Summary
Certain plant-associated microbes can produce gibberellin (GA) phytohormones, as first described for the rice fungal pathogen Gibberella fujikuroi and more recently for bacteria, including several rhizobia and the rice bacterial pathogen Xanthomonas oryzae pv. oryzicola. The relevant enzymes are encoded by a biosynthetic operon that exhibits both a greater phylogenetic range and scattered distribution among plant-associated bacteria. Here the phylogenetic distribution of this operon was investigated. To demonstrate conserved functionality, the enzymes encoded by the disparate operon from Xanthomonas translucens pv. translucens, along with those from the most divergent example, found in Erwinia tracheiphila, were biochemically characterized. In both of these phytopathogens the operon leads to production of the bioactive GA4. Based on these results, it seems that this operon is widely dedicated to GA biosynthesis. However, there is intriguing variation in exact product. In particular, although all plant pathogens seem to produce bioactive GA4, rhizobia generally only produce the penultimate hormonal precursor GA9. This is suggested to reflect their distinct interactions with plants, as production of GA4 counteracts the jasmonic acid-mediated defense response, reflecting the importance of wounds as the entry point for these phytopathogens, while such suppression presumably is detrimental in the rhizobial symbiotic relationship.
Introduction
The development of multicellular organisms is regulated by a multitude of hormones. In plants gibberellins (GAs) control stem elongation, germination, dormancy, flowering, leaf senescence and fruit development (Hedden and Thomas, 2012). Notably, biosynthesis of GAs is not limited to plants. Indeed, GAs were first isolated from the fungus Gibberella fujikuroi, causal agent of the bakanae or foolish seedling disease in rice (Oryza sativa) (Hedden and Sponsel, 2015). This hemibiotrophic fungal phytopathogen seems to produce GAs as a virulence factor (Wiemann et al., 2013). The bioactive GA presumably causes hormonal imbalances in the plant, which negatively affects the defense response, particularly that dependent on jasmonic acid (JA) (Robert-Seilaniantz et al., 2007).
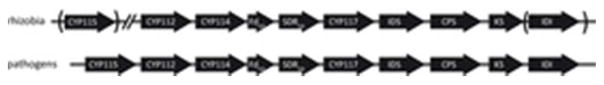
Certain bacteria also produce GAs (Bottini et al., 2004). However, while plant and fungal GA biosynthesis has been extensively characterized (Hedden and Thomas, 2012), the relevant pathway in bacteria has only recently been fully elucidated (Tatsukami and Ueda, 2016; Nett et al., 2017b; Nagel et al., 2017). Bacterial GA biosynthesis is associated with a cytochrome P450 (CYP)-rich operon (Figure 1), as demonstrated by functional characterization of the encoded enzymes. This includes the relevant synthases that together produce ent-kaurene – i.e., an isoprenyl diphosphate synthase (IDS) that produces (E,E,E)-geranylgeranyl diphosphate (GGDP), subsequently acting ent-copalyl diphosphate (ent-CDP) synthase (CPS), and ent-kaurene synthase (KS) – which were first characterized from symbiotic rhizobia that fall within the Rhizobiales order in the α-proteobacteria class (Morrone et al., 2009; Hershey et al., 2014). In addition, the homologous CPS and KS from a more distantly related operon found in Xanthomonas oryzae pv. oryzicola from the separate γ-proteobacteria class were found to be functionally analogous, and the ultimate product of the operon also demonstrated to act as a virulence factor for this rice pathogen (Lu et al., 2015). Even more recently, the three CYPs (CYP112, CYP114 & CYP117), along with ferredoxin (FdGA) and short-chain alcohol dehydrogenase/reductase (SDRGA), found in all copies of the operon were shown to transform ent-kaurene to GA9, although this is not thought to exert hormonal activity (Tatsukami and Ueda, 2016; Nett et al., 2017b; Nagel et al., 2017). Notably, the additional CYP (CYP115) found associated with the operon in the phytopathogen X. oryzae pv. oryzicola carries out the final 3β-hydroxylation reaction necessary to produce the bioactive GA4 (Nagel et al., 2017). Intriguingly, although almost all copies of the operon characterized from rhizobia have only a fragment of CYP115 remaining, it has been shown that some (~16%) also contain a functional copy of CYP115 elsewhere in their genomes, which seems to have been acquired via a separate horizontal gene transfer event from the core GA biosynthetic operon (Nett et al., 2017a).
Figure 1. Schematic representation of the GA biosynthetic operon.
Arrows indicate the direction of translation, abbreviations for the genes are CYP, cytochrome P450; Fd, ferredoxin; SDR, short-chain alcohol dehydrogenase/reductase; IDS, isoprenyl diphosphate synthase; CPS, copalyl diphosphate synthase; KS, ent-kaurene synthase; and IDI, isopentenyl diphosphate isomerase.
