Abstract
Purpose
Pathologic stage II melanoma patients have variable outcomes when divided by sub-stage. We hypothesized that an understanding of the patterns of initial relapse by sub-stage will better inform follow-up guidelines.
Methods
We performed a retrospective review of 738 adult patients with pathologic stage II cutaneous melanoma treated at Memorial Sloan Kettering Cancer Center between 1993 and 2013. Clinical records were reviewed to determine time, location, and method of detection of initial relapse.
Results
At a median follow-up of 52 months, 219 patients relapsed. Relapses were detected more frequently in higher sub-stages. Initial relapses were most commonly local/in-transit for IIA and IIB, and systemic for IIC. Lung and brain were the most frequent sites of systemic relapse. Patient-detection was the most common method of relapse detection (59%) in all sub-stages. The 5-year cumulative incidence for patient-detected relapse was 13.6% for IIA, 18.9% for IIB, and 23.3% for IIC, and for image-detected relapse was 3.4%, 7.9% and 16.6%, respectively. The 5-year cumulative incidence for physician-detected relapse was less than 10% across all sub-stages and leveled off at three years for stage IIA and IIB and two years for stage IIC.
Conclusion
Relapses were most frequently patient-detected in all stage II sub-stages, highlighting the importance of patient education and self-examination. The highest yield for routine imaging is in stage IIC patients during the first four years. Physician examination is unlikely to detect relapses beyond three years for stage IIA and IIB, and beyond two years for stage IIC patients.
Introduction
Pathologic stage II melanoma patients are a group of patients that have, by definition, undergone nodal staging by sentinel lymph node biopsy or elective lymph node dissection and been found to be node negative. Stage IIA includes melanomas 1.01–2.00mm thick with ulceration or 2.01–4.00mm thick without ulceration. Stage IIB includes melanomas 2.01–4.00mm thick with ulceration or >4.00mm thick without ulceration. Stage IIC includes only melanomas >4.00mm thick with ulceration1. Survival for pathologic stage II melanoma patients varies greatly by AJCC sub-stage, making it difficult to define the optimal surveillance schedule for resected pathologic stage II patients. Five-year survival ranges from 80% for stage IIA to 53% for IIC1. In 2010, Romano et al.2 described the site and timing of relapse for pathologic stage III melanoma patients, creating the opportunity to establish evidence-based follow-up guidelines that are sub-stage-specific. This study aims to analyze pathologic stage II melanoma patients by 1) characterizing the site and timing of initial relapse by sub-stage; 2) analyzing the methods of detection for sub-stage-specific initial relapse; 3) recommending evidence-based follow-up guidelines by sub-stage.
Patients and Methods
From a prospectively maintained, single-institution database, we identified 738 adult patients with resected pathologic AJCC (7th ed.)1 stage II primary cutaneous melanoma treated at Memorial Sloan Kettering Cancer Center (MSKCC) between January 1993 and December 2013. All patients underwent pathologic nodal staging by sentinel lymph node biopsy (n=735) or elective lymph node dissection (n=3). From our database and electronic medical record, we extracted demographic information, primary melanoma characteristics, current disease and survival status, and initial relapse descriptors including site, time from initial resection, and method of detection.
Standard follow-up included evaluation by a surgical oncologist, medical oncologist, or dermatologist every three to six months for the first two years, then every six to twelve months thereafter. Serum laboratory values were rarely used for surveillance. CT scans and chest x-rays were performed in asymptomatic patients at the treating physician’s discretion.
Synchronous initial relapses were scored by the most advanced site (systemic sites outranked nodal sites, which outranked local/in-transit). Second primary melanomas were not recorded as relapses. Appropriate symptoms reported at the same time as a corresponding image-detected relapse were recorded as patient-detected.
Access to patient information was approved by the MSKCC Institutional Review Board.
Statistical Analysis
Disease-specific survival (DSS) and time to relapse (TTR) were calculated from the time of operation. Curves were generated using the Kaplan-Meier product-limit method. Patients were censored at the time of non-melanoma death or last follow-up for DSS and at the time of death without relapse or last follow-up for TTR. Cumulative incidence functions were used to estimate the incidence of recurrence by method of detection, using death without recurrence as a competing event. Five-year cumulative incidence and 95% confidence intervals are reported by method of detection and sub-stage.
Results
Clinicopathologic characteristics and outcomes
We identified 738 patients with pathologic stage II cutaneous melanoma: 400 stage IIA, 226 stage IIB, and 112 stage IIC patients (Table 1). Median age was 62 years. The most common primary site was the extremity (45%), followed by trunk (36%), and head and neck (19%). Most had an intermediate thickness (>1 mm ulcerated, or 2–4mm) melanoma (72%) with a Clark level IV (73%). Approximately half (53%) had ulcerated primaries and 79% had ≥1 mitosis/mm2. Thickness and ulceration increased by sub-stage, but presence of mitoses did not.
