Abstract
Macrophages are critical drivers of the immune response during infection and inflammation. The pathogenesis of several inflammatory conditions, such as diabetes, cancer and sepsis has been linked with aldose reductase (AR), a member of the aldo-keto reductase (AKR) superfamily. However, the role of AR in the early stages of innate immunity such as phagocytosis remains unclear. In this study, we examined the role of AR in regulating the growth and the phagocytic activity of bone marrow-derived mouse macrophages (BMMs) from AR-null and wild-type (WT) mice. We found that macrophages derived from AR-null mice were larger in size and had a slower growth rate than those derived from WT mice. The AR-null macrophages also displayed higher basal, and lipopolysaccharide (LPS) stimulated phagocytic activity than WT macrophages. Moreover, absence of AR led to a marked increase in cellular levels of both ATP and NADPH. These data suggest that metabolic pathways involving AR suppress macrophage energy production, and that inhibition of AR could induce a favorable metabolic state that promotes macrophage phagocytosis. Hence, modulation of macrophage metabolism by inhibition of AR might represent a novel strategy to modulate host defense responses and to modify metabolism to promote macrophage hypertrophy and phagocytosis under inflammatory conditions.
Keywords: Aldose reductase, Macrophages, Metabolism, Inflammation, Innate immunity, Phagocytosis
1. Introduction
Macrophages are part of a functionally diverse, heterogeneous, innate myeloid cell population of cells that maintain homeostasis via immune surveillance, tissue remodeling and repair. They are critical drivers of the inflammation-resolution axis and co-ordinate not only removal of pathogens and detritus, but promote tissue repair and regeneration as well [1]. As professional phagocytes, they eliminate bacteria, dead cells, and objects identified as foreign via immune surveillance. They are also responsible for instructing the immune system to mount an appropriate defense response as and when needed [2].
Phagocytosis is important during the acute inflammatory response as well as in the resolution of chronic inflammation conditions. In the early phase of the innate immune response, macrophages produce reactive oxygen species (ROS) and a host of pro-inflammatory cytokines/chemokines to participate in bacterial killing and to regulate inflammatory cascades. Subsequently, they are also capable of being reprogramed to a phenotype capable of resolving inflammation, in part by generating bioactive, pro-resolving lipid species. Although phagocytosis is central to both of these processes, its complex regulatory aspects are less clearly defined. Both the acute and the chronic phases of inflammation are intricately tied to several metabolic pathways, including those in intermediary metabolism such as glycolysis and fatty acid oxidation [3, 4]. Nevertheless, the metabolic pathways that integrate and control macrophage phagocytosis are not well understood.
Aldose reductase (AKR1B) is a multifunctional aldo-keto reductase that catalyzes the reduction of aldehydes ranging from glucose to lipid peroxidation products such as 4-hydroxy-trans-2-nonenal (HNE) and acrolein [5]. Although the primary function of AR is to regulate cellular osmolality by catalyzing the reduction of glucose to sorbitol [6, 7], several studies suggest that AR plays an important role in inflammation [8]. In particular, AR has been shown to regulate the production of key cytokines [9–11], which participate in acute inflammatory responses [12–14]. It has also been shown to mediate endotoxin-induced inflammation and cardiomyopathy [15]. Moreover, inhibition of AR has been shown to protect against the insult of ischemic injury in both the hind limb [16] as well as the heart [17, 18]. Despite this evidence showing that AR regulates inflammatory responses, the role of AR in macrophage function is not known. Therefore, we examined how deletion of AR regulates phagocytosis. We found that the absence of AR is associated with an increase in macrophage phagocytic activity in vitro, and that this phenotype is associated with slow growth and changes in ATP abundance, pyridine nucleotide levels, and cell size. These results suggest that AR controls the immune function of macrophages by regulating their metabolic activity.
2. Materials and methods
All reagents used were of analytical grade and were purchased from the respective vendors as indicated. Alpha tubulin antibody, LPS, TPP tissue culture flasks, 10 cm petridishes, and 6-well plates (Sigma, St. Louis, MO, USA), AR antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA), ATP assay kit, NADP/NADPH assay kit (Abcam, Cambridge, MA, USA), Calcein Green, AM (Molecular Probes, Life Technologies, ThermoFisher Scientific, Waltham, MA, USA), Enhanced chemiluminescence (ECL plus) reagents were from Pierce (Rockford, IL, USA), Fetal bovine serum (Atlanta Biologicals, Flowery Branch, GA, USA), Gentamicin solution, Hepes, Horseradish peroxidase-conjugated anti-mouse and anti-rabbit IgG F(ab′)2 (Cell Signaling, Beverly, MA, USA), Phagocytosis assay kit (Cayman Chemical, Ann Arbor, MI, USA), Protein assay kit (Biorad, Hercules, CA, USA), RPMI-1640 medium (1×) with 2.05 mM L-glutamine and 2.0 g/L glucose (Hyclone Laboratories, GE Healthcare Life Sciences, South Logan, UT, USA), Phosphate-buffered saline were from (Gibco, Life Technologies, Waltham, MA, USA).
