Abstract
Yersinia enterocolitica is a facultative intracellular pathogen and a causative agent of yersiniosis, which can be contracted by ingestion of contaminated food. Yersinia secretes virulence factors to subvert critical pathways in the host cell. In this study we utilized shotgun label-free proteomics to study differential protein expression in epithelial cells infected with Yersinia enterocolitica. We identified a total of 551 proteins, amongst which 42 were downregulated (e.g. Prostaglandin E Synthase 3, POH-1 and Karyopherin alpha) and 22 were upregulated (e.g. Rab1c and RhoA) in infected cells. We validated some of these results by western blot analysis of proteins extracted from Caco-2 and HeLa cells. The proteomic dataset was used to identify host canonical pathways and molecular functions modulated by this infection in the host cells. This study constitutes a proteome of Yersinia-infected cells and can support new discoveries in the area of host-pathogen interactions.
Keywords: label-free proteomics, Yersinia enterocolitica infection, pathway modeling, integrin signaling, protein ubiquitination
3. Introduction
Yersinia enterocolitica, Y. pseudotuberculosis and Y. pestis are pathogenic bacteria, which are of concern due to their biomedical significance. While Y. pseudotuberculosis and Y. pestis are close from the evolutionary standpoint, Y. enterocolitica has a more distant phylogenetic relationship with the other two species [1]. Yersinia pathogens are facultative intracellular pathogens, which translocate their virulence factors, which modify the eukaryotic proteome to interfere with the anti-microbial responses [2], but at the same time the host cell also modifies expression of its proteins to defy the bacterial infection. Proteomics has been utilized to understand the molecular basis of pathogenesis in several bacterial infections, including Listeria monocytogenes [3–5], Salmonella enterica Typhimurium [6, 7], and Mycobacterium tuberculosis [8]. Proteomics has also been a useful tool in characterization of proteins encoded by Yersiniae [9–15]. Furthermore, systems biology-integrated analysis of bacterial proteome, genome and metabolome was performed on Y. pestis and Y. pseudotuberculosis [16]. Finally, two-dimensional gel electrophoresis combined with protein identification was used to identify differentially expressed proteins in human monocytes infected with Y. pestis and Y. pseudotuberculosis, but only 29 proteins have been identified by using this approach [17]. However, label-free proteomics has never been utilized to analyze differential protein expression in cells infected with Y. enterocolitica.
The purpose of our study was to characterize the epithelial cell response to Y. enterocolitica infection by label-free proteomics. The tested non-ionic detergents used for protein extraction, n-dodecyl-beta-D-maltoside and NP-40, gave comparable results in uninfected cells although n-dodecyl-beta-D-maltoside led to a slightly higher number of protein identification reproduced in two biological replicates. Next, we analyzed the proteome of Y. enterocolitica-HeLa cells and compared it to the proteome of uninfected cells. Analysis of the canonical pathways and molecular functions was also performed. The differential expression of selected proteins was confirmed by western blot analysis of proteins extracted from infected HeLa cells, but also from colonic epithelial Caco-2 cells infected with wild-type and virulence plasmid-cured Y. enterocolitica mutant. This is the first report of proteomics analysis of host protein networks altered in response to Y. enterocolitica infection, and it can enable further investigation of the host-pathogen interactions.
4. Materials and Methods
4.1. Overall experimental design
Two different proteomic experiments were performed. First, to test protein extraction methods two biological replicates were used. Second, for analysis of differentially expressed proteins in infected cells three biological replicates were used. Statistical testing was done by using Fisher’s exact test. Independent and complementary validation experiments (western blot analysis) were also performed.
4.2. Culture conditions
Yersinia enterocolitica 8081 wild-type (pYV) and virulence plasmid-cured mutant (8081c) [18] were grown in tryptic soy broth (TSB) overnight at 27°C with aeration. HeLa cells were maintained in Dulbecco’s modified Eagle medium (DMEM) supplemented with 1% penicillin/streptomycin and 10% fetal bovine serum (FBS) and incubated at 37°C under 5% CO2 environment. Caco-2 cells were also maintained similarly, but medium was supplemented with 20% FBS.
4.3. Infection conditions
The overnight culture of wild-type Y. enterocolitica or 8081c mutant grown aerobically in TSB at 27°C was diluted to OD600 of 0.05 and incubated at 27°C. At OD600 of 0.25 the temperature was changed to 37°C. Bacteria were grown until OD600 reached 0.5 (~1.5 hour), centrifuged at 5,000 × g for 10 minutes, washed with phosphate buffered saline (PBS) and resuspended in cell culture medium. One hour before infection, the fresh DMEM medium (Gibco, USA) lacking antibiotics and FBS was added to HeLa or Caco-2 cells. Cells were infected at multiplicity of infection (MOI) of 20:1. One hour post-infection cells were washed with PBS. DMEM medium containing gentamicin (50 μg/ml) was added to eliminate extracellular bacteria, cells were incubated for three hours, and collected by scraping in cold PBS.
4.4. Gentamicin survival assay
2.5×105 HeLa cells per well were plated on a 6-well plate and incubated overnight. On the next day, cells were washed with warmed PBS and counted. 2 mL of DMEM medium with no antibiotics or FBS was added to HeLa cells and the cells were incubated for an hour, followed by infection with Yersinia as described above (MOI of 20:1). One hour post-infection the cells were washed with PBS, and DMEM medium containing gentamicin (50 μg/ml) was added to eliminate extracellular bacteria. Cells were incubated for three hours, after which they were washed three times with warm PBS. Next, cells were counted by detaching them from the cell culture dish by using trypsin, staining with trypan blue and counting by using an automatic TC20 cell counter (Bio-Rad, USA) to establish the total number of HeLa cells. Alternatively, cells well (three biological replicates were used) were lysed as follows: sterile 500 μL Triton-X (0.1% in PBS) was added to each and incubated for 5 minutes, after which PBS was added to bring the final volume to 1 mL. Each well was thoroughly rinsed with the lysis buffer and pipetted into a sterile microcentrifuge tube. Biological triplicates of samples were diluted in LB medium, dilutions of 10−3, 10−4, and 10−5 were plated on LB agar (100 μ//each), incubated at 27 °C for two days, and counted to establish the colony forming units (CFUs).
