Abstract
A multitude of factors, including increased coronary vascular resistance and dysregulated coronary microcirculatory function, contribute to the impairment of coronary blood flow (CBF) regulation and the pathogenesis of myocardial ischemia/reperfusion (I/R) injury. CBF is primarily determined by coronary vascular resistance, which is affected by the balance between various vasodilators and vasoconstrictors. Myocardial I/R causes reduced production of endogenous vasodilators, such as nitric oxide (NO), leaving unopposed vasoconstriction that is caused mainly by continued presence of endothelin-1 (ET-1) and serotonin (5-HT); this imbalance in turn enhances vascular tone, triggers inflammatory response, decreases CBF and exacerbates reperfusion injury. Various inflammatory cytokines participate in the regulation of coronary vasomotor function by affecting the balance between vasodilators and vasoconstrictors. In addition to the enhanced coronary vasoconstriction, coronary microembolization, inflammatory cell infiltration and post-ischemic hyperpermeability contribute to the impairment of coronary microcirculatory function and myocardial perfusion during I/R. Ongoing research examining the role of inflammation in the regulation of CBF and coronary microcirculatory function in myocardial I/R is expected to yield new insights that will lead to therapies for ameliorating the vascular inflammatory response in coronary artery diseases (CADs) in the clinical setting. This article is part of a Special Issue entitled “Coronary Blood Flow”.
Keywords: Coronary vascular resistance, Inflammatory cells, Microcirculation, Myocardial ischemia, Reperfusion injury
1. Introduction
Coronary blood flow (CBF) regulation in myocardial ischemia/reperfusion (I/R), particularly perfusion at the microcirculatory level, is critical to the outcome of ischemic heart disease. Coronary vascular tone is a crucial factor influencing CBF. The impaired vasodilatory response of coronary arteries during I/R injury was first documented by Ku et al. in 1982 [1]. To date, a variety of vasodilators and vasoconstrictors including endothelium-derived vasoactive factors, autacoids, metabolic messengers, and neurohormonal factors are found to be involved in the regulation of coronary vascular resistance and are emerging as therapeutic targets for the restoration of CBF following I/R injury. In addition to the enhanced vascular constriction, the dysregulation of coronary microcirculatory function during myocardial I/R, which is characterized by microembolization, inflammatory cell infiltration and hyperpermeability, lead to reduced CBF and inadequate myocardial reperfusion. A number of inflammatory cytokines and inflammatory cells are actively involved in the regulation of coronary vascular resistance and microcirculatory function. Elucidating the role of inflammation in CBF dysregulation in myocardial I/R would facilitate the development of novel therapeutics and improve clinical outcomes. Within this context, this review focuses on: (1) the role of inflammation in the regulation of coronary vascular resistance and CBF; (2) the role of inflammation in coronary microcirculatory dysfunction in myocardial I/R injury.
2. Inflammation and the regulation of coronary vascular resistance in I/R
Coronary vascular resistance serves as a primary determinant of CBF. Regulation of coronary vascular tone is the result of a balance between a myriad of vasodilator and vasoconstrictor signals, in which endothelium-derived vasoactive factors, metabolic messengers, neurohormones, and various autocoids are crucially involved.
2.1. Endothelium-derived vasoactive factors
2.1.1. Nitric oxide (NO)
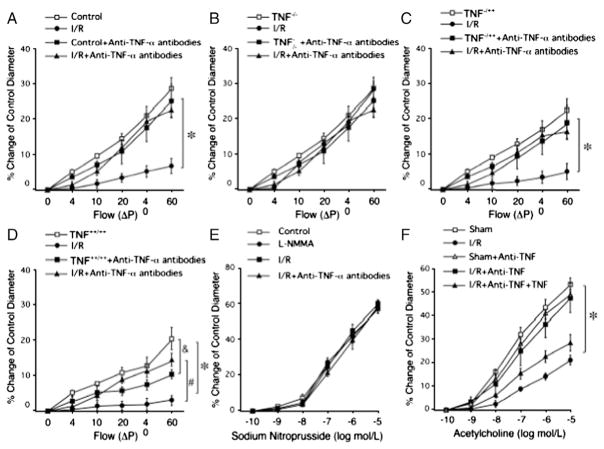
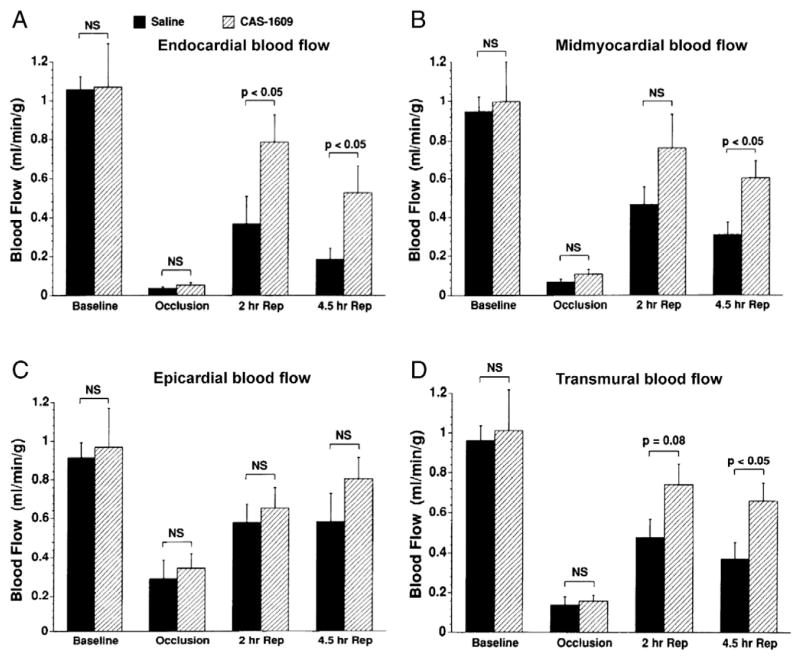
Endothelial nitric oxide synthase (eNOS)-derived NO in coronary resistance vessels plays an essential role in the regulation of myocardial perfusion. Inhibiting NO production decreased CBF [2] and increased the vulnerability of the myocardium to ischemia [3]. Many interventions that stimulate NO production, such as insulin [4,5], calcium channel blocker [6,7], angiotensin converting enzyme inhibitor (ACEI) [8], DiOHF (a synthetic flavonol with antioxidant properties) [9], and raloxifene (a selective estrogen receptor modulator) [10], increased CBF and exert cardioprotective benefits in experimental I/R models. In addition to acting as a potent vasodilator, NO released toward the vascular lumen inhibits platelet aggregation and leukocyte adhesion to endothelium by suppressing CD11/CD18 expression on leukocytes [11]. In the acute myocardial I/R murine model, increased vascular tumor necrosis factor-alpha (TNF-α) expression impaired endothelium-dependent vasodilation of coronary arterioles. Elevated TNF-α induced up-regulation of arginase and enhanced oxidative stress, thereby decreasing NO bioavailability and NO-mediated vasodilation [12–14], and a direct correlation between TNF-α level and reperfusion-induced endothelial dysfunction was demonstrated (Fig. 1) [14]. In a canine model of myocardial I/R, intracoronary administration of NO donor (CAS-1609) augmented postischemic CBF and contractile function, improved acetylcholine (ACh)-induced vasodilation of coronary arteries and reduced neutrophil infiltration into the myocardium (Fig. 2) [15].