Perhaps not surprisingly, although the GA biosynthetic operon exhibits a highly scattered distribution, it seems to be exclusively found in plant-associated bacteria (personal communication, Dr. Asaf Levy, US-DoE Joint Genome Institute). In particular, the operon is found in sub-sets of both symbiotic nitrogen-fixing rhizobia and phytopathogens. It has been suggested that the production of GA9 by rhizobia is followed by conversion to bioactive GA4 by the host plant, and further proposed that this acts to block any further nodulation (Tatsukami and Ueda, 2016), although the investigated Mesorhizobium loti MAFF303099 is capable of producing GA4 directly (Nett et al., 2017a). On the other hand, the direct production of GA4 has been hypothesized to reflect the importance of entry via wounded leaf tissue, with associated JA-dependent defense response, for the relevant phytopathogens (Nagel et al., 2017).
Here bacterial GA biosynthesis is further investigated by functional characterization of all the encoded enzymes from two copies of the relevant operon whose sequences are only distantly related to those already elucidated and each other, covering the full phylogenetic range of this sequence element. These both lead to production of the bioactive GA4, and speculation about the relevance of bacterial production of bioactive GA or the penultimate hormonal precursor is discussed.
Results
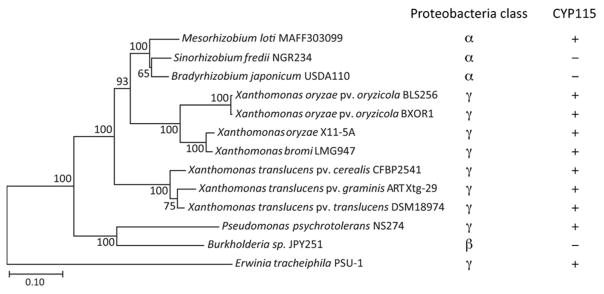
It has been previously reported that the GA biosynthetic operon also is found in various pathovars of Xanthomonas translucens, where it also includes CYP115, but is only distantly related to either those from the Rhizobiales or X. oryzae pv. oryzicola (Lu et al., 2015). To determine if the phylogenetically distinct operon from X. translucens also can impart the ability to produce GA4 (i.e., is functionally analogous to that from X. oryzae pv. oryzicola), the encoded enzymes were investigated here, specifically those from X. translucens pv. translucens DSM18974. In addition, the phylogenetic range of the GA biosynthetic operon was further investigated by searching the publically available sequence databases at NCBI and the Joint Genome Institute, which revealed the presence of a homologous sequence element in a variety of other plant-associated bacteria. In every case, these contained at least the eight genes that compromise the core GA biosynthetic operon (Figure 1). Indeed, while homologs for these genes can be found in other settings (i.e., in operons with different compositions, containing no more than three of these genes), those exhibit much lower homology, < 65% amino acid (aa) sequence identity (Supplemental Table S1), relative to those found in the putative GA operons, which have > 75% aa sequence identity. Beyond the copies of the operon already noted above, even more divergent examples were found here, as demonstrated by phylogenetic analysis (Figure 2 and Supplemental Figure S1). These include examples from not only additional genera in the γ-proteobacteria class, but also rhizobia that fall within the distinct β-proteobacteria class. To determine if the range of operons observed here are involved in GA biosynthesis as expected, the encoded enzymes from the most divergent example, that from Erwinia tracheiphila, which share < 77% aa sequence identity with any homolog, also were investigated here. Specifically, each of the genes from these two copies of the GA biosynthetic operon were expressed in Escherichia coli and the activity of the recombinant enzymes functionally characterized.
Figure 2. Phylogeny of the GA biosynthetic operon.
Unrooted Maximum likelihood phylogenetic tree of the concatenated amino acid sequence of the enzymes from the operon spanning the completely conserved CYP112 to KS. Bootstrap values were determined with MEGA 7 and the scale bar represents substitutions per site. Also indicated are the relevant classes of proteobacteria and the presence of CYP115.
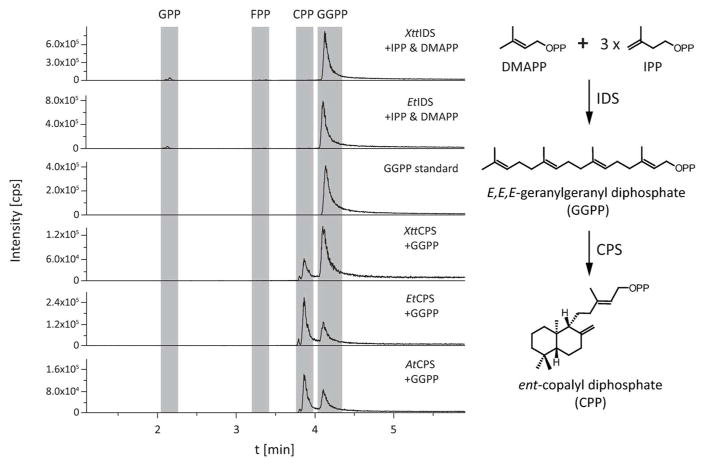
As bacteria do not typically produce diterpenes such as GAs, such biosynthesis requires production of the general diterpene precursor GGDP from the universal isoprenoid 5-carbon precursors isopentenyl diphosphate (IDP) and dimethylallyl diphosphate (DMADP). The IDS from the GA biosynthetic operon has only been indirectly characterized so far, by demonstrating that co-expression of the IDS with the CPS and KS is required for recombinant production of ent-kaurene, specifically using these genes from the α-rhizobia Sinorhizobium fredii (Hershey et al., 2014). Here the activity of the IDSs from X. translucens pv. translucens (XttIDS) and E. tracheiphila (EtIDS) were more directly investigated. In particular, using in vitro assays with purified enzymes and direct detection of the resulting enzymatic products by liquid chromatography with tandem mass spectral detection (LC-MS/MS), which demonstrated that both XttIDS and EtIDS react with IDP and DMADP to specifically produce the 20-carbon GGDP, with only very minor amounts of the initial 10-carbon elongation intermediate geranyl diphosphate, and none of the intermediary 15-carbon intermediate (E,E)-farnesyl diphosphate observed (Figure 3). Thus, these IDSs are specific GGDP synthases.
