Abstract
The prognostic and predictive implications of programmed death-ligand 1 (PD-L1) is unknown in sarcoma. We sought to examine the immune milieu in sarcoma specimens. We evaluated PD-L1 expression by immunohistochemistry (IHC) in sarcoma specimens and quantified tumor infiltrating lymphocytes (TIL). We correlated expression with clinical parameters and outcomes. Fifty sarcoma patients treated at Memorial Sloan Kettering Cancer Center were selected. Using the DAKO PD-L1 IHC assay and archival formalin-fixed paraffin embedded tissue specimens; PD-L1 expression was examined. Macrophage and lymphocyte PD-L1 status was determined qualitatively. TIL was quantified. Associations between PD-L1 expression in tumor, macrophages and lymphocytes, TIL and clinical-pathological characteristics were performed. The median age was 46 years (range, 22 – 76) and 66% of patients were male. Tumor, lymphocyte and macrophage PD-L1 expression was noted in 12%, 30% and 58%, respectively with the highest prevalence in gastrointestinal stromal tumors (GIST) (29%.) Lymphocyte and macrophage infiltration was present in 98% and 90%, respectively. There was no association between clinical features, overall survival and PD-L1 expression in tumor or immune infiltrates. Lymphocyte and macrophage infiltration is common in sarcoma, but PD-L1 tumor expression is uncommon in sarcoma with the highest frequency observed in GIST. There was no association between PD-L1 expression, TIL and clinicopathological features and overall survival, however this is limited by the heterogenous patient sample and minimal death events in the studied cohort.
Keywords: sarcoma, immunotherapy, PD-L1, TIL
Background
Sarcoma is a rare heterogeneous disease with over 70 different subtypes which accounts for 1% of all cancers diagnosed in the United States each year[1]. Unfortunately, close to 50% of patients diagnosed with sarcoma will die from their disease. There remains a paucity of effective therapeutic options for this disease. With the advent of new and exciting immunotherapy agents there is interest in exploring the tumor microenvironment in sarcoma.
The tumor microenvironment has not been well characterized in many sarcoma subtypes. Tumor infiltrating lymphocytes (TIL) have been explored in gastrointestinal stromal tumor (GIST) and Ewing’s sarcoma. Using immunohistochemistry and flow cytometry in a cohort of 91 patients with GIST, cluster differentiation 3 (CD3+) infiltration correlated with improved progression free survival in a multivariate analysis[2]. In Ewing’s sarcoma, high levels of cluster differentiation 8 (CD8+) infiltration correlated with improved survival [3]. In an analysis of 249 sarcoma patients that used tissue microarrays and immunohistochemistry to evaluate CD3+, cluster differentiation 4 (CD4+), CD8+, cluster differentiation 20 (CD20+) and cluster differentiation 45 (CD45+) lymphocytes, only CD20+ lymphocytes were independently associated with improved disease free survival[4]. Nuclear transcription factor forkhead box P3 (FOXP3+) is essential in the development of regulatory T cells and a marker of T regulatory function[5]. High FOXP3+ infiltrates correlated with a high-risk disease and FOXP3+ infiltrates decreased after treatment with imatinib in patients with metastatic GIST[2]. Balachandran and colleagues have demonstrated that imatinib treatment leads to a decrease in FOXP3+ infiltration via a decrease in indoleamine 2, 3-dioxygenase (IDO) levels[6].
Nonetheless, the presence of immune infiltrates is not sufficient in controlling tumor growth. The development of anti-tumor immunity requires activation of cytotoxic T-lymphocytes as well as a balance of positive and negative signals[7]. Negative signals are often generated by cell-surface molecules such as cytotoxic T-lymphocyte antigen-4 (CTLA-4) as well as the programmed cell death 1 (PD-1) [8–10]. PD-1 is an inhibitory receptor that is part of the CD28 family and plays a major role in tumor immune escape[10]. Programmed death ligand-1 (PD-L1) is the ligand for PD-1 and is expressed on T cells, Bcells, macrophages, and dendritic cells as well as non-immune cells. PD-L1 expression is associated with poor prognosis in many different tumors [11–15].