2.1 Macrophage culture
Immortalized murine macrophage cell lines were established by infecting the bone marrow of wild-type C57BL/6J mice and C57BL/6J background AR-null homozygous mice with the murine recombinant J2 retrovirus containing the v-myc and v-raf oncogenes as described previously [19]. The resulting bone-marrow-derived macrophage (BMMs) cell lines were cultured and maintained in RPMI-1640 supplemented with 5% fetal bovine serum (FBS), 1% HEPES, and 0.1% gentamicin, at 37°C and 5% CO2 in a humidified incubator. For each experiment the cells (passages 2–10) were sub-cultured and seeded a day before in either 6-well plates or 10 cm petridishes in 2.0 ml or 10 ml medium respectively and treated as indicated in the text. Where required, BMMs were serum-starved overnight in the media containing 0.1% fetal bovine serum (FBS). Treatment reagents were dissolved in Hank’s balanced salt solution (HBSS, pH 7.4, 20 mM HEPES, 135 mM NaCl, 5.4 mM KCl, 1.0 mM MgCl2, 2.0 mM CaCl2, 2.0 mM NaH2PO4, 5.5 mM glucose). After incubation for indicated times, the cells were washed twice with HBSS, scraped, and resuspended in the lysis buffer and the protein lysates were subjected to Western blotting (10–30 μg of total protein loaded per lane; n=3).
2.2 Western blotting
Total cellular proteins were extracted from WT-BMMs and AR-null BMMs and the expression level of AR was examined following a standard Western blotting protocol. In brief, 10 or 30 μg of total protein amount of each sample was electrophoresed in 4–12% Bis Tris Novex gels (Life Technologies, Waltham, MA, USA) and transferred to PVDF blot membranes (Bio-Rad Laboratories, Hercules, CA, USA). The membranes were blocked with 5% nonfat milk (Bio-Rad Laboratories, Hercules, CA, USA) solution and incubated with primary antibodies at 1:1,000 (anti-AR; Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight at 4° C followed by secondary horseradish peroxidase-conjugated anti-Ig antibodies (Cell Signaling, Beverly, MA, USA) for 1–2 h. The enhanced chemiluminescence detection system (ECL plus) reagents were from Pierce (Rockford, IL, USA) was used to detect bands with peroxidase activity. Alpha tubulin (Sigma, St. Louis, MO, USA) served as an internal reference.
2.3 Measurement of cells’ morphology and size
BMMs were cultured and synchronized using cell culture media containing 0.1% fetal bovine serum (FBS). CellTrace Calcein Green AM (Molecular Probes, Life Technologies, ThermoFisher Scientific, Waltham, MA, USA), a cell-permeant dye was added for labeling viable cells during the experiment in an order to acquire size and shape of both WT and AR-null BMMs. Calcein Green AM (Molecular Probes, Life Technologies, ThermoFisher Scientific, Waltham, MA, USA) is a fluorogenic cell-permeant dye that can be used to determine cell viability and morphology. In living cells, the nonfluorescent Calcein Green AM (Molecular Probes, Life Technologies, ThermoFisher Scientific, Waltham, MA, USA) is converted to an intensely green-fluorescent calcein after intracellular esterases remove the acetoxymethyl (AM) esters. The cells were visualized using Evos, AMG microscope (ThermoFisher Scientific, Grand Island, NY). Briefly, 100 × 100 μm grids having cells were selected to measure the area accurately and the differences in size (cell-area) were measured using the ‘Magic Wand” tool (Image J software).
2.4 Determination of cell population doubling time and the intracellular ATP concentrations
Equal number of WT-BMMs and AR-null BMMs were cultured in the fresh complete medium containing and their growth was synchronized overnight using 0.1% fetal bovine serum (FBS). The next day, cells were counted. The intracellular ATP concentrations were measured using ATP assay kit (Abcam, Cambridge, MA, USA) according to the manufacturer’s instructions. Absorbance was measured at 490 nm using Synergy Mx, BioTek plate reader (BioTek, Winooski, VT).
2.5 Assessing phagocytosis in WT-BMMs versus AR-null BMMs
WT-BMMs and AR-null BMMs were seeded in 6-well plates, and growth synchronized in 0.1% fetal bovine serum (FBS) medium a day before. After 24 h, the cells were treated with or without LPS (100 μM) for 12 h and then incubated the latex beads coated with phycoerythrin (PE) labeled Rabbit-IgG. After 6 h of incubation, the cells were harvested for measuring the kinetics of phagocytosis using a BD LSR II flow cytometer (BD Biosciences, San Jose, CA). Fluorescence microscopy of the macrophages was also carried out after platting them in complete growth medium. Intense red colored particles ingested by phagosomes were photographed. For assessing the phagocytosis under microscope, Calcein Green, AM (Molecular Probes, Life Technologies, ThermoFisher Scientific, Waltham, MA, USA) was used to capture live cell images to detect engulfed fluorescent beads and DAPI (Sigma, St. Louis, MO, USA) was used to stain the nuclei. Pictures were taken using Evos, AMG microscope (ThermoFisher Scientific, Grand Island, NY).
2.6 Determination of NADPH in BMMs
NADP/NADPH levels were assayed by using NADP/NADPH assay kit (Abcam) according to the manufacturer’s instruction. The fluorometric assay offers a sensitive detection of NADP, NADPH and their ratio by recognizing NADP+/NADPH in an enzyme recycling reaction. The assay was performed in a 96-well microtiter-plate and the resultant signal was read by a fluorescence microplate reader at Ex/Em = 530–570/590–600 nm (maximum Ex/Em = 540/590 nm) using Synergy Mx, BioTek plate reader (BioTek, Winooski, VT).
2.7 Statistical analyses
All data are mean ± SEM. Comparisons and the corresponding statistical significance was assessed by ANOVA for multiple samples or Student’s t test for unpaired samples as deemed appropriate. Statistical significance was accepted at P < 0.05.