Table 1.
Clinicopathologic characteristics of patients with pathologic stage II cutaneous melanoma
| All stage II (n=738) |
Stage IIA (n=400) |
Stage IIB (n=226) |
Stage IIC (n=112) |
|
|---|---|---|---|---|
|
| ||||
| Age (years) | ||||
| Median (range) | 62 (17–91) | 61 (17–91) | 62 (18–90) | 67 (26–89) |
|
| ||||
| Sex | ||||
| Male | 454 (61.5%) | 236 (59%) | 148 (65.5%) | 70 (62.5%) |
| Female | 284 (38.5%) | 164 (41%) | 78 (34.5%) | 42 (37.5%) |
|
| ||||
| Location | ||||
| Head and neck | 142 (19.2%) | 69 (17.3%) | 48 (21.2%) | 25 (22.3%) |
| Extremity | 332 (45%) | 189 (47.3%) | 91 (40.3%) | 52 (46.4%) |
| Trunk | 264 (35.8%) | 142 (35.5%) | 87 (38.5%) | 35 (31.3%) |
|
| ||||
| Tumor thickness (mm) | ||||
| Median (range) | 2.8 (1.0–32.0) | 2.3 (1.0–4.0) | 3.5 (2.1–22.0) | 5.7 (4.1–32.0) |
| 1–4 | 535 (72.5%) | 400 (100%) | 135 (69.7%) | 0 (0%) |
| >4 | 203 (27.5%) | 0 (0%) | 91 (40.3%) | 112 (100%) |
|
| ||||
| Clark level | ||||
| II | 3 (0.4%) | 2 (0.5%) | 1 (0.4%) | 0 (0%) |
| III | 46 (6.2%) | 30 (7.5%) | 13 (5.8%) | 3 (2.7%) |
| IV | 540 (73.2%) | 337 (84.3%) | 148 (65.5%) | 55 (49.1%) |
| V | 125 (16.9%) | 21 (5.3%) | 54 (23.9%) | 50 (44.6%) |
| Unknown | 24 (3.3%) | 10 (2.5%) | 10 (4.4%) | 4 (3.6%) |
|
| ||||
| Ulceration | ||||
| Present | 392 (53.1%) | 146 (36.5%) | 134 (59.3%) | 112 (100%) |
| Absent | 302 (40.9%) | 225 (56.3%) | 77 (34.1%) | 0 (0%) |
| Unknown | 44 (6%) | 29 (7.3%) | 15 (6.6%) | 0 (0%) |
|
| ||||
| Mitoses (per mm2) | ||||
| <1 | 19 (2.6%) | 15 (3.8%) | 4 (1.8%) | 0 (0%) |
| ≥1 | 583 (79%) | 319 (79.8%) | 173 (76.5%) | 91 (81.3%) |
| Unknown | 136 (18.4%) | 66 (16.5%) | 49 (21.7%) | 21 (18.8%) |
Median follow-up was 52.1 months for non-relapsing survivors (Table 2). 219 patients relapsed during follow-up. Median time to relapse (TTR) was 195.1 months for IIA, not reached for IIB, and 85.9 months for IIC. One stage IIA patient recurred at 16 years, without which the median TTR would be not reached for IIA. Five-year TTR was 77% for IIA, 62% for IIB, and 53% for IIC. Among patients who relapsed, half of the relapses occurred by 29 months for IIA, 23 months for IIB, and 15 months for IIC. Five-year melanoma-specific survival for IIA, IIB, and IIC was 87%, 78%, and 69%, respectively (Supplemental Figure 1).
Table 2.
