.1016/s0749-3797(01)00329-4. [DOI] [PubMed] [Google Scholar]
- 30.Kurland LT, Molgaard CA. The patient record in epidemiology. Sci Am. 1981;245(4):54–63. doi: 10.1038/scientificamerican1081-54. [DOI] [PubMed] [Google Scholar]
- 31.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71(3):266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 32.St Sauver JL, Grossardt BR, Yawn BP, Meton LJ, Rocca WA. Use of a medical records-linkage system to enumerate a dynamic population over time: The Rochester Epidemiology Project. Am J Epidemiol. 2011;173(9):1059–1068. doi: 10.1093/aje/kwq482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ., 3rd History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87(12):1202–1213. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacobsen SJ, Jacobson DJ, McGree ME, et al. Sixteen-year longitudinal changes in serum prostate-specific antigen levels: the olmsted county study. 2012;87(1):34–40. doi: 10.1016/j.mayocp.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.National Center for Chronic Disease Prevention and Health Promotion. Behavioral Risk Factor Surveillance System. U.S. Department of Health & Human Services; [Accessed July 6, 2016]. http://www.cdc.gov/brfss/ [Google Scholar]
- 36.St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ, 3rd, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. 2012;87(2):151–160. doi: 10.1016/j.mayocp.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rochester Epidemiology Project. REP Population Overview. Rochester Epidemiology Project; [Accessed October 17, 2016]. http://rochesterproject.org/for-researchers/population-overview/ [Google Scholar]
- 38.United States Census Bureau. Data Profiles: 2010–2014 ACS 5-Year Data Profiles. U.S. Department of Commerce. American Community Survey; [Accessed October 17, 2016]. http://www.census.gov/acs/www/data/data-tables-and-tools/data-profiles/2014/ [Google Scholar]
- 39.Richiardi L, Pizzi C, Pearce N. Commentary: Representativeness is usually not necessary and often should be avoided. 2013;42(4):1018–1022. doi: 10.1093/ije/dyt103. [DOI] [PubMed] [Google Scholar]
- 40.Rothman KJ, Gallacher JE, Hatch EE. Why representativeness should be avoided. 2013;42(4):1012–1014. doi: 10.1093/ije/dys223. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.