3. Results
3.1 AR-null macrophages are larger in size
Western blotting results confirmed that AR was abundant in WT-BMMs (upper panel) but was undetectable in AR-null BMMs (Fig. 1A). Deletion of AR did not alter the expression of housekeeping protein; alpha tubulin (lower panel). During regular culturing of the cells, AR-null BMMs appeared to be larger than the WT-BMMs. To confirm this observation, we synchronized both WT-BMMs and AR-null BMMs using cell culture media containing 0.1% fetal bovine serum (FBS). Calcein Green, AM was added and the cell-area was recorded using the ‘Magic Wand” tool (Image J software). As shown in Fig. 1B, the AR-null cells appear larger than WT cells. The mean area of the AR-null BMMs was 192 μm2, whereas that of the WT cells was 138 μm2 (Fig. 1Ci). Frequency distribution of cell size indicated a higher number of cells with a larger cell size (Fig. 1Cii, and 1Ciii). These data indicate that deletion of AR results in a significant increase in BMM cell size.
Fig. 1. Western blotting shows abundance of AR protein in WT-BMMs and its absence in AR-null BMMs.
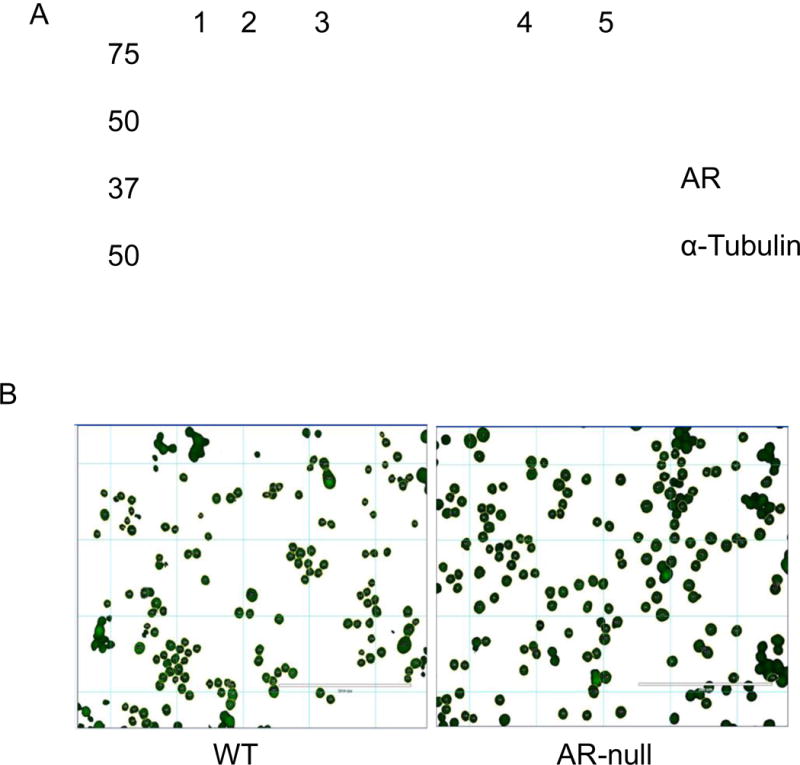
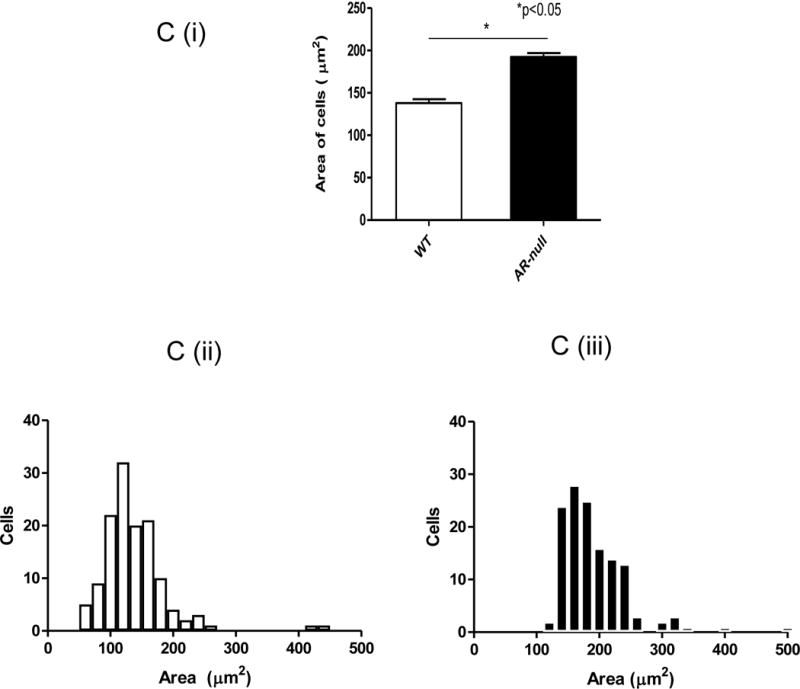
Lysates were prepared from BMMs and probed with a monoclonal antibody against AR (A). Total protein equivalent to 10 μg in lane 2, and 30 μg in lane 4 was loaded from WT-BMMs and an identical amount of 10 μg in lane 3, and 30 μg in lane 5 was loaded from AR-null BMMs (upper panel). Deficiency of AR did not have any effect on the expression of housekeeping protein α-tubulin (lower panel). Lane 1 represents the protein marker. Calcein Green, AM (B) was used to stain live cells and to measure the cell-area employing the ‘Magic Wand” tool (Image J software). The measurements revealed an increase in the mean area of the AR-null BMMs compared to WT-BMMs (Ci, Cii, Ciii). Experiment were repeated a minimum of 3–5 times, and the data represent mean ± SEM, *P<0.05.