Outcomes of patients with pathologic stage II melanoma
| All stage II (n=738) |
Stage IIA (n=400) |
Stage IIB (n=226) |
Stage IIC (n=112) |
|
|---|---|---|---|---|
|
| ||||
| Follow-up (months) | ||||
| All – median (range) | 51.7 (0–215) | 54.2 (0–210) | 50.2 (0–206) | 46.2 (4–215) |
| Survivors – median (range) | 54.7 (0–215) | 54.9 (0–210) | 60.5 (0–206) | 47.9 (6–215) |
| Non-relapsing survivors – median (range) |
52.1 (0–215) | 52.7 (0–210) | 52.5 (0–206) | 49.5 (6–215) |
|
| ||||
| Site of first relapse | ||||
| Local/in-transit | 88 (12%) | 39 (10%) | 34 (15%) | 15 (13%) |
| Nodal | 50 (7%) | 23 (6%) | 17 (7%) | 10 (9%) |
| Systemic | 81 (11%) | 32 (8%) | 22 (10%) | 27 (24%) |
| No recurrence | 519 (70%) | 306 (76%) | 153 (68%) | 60 (54%) |
|
| ||||
| Status at last follow-up | ||||
| Alive with disease | 28 (3.8%) | 11 (2.8%) | 9 (4%) | 8 (7.1%) |
| No evidence of disease | 489 (66.3%) | 287 (71.8%) | 148 (65.5%) | 54 (48.2%) |
| Dead | 221 (29.9%) | 102 (25.5%) | 69 (30.5%) | 50 (44.6%) |
| Died of disease | 124 (16.8%) | 52 (13%) | 37 (16.4%) | 35 (31.3%) |
Patterns of initial relapse by sub-stage
Relapses were divided into three sites (local/in-transit, nodal, and systemic), and analyzed by sub-stage (Figure 1a–c). Among the observed relapses, initial relapses were most frequently local/in-transit in stage IIA (41%) and IIB (47%), while IIC relapses were most frequently systemic (52%).
Figure 1.
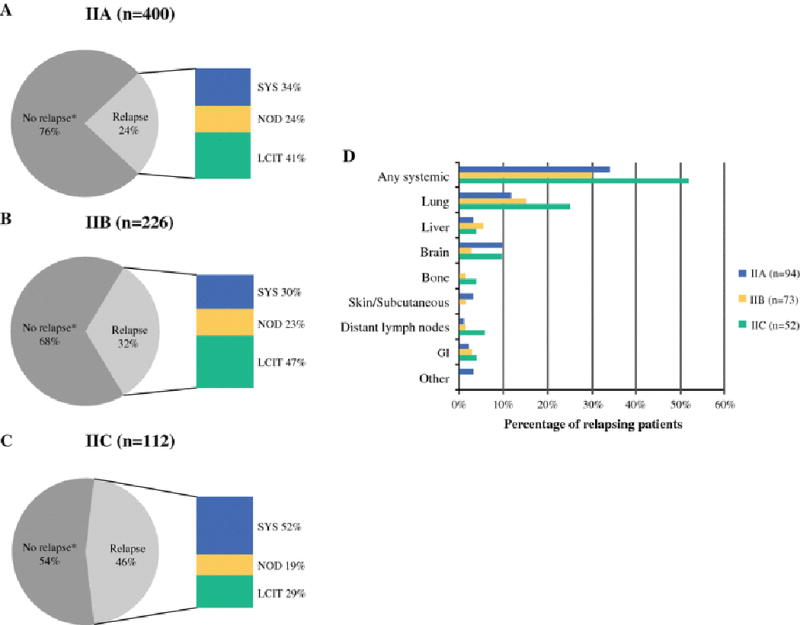
Site of first relapse by sub-stage. Percentage of stage IIA (a), stage IIB (b), or stage IIC (c) patients who relapsed with a local/in-transit (LCIT), regional nodal (NOD), or systemic (SYS) relapse. Percentages of LCIT, NOD, and SYS relapses are represented as percent of relapsing patients. *No relapse detected at the time of last follow-up. (d) Site of first systemic relapse by sub-stage. Percentages represent percentage of relapsing patients within the listed sub-stage.
Systemic relapses were detected in 32 stage IIA patients, 22 stage IIB patients, and 27 stage IIC patients (Figure 1d). Lung was the most frequent site of systemic relapse in all sub-stages (n=11, 11, and 13 for stage IIA, IIB, and IIC, respectively). The second most frequent site was brain for IIA (n=9) and IIC (n=5), and liver for IIB (n=4). Three IIA patients had a rare site of distant metastasis - two to the larynx, and one to the heart.
Of detected relapses, 187 occurred within the first 5 years (supplemental table 1). Of the 32 relapses detected after 5 years, 21 were initially stage IIA, 6 were IIB, and 5 were IIC. Fourteen were systemic, 10 were nodal, and 8 were local/in-transit relapses.
Method of detection of initial relapse
We were able to determine the method of detection for 211 initial relapses, which were divided into three categories: 1) patient-detected, 2) physician-detected, and 3) image-detected. We excluded one patient whose elevated LDH served as the method of detection, leaving 210 patients for final analysis (Figure 2). Patient-detection was the most frequent method across all sites and sub-stages. Patients were able to identify 62% of local/in-transit, 69% of nodal, and 50% of systemic relapses by detection of a visible/palpable lesion or by reporting symptoms that prompted further evaluation. By sub-stage, patient-detection accounted for 66% of IIA, 52% of IIB, and 57% of IIC relapses. Physician examination of an asymptomatic patient detected 36% of local/in-transit, 23% of nodal, and 3% of systemic relapses. By sub-stage, physician-detection accounted for 22% of IIA, 26% of IIB, and 12% of IIC relapses. Surveillance imaging of asymptomatic patients led to identification of 2% of local/in-transit, 8% of nodal, and 47% of systemic relapses. By sub-stage, image-detection accounted for 12% of IIA, 22% of IIB, and 31% of IIC relapses. Of the 42 image-detected recurrences, 24 were detected by CT scans, 14 by chest x-ray, three by PET/CT, and one by nodal basin ultrasound.