4.5. Cell lysis and western blot analysis
The cells were lysed in two different buffers, n-dodecyl-beta-D-maltoside lysis buffer (150 mM NaCl, 20 mM MgCl2, 50 mM Tris-HCl pH 7.4, 0.5% n-dodecyl-beta-D-maltoside), and NP-40 lysis buffer (150 mM NaCl, 20 mM MgCl2, 50 mM Tris-HCl pH 7.4, 0.5% NP-40). Both buffers were supplemented with 1 mM serine protease inhibitor phenylmethylsulfonyl fluoride (PMSF) and lysis was carried out for 30 minutes on ice. The cell debris was removed by centrifugation at 21,000 × g, at 4°C for 10 minutes. The protein concentration was measured and the crude protein extract was subjected to trypsin-based digestion and proteomics, or western blot analysis. Crude protein extracts containing the same amount of protein were resolved by 4–12% polyacrylamide gel electrophoresis (PAGE) and then transferred by western blotting to a polyvinylidene difluoride (PVDF) membrane. The proteins of interest were detected using primary antibodies against RhoA (1:2,000 dilution, Cell Signaling Technology, USA), Rab1c (1:500 dilution, Santa Cruz Biotechnology, USA), Prostaglandin E Synthase 3 (1:1,000 dilution, Santa Cruz Biotechnology, USA), and POH-1 (1:125 dilution, Life Technologies Corporation, USA) and following secondary conjugated antibodies: goat anti-rabbit-HRP (1:7,000 dilution, Santa Cruz Biotechnology, USA) and goat anti-mouse-HRP (1:6,000 dilution, Santa Cruz Biotechnology, USA), and visualized using enhanced chemiluminiscent substrate Luminata Forte Western HRP Substrate (Milipore). Anti-beta-actin antibody (Sigma-Aldrich, USA) was used as a loading control. All antibodies were diluted with 1% milk in Tris buffered saline (TBS) containing 0.1% Tween-20.
4.6. Mass Spectrometry
Proteins (100 μg per sample) were precipitated via chloroform/methanol extraction exactly as we described previously [19]. Protein pellets were suspended in 100 μl of 100 mM Tris-HCl pH containing 6 M urea. Samples were reduced with 5 μl 200 mM dithiothreitol (DTT) for 45 minutes at room temperature, followed by alkylation with 20 μl 200 mM iodoacetamide (IAA) for 45 minutes at room temperature, and addition of 20 μl 200 mM DTT for 45 minutes to quench the remaining IAA. The urea concentration was then reduced by adding 775 μl of milliQ-H2O. Finally, proteins were digested with trypsin (Sequencing Grade Modified Trypsin, Promega) at 1:50 ratio for 18 hours at 37°C. The reaction was stopped by adjusting pH of solution to <6 by addition of acetic acid. The samples were then purified by using C18 SepPak columns (Waters, USA). The peptide samples were dissolved in 98% milliQ-H2O, 2% acetonitrile, 0.1% formic acid. An equivalent of 1 μg of protein content was used for mass spectrometric analysis by in-line HPLC (Dionex, USA) and a linear trap (LTQ Velos) mass spectrometer by using conditions we previously described [20].
4.7. Data analysis
Tandem mass spectra were extracted by Proteome Discoverer (Thermo Scientific, USA), version 1.4. 0.288. Charge state deconvolution and deisotoping were performed and analysis was done by using Sequest (Thermo Fisher Scientific, USA; ver. 1.4.0.288) and X! Tandem (ver. CYCLONE 2010.12.01.1). The databank used was Uniprot human reference proteome (68,949 entries) assuming trypsin as a digestive enzyme. Sequest and X!Tandem were searched with a fragment ion mass tolerance of 0.50 Da and a parent ion tolerance of 2.0 Da (error mass distribution in ppm is shown in the Supplementary Figure 1), which are typical tolerances used when a linear ion trap mass spectrometer is utilized [21]. Oxidation (methionine) and carbamidomethylation (cysteine) were specified in Sequest and X! Tandem as variable modifications. Glu->pyro-Glu (n-terminus), ammonia-loss (n-terminus), gln->pyro-Glu (n-terminus) were also specified in X! Tandem as variable modifications. Scaffold (ver. 4.4.1; Proteome Software Inc., USA) was used to validate MS/MS-based peptide and protein identifications. Peptide identifications achieved False Discovery Rate (FDR) <0.35% by the Scaffold Local FDR algorithm, and protein identifications were accepted at >95.0% probability (assigned by the Protein Prophet algorithm [22]) with a minimum 1 peptide present in a protein. Proteins that could not be differentiated were grouped to satisfy the principles of parsimony. Similar databank search by using Yersinia proteome database (NCBI) was performed but no protein entries were found. Although the bacteria itself were most likely not lysed by using the used protein extraction buffer, there was a possibility that the bacterial proteins were secreted inside the host cell by the use of Yersinia’s secretory system, yet most likely due to their lower abundance no Yersinia-derived proteins were identified.
The spectral counting label-free quantitation was performed in Scaffold (Proteome Software, USA), in which we used the sum of weighted spectra associated with a protein (where the weight is a measure of uniqueness of a given spectrum in terms of its presence in other proteins, this method is briefly described here [23]). This analysis was performed only on proteins, which were identified in minimum two replicates (with minimum 4 exclusive spectra per protein in total), and which passed the Fisher’s Exact Test with a p-value equal or lower than 0.05. Normalization was applied to calculate the fold ratio and a minimum value of 0.2 was used for the samples, in which a protein was not identified. The minimum fold difference was 1.5 for both up- and down-regulated proteins (see Supplementary Table 1 for more information).
4.8. Gene ontology and network analysis
The Database for Annotation, Visualization and Integrated Discovery (DAVID) resource (ver. 6.7) was used for functional annotation. GO terms with a p value<0.005 were taken into consideration, with FDR <5.0% and minimum 10 genes in each category. Statistical significance was determined by EASE Score Threshold (Maximum Probability), which is a modified Fisher Exact p-value along with the FDR correction [24]. The number of molecules was plotted for each GO term in Excel.