3.2 AR deficiency slows macrophage growth despite higher ATP levels
In addition to being larger, the AR-null cells also displayed slower growth. Flow cytometry analysis of doubling time showed that the doubling time of AR-null cells was significantly longer (18 h) than the doubling time of WT cells (16 h; Fig. 2A). To examine whether this slower rate of growth was due to a decrease in energy, we measured ATP levels of WT and AR-null BMM. As shown in Fig. 2B, the ATP levels in AR-null BMM were significantly higher than the ATP levels in WT cells. Taken together, these results indicate that despite higher ATP content in the AR-null BMMs, AR deficiency slowed down the macrophage growth, thus suggesting a regulatory role of AR in macrophage proliferation [20].
Fig. 2. Lack of AR affects macrophages’ rate of division and the ATP levels.
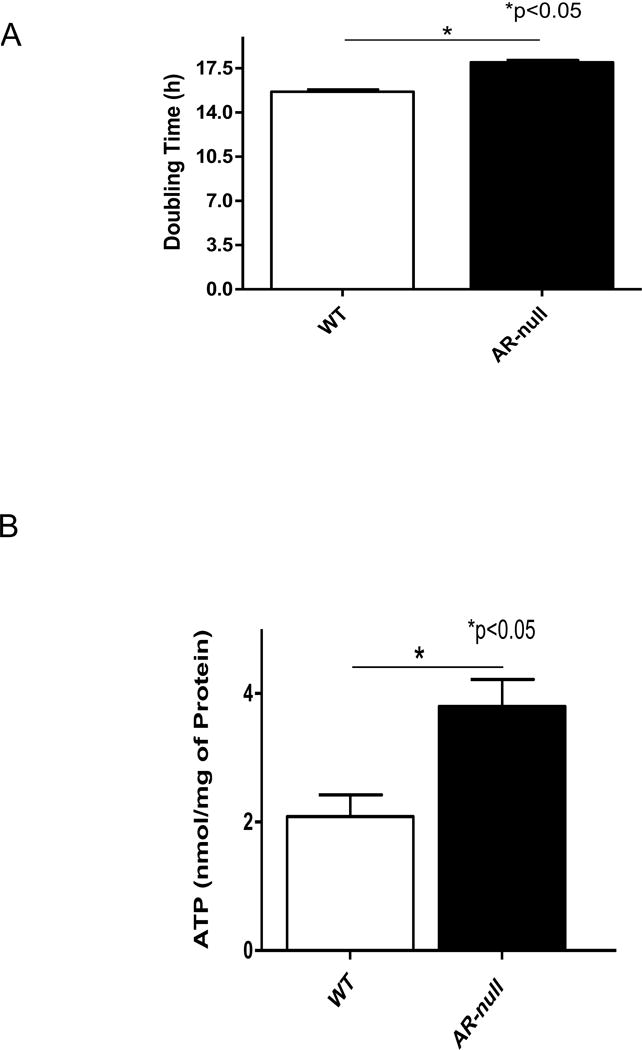
AR-null BMMs grow slowly than WT-BMMs (A) despite having the higher ATP levels (B). Experiment were repeated a minimum of 3–5 times, and the data represent mean ± SEM, *P<0.05.
3.3 Deletion of AR increases macrophage phagocytosis
To measure the phagocytic function of WT and AR-null BMM, IgG coated latex beads labeled with phycoerythrin (PE) were added to medium containing macrophages in 6-well plates and the cells were incubated at 37°C, in 5% CO2. Fluorescence microscopic evaluation showed that there were more phagosomes per cell in AR-null BMMs than in the WT-BMMs, suggesting that AR-null BMM had a higher phagocytic activity (Fig. 3A, lower panel ii). To examine the time, course of this difference, we incubated BMM with the latex beads for different times and measured their uptake. As shown in Fig. 3B, the uptake of fluorescent beads by AR-null BMM was significantly higher at all-time durations examined, such that the total number of beads per cell in AR-null BMM exceeded the number of beads per WT BMMs Fig. 3C). To examine whether in addition to affecting basal phagocytosis, deletion of AR also affects phagocytosis stimulated by LPS, we examined the uptake of latex beads with or without LPS pre-treatment. Calcein green, AM was used to capture live cell images and the comparable data were obtained for both cell types. As shown in Fig. 4, both AR-null and WT-BMMs engulfed the beads but AR-null BMMs exhibited more phagocytosis of beads than the WT-BMMs. LPS treatment further increased their phagocytic activities. Taken together these data suggest that the presence of AR impairs phagocytosis, and that its absence enhances the phagocytic activity of BMM.
Fig. 3. AR-null deficient macrophages show enhanced phagocytosis.