Figure 2.
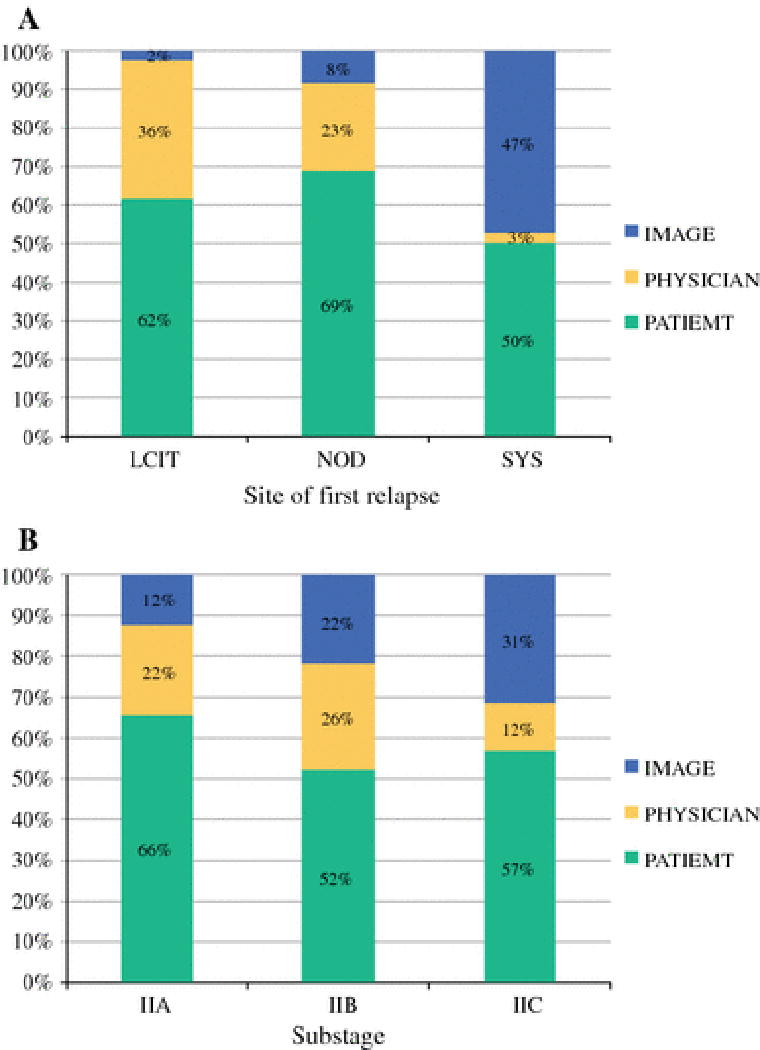
Method of detection of first relapse. a) Divided by site of first relapse: local/in-transit (LCIT), nodal (NOD), and systemic (SYS). b) Divided by pathologic sub-stage.
Cumulative relapse incidence by method of detection
Patient-detected relapses not only had the highest 5-year cumulative incidence in all sub-stages (figure 3d), but also was the only method that did not reach a plateau by five years (figure 3a–c). In contrast, the cumulative incidence of physician-detected relapses levels off by three years for stage IIA and IIB and by two years for IIC. The 5-year cumulative incidence of physician-detected relapses was <10% in all sub-stages. The 5-year cumulative incidence of image-detected relapses was higher for stage IIC (16.6%) than IIB (7.9%) and IIA (3.4%), consistent with higher rates of stage IIC systemic relapses. There was only one image-detected relapse after 5 years.
Figure 3.