Figure 3. IDS and CPS activity. (Left panel).
LC-MS/MS chromatograms from in vitro assays with purified IDS from either X. translucens pv. translucens (XttIDS) or E. tracheiphila (EtIDS) with IDP and DMADP as substrates, with comparison to a GGPP standard; or with purified CPS from either A. thaliana (AtCPS), X. translucens pv. translucens (XttCPS), or E. tracheiphila (EtCPS), with GGPP as substrate. (Right panel) Condensation of DMAPP with three molecules of IPP to form GGPP catalyzed by IDSs, and cyclization of GGPP to ent-CPP catalyzed by CPSs.
Previous characterization of the CPSs from the GA biosynthetic operon relied upon indirect detection of the ent-CDP product, with observation by gas chromatography with mass spectral detection (GC-MS) of the dephosphorylated ent-copalol, or subsequently produced ent-kaurene upon co-expression of a KS, from bacteria also engineered to produce GGDP (Morrone et al., 2009; Hershey et al., 2014; Lu et al., 2015). While analogous studies were carried out with the CPSs from X. translucens pv. translucens (XttCPS) and E. tracheiphila (EtCPS) (Supplemental Figure S2), a more direct approach was taken here to characterize these CPSs. Again, using purified enzymes in in vitro enzyme assays with GGDP as substrate and direct detection of the enzymatic products by LC-MS/MS. This approach was first validated by analysis of the known CPS from Arabidopsis thaliana (AtCPS), which further provided an authentic sample of ent-CDP for comparison to the products of XttCPS and EtCPS, both of which exhibited the predicted activity (Figure 3).
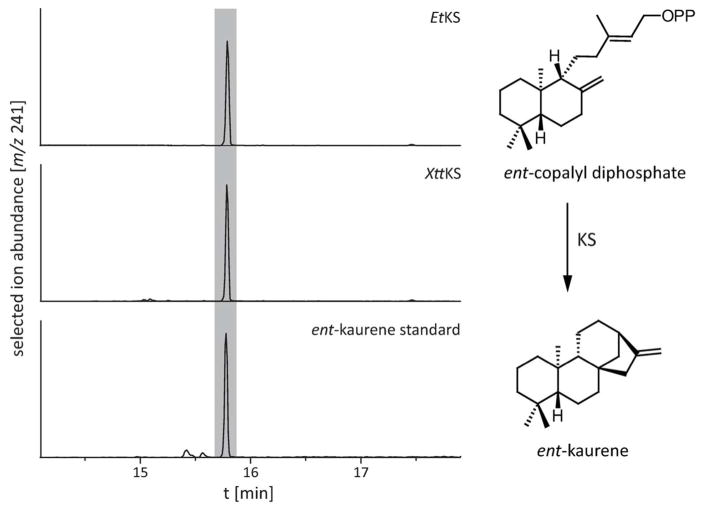
Characterization of the KSs from X. translucens pv. translucens (XttKS) and E. tracheiphila (EtKS) was carried out as previously described (Morrone et al., 2009; Hershey et al., 2014; Lu et al., 2015). In particular, using a metabolic engineering approach in which the KSs were expressed in E. coli also engineered to produce ent-CPP. Such cultures expressing either XttKS or EtKS exhibited the predicted ability to produce ent-kaurene, as detected by extraction and GC-MS analysis (Figure 4).
Figure 4. KS activity. (Left Panel).
GC-MS chromatograms of hexane extract from E. coli metabolically engineered to produce ent-CPP and expressing the KS from either X. translucens pv. translucens (XttKS) or E. tracheiphila (EtKS), and of an authentic ent-kaurene standard. (Right Panel) Cyclization of ent-CPP to ent-kaurene catalyzed by KSs.
The oxidative enzymes catalyzing the remaining steps in GA biosynthesis were functionally characterized by recombinant feeding studies, much as previously described (Nagel et al., 2017). Briefly, each of the CYPs and SDRGA were individually expressed in E. coli, which cannot otherwise catalyze these transformations. The CYP114s also were co-expressed with the accompanying FdGA, as this has been shown to be required for full activity of this CYP – the other CYPs are presumably efficiently reduced by endogenous ferredoxins (Nett et al., 2017b). The resulting recombinant strains were each fed the relevant substrate, and the cultures then extracted for GC-MS analysis to determine if the predicted enzymatic function was observed.