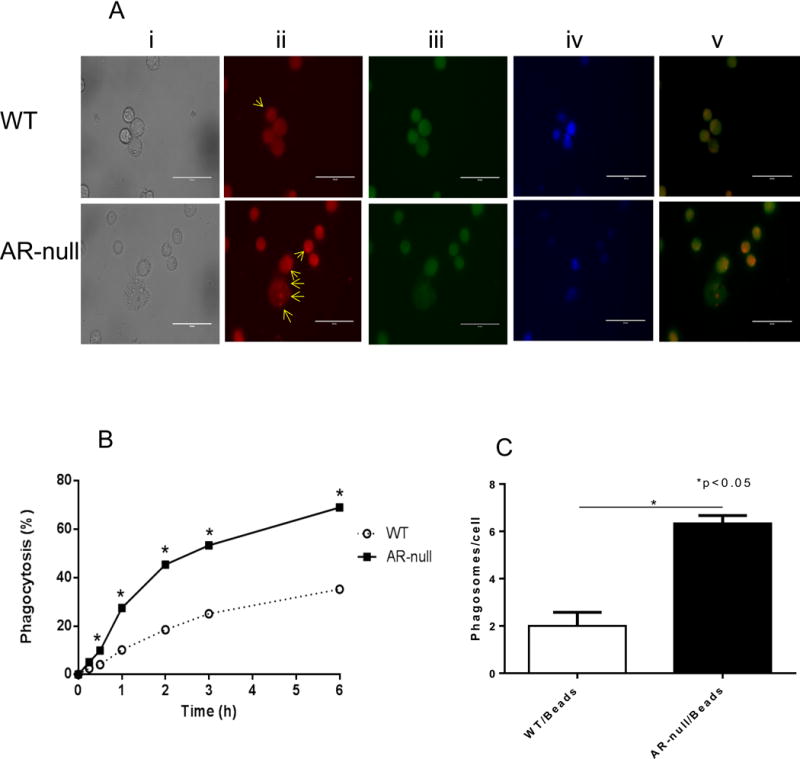
PE-labeled IgG coated latex beads were used to test phagocytosis in live BMMs (A). AR-null BMMs show more phagosomes per cell than WT-BMMs (A; lower panel ii). Percent phagocytosis was quantitated at different time intervals (B) indicating enhanced phagocytosis in AR-null BMMs (C). Scheme in panel (A) represent transmitted image (i), PE-labeled phagosomes (ii), Calcein Green, AM (iii), DAPI (iv) and overlay of PE and Calcein Green, AM (v). Arrows indicate phagosomes in the BMMs). Experiment were repeated a minimum of 3–5 times, and the data represent mean ± SEM, *P<0.05.
Fig. 4. The phagocytosis by BMMs was stimulated by LPS treatment.
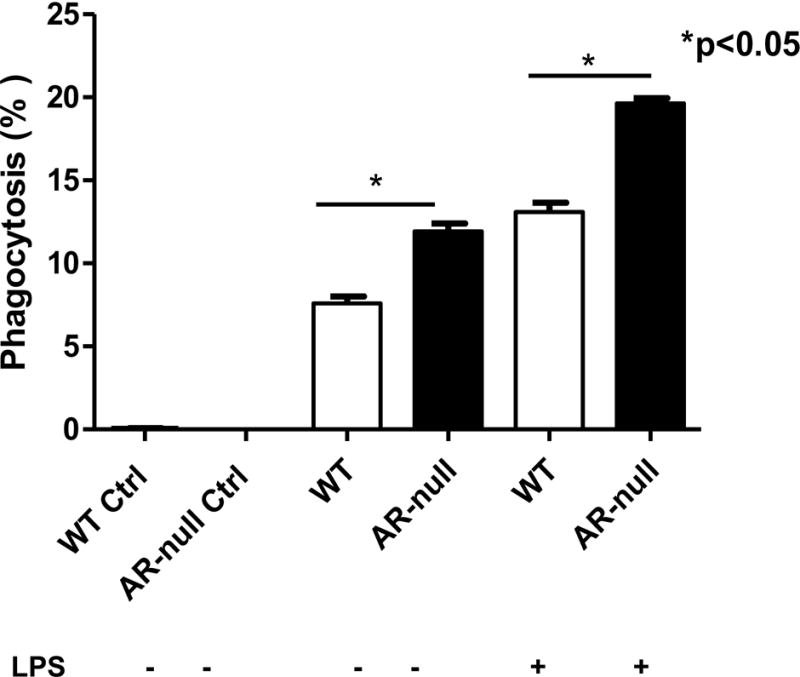
Uptake of latex beads by WT and AR-null BMMs with and without stimulation with LPS. Experiment were repeated a minimum of 3–5 times, and the data represent mean ± SEM, *P<0.05.
3.4 Regulation of NADPH level by AR
Because AR uses NADPH to reduce its aldehydic substrates, increased activity of AR suppresses the cellular levels of NADPH. Hence, to determine whether deletion of AR affects intracellular NADP/NADPH levels we measured the levels of these nucleotides in WT and AR-null BMMs using a NADP/NADPH assay system in which NADPH generated by a glucose dehydrogenase cycling reaction reduces a formazan reagent. The optical density of is the reduced reagent is read at 570 nm. As expected the levels of NADPH contents were lower in WT-BMMs than the AR-null BMMs (Fig. 5A). This increase in cellular NADPH levels led to an overall increase in the total amount of both the NADPH and the NADP (NADPH+NADP) in AR deficient cells. (Fig. 5B). These data demonstrate that AR regulates coenzymes’ levels in macrophages, and this regulatory mechanism may be important in regulating the innate immune responses in macrophages.
Fig. 5. AR regulates NADPH levels in BMMs.
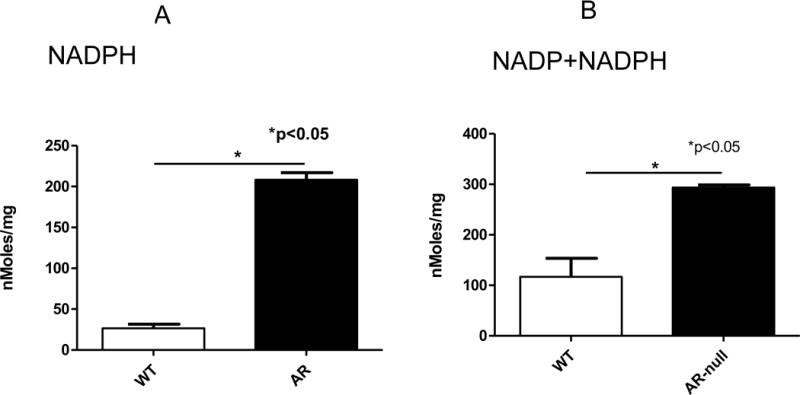
Levels of cellular NADPH in WT-BMMs than AR-null BMMs. Experiment were repeated a minimum of 3 times, and the data represent mean ± SEM, *P<0.05.