The role of PD-L1 expression in soft tissue sarcoma was previously investigated using the Santa Cruz antibody, clone H-130[16]. In an analysis of 105 sarcoma specimens, PD-L1 tumor expression and intra-tumoral PD-1 positive lymphocytes was noted in 58% and 65% of specimens, respectively. Tumor cell PD-L1 positivity and intra-tumoral PD-1 lymphocytes were found to be independent prognostic factors of overall survival (OS) and event free survival.
In an attempt to further characterize the immune milieu of sarcoma, we evaluated PD-L1 and PD-1 expression in sarcoma tumor specimens, using the DAKO 5H-1 antibody. This antibody was used in the prospective clinical trials evaluating PD-1 blockade in melanoma and other malignancies [17, 18]. Our analysis includes certain histologies not previously studied such as chordoma, clear cell chondrosarcoma, desmoid, GIST, myoepithelial tumor, radiation-associated pleomorphic sarcoma and solitary fibrous tumor. We also sought to evaluate the prevalence of macrophages and TIL subsets including CD3+, CD4+, CD8+ and FOXP3+. FOXP3+ has not yet been explored in sarcoma subtypes other than GIST.
Materials/Methods
Cases were selected from sarcoma patients treated at Memorial Sloan Kettering Cancer Center from 2004 – 2013, who were consented to our IRB protocol 06:107 for tissue procurement and collection of correlative clinical information. All procedures were performed in compliance with our institutional guidelines and IRB approval. Original histologic slides were obtained from the archives and reviewed by a pathologist (NA) to confirm the diagnosis. Tumor parameters were recorded. Clinical information and follow up data on selected patients were obtained from the medical records.
Immunohistochemistry
Immunohistochemical staining for PD1 (mouse clone NAT antibody, LOT#GR81330-2 Abcam Cambridge, MA), CD3 (mouse clone LN10 antibody, LOT #6022944, Leica Newcastle Upon Tyne, United Kingdom), CD4 (mouse clone 4B12, Lot #00094580, Dako, Carpinteria, CA), CD8 (mouse clone C8/144B antibody, Lot#00089958, Dako Carpinteria, CA) and FOXP3 (mouse clone 236A/E7, antibody, Lot#GR108410-2, Abcam Cambridge, MA) was performed on 5 µm thick sections obtained from formalin-fixed paraffin embedded tissue of the selected cases. The mouse IgG control antibody was purchased from BD Pharmingen (San Diego, CA.) Multiplex IHC was performed using 3 validated assays provided by Mosaic Laboratories on sections of formalin-fixed, paraffin embedded. The assays selected were 2 dual stains: CD3+PD 1 and CD3+CD8+, and 1 triple stain: CD3+CD4+FOXP3+. Assays were developed using proprietary methodology at Mosaic Laboratories. Each multiplex IHC assay was designed and validated to be compatible with CLIA guideline class I test validation. In the CD3+PD-1 dual stain assay, CD3 was developed with red chromogranin and CD8 was developed with DAB chromogen. In the CD3+CD4+FOXP3+ triple stain assay, CD3 was developed with DAB chromogen, CD4 was developed with purple chromogen, and FOXP3 was developed with red chromogen. A representative 20× field of staining was spectrally imaged using the Nuance Multispectral Imaging System with Software v.2.4.0 (Caliper Life Sciences, Hopkinton, MA) attached to a Nikon 90i microscope. The multispectral image was acquired between 420 and 720 nm using 20 nm wavelength steps. Image cubes were analyzed using inForm software v1.2 (Caliper Life Sciences, Hopkinton, MA). Image cubes were unmixed using the spectral absorbance patterns for each chromogen and Hematoxylin.
Lymphocyte subsets (CD3+, CD4+, CD8+, FOXP3+) were quantified. The percentage of CD3+, CD4+, CD8+, FOXP3+ cells were calculated by dividing the total number of each respective cell type by the total number of cells present in each tissue section. A cutoff of 5% was used to distinguish high density versus low density cells of CD3+, CD4+ and CD8+. For FOXP3+ a cutoff of 1% was used to distinguish high density versus low density cells.