Fig. 1.
Flow-induced vasodilation was impaired after I/R in WT mice (A). In heterozygote TNF−/++ mice underwent I/R (C), flow-induced endothelial-dependent vasodilation was between TNF−/− (B) and TNF++/++ (D) following I/R. Neutralizing antibodies to TNF-α preserved endothelial-dependent dilation following I/R in WT, TNF−/++ and TNF++/++ mice (A, C, D). E shows a dose-response curve for SNP in WT control mice before and following I/R. F shows that endothelial function is restored in I/R for WT mice treated with anti-TNF-α. (Data from Zhang et al. [14]).
Fig. 2.
Blood flow measured using radiolabeled microspheres in ischemia region. Endocardial and midmyocardial blood flow declined sharply following reperfusion in the saline group, while they were preserved through 4.5 h reperfusion by the NO donor (CAS-1609) treatment (A, B). C: There were no significant differences in epicardial blood flow between treatment groups at any of the time points. D: CAS-1609 treatment preserved transmural regional myocardial blood flow at 4.5 h of reperfusion compared with the saline group. (Data from Pabla et al. [15]).
Although deficiency of NO is associated with reduced CBF and impaired myocardial perfusion, excess production of NO due to upregulation of inducible NO synthase (iNOS) [11] may lead to free-radical mediated myocardial damage. Activation of myocardial iNOS during 48 h of reperfusion contributed to a late phase of I/R-induced injury, but inhibition of iNOS increased CBF and improved myocardial performance in rabbits where the circumflex coronary artery was occluded [16]. In a swine model with left anterior descending artery occlusion, sustained regional ischemia induced myocardial iNOS expression, which contributed to contractile dysfunction at the cardiomyocyte level through increased nitrite contents [17].
The availability of L-arginine is also a critical factor that affects the bioavailability of NO, thus affecting the regulation of coronary vascular resistance. L-arginine reduced plasma levels of soluble adhesion molecules and attenuated endothelial injury/inflammatory response following I/R in pig hearts [18]. In patients that underwent coronary artery bypass grafting, addition of L-arginine to cardioplegia resulted in reduced interleukin-6 (IL-6) and TNF-α levels, and decreased platelet and leukocyte counts, thereby contributing to a reduced no-reflow phenomenon and better reperfusion [19]. Another study reported that L-arginine supplementation had no significant effects on CBF, though it reduced myocardial necrosis in a pig I/R model [20]. The dosage of L-arginine supplementation may account for these seemingly contradictory effects. In a pig model of cardiac transplantation, low-dose L-arginine increased CBF and decreased inflammatory cytokine release during donor blood perfusion; however, high-dose L-arginine resulted in severe endothelial injury [21].
Thus, although enhancing NO production and moderate L-arginine supplementation generally exerts beneficial effects on the regulation of CBF, toxic level of NO by iNOS activation or excess dose of L-arginine may exacerbate inflammation and myocardial I/R injury. Anti-inflammatory treatment to counteract the drop in NO levels beyond the normal range of NO homeostasis may represent an efficient strategy to reduce myocardial I/R injury.
2.1.2. Endothelium-derived hyperpolarizing factors (EDHFs)
Endothelium-derived relaxing factors other than NO and prostacyclin (PGI2) also qualify as EDHFs due to their ability to hyperpolarize the vascular smooth muscle cell, via opening of KCa channels to produce vasodilation. C-type natriuretic peptide (CNP) has recently been identified as an EDHF in an in vitro I/R rat model. ACh elicited the release of CNP from coronary endothelium, which locally regulated coronary circulation and protected against I/R injury through activation of CNP receptor-C. Interestingly, the vasorelaxant and protective activity of CNP was enhanced in the absence of endothelium-derived NO, indicating an increased contribution of CNP when NO synthase activity is blunted [22]. Sphingosine kinase is an inflammatory mediator in the pathogenesis of various inflammatory diseases [23]. Activation of sphingosine kinase by methacholine enhanced NO-dependent vasorelaxation, but attenuated EDHF-dependent vasodilation [24]. H2O2 also acts as an EDHF in coronary microvessels and plays a protective role in coronary autoregulation and myocardial I/R injury in dogs [25]. The inflammatory cytokine, IL-6, inhibited H2O2-mediated coronary arteriolar vasodilation to ACh in murine models [26].
2.1.3. Endothelin-1 (ET-1)
In contrast to NO, ET-1 is a potent coronary vasoconstrictor that can provoke marked myocardial ischemia through coronary vasoconstriction [27] and elicit severe ventricular arrhythmias [28]. Elevated ET-1 levels impaired NO production in cultured endothelial cells through a PKC-dependent pathway [29]. In patients undergoing primary angioplasty, a substantial and long lasting rise in ET-1 was documented, which was associated with impaired angiographic reperfusion [30]. Short-term blockade of ET type A receptors after diagnostic coronary arteriography results in decreased coronary vascular resistance and increased CBF in patients with coronary artery disease (CAD) [31]. Long-term (6-month) treatment of ET type A receptor antagonist did not affect the percent change of coronary artery diameter or change in coronary flow reserve, but increased the percent change of CBF in response to ACh in patients with early coronary atherosclerosis, underscoring the therapeutic potential of long-term ET type A receptor antagonism to remediate coronary endothelial dysfunction [32]. The association between ET-1 generation and initiation of the inflammatory process in acute myocardial infarction (AMI) and stable angina has not been completely elucidated. One study sampled coronary sinus and aortic blood during angioplasty for AMI or stable angina to assess the local release of ET-1 and various inflammatory cytokines. The results suggested that local myocardial release of ET-1 is higher in AMI than in stable angina, but local release of inflammatory cytokines is a component of stable coronary artery disease and does not appear acutely enhanced in AMI [33].