The datasets were compared in Blast2Go (ver 3.0.10) [25] by using Fisher’s Exact Test with Multiple Testing Correction of FDR (Benjamini and Hochberg) to establish whether GO terms are enriched in a test group when compared to a reference group while testing effect of a detergent on the subcellular localization of identified proteins. The network analysis was performed by Ingenuity Analysis Pathway (ver. 21901358) software to evaluate the Canonical Pathways, Molecular Functions and Networks; Benjamini and Hochberg multiple testing correction was used. The top two networks were merged, and EIF2 signaling and integrin signaling were overlaid with this network on the basis of the high coverage of identified proteins within the associated canonical pathways (5 for integrin pathway; 9 for EIF2 pathway).
5. Results and discussion
5.1. Proteomic analysis of subcellular localization of proteins obtained by lysis with non-ionic detergents
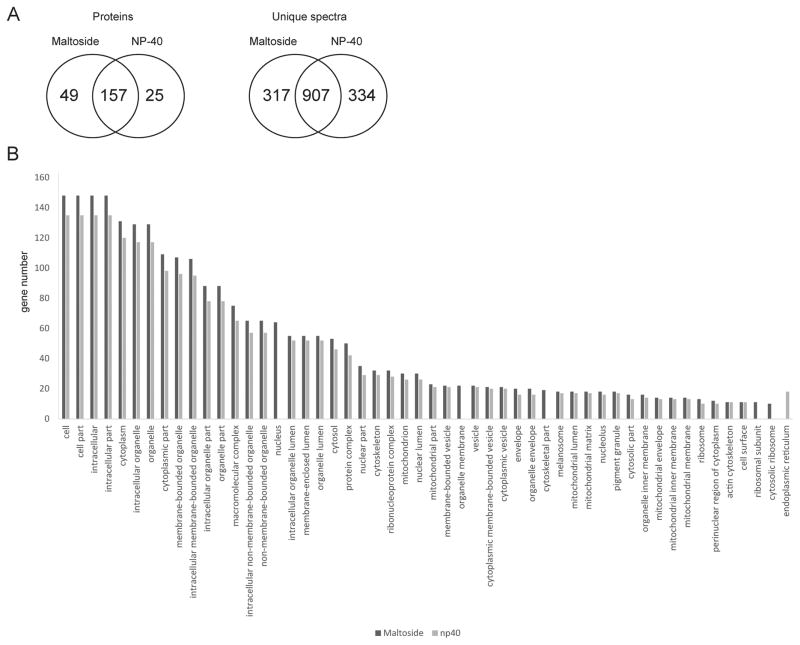
Although there are many detergents available for cell lysis, non-ionic detergents are claimed to be more compatible with the high performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS; [26]). In this study, we lysed HeLa cells with two non-ionic detergents, which were compared to each other, n-dodecyl-beta-D-maltoside and NP-40. The number of the identified proteins and subcellular localizations of the identified proteins yielded by each lysis buffer was evaluated. The n-dodecyl-beta-D-maltoside led to identification of slightly higher number of proteins in comparison to NP-40 (Fig. 1A). DAVID [24] and Blast2GO [25] tools were used for GO term (subcellular localization) analysis of the identified proteins (Fig. 1B). Fisher’s Exact Test established that there were no statistically significant differences in GO terms related to subcellular localization of the proteins in one dataset versus another.
Figure 1. Comparison of proteomics results obtained from HeLa cells lysed by using two different buffers.
(A). The Venn diagrams illustrate the number of proteins identified in at least two of the analyzed biological replicates, and unique spectra detected in each sample type. (B). GO term analysis of subcellular localizations of unique proteins. Proteins identified in each sample type were subjected to the GO term analysis by using The Database for Annotation, Visualization and Integrated Discovery (DAVID).
5.2. Analysis of differentially expressed proteins in epithelial HeLa and Caco-2 cells infected with Yersinia enterocolitica
To quantify the changes in host protein expression during infection with Y. enterocolitica, we infected epithelial HeLa cells for four hours. We tested whether the used infection conditions yield satisfactory number of infected cells by using a gentamicin survival assay. The percentage of infected HeLa cells after 4 hours of infection period was 69.5 % +/− 8.8%. Label-free quantitative proteomic analysis by HPLC-MS-MS/MS led to identification of 551 proteins (FDR<0.35%), amongst which 22 were significantly upregulated (e.g. Rab1c, SUMO-3, and RhoA), and 42 were downregulated (e.g. Karyopherin, POH-1, and Prostaglandin E synthase) in infected cells (Fig. 2A). A subset of these proteins was independently validated by western blot analysis (Fig. 2B). Also, Caco-2 colonic epithelial cells, a frequently used cell line to study host-pathogen interactions in Yersinia infection [27, 28], were used to further confirm these results (Fig. 2C). Caco-2 cells were infected with wild-type Y. enterocolitica or Y. enterocolitica 8081c mutant lacking the virulence plasmid. Upregulation of RhoA and downregulation of POH-1 and Prostaglandin E synthase 3 were observed in Caco-2 cells infected with wild-type Yersinia (Fig. 2C), but protein levels of Prostaglandin E synthase 3 and POH-1 were not affected in cells infected with the 8081c mutant. It could either be caused by an attenuated infection or by one of the virulence factors, which needs to be tested further. Possible functions of some of the identified proteins in regulation of Yersinia infection are discussed below.
Figure 2. Differential protein expression in HeLa cells infected or not infected with Yersinia enterocolitica.
(A). HeLa cells were infected with Y. enterocolitica and analyzed by label-free quantitative proteomics. Significantly regulated proteins are listed, along with their fold change (infected/control), identification number, symbol, Entrez gene name, localization (suggested by Ingenuity Pathway Analysis software) and protein function. (B)–(C) HeLa cells were infected with Y. enterocolitica wild-type (B) and Caco-2 cells were infected with wild-type or plasmid-cured mutant (C) for 4 hours. The extracted proteins were analyzed by SDS-PAGE and western blotting. Anti-beta-actin antibody was used as a loading control.