Immunohistochemical staining for PD-L1 (DAKO, Carpinteria, CA) was performed on 5 µm thick sections obtained from formalin-fixed paraffin embedded tissue of the selected cases. PD-L1 positivity was defined as >1% of tumor cells (minimum of 100 evaluable cells) demonstrating plasma membrane staining. Macrophage and lymphocyte PD-L1 status in the tumor tissue was determined qualitatively and not quantified.
Statistical Analysis
Patient characteristics are defined by frequency and percentage for categorical variables and median and range for continuous variables. Presentation status was defined as metastatic or primary depending on the extent of disease. Overall survival (OS) was calculated from date of primary resection to date of death or last follow up. Patients alive at last followup were censored. The Kaplan-Meier method and log-rank test were used to assess the relationship between overall survival and categorical variables. The p-value from the score test in a univariate Cox proportional hazards regression was used to assess the relationship between overall survival and continuous variables. P-values < 0.05 were considered significant. All analysis was performed using R version 3.0.2 (cran.r-project.org).
Results
Patients were predominantly male (66%) with a median age of 46 years (range 22–76.) At the time of surgical resection, the median primary tumor size was 6.5cm (range 1.0–36.5cm.) Most patients had intermediate/high grade and deep tumors, 39 (78%) and 45 (90%), respectively. A majority of patients did not have metastases at presentation, 32 (64%.) Histologic subtypes of the tumor were comprised of 14(29%) cases of Gastrointestinal Stromal Tumors(GIST), 4(8%) cases of leiomyosarcoma(LMS), 5(10%) cases of liposarcoma, 5(10%) cases of synovial sarcoma, 3 (6%) cases of angiosarcoma, 2 (4%)cases of extraskeletal myxoid chondrosarcoma, 2 (4%) cases solitary fibrous tumor, and 14 (30%) cases of other. (Table 1)
Table 1.
Summary of patient demographics and clinicopathologic features