2.2. Autacoids
2.2.1. Adenosine
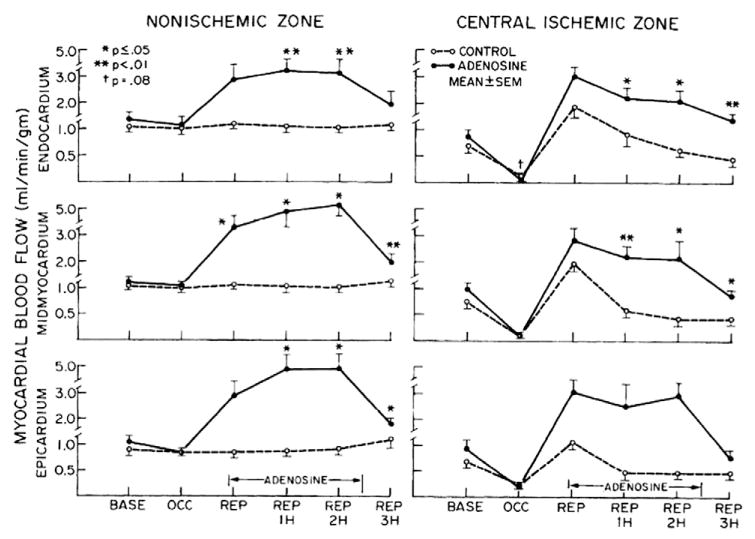
Adenosine, primarily produced extracellularly by CD73 (ecto-5′-nucleotidase), acts as a coronary dilator largely through activation of A2A adenosine receptor [34,35]. CD73−/− mice showed reduced CBF and enhanced leukocyte adhesion to vascular endothelium in a cremaster model of I/R [36]. After coronary microembolization, adenosine released from ischemic areas dilated vessels in adjacent nonembolized myocardium and increased CBF in pigs [37]. Adenosine also inhibits platelet aggregation in an open-chest dog model of myocardial ischemia [38]. Intravenous adenosine administration prevented the progressive decrease in transmural blood flow and preserved cardiac function during reperfusion in dogs subjected to I/R; these effects were associated with reduced neutrophil and erythrocyte plugging of capillaries with relative preservation of endothelial cell structure (Fig. 3) [39]. In a murine model of myocardial I/R, the activation of A2A-adenosine receptor inhibited T lymphocyte activation and the downstream inflammatory response [40]. Therefore, animal studies suggest that adenosine is not a mere vasodilator, but also plays a critical role in downregulating proinflammatory responses like thrombosis, thus mediating cardioprotection against I/R injury. Although animal studies suggest that adenosine is an important therapeutic target for improving coronary perfusion, translation into clinical practice has met with inconsistent results. One study suggested that adenosine adjunct to primary angioplasty prevented the no-reflow phenomenon with a more favorable clinical course in AMI patients [41], while another study demonstrated reduced infarct size, but a lack of improvement in clinical outcomes [42]. Coronary arterial aspirate plasma from patients after percutaneous coronary intervention (PCI) induced vasoconstriction; adenosine was not as potent in attenuating the aspirate-induced vasoconstriction as was nitroprusside or verapamil [43]. In another clinical trial, selective high-dose intracoronary administration of adenosine distal to the occlusion site of the culprit lesion failed to result in myocardial salvage or decrease in microvascular obstruction. The infarct size was also similar compared with the placebo group after 4 months [44]. One explanation for these inconsistent results is that adenosine is only one of a variety of redundant mechanisms involved in the metabolic regulation of CBF; adenosine does not eliminate all active coronary vasomotor tone and may not be the predominant mediator of physiological regulation of CBF [35].
Fig. 3.
Plots of serial regional myocardial blood flow in nonischemic (posterior wall) and central ischemic zones at baseline (BASE), occlusion (OCC), and reperfusion (REP). A progressive decrease in flow in inner two thirds of myocardium is noted in control animals during first 3 h of REP, which was significantly increased during adenosine infusion. (Data from Pitarys et al. [39]).
2.2.2. Prostanoids
Inflammatory cells are capable of releasing arachidonic acid, which may be further metabolized to produce biologically active products, including prostaglandins (PGs), etc. [45]. PGs, together with the thromboxanes and PGI2, form the prostanoid class of fatty acid derivatives. The cardioprotective effects of PGs, especially E-type PGs (PGEs), against myocardial I/R injury have been extensively studied. There are four subtypes of PGE receptors (EP1, EP2, EP3, and EP4). Although EP1, EP2 and EP3 all mediated the cardioprotective benefits of PGEs, a selective agonist of EP3 receptors reduced myocardial infarct size without causing hemodynamic side effects [46]. The attenuation of myocardial ischemic stunning by the ACEI ramiprilat involved a signal cascade of bradykinin and PGs but not NO [47]. Thromboxane A2 (TxA2) is a product of PGs endoperoxide metabolism that is produced by platelets. Cyclic flow variation induced thromboxane synthesis but reduced PGI2 synthesis; these changes led to an imbalance between TxA2 and PGI2, which favors vasoconstriction and myocardial ischemia in dogs [48]. Thus, selective modification of arachidonic acid metabolism may present a useful therapeutic tool to reduce myocardial injury.
2.2.3. Serotonin (5-HT)
5-HT causes vasodilation of small coronary arterioles (<90 μm in diameter) and constriction in larger coronary arterioles and arteries [49]. Intracoronary injection of 5-HT caused a dose-dependent increase in CBF under normal coronary inflow conditions in open-chest dogs, while flow-limiting stenosis enhanced 5-HT-induced constriction of the large coronary arteries and resulted in a reduction in CBF [50,51]. These observations suggest that in the presence of CAD, 5-HT could contribute to reduction in CBF, leading to myocardial ischemia. Indeed, in patients with severe saphenous vein aorto-coronary bypass stenoses, 5-HT was the main coronary vasoconstrictor after stenting, and thromboxane and TNF-α appear to potentiate the 5-HT response [43].
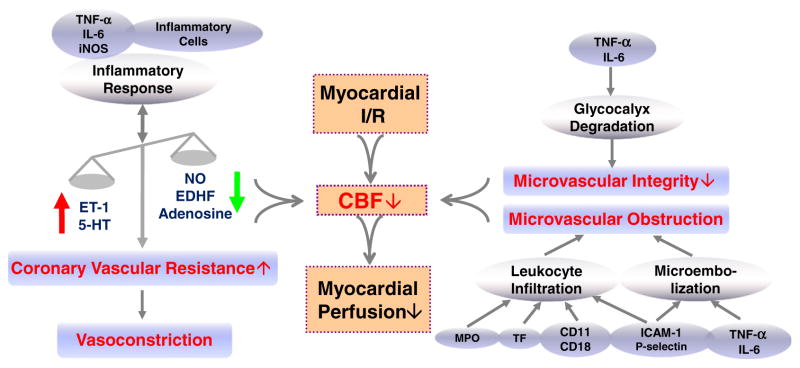
Collectively, persistent increase in vasoconstrictor tone is one of the vital mechanisms responsible for decreased CBF and I/R damage. Myocardial I/R causes reduced production of endogenous vasodilators, such as NO and EDHF, leaving unopposed vasoconstriction that is caused mainly by continued presence of ET-1 and 5-HT, and this imbalance in turn decreases CBF, triggers inflammatory responses, and exacerbates reperfusion injury. Multiple inflammatory cytokines participate in the regulation of coronary vascular tone by modulating the production and signaling of endothelial-derived vasoactive factors and autacoids during myocardial I/R (Fig. 4).
Fig. 4.
Schematic drawing of pathogenesis and mechanisms of decreased coronary blood flow (CBF) and impaired myocardial perfusion in myocardial ischemia/reperfusion (I/R) injury. ET-1, endothelin-1; 5-HT, serotonin; NO, nitric oxide; EDHF, endothelium-dependent hyperpolarizing factor; TNF-α, tumor necrosis factor-alpha; IL-6, interleukin-6; iNOS, inducible NO synthase; ICAM-1, intracellular adhesion molecule-1; MPO, myeloperoxidase; TF, tissue factor.
3. Inflammation and coronary microvascular dysfunction in I/R
The aim of thrombolysis, angioplasty, and coronary artery bypass surgery is to restore CBF; however, successful restoration of epicardial coronary artery patency may not always lead to adequate reperfusion at the microvascular level [52,53], primarily due to microvascular dysfunction caused by coronary microembolization, inflammatory cell infiltration, and impaired microvascular integrity following I/R injury.