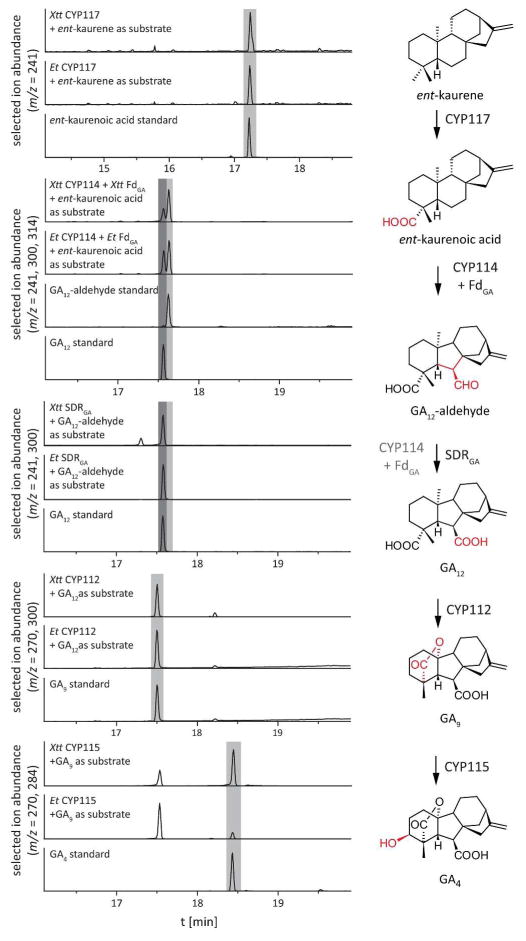
E. coli expressing CYP117 from either X. translucens pv. translucens (XttCYP117) or E. tracheiphila (EtCYP117) efficiently converted ent-kaurene to ent-kauren-19-oic acid (Figure 5 and Supplemental Figure S3). Indeed, this multi-step transformation proceeded to essentially completion, as the intermediates ent-kauren-19-ol and ent-kauren-19-al were not detected.
Figure 5. Activity of the oxidative enzymes. (Left Panel).
GC-MS chromatograms of organic extracts from E. coli cultures expressing the indicated enzymes and fed the indicated substrate, with chromatograms of standard compounds as a reference. Standards and extracts were methylated and in case of GA4 also silylated. Enzymes from X. translucens pv. translucens are indicated by a Xtt prefix, while those from E. tracheiphila by a Et prefix. (Right panel) Reactions catalyzed by the indicated enzymes.
E. coli expressing CYP114 and FdGA from either X. translucens pv. translucens (XttCYP114+XttFdGA) or E. tracheiphila (EtCYP114+EtFdGA) efficiently converted ent-kaurenoic acid to GA12-aldehyde and, less efficiently, to the further oxidized GA12 (Figure 5 and Supplemental Figure S3). Only trace amounts of the intermediate ent-7α-hydroxykaurenoic acid were observed with these cultures, but it was the sole product when E. coli expressing CYP114 only (either XttCYP114 or EtCYP114) were fed ent-kaurenoic acid. Notably, unlike recombinantly expressed CYP114+FdGA from other bacteria, including those from X. hydroxykaurenoic acid to a similar mixture of GA12-aldehyde and lesser amounts of GA12 (Supplemental Figure S4B). Consistent with previous work on the CYP114+FdGA from the α-rhizobia S. fredii (Nett et al., 2016), trace amounts of ent-6α,7α-dihydroxykaurenoic acid was observed here, but neither this (Supplemental Figure S4C+B), nor the closely related kaurenolide or 7β-hydroxykaurenolide (neither of which were observed here) appeared to be further transformed by EtCYP114+EtFdGA (data not shown).
While CYP114+FdGA is able to further oxidize GA12-aldehyde to GA12, this transformation does not seem to be efficiently catalyzed. By contrast, this reaction is efficiently catalyzed by the SDRGA from either X. translucens pv. translucens (XttSDRGA) or E. tracheiphila (EtSDRGA), as E. coli expressing either completely converted GA12-aldehyde to GA12 (Figure 5 and Supplemental Figure S3).
E. coli expressing CYP112 from either X. translucens pv. translucens (XttCYP112) or E. tracheiphila (EtCYP112) efficiently convert GA12 to GA9 (Figure 5 and Supplemental Figure S3). This multi-step transformation was catalyzed almost to completion, as GA15 (formed by initial hydroxylation) is not observed, and only small amounts of the intermediary GA24 are observed (Supplemental Figure S5).
Finally, just as in X. oryzae pv. oryzae, the GA biosynthetic operons in both X. translucens pv. translucens and E. tracheiphila contain a full-length CYP115. E. coli expressing CYP115 either from X. translucens pv. translucens (XttCYP115) or E. tracheiphila (EtCYP115) catalyzed 3β-hydroxylation of GA9 to form the bioactive GA4 (Figure 5 and Supplemental Figure S3).