4. Discussion
The major findings of this study are that deletion of AR increases cell size and doubling time of BMM. These changes were accompanied by an increase in the phagocytic capacity in AR-null cells, which had higher levels of ATP and NADPH than WT cells. These results suggest that AR regulates both the growth and the phagocytic function of macrophages; and therefore, may be an important modulator of immune responses.
Aldose reductase is an aldo-keto reductase that catalyzes NADPH-dependent reduction of aldehydes [21, 22]. Although the active site of the enzyme is not well-suited for glucose binding, the enzyme displays low activity with glucose. Under normal glucose conditions, reduction of glucose by AR represents a minor pathway of glucose metabolism, however, under conditions of hyperglycemia, a significant proportion of glucose is reduced by AR to sorbitol. Accumulation of glucose-derived sorbitol has been linked to osmoregulation, particularly in renal tubular cells, which upon high osmotic conditions, accumulate sorbitol to balance the osmotic gap [23]. The contribution of AR in osmotic regulation in other cells is not well known. Because AR reduces un-phosphorylated glucose, it completes with glycolysis for glucose, and therefore an increase in AR activity decreases the flux of glucose through the glycolytic pathway [24] by promoting sorbitol synthesis. Moreover, conversion of sorbitol to fructose converts NAD+ to NADH, resulting the accumulation of NADH, which would further inhibit glycolytic activity by creating a state of pseudo-hypoxia [25, 26]. In agreement with this view, inhibition of AR has been found to increase glycolytic activity [27, 28], presumably by making more glucose available for glycolysis and increasing the NAD+/NADH ratio. The regulation of glycolysis by AR has cell-specific effects; in cells that rely largely on glycolysis for energy production, increased activity of AR could induce a state of energy deprivation. Moreover, even in tissues that do not derive most of their energy from glucose, increased AR activity could compromise energy production under conditions that need increased glycolytic activity such as hypoxia or ischemia.
Since glycolysis plays an important role in fueling initial response to inflammatory challenges in macrophages, which switch from oxidative phosphorylation to glycolysis [29], we examined how deletion of AR would affect macrophage function and growth. We found that inhibition of AR slowed macrophage growth, but it increased their phagocytic activity. These findings are consistent with previous observations showing that upon inflammatory challenge, such as stimulation with the TLR4 agonist LPS, macrophages upregulate their glycolytic activity, which is required not only to fuel increased cytokine production, but also phagocytosis [30]. These studies have shown that different macrophage activities, like spreading, formation of cell protrusions, as a cell phagocytosis of complement-opsonized particles rely on extracellular glucose and are fueled by glycolysis, not oxidative phosphorylation [30]. Hence, the increase phagocytosis in AR-null macrophages is consistent with increased glycolysis and elevated levels of ATP in these cells. During their phagocytic activity, macrophages have high energy demand [31], and reduced phagocytosis in macrophages has been associated with a reduction in intracellular ATP contents [32]. Thus, elevated levels of ATP in AR-null macrophages could explain, at least in part, the high phagocytic activity of these cells. Similarly, an increase in NADPH levels in the AR-null mice would likely provide reducing equivalents for protection of macrophages against reactive oxygen species and for biosynthesis required for phagocytosis and microbial clearance.
Our data showing that AR deficiency up-regulates phagocytosis has wide implications in understanding the metabolic dependence of inflammatory states such as sepsis, which is a life-threatening dysregulated host response that triggers serious inflammation and multi-organ failure. During sepsis, hypo-responsive immune cells struggle to clear infections. Thus, inhibition of AR, leading to an increase in the phagocytic capacity of macrophages may improve survival during sepsis. Also, improved immunity by AR-null macrophage may reverse/replace the hypo-responsive endotoxin-tolerant cells that develop and persist during sepsis. Indeed, our previous studies have shown that inhibition of AR prevents LPS-induced sepsis, cardiac dysfunction, and mortality in mice [11, 15].
In the past, AR has received considerable attention as a potential therapeutic target for preventing secondary diabetic complications [21, 22]. However, our data suggest that regulation of macrophage function may be an equally important aspect of the physiological role of AR. If such a role could be substantiated in vivo, it would indicate that pharmacological inhibition of AR could help control inflammatory milieu stemming from either injury or pathogens. In this regard, systematic examination of metabolic capacity of cells from sepsis patients and comparative analysis of cell metabolism in healthy subjects will be informative to help treat patients with aggressive and systemic inflammation, particularly in the setting of metabolic diseases. Nonetheless, because AR can catalyze the reduction of a wide range of aldehydes, the entirety of its function cannot be assessed from studying one specific pathway. For instance, even though in our experiments inhibition of AR acutely increased macrophage phagocytosis, chronically, loss of AR activity could result in the accumulation of toxic lipid peroxidation products that could induce cell dysfunction and death. In vitro experiments show that AR displays much higher catalytic activity with products of lipid peroxidation such as HNE and acrolein and their glutathione conjugates and chronic treatment with AR inhibitors or deletion of the AR gene promotes the accumulation of aldehyde-protein adducts [33] as well as advanced glycosylation end products [34]. Hence, further studies are required to understand the role of AR in regulating immune response in the context of its function as a detoxification enzyme and to develop treatment strategies that maximize the beneficial, while minimizing the potentially harmful effects of AR inhibition.