3.1. Coronary microembolization
Coronary microembolization from the erosion or rupture of a vulnerable atherosclerotic plaque occurs spontaneously in acute coronary syndromes or during PCI. Typical consequences of coronary microembolization are microinfarcts with an inflammatory response, contractile dysfunction, and reduced coronary reserve as summarized by Heusch et al. [54]. The incidence of cardiac enzyme elevation is associated with a persistent reduction of relative coronary flow velocity reserve, and serve as a marker of underlying embolic myocardial injury in patients with successful coronary stenting [55]. Patients with periprocedural non-ST-segment elevation myocardial infarctions had a significantly higher frequency of coronary microembolization during primary angioplasty, and their systemic inflammatory response and microvascular impairment after PCI were more pronounced [56]. Intracoronary infusion of low-dose streptokinase (a clot-resolving medication) immediately after primary PCI improved myocardial perfusion by dissolving thrombi in the microvasculature, which was therapeutic [57]. Experimental data indicate that, in a rat model of coronary microembolization, activity of NF-κB/DNA-binding was markedly increased; TNF-α, IL-6 and intercellular adhesion molecule-1 (ICAM-1) expressions were upregulated. NF-κB inhibitor pyrrolidine dithiocarbamate suppressed the myocardial inflammatory cytokine transcriptions, and improved cardiac function, suggesting that patients with or at risk of coronary microembolization may benefit from acute anti-inflammatory treatment [58].
3.2. Inflammatory cell infiltration
Postischemic reperfusion is accompanied by reintroduction of inflammatory cells. Neutrophils are the most abundant of the infiltrated immune cells during reperfusion; they impair endothelial function and reduce coronary vasodilator reserve by promoting the procoagulant and proinflammatory milieu, and cause mechanical plugging at the microvascular level [39,59,60]. The mechanisms responsible for leukocyte accumulation in reperfused heart are not well understood. Reduced coronary reflow during reperfusion may be an important factor enhancing leukocyte trapping in capillaries and leukocyte adhesion in venules [61]. The critical step in this inflammatory cascade is the firm adhesion of these leukocytes to the activated or dysfunctional endothelium, which is governed by the beta2-integrins (e.g., CD11/CD18) on the leukocytes and the adhesion molecules on the activated endothelium; these leukocytes transmigrate across the endothelium to the site of the reperfused myocardium [62]. Indeed, myocardial I/R induced coronary vascular expression of P-selectin, E-selectin, and ICAM-1 through oxygen radicals triggered activation of NF-κB and AP-1 [63–65]. Accordingly, extravascular neutrophils in ischemic areas were localized with endothelium bearing high levels of ICAM-1 within 1 h of reperfusion [66]. Similarly, in reperfused canine myocardium, margination and emigration of neutrophils originated from thin-walled venous cisterns with increased expression of ICAM-1 and P-selectin [67].
Genetic deficiencies of P-selectin, E-selectin, ICAM-1, or CD18 attenuated polymorphonuclear leukocytes (PMNs) sequestration into I/R myocardium [63]. A neutralizing antibody to P-selectin reduced endothelial adherence of PMNs and improved ACh-induced vasorelaxation in ischemic-reperfused coronary arteries in felines [68]. CD18 blockade and NF-κB inhibition prevented subsequent inflammatory activation and exerted postischemic cardioprotection in pig I/R hearts [69]. Furthermore, c-Jun DNAzymes attenuated c-Jun and ICAM-1 expression in vascular endothelium, and neutrophil infiltration following myocardial I/R [70]. In addition, simultaneous inhibition of glycoprotein IIb/IIIa and integrin alpha(v)beta(3) in dogs [71], and inhibiting tissue factor (TF)-thrombin pathway in rabbits [72] decreased leukocyte recruitment, reduced infarct size, and improved CBF following I/R.
Clinical studies reveal that, in patients receiving coronary bypass grafts, transcardiac veno–arterial differences in CD11b-positive neutrophils decreased gradually with the cardiac reperfusion underway, suggesting sequestration of activated neutrophils following reperfusion [73]. Myocardial tissue from patients with AMI revealed intense recruitment of myeloperoxidase (MPO)-positive PMN localized along infarct-related vessels [74]. Regression analysis revealed that neutrophil count negatively correlated with coronary flow reserve, and neutrophil count on admission was an independent predictor of severe microvascular injury in patients with first anterior wall AMI [75].
3.3. Microvascular integrity
I/R injury impairs microcirculatory integrity and causes hyperpermeability, in which various inflammatory cytokines and inflammatory cells are crucially involved. Activated neutrophils induce hyperpermeability and phosphorylation of adherens junction proteins in cultured coronary endothelial cells [76]. In isolated heart subjected to global I/R, endothelial STAT3 ablation resulted in increased myocardial IL-6 expression and enhanced apoptosis in the myocardium around the capillary, thereby affecting capillary network integrity [77]. CBF and inflammatory indices are also associated with microvascular integrity. Peak and mean diastolic flow changes and coronary sinus IL-6 were inversely correlated with changes in total microvascular cross-sectional area; thus changes in the integrity of the coronary microcirculation after I/R [78]. Furthermore, healthy vascular endothelium is clothed by the endothelial glycocalyx, which plays a vital role in the regulation of inflammation and vascular permeability by constituting the vascular barrier together with the endothelial cells themselves [79]. Myocardial I/R resulted in severe degradation of the glycocalyx, shedding adhesion molecules, whereas preservation of the glycocalyx mitigated postischemic PMNs adhesion [80]. In isolated pig hearts, TNF-α induced severe inflammatory degradation of the endothelial glycocalyx, increased coronary resistance, heightened vascular leak and permeability to hydroxyethyl starch and caused mast-cell degranulation [81]. In addition to the results in animal studies, endothelial glycocalyx shedding was also reported in patients undergoing I/R procedures [82].
Thus, coronary microembolization, inflammatory cell infiltration, and hyperpermealization play critical roles in the initiation and perpetuation of coronary microvascular obstruction and impaired vascular integrity, which lead to reduced CBF and inadequate microcirculatory perfusion (Fig. 4). Protection of the coronary microvascular function should be included in any attempt to improve treatment of occlusive CAD and I/R injury.
4. Perspectives
Although great progress has been made in elucidating the mechanisms of CBF regulation in myocardial I/R injury, we are experiencing a failure in the translation of these exciting scientific results to patients. First, it remains unclear if myocardial I/R injury occurs to the same extent in man as it does in animal models; the experimental models of myocardial I/R are lacking certain aspects that are frequently present in the clinical environment [52]. Second, results from mice and rats may not be properly translated to the human [53] because I/R studies utilize healthy young animals without previous cardiovascular risk that are provided with timely reperfusion. Human patients that present with AMI may possess a number of cardiovascular risk factors, such as diabetes, hypertension, and aging, and may receive a number of drugs to maximize reperfusion and minimize cardiac injury prior to the onset of reperfusion. Determining which animal models feature cardiovascular risk factors that best mimic reperfusion injury in man will aid translation of I/R results to the clinic.
To sum up, vascular inflammatory response is actively involved in the enhanced coronary and microvascular constriction, microcirculatory obstruction and impaired microvascular integrity during myocardial I/R. The worldwide increase of occlusive CAD shows that elucidating the role of inflammation in CBF dysregulation is needed. The discovery of potential therapeutic targets and advanced therapeutic interventions pose significant challenges in this exciting field. Success will significantly impact the prognosis of myocardial I/R injury and improve clinical outcomes in patients with CADs.