| Patient Characteristic | N (%) |
|---|---|
|
| |
| Age (range) | 46 (22 – 76) |
|
| |
| Primary Tumor Size (cm) | 6.5 (1 – 36.5) |
|
| |
| Gender | |
|
| |
| Female | 17 (34) |
| Male | 33 (66) |
|
| |
| Histology | |
|
| |
| Gastrointestinal stromal tumor | 14 (28) |
| Leiomyosarcoma | 4 (8) |
| Liposarcoma* | 5 (10) |
| Synovial | 3 (6) |
| Chondrosarcoma | 3 (6) |
| Other | 21 (42) |
|
| |
| Original Location | |
|
| |
| Trunk | 6 (12) |
| Abdomen/Pelvis/Retroperitoneum | 30 (60) |
| Head & Neck | 6 (12) |
| Extremities | 8 (16) |
|
| |
| Grade | |
|
| |
| Low | 5 (10) |
| Intermediate/High | 39 (78) |
|
| |
| Depth | |
|
| |
| Superficial | 3 (6) |
| Deep | 45 (90) |
|
| |
| Margins | |
|
| |
| Negative | 34 (68) |
| Positive | 13 (26) |
|
| |
| Adjuvant Treatment | |
|
| |
| None | 24 (48) |
| Yes | 22 (44) |
| No Surgery | 3 (6) |
|
| |
| Disease status at MSKCC presentation | |
|
| |
| No metastases | 32 (64) |
| Metastases | 18 (36) |
|
| |
| Tumor infiltrating lymphocytes | |
|
| |
| CD3+ (%) | 3.3 (0–33.2) |
| CD4+ (%) | 0.2 (0–13.6) |
| CD8+ (%) | 1.2 (0–14) |
| FOXP3+ (%) | 0.1 (0–3.6) |
Abbreviations: MSKCC Memorial Sloan Kettering Cancer Center, CD 3 cluster differentiation 3, CD 4 cluster differentiation 4, CD 8 cluster differentiation 8, Nuclear transcription factor forkhead box P3 (FOXP3)
subtypes of liposarcoma included myxoid liposarcoma-2, dedifferentiated liposarcoma-2, well differentiated liposarcoma -1
Tumor infiltrating lymphocytes
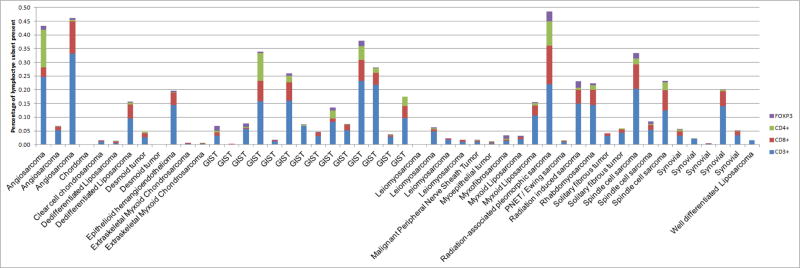
Tumor infiltrating lymphocytes were quantified. (Figure 1) The median number of each respective lymphocyte subset was as follows: CD3+ cells 3.3% (range 0–33.2%,) CD8+ cells 1.2% (range 0–14%,) CD4+ 0.2 (range 0–13.6%,) FOXP3+ 0.1 (range 0–3.6%.) (Figure 2)
Figure 1. Percentages of respective TIL.
This is the percentage of the respective lymphocyte subset (CD3+, CD4+, CD8+ and FOXP3+) of the total cells present.
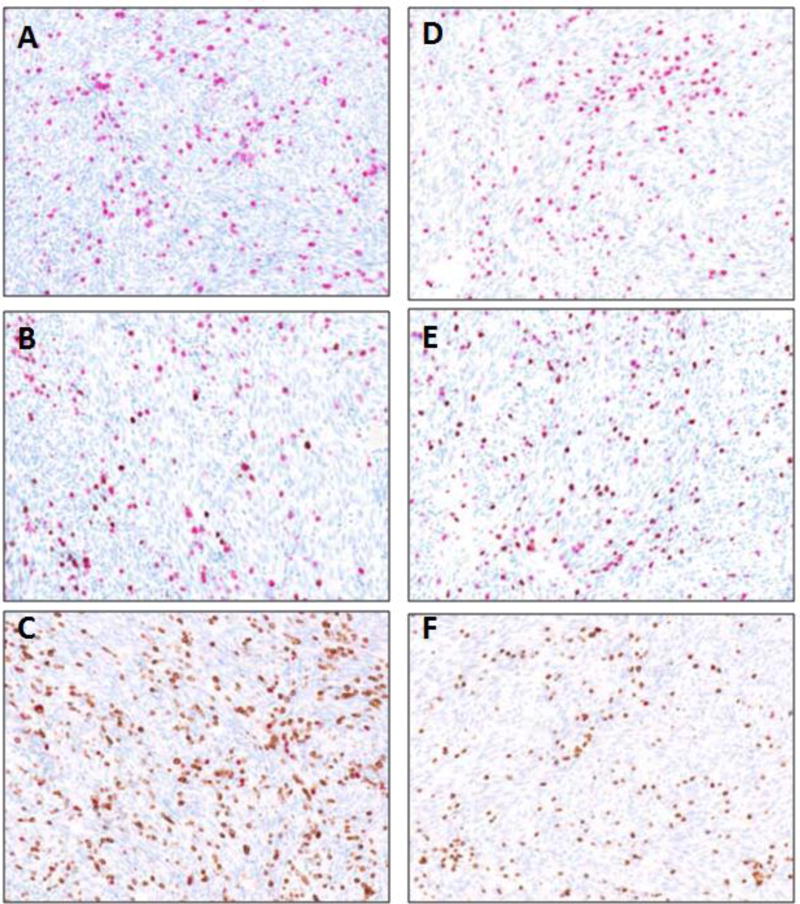
Figure 2. Representative images of multiplex TIL staining.