Rab1c is a small GTPase with unknown functions, and a significant homologue of Rab1b (Rab1) responsible for maintaining transport between endoplasmic reticulum and Golgi complex [29]. Rab1 is hijacked by Legionella pneumophila’s secreted proteins (SidM, SidD, and LepB), which leads to inactivation of Rab1 [30]. In Salmonella infection, knockdown of Rab1 causes attenuated replication of this bacterium in HeLa cells due to the interference with the autophagic process [31]. To date, no specific function of Rab1 in Yersinia infection has been proposed, but up-regulation of Rab1 could potentially lead to increased autophagy of Yersinia [32].
RhoA is another small GTPase, which controls the actin cytoskeleton assembly and is crucial in regulation of phagocytosis, or integrity of the epithelial cell monolayer. RhoA is known to regulate the host cell response to Yersinia infection, and there are several Yersinia virulence factors that bind and alter the function or activity state of this protein [33]. Moreover, during infection with Y. enterocolitica RhoA accumulates on membranes of HeLa cells, which corroborates our results [34].
SUMO-3, SUMO-1 and SUMO-2 are ubiquitin-like proteins that form a covalent post-translational modification on proteins [35]. SUMO-3 and SUMO-2 are often referred to as SUMO-2/3 due to their similarity. SUMO molecules modify the components of the NF-kappaB pathway, and together with ubiquitin they lead to the TNF-alpha-mediated activation of NF-kappaB [36]. It has been recently shown that that during infection with another gram-negative bacterium, Salmonella Typhimurium, the overall SUMOylome was significantly reduced at early stages of infection [37]. Moreover, sumoylation is required for restriction of epithelial invasion and pro-inflammatory transcriptional response in Shigella flexneri infection [38]. It was initially reported that Yersinia’s virulence factor YopJ inhibits conjugation of SUMO-1 to host proteins [39], but it was subsequently shown that this protein rather functions as an acetyltransferase [40, 41], therefore its desumoylating function was questioned. However, it cannot be completely ruled out that YopJ directly or indirectly leads to removal of SUMO modification from protein substrates during infection [39].
Prostaglandin E Synthase 3 (p23) is a component of the cyclooxygenase (COX)-1 pathway of prostaglandin E2 (PGE2) biosynthesis [42]. As such, it is involved in a variety of processes, which include regulation of immune responses [43]. Documentation of downregulation of this synthase in epithelial cells infected with Y. enterocolitica could point towards novel function of eicosanoids in the host-pathogen interactions.
Karyopherin alpha belongs to the family of karyopherins (importins), which are adaptor proteins that recognize the nuclear localization signals critical in the protein export to the nucleus [44]. Karyopherin alpha is involved in the NF-kappaB p50/p65 heterodimer translocation upon TNF-alpha stimulation [45]. Together with Importin beta 1, Karyopherin alpha activates a large number of genes encoding pro-inflammatory cytokines and chemokines, and other proteins critical in host responses to infection [46]. Down-regulation of karyopherin alpha in response to Yersinia infection is interesting, as Yersinia evades immune response via inhibition of key inflammatory regulators by its virulence factors [47], some of which might be also regulated by karyopherin alpha [46]
Finally, POH-1 (RPN11, PSMD14) is a Zn2+-dependent deubiquitinating enzyme important in proteolysis as a component of the proteasome [48]. POH-1 is most likely involved in cleavage of the ubiquitin chains from protein substrates prior to their processing by the proteasome [49]. Although POH-1’s precise function in Yersinia infection is cryptic, proteasome-dependent degradation of certain virulence proteins has been described, e.g. YopE [50].
5.3. Network analysis
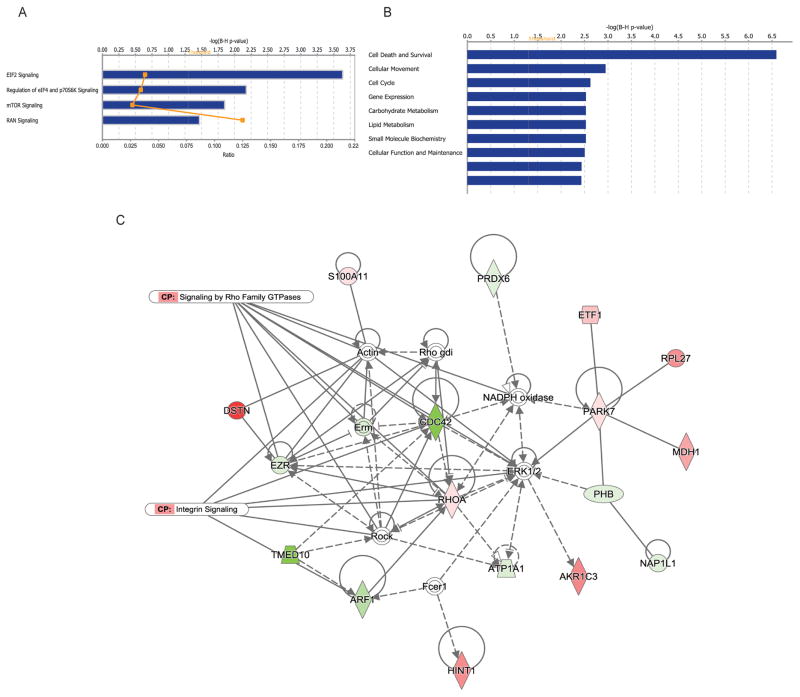
The canonical pathways altered in infected cells were EIF2, RAN, regulation of eIF4/p70S6K signaling, and mTOR signaling pathways (Fig. 3A). EIF2 pathway regulates translation initiation in response to stress. This pathway is important in the functions of bacterial virulence factors, including Yersinia protein kinase A (YpkA) and Yersinia outer protein J (YopJ) [51] as described below. Specifically, EIF2 signaling is disrupted by Yersinia virulence factor YopJ, which thereby alters the expression of pro-inflammatory cytokines and bacterial invasion [52]. Another pathway significantly altered in infected cells was RAN (Ran) pathway. Ran is a small GTPase predominantly localized to nucleus and it has important functions in nucleo-cytoplasmic transport [53]. Yersinia infection also affected proteins involved in such molecular functions as death and survival, cellular movement, cell cycle, gene expression, carbohydrate metabolism, lipid metabolism, small molecule biochemistry, as well as cellular function and maintenance (Fig. 3B). Inducing cell death is a common tactic used by bacterial pathogens for effective spread in the host, and Yersinia is not an exception [54]. It would be interesting to establish whether Yersinia promotes cell death in epithelial cells by targeting any of the identified molecules specifically. For example, Yersinia is already known to modify Cdc42 via its toxin CNF-gamma, which activates several other GTPases, and this is proposed to lead to stronger pro-inflammatory responses [55, 56]. Cdc42 and Ezrin (both present in our dataset) are GTPases involved in modification of actin cytoskeleton and they both regulate cell viability [57, 58].