Acknowledgments
Sources of funding
This study was supported by grants from NIH grants (RO1-HL077566 and RO1-HL085119 to C.Z.) and American Heart Association Predoctoral Fellowship (10PRE4300043 to H.Z.).
Abbreviations
- 5-HT
serotonin
- ACEI
angiotensin converting enzyme inhibitor
- ACh
acetylcholine
- AMI
acute myocardial infarction
- CAD
coronary artery disease
- CBF
coronary blood flow
- CNP
C-type natriuretic peptide
- EDHF
endothelium-derived hyperpolarizing factor
- eNOS
endothelial NO synthase
- ET-1
endothelin-1
- ICAM-1
intercellular adhesion molecule-1
- IL-6
interleukin-6
- iNOS
inducible NO synthase
- I/R
ischemia/reperfusion
- MPO
myeloperoxidase
- NO
nitric oxide
- PCI
percutaneous coronary intervention
- PGs
prostaglandins
- PGEs
E-type PGs
- PGI2
prostacyclin
- PMNs
polymorphonuclear leukocytes
- TF
tissue factor
- TNF-α
tumor necrosis factor-alpha
- TxA2
thromboxane A2
Footnotes
Disclosures
None.
References
- 1.Ku DD. Coronary vascular reactivity after acute myocardial ischemia. Science. 1982;218:576–8. doi: 10.1126/science.7123259. [DOI] [PubMed] [Google Scholar]
- 2.Node K, Kitakaze M, Kosaka H, Komamura K, Minamino T, Tada M, et al. Plasma nitric oxide end products are increased in the ischemic canine heart. Biochem Biophys Res Commun. 1995;211:370–4. doi: 10.1006/bbrc.1995.1823. [DOI] [PubMed] [Google Scholar]
- 3.Smith TP, Jr, Canty JM., Jr Modulation of coronary autoregulatory responses by nitric oxide. Evidence for flow-dependent resistance adjustments in conscious dogs. Circ Res. 1993;73:232–40. doi: 10.1161/01.res.73.2.232. [DOI] [PubMed] [Google Scholar]
- 4.Zhang HX, Zang YM, Huo JH, Liang SJ, Zhang HF, Wang YM, et al. Physiologically tolerable insulin reduces myocardial injury and improves cardiac functional recovery in myocardial ischemic/reperfused dogs. J Cardiovasc Pharmacol. 2006;48:306–13. doi: 10.1097/01.fjc.0000249873.73197.c3. [DOI] [PubMed] [Google Scholar]
- 5.Ma H, Zhang HF, Yu L, Zhang QJ, Li J, Huo JH, et al. Vasculoprotective effect of insulin in the ischemic/reperfused canine heart: role of Akt-stimulated NO production. Cardiovasc Res. 2006;69:57–65. doi: 10.1016/j.cardiores.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 6.Kitakaze M, Node K, Minamino T, Asanuma H, Kuzuya T, Hori M. A Ca channel blocker, benidipine, increases coronary blood flow and attenuates the severity of myocardial ischemia via NO-dependent mechanisms in dogs. J Am Coll Cardiol. 1999;33:242–9. doi: 10.1016/s0735-1097(98)00556-7. [DOI] [PubMed] [Google Scholar]
- 7.Kitakaze M, Asanuma H, Takashima S, Minamino T, Ueda Y, Sakata Y, et al. Nifedipine-induced coronary vasodilation in ischemic hearts is attributable to bradykinin- and NO-dependent mechanisms in dogs. Circulation. 2000;101:311–7. doi: 10.1161/01.cir.101.3.311. [DOI] [PubMed] [Google Scholar]
- 8.Piana RN, Wang SY, Friedman M, Sellke FW. Angiotensin-converting enzyme inhibition preserves endothelium-dependent coronary microvascular responses during short-term ischemia–reperfusion. Circulation. 1996;93:544–51. doi: 10.1161/01.cir.93.3.544. [DOI] [PubMed] [Google Scholar]
- 9.Wang S, Dusting GJ, May CN, Woodman OL. 3′,4′-Dihydroxyflavonol reduces infarct size and injury associated with myocardial ischaemia and reperfusion in sheep. Br J Pharmacol. 2004;142:443–52. doi: 10.1038/sj.bjp.0705815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogita H, Node K, Asanuma H, Sanada S, Kim J, Takashima S, et al. Raloxifene improves coronary perfusion, cardiac contractility, and myocardial metabolism in the ischemic heart: role of phosphatidylinositol 3-kinase/Akt pathway. J Cardiovasc Pharmacol. 2004;43:821–9. doi: 10.1097/00005344-200406000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Forstermann U, Munzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006;113:1708–14. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- 12.Zhang C, Xu X, Potter BJ, Wang W, Kuo L, Michael L, et al. TNF-alpha contributes to endothelial dysfunction in ischemia/reperfusion injury. Arterioscler Thromb Vasc Biol. 2006;26:475–80. doi: 10.1161/01.ATV.0000201932.32678.7e. [DOI] [PubMed] [Google Scholar]
- 13.Gao X, Xu X, Belmadani S, Park Y, Tang Z, Feldman AM, et al. TNF-alpha contributes to endothelial dysfunction by upregulating arginase in ischemia/reperfusion injury. Arterioscler Thromb Vasc Biol. 2007;27:1269–75. doi: 10.1161/ATVBAHA.107.142521. [DOI] [PubMed] [Google Scholar]
- 14.Zhang C, Wu J, Xu X, Potter BJ, Gao X. Direct relationship between levels of TNF-alpha expression and endothelial dysfunction in reperfusion injury. Basic Res Cardiol. 2010;105:453–64. doi: 10.1007/s00395-010-0083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pabla R, Buda AJ, Flynn DM, Salzberg DB, Lefer DJ. Intracoronary nitric oxide improves postischemic coronary blood flow and myocardial contractile function. Am J Physiol. 1995;269:H1113–21. doi: 10.1152/ajpheart.1995.269.3.H1113. [DOI] [PubMed] [Google Scholar]
- 16.Wildhirt SM, Weismueller S, Schulze C, Conrad N, Kornberg A, Reichart B. Inducible nitric oxide synthase activation after ischemia/reperfusion contributes to myocardial dysfunction and extent of infarct size in rabbits: evidence for a late phase of nitric oxide-mediated reperfusion injury. Cardiovasc Res. 1999;43:698–711. doi: 10.1016/s0008-6363(99)00080-2. [DOI] [PubMed] [Google Scholar]
- 17.Heinzel FR, Gres P, Boengler K, Duschin A, Konietzka I, Rassaf T, et al. Inducible nitric oxide synthase expression and cardiomyocyte dysfunction during sustained moderate ischemia in pigs. Circ Res. 2008;103:1120–7. doi: 10.1161/CIRCRESAHA.108.186015. [DOI] [PubMed] [Google Scholar]
- 18.Engelman DT, Watanabe M, Maulik N, Cordis GA, Engelman RM, Rousou JA, et al. L-arginine reduces endothelial inflammation and myocardial stunning during ischemia/reperfusion. Ann Thorac Surg. 1995;60:1275–81. doi: 10.1016/0003-4975(95)00614-Q. [DOI] [PubMed] [Google Scholar]