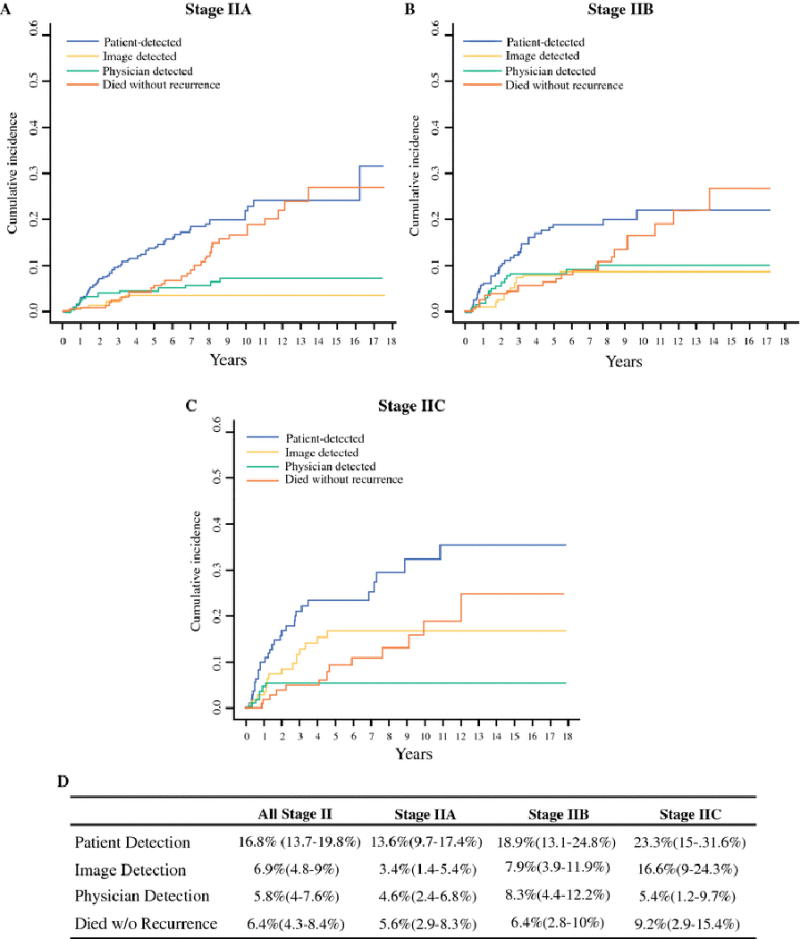
Cumulative incidence of relapse by method of detection for stage IIA (a), stage IIB (b), and stage IIC (c) patients. d) 5-year cumulative incidence and 95% confidence interval (in parentheses) for relapse and death without relapse. Relapses are broken down by method of detection and sub-stage.
Discussion
Current NCCN guidelines divide primary melanoma patients into two follow-up groups: stage IA–IIA and stage IIB-IV3. The latter represents a broad range of relapse risks. Combined with the lack of actionable prospective studies on optimal follow-up strategies, this has led to guidelines that allow variable visit intervals and a wide range of acceptable imaging strategies. International melanoma guidelines suffer from a similar lack of data. A 2012 systematic review of stage-specific surveillance practices found wide variability in visit frequency and use of surveillance imaging and blood tests across countries with consensus guidelines4.
There have been two prospective studies examining melanoma follow-up schedules. Garbe et al. followed 2008 consecutive stage I–IV cutaneous melanoma patients with physical examinations every three months and routine nodal basin ultrasounds. In their stage I/II cohort, early relapse detection was associated with improved 3-year overall survival (76% vs. 38%)5. However, it is unclear how much of the survival advantage was attributable to lead-time bias versus earlier treatment opportunities. In a German multi-center 4-year prospective study, Livingstone et al. found that only 7.7% of stage I–III patients (n=555) developed first or additional metastases after 3 years6. They recommended reduced follow-up for low-risk patients, but were unable to draw conclusions for the stage II cohort, who represented only 13% of their study population. The Melanoma Follow-up (MELFO) trial in the Netherlands has accrued 180 stage IB/II patients and prospectively randomized them to either a conventional follow-up schedule (4 visits yearly) or a less frequent “experimental” schedule. Preliminary results after one year showed no difference in recurrence and self-detection rates, no adverse effect on mental well-being, and a 45% reduction in follow-up cost7.
We present here the largest series dedicated to outcomes of pathologic stage II melanoma patients. Our first aim was to characterize the site and timing of initial relapse by sub-stage. Our data confirm that increasing sub-stage is associated with progressively worse rates of relapse and melanoma-specific survival. Stage IIC patients relapsed more frequently, earlier, and were more likely to relapse systemically. Lung was the most common site of metastasis, followed by brain. Low rates of other site-specific systemic relapse suggest that routine imaging targeted at other sites or in stage IIA patients is of exceedingly low yield.
Our second aim was to analyze the method of detection of initial relapse by sub-stage. Shumate et al.8 found that symptoms were present in 90% of patients with recurrent melanoma and accurately predicted the site of recurrence. Furthermore, overall survival was not affected by whether the recurrence was detected during a routine versus unscheduled visit. Additional studies have confirmed that most relapses are patient-detected 2,9–12, and demonstrated no survival difference between symptomatic and asymptomatic patients 10,11,13. In our cohort, relapses were most frequently patient-detected in all sub-stages, and the cumulative incidence of patient-detected relapse continues to rise past five years. The cumulative incidence of physician-detected relapses plateaus around three years for IIA and IIB and by two years for IIC, providing a natural time point for patients to transition to annual or as-needed follow-up. Image-detected relapses plateau at three years for IIB and four years for IIC. The cumulative incidence of death without recurrence continues to rise in all sub-stages, implying that while the decrease in relapse incidence is in part due to less intensive follow-up, it is also due to ongoing all-cause mortality.