Discussion
The results reported here strengthen the association of this operon with GA biosynthesis. For example, while previous characterization of the enzymes producing isoprenyl diphosphates (i.e., IDS and CPS) has relied on indirect observations, using GC-MS to analyze the dephosphorylated products, these have been directly characterized here via LC-MS/MS (Figure 3). This enabled demonstration of the specific production of GGDP by the associated IDSs, rather than a mixture of different chain length products as seen with other such enzymes (Schmidt et al., 2010; Nagel et al., 2015), which is consistent with dedicated production of the derived GAs. In addition, the ability of EtCYP114+EtFdGA to convert ent-7α-hydroxykaurenoic acid to GA12-aldehyde further supports the relevance of this intermediate, consistent with recent labeling experiments (Nett et al., 2016).
More importantly, the results presented here demonstrate the conserved function for production of GAs of this operon across the full phylogenetic range that is currently available (Figure 2). Accordingly, bacteria containing this operon are then predicted to produce GAs. This includes species not only from additional α-rhizobia genera within the Rhizobiales (i.e., Microvirga and Azorhizobium), but also from the Pseudomonas genus in the γ-proteobacteria class, as well as β-rhizobia from the Burkholderia genus in the distinct β-proteobacteria class (albeit only in three strains). Hence, plant-associated bacteria spanning three orders of Proteobacteria have acquired the GA biosynthetic operon, almost certainly via horizontal gene transfer (Figure 2). However, the scattered distribution of the operon within these plant-associated bacteria indicates that production of GAs only provides a selective advantage under certain circumstances.
Intriguingly, there seems to be a correlation between the plant-microbe relationship, specifically pathogenic versus symbiotic, and presence or absence of CYP115, with associated production of bioactive GA4 or the penultimate precursor GA9, respectively. In particular, phytopathogens seem to invariably contain the full GA biosynthetic operon (i.e., including CYP115) and, thus, directly produce the bioactive GA4, while rhizobia generally (although not always) contain only the core GA biosynthetic operon (i.e., without CYP115) and only produce the penultimate hormonal precursor GA9. Indeed, beyond the previously noted loss of CYP115 in more than 80% of α-rhizobia (Nett et al., 2017a), its absence in all the β-rhizobia containing the GA biosynthetic operon (i.e., the core operon) further supports the hypothesis that production of bioactive GA4 may be deleterious to the symbiotic relationship between these rhizobia and their leguminous host plants. In particular, given that the bioactive GA4 produced by X. oryzae pv. oryzicola acts as a virulence factor by suppressing the JA-dependent defense response (Lu et al., 2015), it has been hypothesized that the rhizobia may leave the final step in bioactive GA production to their hosts in order to not compromise the ability of the plant to respond to infection by pathogenic microbes (Nett et al., 2017a). However, although it has been proposed that rhizobial production of GAs affects nodulation by limiting nodule numbers (Tatsukami and Ueda, 2016), it remains unclear why only certain rhizobia contain the GA biosynthetic operon, particularly given the much wider phylogenetic distribution of this sequence element shown here.
The presence of the (full) GA biosynthetic operon in phytopathogenic bacteria has been hypothesized to reflect the importance of entry via wounded leaf tissue for that bacterial species (Nagel et al., 2017). The basis for this hypothesis arose from the similar lifestyles of X. oryzae pv. oryzicola and X. translucens. Although not otherwise closely related (Naushad et al., 2015), these nominally enter via stomata and propagate throughout the mesophyll parenchyma, causing leaf streak disease, in small grain cereals and forage grasses from a sub-set of the Poaceae/grass plant family, specifically the BEP clade (GPWGII, 2012). However, these plants have highly water-repellent leaves (Neinhuis and Barthlott, 1997), a function of their vertical/upright nature and hydrophobic surface (Koch et al., 2008), which limits bacterial colonization and stomatal access (Beattie, 2011). Hence, it seems likely that these phytopathogens are largely dependent on entry via wounded tissue, such that the GA biosynthetic operon provides a means for suppressing the JA response associated with wounding (Nagel et al., 2017), consistent with the known antagonistic interactions between GA and JA (Robert-Seilaniantz et al., 2007; Bari and Jones, 2009; Wasternack and Hause, 2013). The appearance of the full GA biosynthetic operon in Xanthomonas bromi noted here (Figure 2) falls neatly within this paradigm, as X. bromi also nominally enters via stomata and causes leaf streak disease in grasses from the Poaceae BEP clade (Hersemann et al., 2016). Although little is known about the lifestyle of the Pseudomonas psychrotolerans strain that was also found here to contain the full GA biosynthetic operon, this genus contains many phytopathogens, and this strain was isolated from rice (Midha et al., 2015), consistent with the overall scenario presented above.
By contrast, the very different lifestyle of the cucurbit pathogen E. tracheiphila might seem more difficult to reconcile. Nevertheless, this phytopathogen also seems to depend on entry via wounded tissue. In particular, E. tracheiphila is transmitted by herbivorous beetles via deposition of bacteria-infested frass on fresh feeding wounds (Rojas et al., 2015). Thus, the production of bioactive GA4 imparted by the operon, as shown here, presumably provides E. tracheiphila a means of suppressing the JA response induced by feeding.