- 19.Colagrande L, Formica F, Porta F, Brustia M, Avalli L, Sangalli F, et al. L-arginine effects on myocardial stress in cardiac surgery: preliminary results. Ital Heart J. 2005;6:904–10. [PubMed] [Google Scholar]
- 20.Padilla F, Garcia-Dorado D, Agullo L, Inserte J, Paniagua A, Mirabet S, et al. L-arginine administration prevents reperfusion-induced cardiomyocyte hypercontracture and reduces infarct size in the pig. Cardiovasc Res. 2000;46:412–20. doi: 10.1016/s0008-6363(00)00048-1. [DOI] [PubMed] [Google Scholar]
- 21.Ramzy D, Rao V, Mallidi H, Tumiati LC, Xu N, Miriuka S, et al. Cardiac allograft preservation using donor-shed blood supplemented with L-arginine. J Heart Lung Transplant. 2005;24:1665–72. doi: 10.1016/j.healun.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 22.Hobbs A, Foster P, Prescott C, Scotland R, Ahluwalia A. Natriuretic peptide receptor-C regulates coronary blood flow and prevents myocardial ischemia/reperfusion injury: novel cardioprotective role for endothelium-derived C-type natriuretic peptide. Circulation. 2004;110:1231–5. doi: 10.1161/01.CIR.0000141802.29945.34. [DOI] [PubMed] [Google Scholar]
- 23.Lai WQ, Irwan AW, Goh HH, Howe HS, Yu DT, Valle-Onate R, et al. Anti-inflammatory effects of sphingosine kinase modulation in inflammatory arthritis. J Immunol. 2008;181:8010–7. doi: 10.4049/jimmunol.181.11.8010. [DOI] [PubMed] [Google Scholar]
- 24.Mulders AC, Mathy MJ, Meyer zu Heringdorf D, ter Braak M, Hajji N, Olthof DC, et al. Activation of sphingosine kinase by muscarinic receptors enhances NO-mediated and attenuates EDHF-mediated vasorelaxation. Basic Res Cardiol. 2009;104:50–9. doi: 10.1007/s00395-008-0744-x. [DOI] [PubMed] [Google Scholar]
- 25.Shimokawa H, Morikawa K. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in animals and humans. J Mol Cell Cardiol. 2005;39:725–32. doi: 10.1016/j.yjmcc.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 26.Park Y, Capobianco S, Gao X, Falck JR, Dellsperger KC, Zhang C. Role of EDHF in type 2 diabetes-induced endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2008;295:H1982–8. doi: 10.1152/ajpheart.01261.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Egashira K, Pipers FS, Rush JE, Morgan JP. Effects of calcium channel blockers on coronary vasoconstriction induced by endothelin-1 in closed chest pigs. J Am Coll Cardiol. 1990;16:1296–303. doi: 10.1016/0735-1097(90)90568-a. [DOI] [PubMed] [Google Scholar]
- 28.Szabo T, Geller L, Merkely B, Selmeci L, Juhasz-Nagy A, Solti F. Investigating the dual nature of endothelin-1: ischemia or direct arrhythmogenic effect? Life Sci. 2000;66:2527–41. doi: 10.1016/s0024-3205(00)00587-7. [DOI] [PubMed] [Google Scholar]
- 29.Ramzy D, Rao V, Tumiati LC, Xu N, Sheshgiri R, Miriuka S, et al. Elevated endothelin-1 levels impair nitric oxide homeostasis through a PKC-dependent pathway. Circulation. 2006;114:I319–26. doi: 10.1161/CIRCULATIONAHA.105.001503. [DOI] [PubMed] [Google Scholar]
- 30.Schafer U, Kurz T, Jain D, Hartmann F, Dendorfer A, Tolg R, et al. Impaired coronary flow and left ventricular dysfunction after mechanical recanalization in acute myocardial infarction: role of neurohumoral activation? Basic Res Cardiol. 2002;97:399–408. doi: 10.1007/s003950200049. [DOI] [PubMed] [Google Scholar]
- 31.Kyriakides ZS, Kremastinos DT, Bofilis E, Tousoulis D, Antoniadis A, Webb DJ. Endogenous endothelin maintains coronary artery tone by endothelin type A receptor stimulation in patients undergoing coronary arteriography. Heart. 2000;84:176–82. doi: 10.1136/heart.84.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reriani M, Raichlin E, Prasad A, Mathew V, Pumper GM, Nelson RE, et al. Long-term administration of endothelin receptor antagonist improves coronary endothelial function in patients with early atherosclerosis. Circulation. 2010;122:958–66. doi: 10.1161/CIRCULATIONAHA.110.967406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taylor AJ, Bobik A, Richards M, Kaye D, Raines G, Gould P, et al. Myocardial endothelin-1 release and indices of inflammation during angioplasty for acute myocardial infarction and stable coronary artery disease. Am Heart J. 2004;148:e10. doi: 10.1016/j.ahj.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 34.Mubagwa K, Mullane K, Flameng W. Role of adenosine in the heart and circulation. Cardiovasc Res. 1996;32:797–813. [PubMed] [Google Scholar]
- 35.Heusch G. Adenosine and maximum coronary vasodilation in humans: myth and misconceptions in the assessment of coronary reserve. Basic Res Cardiol. 2010;105:1–5. doi: 10.1007/s00395-009-0074-7. [DOI] [PubMed] [Google Scholar]
- 36.Koszalka P, Ozuyaman B, Huo Y, Zernecke A, Flogel U, Braun N, et al. Targeted disruption of cd73/ecto-5′-nucleotidase alters thromboregulation and augments vascular inflammatory response. Circ Res. 2004;95:814–21. doi: 10.1161/01.RES.0000144796.82787.6f. [DOI] [PubMed] [Google Scholar]
- 37.Skyschally A, Schulz R, Gres P, Konietzka I, Martin C, Haude M, et al. Coronary microembolization does not induce acute preconditioning against infarction in pigs—the role of adenosine. Cardiovasc Res. 2004;63:313–22. doi: 10.1016/j.cardiores.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 38.Kitakaze M, Hori M, Sato H, Takashima S, Inoue M, Kitabatake A, et al. Endogenous adenosine inhibits platelet aggregation during myocardial ischemia in dogs. Circ Res. 1991;69:1402–8. doi: 10.1161/01.res.69.5.1402. [DOI] [PubMed] [Google Scholar]
- 39.Pitarys CJ, 2nd, Virmani R, Vildibill HD, Jr, Jackson EK, Forman MB. Reduction of myocardial reperfusion injury by intravenous adenosine administered during the early reperfusion period. Circulation. 1991;83:237–47. doi: 10.1161/01.cir.83.1.237. [DOI] [PubMed] [Google Scholar]
- 40.Yang Z, Day YJ, Toufektsian MC, Ramos SI, Marshall M, Wang XQ, et al. Infarct-sparing effect of A2A-adenosine receptor activation is due primarily to its action on lymphocytes. Circulation. 2005;111:2190–7. doi: 10.1161/01.CIR.0000163586.62253.A5. [DOI] [PubMed] [Google Scholar]