Figure 3. Network and pathway analysis of differentially regulated proteins in HeLa cells subjected to Yersinia infection.
(A)–(B). Differentially expressed proteins with protein level affected by Yersinia infection (Fig. 2A) were analyzed by Ingenuity Pathway Analysis to identify canonical pathways (A) and molecular functions (B). (C). The top protein network identified from differentially regulated proteins in infected cells is associated with the integrin signaling and Signaling by Rho family of GTPases. Expression of proteins is indicated by colors (red for downregulated and green for upregulated proteins, white for proteins that were not on a differentially expressed protein list). Different shades of color represent the level of regulation.
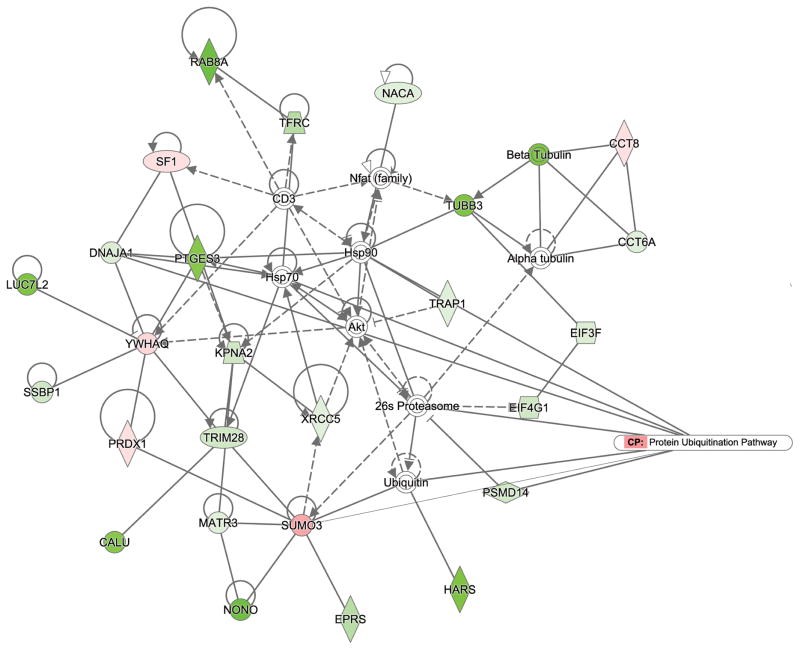
Moreover, the differentially regulated proteins were placed into two networks (Fig. 3C and Fig. 4) to illustrate significant relationships between these molecules. One of the canonical pathways associated with this network was integrin signaling, which role in Yersinia infection is already known [59], [60]. Moreover, several molecules in this network were associated with “signaling by Rho family of GTPases” canonical pathway, such as Cdc42 (downregulated in infected cells) and RhoA (up-regulated in infected cells), both of which are small GTPases controlled by guanine nucleotide dissociation inhibitors (GDI), which prevent GTPase activation [61]. Yersinia encodes YopO (YpkA) that mimics host guanidine nucleotide dissociation inhibitors, and it has affinity for Rac1 and RhoA but not for Cdc42 [62, 63]. Also, cytotoxic necrotizing factor-Y (CNF-gamma) activates Rac1 and Cdc42, but not RhoA [55]. Another interesting interaction found in the second identified protein network (Fig. 4) is association of the differentially expressed proteins to the protein ubiquitination pathway. Additionally, this network predicted binding of a ubiquitin-like protein SUMO-3 (up-regulated in infected cells) to several proteins identified as downregulated in infected cells, including NONO, EPRS, MATR3, TRIM28, and XRCC5. It would be interesting to establish whether SUMO-3 modification is regulated during infection with Yersinia and identify its specific protein substrates, since it is clear that this ubiquitin-like modifier affects critical regulators of the host immune responses [36, 38, 39]. The function of SUMO modification in Yersinia enterocolitica infection of epithelial cells will be a subject of future studies, especially in the light of a study, which describes the role of SUMO in intracellular survival of another gram-negative bacterium, Salmonella Typhimurium [37].
Figure 4. Protein network analysis of differentially regulated proteins in HeLa cells subjected to Yersinia infection.
The second top network identified from differentially regulated proteins in Yersinia-infected HeLa cells is associated with the protein ubiquitination pathway. Expression of proteins is indicated by colors (red for downregulated and green for upregulated proteins, white for proteins that were not on a differentially expressed protein list). Different shades of color represent the level of regulation.
6. Concluding remarks
Label-free quantitative proteomics has been applied to understand the cell host response to various bacterial pathogens [3–8], but this is the first description of differentially expressed proteome of Y. enterocolitica-infected and uninfected HeLa cells, which is likely to contribute to a better understanding of Y. enterocolitica pathogenesis.
Supplementary Material
2. Statement of significance of the study.
We describe a proteome of Yersinia enterocolitica-infected HeLa cells, including description of specific proteins differentially expressed upon infection, molecular functions as well as pathways altered during infection. This proteomic study can lead to a better understanding of Y. enterocolitica pathogenesis in human epithelial cells.
Acknowledgments
We would like to thank Dr. James Bliska (Stony Brook University, USA) for the Yersinia strains and Dr. Tibor Pechan (Institute for Genomics, Biocomputing and Biotechnology, Mississippi State University, USA), for technical assistance. This study was funded by the NIH fund number 5P20GM103646-02.