In conclusion, the results reported here demonstrate functional conservation of the gibberellin biosynthetic operon across a wide phylogenetic range of plant-associated bacteria, both rhizobia and plant pathogens. Intriguingly, based on the current distribution of this operon, as well as the CYP115 that catalyzes the final step in biosynthesis of the bioactive GA4, such hormone production seems to increase the virulence of plant pathogens that must attenuate a pre-existing JA-mediated defense response, while leaving the final step to the host plant may be beneficial in the rhizobia-legume symbiotic relationship. Thus, it will be interesting to see where else the operon will be found in the future, along with more direct investigation of the physiological relevance of GA production, either the bioactive GA4 or penultimate hormonal precursor GA9, in phytopathogens and rhizobial symbionts.
Material and Methods
Chemicals were purchased from Sigma and Fisher Scientific if not otherwise specified. Authentic GA standards (GA12-Aldehyde, GA12, GA9 and GA4) were purchased from OlChemIm Ltd., while authentic ent-7α-hydroxykaurenoic acid, GA15, and GA24 were kindly provided by Dr. Peter Hedden (Rothamsted Research).
To investigate the currently available phylogenetic range of the GA biosynthetic operon, the protein sequence of CYP114 from Xanthomonas oryzae pv. orizicola BLS256 was used in a BLASTP search against the NCBI non-redundant protein sequences database. Homologs in other bacteria with known genome sequences provided an entry point for examination of the presence of the other relevant genes in the vicinity (Figure 1). To investigate homologs of each gene from the operon, those from E. tracheiphila were used for a BLAST search against the protein database at NCBI, with the default number of target sequences increased from 100 to 1000. The closest homolog that is not part of a GA operon is noted in Supplemental Table S1.
For phylogenetic analysis, the protein sequences for CYP112, CYP114, FdGA, SDRGA, CYP117, GGPS, CPS and KS from a representative sub-set of species were downloaded from GenBank for Sinorhizobium fredii NGR234, Bradyrhizobium japonicum USDA110, Mesorhizobium loti MAFF303099, Xanthomonas oryzae pv. orizicola BLS256, Xanthomonas oryzae pv. orizicola BOXR1, Xanthomonas oryzae X11-5A, Xanthomonas bromi LMG 947, Xanthomonas translucens pv. translucens DSM18974, Xanthomonas translucens pv. cerealis CFBP2541, Xanthomonas translucens pv. graminis ART-Xtg29, Burkholderia sp. JPY251, Pseudomonas psychrotolerans NS274 and Erwinia tracheiphila PSU-1. CYP115 and the isopentenyl diphosphate isomerase (IDI) were not included due to their absence in certain rhizobia. Sequences were aligned in MEGA 7 using the Muscle algorithm, the phylogenetic tree was constructed and tested with the bootstrap method in the same program. For the Maximum Likelihood, Neighbor Joining and Minimum Evolution algorithms the JTT model was used with a gamma distribution. With the Maximum Likelihood algorithm all sites were used, while for the other algorithms gaps were deleted.
The targeted enzymatic genes were cloned by amplification from genomic DNA of Xanthomonas High-Fidelity DNA polymerase (NEB) according to the product manual, using 5 μL of the high GC-content enhancer for genes from Xanthomonas translucens pv. translucens and gene specific primers (Supplemental Table S2). The forward primer included a CACC sequence before the start codon to allow cloning into pET100 (Invitrogen) according to the instructions supplied by the manufacturer. Clones were sequenced to verify the correct DNA sequence. CYP114 was cloned either alone or in tandem with the neighboring FdGA, using the forward primer of CYP114 and the reverse primer of FdGA.
For recombinant expression, the pET100 constructs containing either GGPS or CPS were transformed into E. coli strain BL21 Star (Invitrogen) for expression. Starter cultures were grown in 10 mL NZY media (10 g/L NaCl, 10 g /L casein, 5 g/L yeast extract, 1 g/L MgSO4 (anhydrous), pH 7.0) with 50 μg/mL carbenicillin at 18 °C and 200 rpm for 3 days. 5 ml of the starter culture were used to inoculate 100 mL fresh NZY media, with 50 μg/mL carbenicillin, after the cultures reached an OD of 0.6 they were induced with 1 mM IPTG and grown under continuous shaking at 200 rpm at 18 °C for one day. Cells were harvested by centrifugation at 3000 x g for 20 min. The cell pellet was re-suspended in 5 mL buffer (25 mM 3-(N-morpholino)-2-hydroxypropanesulfonic acid (MOPSO), pH 7.2, 10 mM MgCl2, 10% (v/v) glycerol) with 20 mM imidazole, and homogenized using an EmulsiFlex C-5 (Avestin, Canada). The homogenized suspensions were centrifuged at 14,000 x g for 30 min. The supernatant was mixed with 1 mL Ni-NTA agarose (Qiagen), incubated under gentle shaking for 1 hour at 4°C and washed with 5 mL buffer containing 20 mM imidazole and an additional 5 mL with 60 mM imidazole. Proteins were eluted with 2 mL buffer containing 250 mM imidazole.