- 41.Marzilli M, Orsini E, Marraccini P, Testa R. Beneficial effects of intracoronary adenosine as an adjunct to primary angioplasty in acute myocardial infarction. Circulation. 2000;101:2154–9. doi: 10.1161/01.cir.101.18.2154. [DOI] [PubMed] [Google Scholar]
- 42.Ross AM, Gibbons RJ, Stone GW, Kloner RA, Alexander RW. A randomized, double-blinded, placebo-controlled multicenter trial of adenosine as an adjunct to reperfusion in the treatment of acute myocardial infarction (AMISTAD-II) J Am Coll Cardiol. 2005;45:1775–80. doi: 10.1016/j.jacc.2005.02.061. [DOI] [PubMed] [Google Scholar]
- 43.Kleinbongard P, Bose D, Baars T, Mohlenkamp S, Konorza T, Schoner S, et al. Vasoconstrictor potential of coronary aspirate from patients undergoing stenting of saphenous vein aortocoronary bypass grafts and its pharmacological attenuation. Circ Res. 2011;108:344–52. doi: 10.1161/CIRCRESAHA.110.235713. [DOI] [PubMed] [Google Scholar]
- 44.Desmet W, Bogaert J, Dubois C, Sinnaeve P, Adriaenssens T, Pappas C, et al. High-dose intracoronary adenosine for myocardial salvage in patients with acute ST-segment elevation myocardial infarction. Eur Heart J. 2011;32:867–77. doi: 10.1093/eurheartj/ehq492. [DOI] [PubMed] [Google Scholar]
- 45.Levick SP, Loch DC, Taylor SM, Janicki JS. Arachidonic acid metabolism as a potential mediator of cardiac fibrosis associated with inflammation. J Immunol. 2007;178:641–6. doi: 10.4049/jimmunol.178.2.641. [DOI] [PubMed] [Google Scholar]
- 46.Thiemermann C, Zacharowski K. Selective activation of E-type prostanoid(3)-receptors reduces myocardial infarct size. A novel insight into the cardioprotective effects of prostaglandins. Pharmacol Ther. 2000;87:61–7. doi: 10.1016/s0163-7258(00)00069-3. [DOI] [PubMed] [Google Scholar]
- 47.Ehring T, Baumgart D, Krajcar M, Hummelgen M, Kompa S, Heusch G. Attenuation of myocardial stunning by the ACE inhibitor ramiprilat through a signal cascade of bradykinin and prostaglandins but not nitric oxide. Circulation. 1994;90:1368–85. doi: 10.1161/01.cir.90.3.1368. [DOI] [PubMed] [Google Scholar]
- 48.Schmitz JM, Apprill PG, Buja LM, Willerson JT, Campbell WB. Vascular prostaglandin and thromboxane production in a canine model of myocardial ischemia. Circ Res. 1985;57:223–31. doi: 10.1161/01.res.57.2.223. [DOI] [PubMed] [Google Scholar]
- 49.Lamping KG, Kanatsuka H, Eastham CL, Chilian WM, Marcus ML. Nonuniform vasomotor responses of the coronary microcirculation to serotonin and vasopressin. Circ Res. 1989;65:343–51. doi: 10.1161/01.res.65.2.343. [DOI] [PubMed] [Google Scholar]
- 50.Ichikawa Y, Yokoyama M, Akita H, Fukuzaki H. Constriction of a large coronary artery contributes to serotonin-induced myocardial ischemia in the dog with pliable coronary stenosis. J Am Coll Cardiol. 1989;14:449–59. doi: 10.1016/0735-1097(89)90201-5. discussion 60–1. [DOI] [PubMed] [Google Scholar]
- 51.Woodman OL. Enhanced coronary vasoconstrictor responses to 5-hydroxytryptamine in the presence of a coronary artery stenosis in anaesthetized dogs. Br J Pharmacol. 1990;100:153–7. doi: 10.1111/j.1476-5381.1990.tb12068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bekkers SC, Yazdani SK, Virmani R, Waltenberger J. Microvascular obstruction: underlying pathophysiology and clinical diagnosis. J Am Coll Cardiol. 2010;55:1649–60. doi: 10.1016/j.jacc.2009.12.037. [DOI] [PubMed] [Google Scholar]
- 53.Prasad A, Stone GW, Holmes DR, Gersh B. Reperfusion injury, microvascular dysfunction, and cardioprotection: the “dark side” of reperfusion. Circulation. 2009;120:2105–12. doi: 10.1161/CIRCULATIONAHA.108.814640. [DOI] [PubMed] [Google Scholar]
- 54.Heusch G, Kleinbongard P, Bose D, Levkau B, Haude M, Schulz R, et al. Coronary microembolization: from bedside to bench and back to bedside. Circulation. 2009;120:1822–36. doi: 10.1161/CIRCULATIONAHA.109.888784. [DOI] [PubMed] [Google Scholar]
- 55.Herrmann J, Haude M, Lerman A, Schulz R, Volbracht L, Ge J, et al. Abnormal coronary flow velocity reserve after coronary intervention is associated with cardiac marker elevation. Circulation. 2001;103:2339–45. doi: 10.1161/01.cir.103.19.2339. [DOI] [PubMed] [Google Scholar]
- 56.Bahrmann P, Werner GS, Heusch G, Ferrari M, Poerner TC, Voss A, et al. Detection of coronary microembolization by Doppler ultrasound in patients with stable angina pectoris undergoing elective percutaneous coronary interventions. Circulation. 2007;115:600–8. doi: 10.1161/CIRCULATIONAHA.106.660779. [DOI] [PubMed] [Google Scholar]
- 57.Sezer M, Oflaz H, Goren T, Okcular I, Umman B, Nisanci Y, et al. Intracoronary streptokinase after primary percutaneous coronary intervention. N Engl J Med. 2007;356:1823–34. doi: 10.1056/NEJMoa054374. [DOI] [PubMed] [Google Scholar]
- 58.Li S, Zhong S, Zeng K, Luo Y, Zhang F, Sun X, et al. Blockade of NF-kappaB by pyrrolidine dithiocarbamate attenuates myocardial inflammatory response and ventricular dysfunction following coronary microembolization induced by homologous microthrombi in rats. Basic Res Cardiol. 2010;105:139–50. doi: 10.1007/s00395-009-0067-6. [DOI] [PubMed] [Google Scholar]
- 59.Kloner RA, Giacomelli F, Alker KJ, Hale SL, Matthews R, Bellows S. Influx of neutrophils into the walls of large epicardial coronary arteries in response to ischemia/reperfusion. Circulation. 1991;84:1758–72. doi: 10.1161/01.cir.84.4.1758. [DOI] [PubMed] [Google Scholar]
- 60.Ambrosio G, Tritto I. Reperfusion injury: experimental evidence and clinical implications. Am Heart J. 1999;138:S69–75. doi: 10.1016/s0002-8703(99)70323-6. [DOI] [PubMed] [Google Scholar]
- 61.Ritter LS, McDonagh PF. Low-flow reperfusion after myocardial ischemia enhances leukocyte accumulation in coronary microcirculation. Am J Physiol. 1997;273:H1154–65. doi: 10.1152/ajpheart.1997.273.3.H1154. [DOI] [PubMed] [Google Scholar]
- 62.Lefer AM. Role of the beta2-integrins and immunoglobulin superfamily members in myocardial ischemia–reperfusion. Ann Thorac Surg. 1999;68:1920–3. doi: 10.1016/s0003-4975(99)01017-6. [DOI] [PubMed] [Google Scholar]