A representative 20× field of staining was spectrally imaged. CD3+ was stained in red chromogen in figures A,B,D,E and DAB chromogen in figures C and F. CD8+ was stained in DAB in figures B and E and in purple chromogen in figure F. PD-1 was stained in DAB in figures A and D. FOXP3+ was stained in red chromogen in figures C and F.
A: CD3+PD-1 multiplex IHC in angiosarcoma patient.
B: CD3+CD8+ multiplex IHC in angiosarcoma patient.
C: CD3+CD4+FOXP3+ multiplex IHC in angiosarcoma patient.
D: CD3+PD-1 multiplex IHC in GIST patient.
E: CD3+CD8+ multiplex IHC in GIST patient.
F: CD3+CD4+FOXP3+ multiplex IHC in GIST patient.
Abbreviations: GIST- gastrointestinal stromal tumor
As a dichotomous variable, CD3+, CD8+, and CD4+ expression was defined as “low density” if the percentage of cells was <5% and ”high density” if the percentage of cells was ≥ 5%. For FOXP3+, a cutoff of 1% of cells was used to define low versus high density.
For CD3+, 27 patients (54%) had low density cells while 22 patients (44%) had high density of cells. Histological subtypes such as LMS, synovial sarcoma, chondrosarcoma and liposarcoma generally had low density CD3+ infiltration. Of those patients with high density CD3+ infiltration, 9/22 (41%) had GIST. (Table 2)
Table 2.
Tumor infiltrating lymphocyte subsets by histology
| Histology | n (%) |
CD3 | CD4 | CD8 | FOX3 | ||||
|---|---|---|---|---|---|---|---|---|---|
| 0–5% (n = 27) |
> 5% (n = 22) |
0–5% (n = 45) |
5% (n = 4) |
0–5% (n = 38) |
> 5% (n = 11) |
0% (n = 16) |
1–5% (n = 33) |
||
| Angiosarcoma | 3 (6) | 0 (0) | 3 (14) | 2 (4) | 1 (25) | 2 (5) | 1 (9) | 0 (0) | 3 (9) |
| Clear Cell Chondrosarcoma | 1 (2) | 1 (4) | 0 (0) | 1 (2) | 0 (0) | 1 (3) | 0 (0) | 1 (6) | 0 (0) |
| Chordoma | 1 (2) | 1 (4) | 0 (0) | 1 (2) | 0 (0) | 1 (3) | 0 (0) | 1 (6) | 0 (0) |
| Dedifferentiated Liposarcoma | 2 (4) | 1 (4) | 1 (5) | 2 (4) | 0 (0) | 1 (3) | 1 (9) | 0 (0) | 2 (6) |
| Desmoid | 2 (4) | 2 (7) | 0 (0) | 2 (4) | 0 (0) | 2 (5) | 0 (0) | 2 (12) | 0 (0) |
| Epithelioid hemangioendothelioma | 1 (2) | 0 (0) | 1 (5) | 1 (2) | 0 (0) | 1 (3) | 0 (0) | 0 (0) | 1 (3) |
| Extraskeletal myxoid chondrosarcoma | 1 (2) | 1 (4) | 0 (0) | 1 (2) | 0 (0) | 1 (3) | 0 (0) | 1 (6) | 0 (0) |
| Gastrointestinal stromal tumor | 14 (28) | 5 (19) | 9 (41) | 12 (27) | 2 (50) | 11 (29) | 3 (27) | 2 (12) | 12 (36) |
| Leiomyosarcoma | 4 (8) | 3 (11) | 0 (0) | 3 (7) | 0 (0) | 3 (8) | 0 (0) | 1 (6) | 2 (6) |
| Malignant Peripheral Nerve Sheath Tumor | 1 (2) | 1 (4) | 0 (0) | 1 (2) | 0 (0) | 1 (3) | 0 (0) | 0 (0) | 1 (3) |
| Myoepithelial tumor | 1 (2) | 1 (4) | 0 (0) | 1 (2) | 0 (0) | 1 (3) | 0 (0) | 0 (0) | 1 (3) |
| Myxofibrosarcoma | 1 (2) | 1 (4) | 0 (0) | 1 (2) | 0 (0) | 1 (3) | 0 (0) | 0 (0) | 1 (3) |