Acknowledgments
Authors are grateful to Lihua Zhang for her help in generating bone marrow-derived macrophage (BMM) cell lines, Susan Dougherty, and Nalinie Wickramasinghe for maintaining initial BMMs cultures. This work was supported by National Institutes of Health grants HL55477, HL59378, HL65660, HL78825, and GM103492.
Abbreviations
- AR
aldose reductase
- ATP
adenosine triphosphate
- BMMs
bone marrow derived macrophages
- FcγRs
Fc gamma receptors
- FBS
fetal bovine serum
- IgG
immunoglobulin G
- LPS
lipopolysaccharides
- NADP
nicotinamide adenine dinucleotide
- NADPH
nicotinamide adenine dinucleotide phosphate
- PE
phycoerythrin
- ROS
reactive oxygen species
Footnotes
Conflict of interest statement
The authors have nothing to declare.
References
- 1.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation, Nature reviews. Immunology. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stefater JA, 3rd, Ren S, Lang RA, Duffield JS. Metchnikoff’s policemen: macrophages in development, homeostasis and regeneration. Trends in molecular medicine. 2011;17:743–752. doi: 10.1016/j.molmed.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newsholme P, Curi R, Gordon S, Newsholme EA. Metabolism of glucose, glutamine, long-chain fatty acids and ketone bodies by murine macrophages. The Biochemical journal. 1986;239:121–125. doi: 10.1042/bj2390121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guminska M, Ptak W, Zembala M. Macrophage metabolism during phagocytosis and digestion of normal and IgG antibody-coated sheep erythrocytes. Enzyme. 1975;19:24–37. doi: 10.1159/000458969. [DOI] [PubMed] [Google Scholar]
- 5.Singh M, Kapoor A, Bhatnagar A. Oxidative and reductive metabolism of lipid-peroxidation derived carbonyls. Chemico-biological interactions. 2015;234:261–273. doi: 10.1016/j.cbi.2014.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee AY, Chung SS. Contributions of polyol pathway to oxidative stress in diabetic cataract. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1999;13:23–30. doi: 10.1096/fasebj.13.1.23. [DOI] [PubMed] [Google Scholar]
- 7.Hamada Y, Kitoh R, Raskin P. Crucial role of aldose reductase activity and plasma glucose level in sorbitol accumulation in erythrocytes from diabetic patients. Diabetes. 1991;40:1233–1240. doi: 10.2337/diab.40.10.1233. [DOI] [PubMed] [Google Scholar]
- 8.Gleissner CA, Sanders JM, Nadler J, Ley K. Upregulation of aldose reductase during foam cell formation as possible link among diabetes, hyperlipidemia, and atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2008;28:1137–1143. doi: 10.1161/ATVBAHA.107.158295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramana KV, Fadl AA, Tammali R, Reddy AB, Chopra AK, Srivastava SK. Aldose reductase mediates the lipopolysaccharide-induced release of inflammatory mediators in RAW264.7 murine macrophages. The Journal of biological chemistry. 2006;281:33019–33029. doi: 10.1074/jbc.M603819200. [DOI] [PubMed] [Google Scholar]
- 10.Ramana KV, Srivastava SK. Mediation of aldose reductase in lipopolysaccharide-induced inflammatory signals in mouse peritoneal macrophages. Cytokine. 2006;36:115–122. doi: 10.1016/j.cyto.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reddy AB, Srivastava SK, Ramana KV. Anti-inflammatory effect of aldose reductase inhibition in murine polymicrobial sepsis. Cytokine. 2009;48:170–176. doi: 10.1016/j.cyto.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ravindranath TM, Mong PY, Ananthakrishnan R, Li Q, Quadri N, Schmidt AM, Ramasamy R, Wang Q. Novel role for aldose reductase in mediating acute inflammatory responses in the lung. Journal of immunology. 2009;183:8128–8137. doi: 10.4049/jimmunol.0900720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yadav UC, Ramana KV, Aguilera-Aguirre L, Boldogh I, Boulares HA, Srivastava SK. Inhibition of aldose reductase prevents experimental allergic airway inflammation in mice. PloS one. 2009;4:e6535. doi: 10.1371/journal.pone.0006535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yadav UC, Ramana KV, Srivastava SK. Aldose reductase inhibition suppresses airway inflammation. Chemico-biological interactions. 2011;191:339–345. doi: 10.1016/j.cbi.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramana KV, Willis MS, White MD, Horton JW, DiMaio JM, Srivastava D, Bhatnagar A, Srivastava SK. Endotoxin-induced cardiomyopathy and systemic inflammation in mice is prevented by aldose reductase inhibition. Circulation. 2006;114:1838–1846. doi: 10.1161/CIRCULATIONAHA.106.630830. [DOI] [PubMed] [Google Scholar]
- 16.Yagihashi S, Mizukami H, Ogasawara S, Yamagishi S, Nukada H, Kato N, Hibi C, Chung S, Chung S. The role of the polyol pathway in acute kidney injury caused by hindlimb ischaemia in mice. The Journal of pathology. 2010;220:530–541. doi: 10.1002/path.2671. [DOI] [PubMed] [Google Scholar]
- 17.Ramasamy R, Oates PJ, Schaefer S. Aldose reductase inhibition protects diabetic and nondiabetic rat hearts from ischemic injury. Diabetes. 1997;46:292–300. doi: 10.2337/diab.46.2.292. [DOI] [PubMed] [Google Scholar]