Our final aim was to propose sub-stage-specific guidelines for the frequency and duration of physician visits and routine imaging based the above results (Supplemental Table 2). We recommend routine visits for three years for stage IIA and IIB, with less frequency for IIA. For stage IIC, we recommend routine visits for the first four years, with the latter two years primarily to assist in imaging interpretation. We recommend against routine imaging for stage IIA, but would consider annual surveillance imaging for three years for IIB, and every 6–12 months for four years for IIC. Imaging should target the most frequent sites of systemic relapse: lung for IIB/IIC with consideration of brain imaging for IIC. Once on annual visits and not undergoing surveillance imaging, we recommend transition into a survivorship program. This has the added benefits of ongoing education on prevention and self-examination and identifying and managing treatment complications and toxicities, all while facilitating systematic data recording for future studies. This is not, however, to diminish the importance of routine dermatologic surveillance, as melanoma patients have a 9-fold increased risk of a second invasive primary melanoma14 and a 4-fold increased risk of a non-melanoma skin cancer15. These recommendations intentionally leave flexibility for individual tailoring based on additional patient risk factors, such as age16–18, sex17–19, and tumor location17–19. It should be emphasized that these are only guidelines, and clinical judgement must be used to adapt them to an individual patient based on the previously mentioned risk factors, as well as patient anxiety level, personal history of melanoma, and availability of resources. Results of the MELFO trial will further guide follow-up for stage II patients; however, prospective randomized trials of multiple follow-up strategies will be exceedingly difficult due to the number of patients required.
In a single-center cost evaluation, Hofmann et al. found that routine imaging in stage I/II patients (n=554) detected only 21% of initial relapses, but accounted for almost half the cost13. In this study, routine imaging included nodal basin ultrasound and chest x-ray, but not PET/CT, brain MRI, or chest CT. Use of the latter would likely increase costs with little additional yield. Rueth et al. did a comparative analysis of stage-specific surveillance strategies and found in pathologic stage II melanoma patients, the positive predictive value was 12.7% for annual PET/CT and 4.5% for annual CT20. These are low in part due to a higher false positive rate, which invariably adds cost by stimulating additional testing. The expected average life expectancy increase with image-detected relapse treatment was only one month. This study divided patients by stage III sub-stage, but was unable to do so for the smaller stage II cohort (n=72). A German cost analysis for stage II melanoma patients demonstrated the median cost to detect a recurrence with clinical examination was €2,715 ($3,910US) versus €32,007 ($46,090US) when using surveillance imaging21. Although our study does not directly address the cost-effectiveness of various follow-up strategies, our data suggests that we can implement a more efficient, cost-effective follow-up strategy by decreasing use of routine imaging in low-risk patients, increasing patient education on symptom identification and self-examination, and decreasing the frequency of physician visits.
Although these data were obtained from a prospectively maintained database, it is still susceptible to the usual limitations of a retrospective study. We do not have full data in regards to the frequency and type of surveillance imaging performed that did not detect any relapses. This is not a prospectively collected data point in our database, and because care of our patients is often shared with their local physicians, we would not be able to accurately retrieve this information retrospectively. This limits our ability to draw conclusions on the type of surveillance imaging with highest yield and the optimal frequency for imaging. However, the data regarding where and when patients relapse can help guide physicians in planning their follow-up strategies.
Our median follow-up is just short of 5 years. While this may underestimate the true long-term risk of relapse, previous studies have also shown that a majority of relapses occur within the first five years12,22,23. Our study is naturally biased towards patient-detection in the later years, when physician visits and imaging are performed less frequently. One could argue that some of these patient-detected relapses could have been detected earlier had imaging been performed. However, even if imaging could have led to earlier detection of all late (>5 year) patient-detected relapses, this would have only affected 14 stage IIA, 2 IIB, and 5 IIC patients. It would be difficult to justify continued radiographic surveillance of the entire group for such a low yield; especially given the lack of evidence that earlier image-detection affects overall survival.