Enzyme assays were carried out with 10 μg of purified heterologously expressed protein in 300 μL of buffer. GGPS assays were run with 50 μM IPP and 50 μM DMAPP as substrates, while CPS assays were run with 20 μM GGPP as substrates. Assays were incubated for 1 h at 30 °C, then flash frozen in liquid nitrogen and kept at -80°C until their analysis by LC-MS/MS, which was carried out as previously described (Nagel et al., 2015). Metabolic engineering to test the activity of the CPS and KS from X. translucens pv. translucens was carried out using a modular expression system in E. coli (Cyr et al., 2007), with analysis by GC-MS, as previously described (Morrone et al., 2009; Hershey et al., 2014; Lu et al., 2015).
For recombinant feeding studies the pPET100 constructs with CYP117, CYP112, CYP114, CYP114+FdGA, CYP115 or SDRGA were transformed into E. coli strain BL21 Star (Invitrogen). For each of the resulting recombinant strains, three colonies were inoculated into 10 mL TB broth (12 g/L casein, 24 g/L yeast extract, 0.4% glycerol), including 50 μg/mL carbenicillin, and grown at 18 °C for 2 days with constant shaking at 200 rpm. From the starter culture 2 mL were transferred into 25 mL fresh TB broth, including 50 μg/mL carbenicillin. Cultures were induced with 1 mM IPTG. Those expressing CYPs were induced upon reaching an OD of 0.8, while those expressing SDRGA cultures were induced at an OD 0.6. Upon induction 5 mL 1 M phosphate buffer pH 7.5 with 10 μM specific substrates were added to all cultures, while for cultures expressing CYPs this further contained 1 mM aminolevulinic acid, 1 mM riboflavin and 0.1 mM FeCl3. The induced and fed cultures were then grown for 3 days at 18 °C with constant shaking at 200 rpm. Products were then extracted and analyzed by GC-MS as previously described (Nagel et al., 2017). For detection of ent-6α,7α-dihydroxykaurenoic acid, the relevant hexane extracts were silylated with N-Methyl-N-(trimethylsilyl) trifluoroacetamide (MSTFA) after methylation.
Supplementary Material
Acknowledgments
The authors thank Dr. Roland Kolliker (Agroscope) for genomic DNA of Xanthomonas translucens pv. translucens strain DSM 18974, Prof. Gwyn A. Beattie (Iowa State University) for genomic DNA of Erwinia tracheiphila PSU-1 and insightful discussion of the lifestyle of this phytopathogen, Dr. Peter Hedden (Rothamsted Research) for authentic gibberellin standards, and Dr. Axel Schmidt and Prof. Jonathan Gershenzon (Max Planck Institute for Chemical Ecology) for use of their LC-MS/MS instrument. This work was supported by grants to R.J.P. from the NIH (GM109773) and USDA (NIFA-AFRI grant 2014-67013-21720), along with a postdoctoral fellowship to R.N. from the Deutsche Forschungsgemeinschaft (DFG) NA 1261/1-1.
Footnotes
Author contributions
RN cloned the genes, performed the heterologous expression, analyzed data and wrote the manuscript, RJP aided in data analysis and writing the manuscript.
Literature/References
- Bari R, Jones JD. Role of plant hormones in plant defence responses. Plant Mol Biol. 2009;69:473–488. doi: 10.1007/s11103-008-9435-0. [DOI] [PubMed] [Google Scholar]
- Beattie GA. Water relations in the interaction of foliar bacterial pathogens with plants. Annual review of phytopathology. 2011;49:533–555. doi: 10.1146/annurev-phyto-073009-114436. [DOI] [PubMed] [Google Scholar]
- Bottini R, Cassan F, Piccoli P. Gibberellin production by bacteria and its involvement in plant growth promotion and yield increase. Appl Microbiol Biotechnol. 2004;65:497–503. doi: 10.1007/s00253-004-1696-1. [DOI] [PubMed] [Google Scholar]
- Cyr A, Wilderman PR, Determan M, Peters RJ. A Modular Approach for Facile Biosynthesis of Labdane-Related Diterpenes. J Am Chem Soc. 2007;129:6684–6685. doi: 10.1021/ja071158n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grass Phylogeny Working Group II. New grass phylogeny resolves deep evolutionary relationships and discovers C4 origins. New Phytol. 2012;193:304–312. doi: 10.1111/j.1469-8137.2011.03972.x. [DOI] [PubMed] [Google Scholar]
- Hedden P, Thomas SG. Gibberellin biosynthesis and its regulation. The Biochemical journal. 2012;444:11–25. doi: 10.1042/BJ20120245. [DOI] [PubMed] [Google Scholar]
- Hedden P, Sponsel V. A Century of Gibberellin Research. J Plant Growth Regul. 2015;34:740–760. doi: 10.1007/s00344-015-9546-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersemann L, Wibberg D, Blom J, Widmer F, Kolliker R. Draft Genome Sequence of the Xanthomonas bromi Type Strain LMG 947. Genome announcements. 2016:4. doi: 10.1128/genomeA.00961-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershey DM, Lu X, Zi J, Peters RJ. Functional conservation of the capacity for ent-kaurene biosynthesis and an associated operon in certain rhizobia. J Bact. 2014;196:100–106. doi: 10.1128/JB.01031-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch K, Bhushan B, Barthlott W. Diversity of structure, morphology and wetting of plant surfaces. Soft Matter. 2008;4:1943–1963. [Google Scholar]