| Myxoid Liposarcoma | 2 (4) | 1 (4) | 1 (5) | 2 (4) | 0 (0) | 2 (5) | 0 (0) | 0 (0) | 2 (6) |
| Pleomorphic rhabdomyosarcoma | 1 (2) | 0 (0) | 1 (5) | 0 (0) | 1 (25) | 0 (0) | 1 (9) | 0 (0) | 1 (3) |
| Primitive Neuroectodermal Tumor /Ewings | 1 (2) | 1 (4) | 0 (0) | 1 (2) | 0 (0) | 1 (3) | 0 (0) | 1 (6) | 0 (0) |
| Radiation-associated pleomorphic sarcoma | 1 (2) | 0 (0) | 1 (5) | 1 (2) | 0 (0) | 0 (0) | 1 (9) | 0 (0) | 1 (3) |
| Rhabomyosarcoma | 1 (2) | 0 (0) | 1 (5) | 1 (2) | 0 (0) | 0 (0) | 1 (9) | 0 (0) | 1 (3) |
| Solitary Fibrous tumor | 2 (4) | 2 (7) | 0 (0) | 2 (4) | 0 (0) | 2 (5) | 0 (0) | 2 (12) | 0 (0) |
| Spindle cell sarcoma | 3 (6) | 0 (0) | 3 (14) | 3 (7) | 0 (0) | 1 (3) | 2 (18) | 0 (0) | 3 (9) |
| Synovial sarcoma | 5 (10) | 4 (15) | 1 (5) | 5 (11) | 0 (0) | 4 (11) | 1 (9) | 4 (25) | 1 (3) |
| Well differentiated liposarcoma | 1 (2) | 1 (4) | 0 (0) | 1 (2) | 0 (0) | 1 (3) | 0 (0) | 1 (6) | 0 (0) |
Abbreviations: CD 3 cluster differentiation 3, CD 4 cluster differentiation 4, CD 8 cluster differentiation 8, Nuclear transcription factor forkhead box P3 (FOXP3)
For CD4+, 45 patients (90%) had low density cells while 4 patients (8%) had high density cells. There were 2/4 patients with GIST that had high density CD4+ infiltration. However most GIST patients (12/14) had low percentage CD4+ cells. (Table 2)
For CD8+, there were 11 patients with high density cells compared to 38 patients with low density cells. Therefore, many histological subtypes such as GIST, LMS, liposarcoma, synovial sarcoma and chondrosarcoma generally had low density CD8+ cells.
There were 16 patients that had no FOXP3+ cells present, while the remaining 33 patients (75%) had >1% cells. The highest percentage of FOXP3+ cells were found in GIST 12/33 (36%.) (Table 2)
There was no clear correlation noted between initial tumor size, tumor, lymphocyte or macrophage PD-L1 status and initial tumor characteristics for patients with GIST. In addition, there was no clear trend noted between TIL and tumor characteristics. (Supplemental Table 1)
The relationship between low versus high density TIL and clinicopathological features was evaluated. Median tumor size for patients with >5% CD8+ cells was 10.8cm versus 6.1cm for those with <5% cells, p=0.022. Patients who presented with metastases were more likely to have high CD8+ cells (7/11, 64%) versus low CD8+ cells (10/38, 26%), p=0.033. Tumors with GIST histology were more likely to have >1% FOXP3+ infiltration compared to other histologies, p=0.053. Deep tumors were more likely to be associated with high FOXP3+ (32/33, 97%) compared to superficial tumors (0), p=0.028. For the survival analysis, low CD3+ p=0.050 and low CD4+ p=0.050 appeared to correlate with better overall survival.