Abbreviations
- TSB
Tryptic soy broth
- DMEM
Dulbecco’s modified Eagle medium
- FBS
Fetal bovine serum
- PBS
Phosphate buffered saline
- MOI
Multiplicity of infection
- PVDF
Polyvinylidene difluoride
- TBS
Tris buffered saline
- DTT
Dithiothreitol
- IAA
Iodoacetamide
Footnotes
8. Conflict of interest statement
The authors have declared no conflict of interest.
References
- 1.Duan R, Liang J, Shi G, Cui Z, et al. Homology analysis of pathogenic Yersinia species Yersinia enterocolitica, Yersinia pseudotuberculosis, and Yersinia pestis based on multilocus sequence typing. Journal of clinical microbiology. 2014;52:20–29. doi: 10.1128/JCM.02185-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bliska JB, Wang X, Viboud GI, Brodsky IE. Modulation of innate immune responses by Yersinia type III secretion system translocators and effectors. Cellular microbiology. 2013;15:1622–1631. doi: 10.1111/cmi.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Troys M, Lambrechts A, David V, Demol H, et al. The actin propulsive machinery: the proteome of Listeria monocytogenes tails. Biochem Biophys Res Commun. 2008;375:194–199. doi: 10.1016/j.bbrc.2008.07.152. [DOI] [PubMed] [Google Scholar]
- 4.Pizarro-Cerda J, Jonquieres R, Gouin E, Vandekerckhove J, et al. Distinct protein patterns associated with Listeria monocytogenes InlA- or InlB-phagosomes. Cellular microbiology. 2002;4:101–115. doi: 10.1046/j.1462-5822.2002.00169.x. [DOI] [PubMed] [Google Scholar]
- 5.Reinl T, Nimtz M, Hundertmark C, Johl T, et al. Quantitative phosphokinome analysis of the Met pathway activated by the invasin internalin B from Listeria monocytogenes. Mol Cell Proteomics. 2009;8:2778–2795. doi: 10.1074/mcp.M800521-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vogels MW, van Balkom BW, Heck AJ, de Haan CA, et al. Quantitative proteomic identification of host factors involved in the Salmonella typhimurium infection cycle. Proteomics. 2011;11:4477–4491. doi: 10.1002/pmic.201100224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vorwerk S, Krieger V, Deiwick J, Hensel M, Hansmeier N. Proteomes of Host Cell Membranes Modified by Intracellular Activities of Salmonella enterica. Molecular & cellular proteomics : MCP. 2015;14:81–92. doi: 10.1074/mcp.M114.041145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ragno S, Romano M, Howell S, Pappin DJ, et al. Changes in gene expression in macrophages infected with Mycobacterium tuberculosis: a combined transcriptomic and proteomic approach. Immunology. 2001;104:99–108. doi: 10.1046/j.0019-2805.2001.01274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pieper R, Huang ST, Parmar PP, Clark DJ, et al. Proteomic analysis of iron acquisition, metabolic and regulatory responses of Yersinia pestis to iron starvation. BMC microbiology. 2010;10:30. doi: 10.1186/1471-2180-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsumoto H, Young GM. Proteomic and functional analysis of the suite of Ysp proteins exported by the Ysa type III secretion system of Yersinia enterocolitica Biovar 1B. Molecular microbiology. 2006;59:689–706. doi: 10.1111/j.1365-2958.2005.04973.x. [DOI] [PubMed] [Google Scholar]
- 11.Mahdavi A, Szychowski J, Ngo JT, Sweredoski MJ, et al. Identification of secreted bacterial proteins by noncanonical amino acid tagging. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:433–438. doi: 10.1073/pnas.1301740111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gu W, Wang X, Qiu H, Luo X, et al. Comparative antigenic proteins and proteomics of pathogenic Yersinia enterocolitica bio-serotypes 1B/O: 8 and 2/O: 9 cultured at 25 degrees C and 37 degrees C. Microbiology and immunology. 2012;56:583–594. doi: 10.1111/j.1348-0421.2012.00478.x. [DOI] [PubMed] [Google Scholar]
- 13.Ponnusamy D, Hartson SD, Clinkenbeard KD. Intracellular Yersinia pestis expresses general stress response and tellurite resistance proteins in mouse macrophages. Veterinary microbiology. 2011;150:146–151. doi: 10.1016/j.vetmic.2010.12.025. [DOI] [PubMed] [Google Scholar]
- 14.Pieper R, Huang ST, Robinson JM, Clark DJ, et al. Temperature and growth phase influence the outer-membrane proteome and the expression of a type VI secretion system in Yersinia pestis. Microbiology. 2009;155:498–512. doi: 10.1099/mic.0.022160-0. [DOI] [PubMed] [Google Scholar]
- 15.Pieper R, Huang ST, Clark DJ, Robinson JM, et al. Characterizing the dynamic nature of the Yersinia pestis periplasmic proteome in response to nutrient exhaustion and temperature change. Proteomics. 2008;8:1442–1458. doi: 10.1002/pmic.200700923. [DOI] [PubMed] [Google Scholar]
- 16.Ansong C, Schrimpe-Rutledge AC, Mitchell HD, Chauhan S, et al. A multi-omic systems approach to elucidating Yersinia virulence mechanisms. Molecular bioSystems. 2013;9:44–54. doi: 10.1039/c2mb25287b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang CG, Gonzales AD, Choi MW, Chromy BA, et al. Subcellular proteomic analysis of host-pathogen interactions using human monocytes exposed to Yersinia pestis and Yersinia pseudotuberculosis. Proteomics. 2005;5:1877–1888. doi: 10.1002/pmic.200401083. [DOI] [PubMed] [Google Scholar]
- 18.Portnoy DA, Moseley SL, Falkow S. Characterization of plasmids and plasmid-associated determinants of Yersinia enterocolitica pathogenesis. Infect Immun. 1981;31:775–782. doi: 10.1128/iai.31.2.775-782.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edelmann MJ, Kramer HB, Altun M, Kessler BM. Post-translational modification of the deubiquitinating enzyme otubain 1 modulates active RhoA levels and susceptibility to Yersinia invasion. The FEBS journal. 2010;277:2515–2530. doi: 10.1111/j.1742-4658.2010.07665.x. [DOI] [PubMed] [Google Scholar]