- Lu X, Hershey DM, Wang L, Bogdanove AJ, Peters RJ. An ent-kaurene derived diterpenoid virulence factor from Xanthomonas oryzae pv. oryzicola. New Phytol. 2015;406:295–302. doi: 10.1111/nph.13187. [DOI] [PubMed] [Google Scholar]
- Midha S, Bansal K, Sharma S, Kumar N, Patil PP, Chaudhry V, Patil PB. Genomic Resource of Rice Seed Associated Bacteria. Front Microbiol. 2015;6:1551. doi: 10.3389/fmicb.2015.01551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrone D, Chambers J, Lowry L, Kim G, Anterola A, Bender K, Peters RJ. Gibberellin biosynthesis in bacteria: Separate ent-copalyl diphosphate and ent-kaurene synthases in Bradyrhizobium japonicum. FEBS Lett. 2009;583:475–480. doi: 10.1016/j.febslet.2008.12.052. [DOI] [PubMed] [Google Scholar]
- Nagel R, Turrini PCG, Nett RS, Leach JE, Verdier V, Van Sluys MA, Peters RJ. An operon for production of bioactive gibberellin A4 phytohormone with wide distribution in the bacterial rice leaf streak pathogen Xanthomonas oryzae pv. oryzicola. New Phytol. 2017 doi: 10.1111/nph.14441.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel R, Bernholz C, Vranova E, Kosuth J, Bergau N, Ludwig S, Wessjohann L, Gershenzon J, Tissier A, Schmidt A. Arabidopsis thaliana isoprenyl diphosphate synthases produce the C25 intermediate geranylfarnesyl diphosphate. Plant J. 2015;84:847–859. doi: 10.1111/tpj.13064. [DOI] [PubMed] [Google Scholar]
- Naushad S, Adeolu M, Wong S, Sohail M, Schellhorn HE, Gupta RS. A phylogenomic and molecular marker based taxonomic framework for the order Xanthomonadales: proposal to transfer the families Algiphilaceae and Solimonadaceae to the order Nevskiales ord. nov. and to create a new family within the order Xanthomonadales, the family Rhodanobacteraceae fam. nov., containing the genus Rhodanobacter and its closest relatives. Antonie Van Leeuwenhoek. 2015;107:467–485. doi: 10.1007/s10482-014-0344-8. [DOI] [PubMed] [Google Scholar]
- Neinhuis C, Barthlott W. Characterization and Distribution of Water-repellent, Self-cleaning Plant Surfaces. Ann Bot. 1997;79:667–677. [Google Scholar]
- Nett RS, Dickschat JS, Peters RJ. Labeling Studies Clarify the Committed Step in Bacterial Gibberellin Biosynthesis. Org Lett. 2016;18:5974–5977. doi: 10.1021/acs.orglett.6b02569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nett RS, Contreras T, Peters RJ. Characterization of CYP115 as a gibberellin 3-oxidase indicates that certain rhizobia can produce bioactive gibberellin A4. ACS Chem Biol. 2017a doi: 10.1021/acschembio.6b01038.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nett RS, Montanares M, Marcassa A, Lu X, Nagel R, Charles TC, Hedden P, Rojas MC, Peters RJ. Elucidation of gibberellin biosynthesis in bacteria reveals convergent evolution. Nat Chem Biol. 2017b;13:69–74. doi: 10.1038/nchembio.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert-Seilaniantz A, Navarro L, Bari R, Jones JDG. Pathological hormone imbalances. Curr Opin Plant Biol. 2007;10:372–379. doi: 10.1016/j.pbi.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Rojas ES, Batzer JC, Beattie GA, Fleischer SJ, Shapiro LR, Williams MA, Bessin R, Bruton BD, Boucher TJ, Jesse LCH, Gleason ML. Bacterial Wilt of Cucurbits: Resurrecting a Classic Pathosystem. Plant Dis. 2015;99:564–574. doi: 10.1094/PDIS-10-14-1068-FE. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Wachtler B, Temp U, Krekling T, Seguin A, Gershenzon J. A Bifunctional Geranyl and Geranylgeranyl Diphosphate Synthase Is Involved in Terpene Oleoresin Formation in Picea abies. Plant Physiology. 2010;152:639–655. doi: 10.1104/pp.109.144691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsukami Y, Ueda M. Rhizobial gibberellin negatively regulates host nodule number. Scientific Reports. 2016;6:27998. doi: 10.1038/srep27998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasternack C, Hause B. Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Annals of Botany. 2013;111:1021–1058. doi: 10.1093/aob/mct067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiemann P, Sieber CM, von Bargen KW, Studt L, Niehaus EM, Espino JJ, Huss K, Michielse CB, Albermann S, Wagner D, Bergner SV, Connolly LR, Fischer A, Reuter G, Kleigrewe K, Bald T, Wingfield BD, Ophir R, Freeman S, Hippler M, Smith KM, Brown DW. Deciphering the cryptic genome: genome-wide analyses of the rice pathogen Fusarium fujikuroi reveal complex regulation of secondary metabolism and novel metabolites. PLoS Pathog. 9:e1003475. doi: 10.1371/journal.ppat.1003475. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.