PD-L1/PD-1 expression in tumor specimens, lymphocytes and macrophages
Tumor cell PD-L1 expression was observed in 6 (12%) of cases. (Figure 3) Lymphocytic and macrophagic PD-L1 expression was identified in 15 (30%) and 29 (58%) of specimens, respectively. PD-1 expression was observed in 11 (22%) of specimens. Of all sarcoma subtypes sampled, GIST had the highest prevalence of PD-L1 expression noted in 4/14 (29%) of samples. In addition there was 1 radiation associated pleomorphic sarcoma and 1 spindle cell sarcoma with tumor positive for PD-L1 expression. (Table 3)
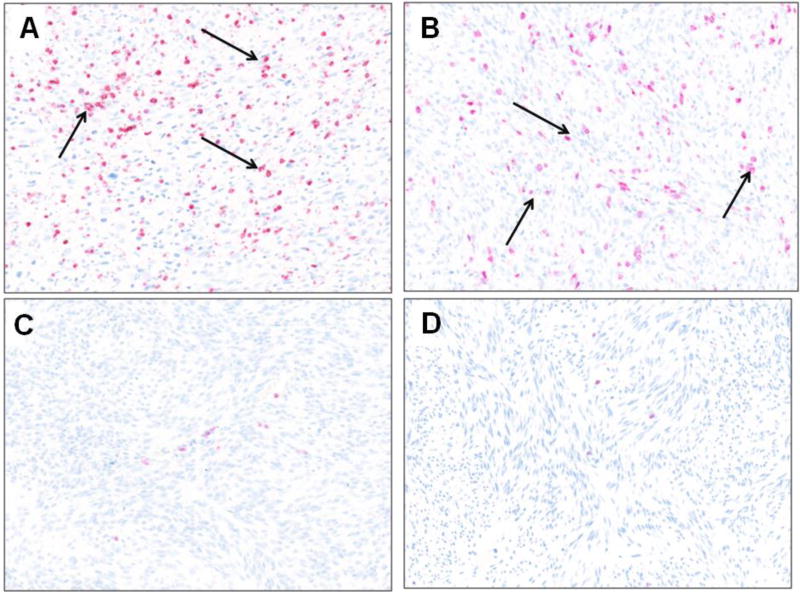
Figure 3. Representative images of positive PD-L1 staining by IHC in (A) Gastrointestinal stromal tumor and (B) Radiation-associated pleomorphic sarcoma, arrows indicate PD-L1 positive cells.
A representative 20× field of staining was spectrally imaged. Negative PD-L1 staining in (C) Gastrointestinal stromal tumor and (D) synovial sarcoma. PD-L1 staining is indicated by red chromogen.
Abbreviations: GIST- gastrointestinal stromal tumor, PNET peripheral nerve sheath tumor
Table 3.
PD-L1 expression in tumor, lymphocytes and macrophages by histology
| Histology | n (%) |
Tumor PD-L1 expression |
Lymphocyte infiltration |
Lymphocyte PD-L1 expression |
Macrophage infiltration |
Macrophage PD-L1 expression |
|---|---|---|---|---|---|---|
| Angiosarcoma | 3 | 0 | 3 (100%) | 3 (100%) | 3 (100%) | 3 (100%) |
| Clear Cell Chondrosarcoma | 1 | 0 | 1 (100%) | 0 | 1 (100%) | 1 (100%) |
| Chordoma | 1 | 0 | 1 (100%) | 0 | 1 (100%) | 0 |
| Dedifferentiated Liposarcoma | 2 | 0 | 2 (100%) | 1 (50%) | 1 (50%) | 1 (50%) |
| Desmoid | 2 | 0 | 2 (100%) | 0 | 2 (100%) | 2 (100%) |
| Epithelioid hemangioendothelioma | 1 | 0 | 1 (100%) | 0 | 1 (100%) | 1 (100%) |
| Extraskeletal myxoid chondrosarcoma | 2 | 0 | 2 (100%) | 0 | 2 (100%) | 1 (100%) |
| Gastrointestinal stromal tumor | 14 | 4 (29%) | 14 (100%) | 7 (50%) | 13 (93%) | 7 (50%) |
| Leiomyosarcoma | 4 | 0 | 3 (75%) | 0 | 3 (75%) | 1 (25%) |
| Malignant Peripheral Nerve Sheath Tumor | 1 | 0 | 1 (100%) | 0 | 1 (100%) | 1 (100%) |
| Myoepithelial tumor | 1 | 0 | 1 (100%) | 0 | 1 (100%) | 0 |
| Myxofibrosarcoma | 1 | 0 | 1 (100%) | 0 | 1 (100%) | 1 (100%) |
| Myxoid Liposarcoma | 2 | 0 | 2 (100%) | 0 | 2 (100%) | 2 (100%) |
| Pleomorphic rhabdomyosarcoma | 1 | 0 | 1 (100%) | 0 | 1 (100%) | 1 (100%) |