- 20.Wang R, Borazjani A, Matthews AT, Mangum LC, et al. Identification of palmitoyl protein thioesterase 1 in human THP1 monocytes and macrophages and characterization of unique biochemical activities for this enzyme. Biochemistry. 2013;52:7559–7574. doi: 10.1021/bi401138s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haas W, Faherty BK, Gerber SA, Elias JE, et al. Optimization and use of peptide mass measurement accuracy in shotgun proteomics. Mol Cell Proteomics. 2006;5:1326–1337. doi: 10.1074/mcp.M500339-MCP200. [DOI] [PubMed] [Google Scholar]
- 22.Nesvizhskii AI, Keller A, Kolker E, Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Analytical chemistry. 2003;75:4646–4658. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- 23.Choi H, Fermin D, Nesvizhskii AI. Significance analysis of spectral count data in label-free shotgun proteomics. Mol Cell Proteomics. 2008;7:2373–2385. doi: 10.1074/mcp.M800203-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dennis G, Jr, Sherman BT, Hosack DA, Yang J, et al. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome biology. 2003;4:P3. [PubMed] [Google Scholar]
- 25.Conesa A, Gotz S, Garcia-Gomez JM, Terol J, et al. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21:3674–3676. doi: 10.1093/bioinformatics/bti610. [DOI] [PubMed] [Google Scholar]
- 26.Canas B, Pineiro C, Calvo E, Lopez-Ferrer D, Gallardo JM. Trends in sample preparation for classical and second generation proteomics. Journal of chromatography A. 2007;1153:235–258. doi: 10.1016/j.chroma.2007.01.045. [DOI] [PubMed] [Google Scholar]
- 27.Habyarimana F, Swearingen MC, Young GM, Seveau S, Ahmer BM. Yersinia enterocolitica inhibits Salmonella enterica serovar Typhimurium and Listeria monocytogenes cellular uptake. Infection and immunity. 2014;82:174–183. doi: 10.1128/IAI.00984-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sugiura Y, Kamdar K, Khakpour S, Young G, et al. TLR1-induced chemokine production is critical for mucosal immunity against Yersinia enterocolitica. Mucosal immunology. 2013;6:1101–1109. doi: 10.1038/mi.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monetta P, Slavin I, Romero N, Alvarez C. Rab1b interacts with GBF1 and modulates both ARF1 dynamics and COPI association. Molecular biology of the cell. 2007;18:2400–2410. doi: 10.1091/mbc.E06-11-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ham H, Orth K. De-AMPylation unmasked: modulation of host membrane trafficking. Science signaling. 2011;4:pe42. doi: 10.1126/scisignal.2002458. [DOI] [PubMed] [Google Scholar]
- 31.Huang J, Birmingham CL, Shahnazari S, Shiu J, et al. Antibacterial autophagy occurs at PI(3)P-enriched domains of the endoplasmic reticulum and requires Rab1 GTPase. Autophagy. 2011;7:17–26. doi: 10.4161/auto.7.1.13840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deuretzbacher A, Czymmeck N, Reimer R, Trulzsch K, et al. Beta1 integrin-dependent engulfment of Yersinia enterocolitica by macrophages is coupled to the activation of autophagy and suppressed by type III protein secretion. Journal of immunology. 2009;183:5847–5860. doi: 10.4049/jimmunol.0804242. [DOI] [PubMed] [Google Scholar]
- 33.Lemichez E, Aktories K. Hijacking of Rho GTPases during bacterial infection. Experimental cell research. 2013;319:2329–2336. doi: 10.1016/j.yexcr.2013.04.021. [DOI] [PubMed] [Google Scholar]
- 34.Aepfelbacher M, Trasak C, Wilharm G, Wiedemann A, et al. Characterization of YopT effects on Rho GTPases in Yersinia enterocolitica-infected cells. The Journal of biological chemistry. 2003;278:33217–33223. doi: 10.1074/jbc.M303349200. [DOI] [PubMed] [Google Scholar]
- 35.Saitoh H, Hinchey J. Functional heterogeneity of small ubiquitin-related protein modifiers SUMO-1 versus SUMO-2/3. The Journal of biological chemistry. 2000;275:6252–6258. doi: 10.1074/jbc.275.9.6252. [DOI] [PubMed] [Google Scholar]
- 36.Aillet F, Lopitz-Otsoa F, Egana I, Hjerpe R, et al. Heterologous SUMO-2/3-ubiquitin chains optimize IkappaBalpha degradation and NF-kappaB activity. PloS one. 2012;7:e51672. doi: 10.1371/journal.pone.0051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verma S, Mohapatra G, Ahmad SM, Rana S, et al. Salmonella Engages Host MicroRNAs To Modulate SUMOylation: a New Arsenal for Intracellular Survival. Mol Cell Biol. 2015;35:2932–2946. doi: 10.1128/MCB.00397-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fritah S, Lhocine N, Golebiowski F, Mounier J, et al. Sumoylation controls host anti-bacterial response to the gut invasive pathogen Shigella flexneri. EMBO reports. 2014;15:965–972. doi: 10.15252/embr.201338386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Orth K, Xu Z, Mudgett MB, Bao ZQ, et al. Disruption of signaling by Yersinia effector YopJ, a ubiquitin-like protein protease. Science. 2000;290:1594–1597. doi: 10.1126/science.290.5496.1594. [DOI] [PubMed] [Google Scholar]
- 40.Mittal R, Peak-Chew SY, McMahon HT. Acetylation of MEK2 and I kappa B kinase (IKK) activation loop residues by YopJ inhibits signaling. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:18574–18579. doi: 10.1073/pnas.0608995103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mukherjee S, Keitany G, Li Y, Wang Y, et al. Yersinia YopJ acetylates and inhibits kinase activation by blocking phosphorylation. Science. 2006;312:1211–1214. doi: 10.1126/science.1126867. [DOI] [PubMed] [Google Scholar]