In the new era of systemic treatment for unresectable or metastatic melanoma, immunotherapy and targeted therapies have shown improved rates of objective response, prolonged duration of response, and a favorable overall survival rate compared with traditional chemotherapy24–28. Naturally, these new therapies are being tested in the adjuvant setting. However, it has yet to be shown that survival is improved with earlier detection and treatment using these newer agents in the minimal residual disease setting. The current adjuvant trials with these highly active therapies compare adjuvant therapy vs. no adjuvant therapy, but the real question will be whether adjuvant therapy for all patients after surgery (i.e. treatment now) is better than treating only relapsed patients at the time of relapse (i.e. treatment later if needed). As we gain more long-term experience with these treatments, we anticipate that the national guidelines will be revisited and updated to reflect the most current data.
Conclusion
Rates of melanoma have been increasing approximately 3% per year despite stable mortality rates, which cannot be explained entirely by increases in thin melanomas secondary to screening29. With increasing numbers of melanoma survivors, there is an increased burden on the system for surveillance. This study found low rates of image-detected relapse in stage IIA, supporting current NCCN recommendation against routine imaging for stage IIA. The kinetics of initial relapse reveal that a majority of physician-detect relapses occur within three years for stage IIA/IIB and two years for IIC, allowing for earlier transition to annual visits with a survivorship focus than currently recommended. We verified that stage IIC represents a distinct higher risk group that deserves close follow-up in the early years when overall and systemic relapse rates are highest. Early relapse detection must be balanced against surveillance costs, and based on these data we suggest that changes in follow-up guidelines must be sub-stage-specific and account for the yield of physician follow-up and imaging.
Supplementary Material
Synopsis.
We reviewed 738 pathologic stage II cutaneous melanoma patients and determined their patterns of relapse and method of relapse detection by sub-stage. Based on results from this descriptive analysis, we make sub-stage-specific recommendations for follow-up guidelines in stage II melanoma patients.
Acknowledgments
We thank Shreya Balakrishna of MSKCC for her assistance in managing our database and editing the manuscript.
Research support: This work was supported by the Memorial Sloan Kettering Cancer Center Core Grant (P30 CA008748).
Footnotes
Conflicts of interest: none
References
- 1.Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009 Dec 20;27(36):6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Romano E, Scordo M, Dusza SW, Coit DG, Chapman PB. Site and timing of first relapse in stage III melanoma patients: implications for follow-up guidelines. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010 Jun 20;28(18):3042–3047. doi: 10.1200/JCO.2009.26.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.NCCN Clinical Practice Guidelines in Oncology: Melanoma, Version 2.2016. 2016 http://www.nccn.org/professionals/physician_gls/pdf/melanoma.pdf.
- 4.Xing Y, Cromwell KD, Cormier JN. Review of diagnostic imaging modalities for the surveillance of melanoma patients. Dermatology research and practice. 2012;2012:941921. doi: 10.1155/2012/941921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garbe C, Paul A, Kohler-Spath H, et al. Prospective evaluation of a follow-up schedule in cutaneous melanoma patients: recommendations for an effective follow-up strategy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003 Feb 1;21(3):520–529. doi: 10.1200/JCO.2003.01.091. [DOI] [PubMed] [Google Scholar]
- 6.Livingstone E, Eigentler TK, Windemuth-Kieselbach C, et al. Actual practice of melanoma follow-up and treatment in Germany: results of a prospective, longitudinal cohort study. The British journal of dermatology. 2015 Jun;172(6):1646–1650. doi: 10.1111/bjd.13612. [DOI] [PubMed] [Google Scholar]
- 7.Damude S, Hoekstra-Weebers JE, Francken AB, Ter Meulen S, Bastiaannet E, Hoekstra HJ. The MELFO-Study: Prospective, Randomized, Clinical Trial for the Evaluation of a Stage-adjusted Reduced Follow-up Schedule in Cutaneous Melanoma Patients-Results after 1 Year. Annals of surgical oncology. 2016 May 18; doi: 10.1245/s10434-016-5263-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shumate CR, Urist MM, Maddox WA. Melanoma recurrence surveillance. Patient or physician based? Annals of surgery. 1995 May;221(5):566–569. doi: 10.1097/00000658-199505000-00014. discussion 569–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore Dalal K, Zhou Q, Panageas KS, Brady MS, Jaques DP, Coit DG. Methods of detection of first recurrence in patients with stage I/II primary cutaneous melanoma after sentinel lymph node biopsy. Annals of surgical oncology. 2008 Aug;15(8):2206–2214. doi: 10.1245/s10434-008-9985-z. [DOI] [PubMed] [Google Scholar]