- 63.Jones SP, Trocha SD, Strange MB, Granger DN, Kevil CG, Bullard DC, et al. Leukocyte and endothelial cell adhesion molecules in a chronic murine model of myocardial reperfusion injury. Am J Physiol Heart Circ Physiol. 2000;279:H2196–201. doi: 10.1152/ajpheart.2000.279.5.H2196. [DOI] [PubMed] [Google Scholar]
- 64.Sun B, Fan H, Honda T, Fujimaki R, Lafond-Walker A, Masui Y, et al. Activation of NF kappa B and expression of ICAM-1 in ischemic–reperfused canine myocardium. J Mol Cell Cardiol. 2001;33:109–19. doi: 10.1006/jmcc.2000.1280. [DOI] [PubMed] [Google Scholar]
- 65.Fan H, Sun B, Gu Q, Lafond-Walker A, Cao S, Becker LC. Oxygen radicals trigger activation of NF-kappaB and AP-1 and upregulation of ICAM-1 in reperfused canine heart. Am J Physiol Heart Circ Physiol. 2002;282:H1778–86. doi: 10.1152/ajpheart.00796.2000. [DOI] [PubMed] [Google Scholar]
- 66.Kukielka GL, Hawkins HK, Michael L, Manning AM, Youker K, Lane C, et al. Regulation of intercellular adhesion molecule-1 (ICAM-1) in ischemic and reperfused canine myocardium. J Clin Invest. 1993;92:1504–16. doi: 10.1172/JCI116729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hawkins HK, Entman ML, Zhu JY, Youker KA, Berens K, Dore M, et al. Acute inflammatory reaction after myocardial ischemic injury and reperfusion. Development and use of a neutrophil-specific antibody. Am J Pathol. 1996;148:1957–69. [PMC free article] [PubMed] [Google Scholar]
- 68.Weyrich AS, Ma XY, Lefer DJ, Albertine KH, Lefer AM. In vivo neutralization of P-selectin protects feline heart and endothelium in myocardial ischemia and reperfusion injury. J Clin Invest. 1993;91:2620–9. doi: 10.1172/JCI116501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kupatt C, Wichels R, Deiss M, Molnar A, Lebherz C, Raake P, et al. Retroinfusion of NFkappaB decoy oligonucleotide extends cardioprotection achieved by CD18 inhibition in a preclinical study of myocardial ischemia and retroinfusion in pigs. Gene Ther. 2002;9:518–26. doi: 10.1038/sj.gt.3301673. [DOI] [PubMed] [Google Scholar]
- 70.Luo X, Cai H, Ni J, Bhindi R, Lowe HC, Chesterman CN, et al. c-Jun DNAzymes inhibit myocardial inflammation, ROS generation, infarct size, and improve cardiac function after ischemia–reperfusion injury. Arterioscler Thromb Vasc Biol. 2009;29:1836–42. doi: 10.1161/ATVBAHA.109.189753. [DOI] [PubMed] [Google Scholar]
- 71.Sakuma T, Sari I, Goodman CN, Lindner JR, Klibanov AL, Kaul S. Simultaneous integrin alphavbeta3 and glycoprotein IIb/IIIa inhibition causes reduction in infarct size in a model of acute coronary thrombosis and primary angioplasty. Cardiovasc Res. 2005;66:552–61. doi: 10.1016/j.cardiores.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 72.Erlich JH, Boyle EM, Labriola J, Kovacich JC, Santucci RA, Fearns C, et al. Inhibition of the tissue factor-thrombin pathway limits infarct size after myocardial ischemia–reperfusion injury by reducing inflammation. Am J Pathol. 2000;157:1849–62. doi: 10.1016/S0002-9440(10)64824-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zahler S, Massoudy P, Hartl H, Hahnel C, Meisner H, Becker BF. Acute cardiac inflammatory responses to postischemic reperfusion during cardiopulmonary bypass. Cardiovasc Res. 1999;41:722–30. doi: 10.1016/s0008-6363(98)00229-6. [DOI] [PubMed] [Google Scholar]
- 74.Baldus S, Heitzer T, Eiserich JP, Lau D, Mollnau H, Ortak M, et al. Myeloperoxidase enhances nitric oxide catabolism during myocardial ischemia and reperfusion. Free Radic Biol Med. 2004;37:902–11. doi: 10.1016/j.freeradbiomed.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 75.Takahashi T, Hiasa Y, Ohara Y, Miyazaki S, Ogura R, Miyajima H, et al. Relation between neutrophil counts on admission, microvascular injury, and left ventricular functional recovery in patients with an anterior wall first acute myocardial infarction treated with primary coronary angioplasty. Am J Cardiol. 2007;100:35–40. doi: 10.1016/j.amjcard.2007.02.049. [DOI] [PubMed] [Google Scholar]
- 76.Tinsley JH, Wu MH, Ma W, Taulman AC, Yuan SY. Activated neutrophils induce hyperpermeability and phosphorylation of adherens junction proteins in coronary venular endothelial cells. J Biol Chem. 1999;274:24930–4. doi: 10.1074/jbc.274.35.24930. [DOI] [PubMed] [Google Scholar]
- 77.Wang M, Zhang W, Crisostomo P, Markel T, Meldrum KK, Fu XY, et al. Endothelial STAT3 plays a critical role in generalized myocardial proinflammatory and proapoptotic signaling. Am J Physiol Heart Circ Physiol. 2007;293:H2101–8. doi: 10.1152/ajpheart.00125.2007. [DOI] [PubMed] [Google Scholar]
- 78.Bramos D, Ikonomidis I, Tsirikos N, Kottis G, Kostopoulou V, Pamboucas C, et al. The association of coronary flow changes and inflammatory indices to ischaemia–reperfusion microvascular damage and left ventricular remodelling. Basic Res Cardiol. 2008;103:345–55. doi: 10.1007/s00395-008-0720-5. [DOI] [PubMed] [Google Scholar]
- 79.Becker BF, Chappell D, Jacob M. Endothelial glycocalyx and coronary vascular permeability: the fringe benefit. Basic Res Cardiol. 2010;105:687–701. doi: 10.1007/s00395-010-0118-z. [DOI] [PubMed] [Google Scholar]
- 80.Chappell D, Dorfler N, Jacob M, Rehm M, Welsch U, Conzen P, et al. Glycocalyx protection reduces leukocyte adhesion after ischemia/reperfusion. Shock. 2010;34:133–9. doi: 10.1097/SHK.0b013e3181cdc363. [DOI] [PubMed] [Google Scholar]
- 81.Chappell D, Hofmann-Kiefer K, Jacob M, Rehm M, Briegel J, Welsch U, et al. TNF-alpha induced shedding of the endothelial glycocalyx is prevented by hydrocortisone and antithrombin. Basic Res Cardiol. 2009;104:78–89. doi: 10.1007/s00395-008-0749-5. [DOI] [PubMed] [Google Scholar]
- 82.Rehm M, Bruegger D, Christ F, Conzen P, Thiel M, Jacob M, et al. Shedding of the endothelial glycocalyx in patients undergoing major vascular surgery with global and regional ischemia. Circulation. 2007;116:1896–906. doi: 10.1161/CIRCULATIONAHA.106.684852. [DOI] [PubMed] [Google Scholar]