- 42.Tanioka T, Nakatani Y, Semmyo N, Murakami M, Kudo I. Molecular identification of cytosolic prostaglandin E2 synthase that is functionally coupled with cyclooxygenase-1 in immediate prostaglandin E2 biosynthesis. The Journal of biological chemistry. 2000;275:32775–32782. doi: 10.1074/jbc.M003504200. [DOI] [PubMed] [Google Scholar]
- 43.Kalinski P. Regulation of immune responses by prostaglandin E2. Journal of immunology. 2012;188:21–28. doi: 10.4049/jimmunol.1101029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goldfarb DS, Corbett AH, Mason DA, Harreman MT, Adam SA. Importin alpha: a multipurpose nuclear-transport receptor. Trends in cell biology. 2004;14:505–514. doi: 10.1016/j.tcb.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 45.Fagerlund R, Kinnunen L, Kohler M, Julkunen I, Melen K. NF-{kappa}B is transported into the nucleus by importin {alpha}3 and importin {alpha}4. The Journal of biological chemistry. 2005;280:15942–15951. doi: 10.1074/jbc.M500814200. [DOI] [PubMed] [Google Scholar]
- 46.DiGiandomenico A, Veach RA, Zienkiewicz J, Moore DJ, et al. The “genomic storm” induced by bacterial endotoxin is calmed by a nuclear transport modifier that attenuates localized and systemic inflammation. PloS one. 2014;9:e110183. doi: 10.1371/journal.pone.0110183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Asrat S, Davis KM, Isberg RR. Modulation of the host innate immune and inflammatory response by translocated bacterial proteins. Cellular microbiology. 2015;17:785–795. doi: 10.1111/cmi.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lander GC, Estrin E, Matyskiela ME, Bashore C, et al. Complete subunit architecture of the proteasome regulatory particle. Nature. 2012;482:186–191. doi: 10.1038/nature10774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yao T, Cohen RE. A cryptic protease couples deubiquitination and degradation by the proteasome. Nature. 2002;419:403–407. doi: 10.1038/nature01071. [DOI] [PubMed] [Google Scholar]
- 50.Hentschke M, Trulzsch K, Heesemann J, Aepfelbacher M, Ruckdeschel K. Serogroup-related escape of Yersinia enterocolitica YopE from degradation by the ubiquitin-proteasome pathway. Infection and immunity. 2007;75:4423–4431. doi: 10.1128/IAI.00528-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wiley DJ, Shrestha N, Yang J, Atis N, et al. The activities of the Yersinia protein kinase A (YpkA) and outer protein J (YopJ) virulence factors converge on an eIF2alpha kinase. The Journal of biological chemistry. 2009;284:24744–24753. doi: 10.1074/jbc.M109.010140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shrestha N, Bahnan W, Wiley DJ, Barber G, et al. Eukaryotic initiation factor 2 (eIF2) signaling regulates proinflammatory cytokine expression and bacterial invasion. The Journal of biological chemistry. 2012;287:28738–28744. doi: 10.1074/jbc.M112.375915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gorlich D, Pante N, Kutay U, Aebi U, Bischoff FR. Identification of different roles for RanGDP and RanGTP in nuclear protein import. The EMBO journal. 1996;15:5584–5594. [PMC free article] [PubMed] [Google Scholar]
- 54.Novikova L, Czymmeck N, Deuretzbacher A, Buck F, et al. Cell death triggered by Yersinia enterocolitica identifies processing of the proinflammatory signal adapter MyD88 as a general event in the execution of apoptosis. Journal of immunology. 2014;192:1209–1219. doi: 10.4049/jimmunol.1203464. [DOI] [PubMed] [Google Scholar]
- 55.Wolters M, Boyle EC, Lardong K, Trulzsch K, et al. Cytotoxic necrotizing factor-Y boosts Yersinia effector translocation by activating Rac protein. The Journal of biological chemistry. 2013;288:23543–23553. doi: 10.1074/jbc.M112.448662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schweer J, Kulkarni D, Kochut A, Pezoldt J, et al. The cytotoxic necrotizing factor of Yersinia pseudotuberculosis (CNFY) enhances inflammation and Yop delivery during infection by activation of Rho GTPases. PLoS pathogens. 2013;9:e1003746. doi: 10.1371/journal.ppat.1003746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.AM, CT, PM, CP, et al. Differential roles of JNK and Smad2 signaling pathways in the inhibition of c-Myc-induced cell death by TGF-beta. Oncogene. 2000;19:1277–1287. doi: 10.1038/sj.onc.1203420. [DOI] [PubMed] [Google Scholar]
- 58.KLW, SK, SLR, GJ, et al. The NHE1 Na+/H+ exchanger recruits ezrin/radixin/moesin proteins to regulate Akt-dependent cell survival. The Journal of biological chemistry. 2004;279:26280–26286. doi: 10.1074/jbc.M400814200. Epub 22004 Apr 26219. [DOI] [PubMed] [Google Scholar]
- 59.Cossart P, Sansonetti PJ. Bacterial invasion: the paradigms of enteroinvasive pathogens. Science. 2004;304:242–248. doi: 10.1126/science.1090124. [DOI] [PubMed] [Google Scholar]
- 60.Bliska JB, Copass MC, Falkow S. The Yersinia pseudotuberculosis adhesin YadA mediates intimate bacterial attachment to and entry into HEp-2 cells. Infection and immunity. 1993;61:3914–3921. doi: 10.1128/iai.61.9.3914-3921.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schmidt A, Hall A. Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes & development. 2002;16:1587–1609. doi: 10.1101/gad.1003302. [DOI] [PubMed] [Google Scholar]
- 62.Prehna G, Ivanov MI, Bliska JB, Stebbins CE. Yersinia virulence depends on mimicry of host Rho-family nucleotide dissociation inhibitors. Cell. 2006;126:869–880. doi: 10.1016/j.cell.2006.06.056. [DOI] [PubMed] [Google Scholar]
- 63.Groves E, Rittinger K, Amstutz M, Berry S, et al. Sequestering of Rac by the Yersinia effector YopO blocks Fcgamma receptor-mediated phagocytosis. The Journal of biological chemistry. 2010;285:4087–4098. doi: 10.1074/jbc.M109.071035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.