- 10.Francken AB, Shaw HM, Accortt NA, Soong SJ, Hoekstra HJ, Thompson JF. Detection of first relapse in cutaneous melanoma patients: implications for the formulation of evidence-based follow-up guidelines. Annals of surgical oncology. 2007 Jun;14(6):1924–1933. doi: 10.1245/s10434-007-9347-2. [DOI] [PubMed] [Google Scholar]
- 11.Meyers MO, Yeh JJ, Frank J, et al. Method of detection of initial recurrence of stage II/III cutaneous melanoma: analysis of the utility of follow-up staging. Annals of surgical oncology. 2009 Apr;16(4):941–947. doi: 10.1245/s10434-008-0238-y. [DOI] [PubMed] [Google Scholar]
- 12.Regan MW, Reid CD, Griffiths RW, Briggs JC. Malignant melanoma, evaluation of clinical follow up by questionnaire survey. British journal of plastic surgery. 1985 Jan;38(1):11–14. doi: 10.1016/0007-1226(85)90080-3. [DOI] [PubMed] [Google Scholar]
- 13.Hofmann U, Szedlak M, Rittgen W, Jung EG, Schadendorf D. Primary staging and follow-up in melanoma patients–monocenter evaluation of methods, costs and patient survival. British journal of cancer. 2002 Jul 15;87(2):151–157. doi: 10.1038/sj.bjc.6600428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bradford PT, Freedman DM, Goldstein AM, Tucker MA. Increased risk of second primary cancers after a diagnosis of melanoma. Archives of dermatology. 2010 Mar;146(3):265–272. doi: 10.1001/archdermatol.2010.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caini S, Boniol M, Botteri E, et al. The risk of developing a second primary cancer in melanoma patients: a comprehensive review of the literature and meta-analysis. Journal of dermatological science. 2014 Jul;75(1):3–9. doi: 10.1016/j.jdermsci.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 16.Austin PF, Cruse CW, Lyman G, Schroer K, Glass F, Reintgen DS. Age as a prognostic factor in the malignant melanoma population. Annals of surgical oncology. 1994 Nov;1(6):487–494. doi: 10.1007/BF02303614. [DOI] [PubMed] [Google Scholar]
- 17.Schuchter L, Schultz DJ, Synnestvedt M, et al. A prognostic model for predicting 10-year survival in patients with primary melanoma. The Pigmented Lesion Group. Annals of internal medicine. 1996 Sep 1;125(5):369–375. doi: 10.7326/0003-4819-125-5-199609010-00003. [DOI] [PubMed] [Google Scholar]
- 18.Balch CM, Soong SJ, Gershenwald JE, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2001 Aug 15;19(16):3622–3634. doi: 10.1200/JCO.2001.19.16.3622. [DOI] [PubMed] [Google Scholar]
- 19.Masback A, Olsson H, Westerdahl J, Ingvar C, Jonsson N. Prognostic factors in invasive cutaneous malignant melanoma: a population-based study and review. Melanoma research. 2001 Oct;11(5):435–445. doi: 10.1097/00008390-200110000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Rueth NM, Xing Y, Chiang YJ, et al. Is surveillance imaging effective for detecting surgically treatable recurrences in patients with melanoma? A comparative analysis of stage-specific surveillance strategies. Annals of surgery. 2014 Jun;259(6):1215–1222. doi: 10.1097/SLA.0000000000000233. [DOI] [PubMed] [Google Scholar]
- 21.Hengge UR, Wallerand A, Stutzki A, Kockel N. Cost-effectiveness of reduced follow-up in malignant melanoma. Journal der Deutschen Dermatologischen Gesellschaft = Journal of the German Society of Dermatology : JDDG. 2007 Oct;5(10):898–907. doi: 10.1111/j.1610-0387.2007.06454.x. [DOI] [PubMed] [Google Scholar]
- 22.Dicker TJ, Kavanagh GM, Herd RM, et al. A rational approach to melanoma follow-up in patients with primary cutaneous melanoma. Scottish Melanoma Group. The British journal of dermatology. 1999 Feb;140(2):249–254. doi: 10.1046/j.1365-2133.1999.02657.x. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen T, Kuo C, Nicholl MB, et al. Downregulation of microRNA-29c is associated with hypermethylation of tumor-related genes and disease outcome in cutaneous melanoma. Epigenetics. 2011 Mar;6(3):388–394. doi: 10.4161/epi.6.3.14056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. The New England journal of medicine. 2010 Aug 19;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. The New England journal of medicine. 2015 Jul;Feb;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. The New England journal of medicine. 2015 May 21;372(21):2006–2017. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. The New England journal of medicine. 2011 Jun 30;364(26):2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sosman JA, Kim KB, Schuchter L, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. The New England journal of medicine. 2012 Feb 23;366(8):707–714. doi: 10.1056/NEJMoa1112302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Linos E, Swetter SM, Cockburn MG, Colditz GA, Clarke CA. Increasing burden of melanoma in the United States. The Journal of investigative dermatology. 2009;129(7):1666–1674. doi: 10.1038/jid.2008.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