- 18.Tracey WR, Magee WP, Ellery CA, MacAndrew JT, Smith AH, Knight DR, Oates PJ. Aldose reductase inhibition alone or combined with an adenosine A(3) agonist reduces ischemic myocardial injury. American journal of physiology Heart and circulatory physiology. 2000;279:H1447–1452. doi: 10.1152/ajpheart.2000.279.4.H1447. [DOI] [PubMed] [Google Scholar]
- 19.Clemons-Miller AR, Cox GW, Suttles J, Stout RD. LPS stimulation of TNF-receptor deficient macrophages: a differential role for TNF-alpha autocrine signaling in the induction of cytokine and nitric oxide production. Immunobiology. 2000;202:477–492. doi: 10.1016/s0171-2985(00)80105-9. [DOI] [PubMed] [Google Scholar]
- 20.Tarrats N, Moles A, Morales A, Garcia-Ruiz C, Fernandez-Checa JC, Mari M. Critical role of tumor necrosis factor receptor 1, but not 2, in hepatic stellate cell proliferation, extracellular matrix remodeling, and liver fibrogenesis. Hepatology. 2011;54:319–327. doi: 10.1002/hep.24388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Srivastava SK, Ramana KV, Bhatnagar A. Role of aldose reductase and oxidative damage in diabetes and the consequent potential for therapeutic options. Endocrine reviews. 2005;26:380–392. doi: 10.1210/er.2004-0028. [DOI] [PubMed] [Google Scholar]
- 22.Barski OA, Tipparaju SM, Bhatnagar A. The aldo-keto reductase superfamily and its role in drug metabolism and detoxification. Drug metabolism reviews. 2008;40:553–624. doi: 10.1080/03602530802431439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia-Perez A, Burg MB. Renal medullary organic osmolytes. Physiological reviews. 1991;71:1081–1115. doi: 10.1152/physrev.1991.71.4.1081. [DOI] [PubMed] [Google Scholar]
- 24.Hwang YC, Sato S, Tsai JY, Yan S, Bakr S, Zhang H, Oates PJ, Ramasamy R. Aldose reductase activation is a key component of myocardial response to ischemia. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2002;16:243–245. doi: 10.1096/fj.01-0368fje. [DOI] [PubMed] [Google Scholar]
- 25.Tilton RG, Baier LD, Harlow JE, Smith SR, Ostrow E, Williamson JR. Diabetes-induced glomerular dysfunction: links to a more reduced cytosolic ratio of NADH/NAD+ Kidney international. 1992;41:778–788. doi: 10.1038/ki.1992.121. [DOI] [PubMed] [Google Scholar]
- 26.Van den Enden MK, Nyengaard JR, Ostrow E, Burgan JH, Williamson JR. Elevated glucose levels increase retinal glycolysis and sorbitol pathway metabolism. Implications for diabetic retinopathy. Investigative ophthalmology & visual science. 1995;36:1675–1685. [PubMed] [Google Scholar]
- 27.Trueblood N, Ramasamy R. Aldose reductase inhibition improves altered glucose metabolism of isolated diabetic rat hearts. The American journal of physiology. 1998;275:H75–83. doi: 10.1152/ajpheart.1998.275.1.H75. [DOI] [PubMed] [Google Scholar]
- 28.Ramasamy R, Trueblood N, Schaefer S. Metabolic effects of aldose reductase inhibition during low-flow ischemia and reperfusion. The American journal of physiology. 1998;275:H195–203. doi: 10.1152/ajpheart.1998.275.1.H195. [DOI] [PubMed] [Google Scholar]
- 29.Mills EL, Kelly B, Logan A, Costa AS, Varma M, Bryant CE, Tourlomousis P, Dabritz JH, Gottlieb E, Latorre I, Corr SC, McManus G, Ryan D, Jacobs HT, Szibor M, Xavier RJ, Braun T, Frezza C, Murphy MP, O’Neill LA. Succinate Dehydrogenase Supports Metabolic Repurposing of Mitochondria to Drive Inflammatory Macrophages. Cell. 2016;167:457–470.e413. doi: 10.1016/j.cell.2016.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Venter G, Oerlemans FT, Wijers M, Willemse M, Fransen JA, Wieringa B. Glucose controls morphodynamics of LPS-stimulated macrophages. PloS one. 2014;9:e96786. doi: 10.1371/journal.pone.0096786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oorni K, Rajamaki K, Nguyen SD, Lahdesmaki K, Plihtari R, Lee-Rueckert M, Kovanen PT. Acidification of the intimal fluid: the perfect storm for atherogenesis. Journal of lipid research. 2015;56:203–214. doi: 10.1194/jlr.R050252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu BF, Miyata S, Kojima H, Uriuhara A, Kusunoki H, Suzuki K, Kasuga M. Low phagocytic activity of resident peritoneal macrophages in diabetic mice: relevance to the formation of advanced glycation end products. Diabetes. 1999;48:2074–2082. doi: 10.2337/diabetes.48.10.2074. [DOI] [PubMed] [Google Scholar]
- 33.Srivastava S, Vladykovskaya E, Barski OA, Spite M, Kaiserova K, Petrash JM, Chung SS, Hunt G, Dawn B, Bhatnagar A. Aldose reductase protects against early atherosclerotic lesion formation in apolipoprotein E-null mice. Circulation research. 2009;105:793–802. doi: 10.1161/CIRCRESAHA.109.200568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baba SP, Hellmann J, Srivastava S, Bhatnagar A. Aldose reductase (AKR1B3) regulates the accumulation of advanced glycosylation end products (AGEs) and the expression of AGE receptor (RAGE) Chemico-biological interactions. 2011;191:357–363. doi: 10.1016/j.cbi.2011.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]