| Primitive Neuroectodermal Tumor /Ewings | 1 | 0 | 1 (100%) | 0 | 1 (100%) | 1 (100%) |
| Radiation-associated pleomorphic sarcoma | 1 | 1 (100%) | 1 (100%) | 1 (100%) | 1 (100%) | 1 (100%) |
| Rhabomyosarcoma | 1 | 0 | 1 (100%) | 1 (100%) | 1 (100%) | 1 (100%) |
| Solitary Fibrous tumor | 2 | 0 | 2 (100%) | 0 | 2 (100%) | 1 (50%) |
| Spindle cell sarcoma | 3 | 1 (33%) | 3 (100%) | 0 | 2 (67%) | 2 (67%) |
| Synovial sarcoma | 5 | 0 | 5 (100%) | 2 (40%) | 5 (100%) | 1 (20%) |
| Well differentiated liposarcoma | 1 | 0 | 1 (100%) | 0 | 0 | 0 |
| TOTAL | 50 | 6 (12%) | 49 (98%) | 15 (30%) | 45 (90%) | 29 (58%) |
Abbreviation: PD-L1 programmed death ligand -1
Evaluating relationship between PD-L1 expression and TIL
Among the 6 specimens containing PD-L1 + tumor cells, 100% harbored tumor infiltrating lymphocytes (TIL.) In the remaining 44 (88%) specimens which were negative for tumor PD-L1 expression, 43 (98%) contained TIL. The relationship between the TIL and PD-L1 status was evaluated. Positive tumor PD-L1 expression was associated with high density CD8+ cells, p=0.020. (Table 4) Positive tumor PD-1 was significantly associated with high density CD3+ p <0.001 and high density CD8+ p=0.009. Positive lymphocytic PD-L1 expression was significantly associated with high CD8+ p= 0.018. (Table 4)
Table 4.
relationship between Tumor infiltrating lymphocyte subsets and Tumor/Lymph PD-L1 status
| Tumor PDL-1 | Lymph PDL-1 | Tumor PD1 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Negative (n=44) |
Positive (n=6) |
p- valuea |
Negative (n=33) |
Positive (n=15) |
p- valuea |
Negative (n=38) |
Positive (n=11) |
p- valuea |
|
| Lymphocyte Infiltration | |||||||||
| No | 1 (2) | 0 (0) | 1 | 0 (0) | 0 (0) | 1 | 0 (0) | 0 (0) | 1 |
| Yes | 43 (98) | 6 (100) | 33 (100) | 15 (100) | 38 (100) | 11 (100) | |||
|
| |||||||||
| Macrophage Infiltration | |||||||||
| No | 3 (7) | 2 (33) | 0.103 | 3 (9) | 1 (7) | 1 | 3 (8) | 1 (9) | 1 |
| Yes | 41 (93) | 4 (67) | 30 (91) | 14 (93) | 35 (92) | 10 (91) | |||
|
| |||||||||
| CD3 | |||||||||
| 0–5% | 26 (59) | 1 (17) | 0.0773 | 24 (73) | 2 (13) | <0.001 | 27 (71) | 0 (0) | <0.001 |
| >5% | 17 (39) | 5 (83) | 9 (27) | 13 (87) | 11 (29) | 11 (100) | |||
|
| |||||||||
| CD4 | |||||||||
| 0–5% | 40 (91) | 5 (83) | 0.4175 | 31 (94) | 13 (87) | 0.5794 | 34 (89) | 11 (100) | 0.562 |
| >5% | 3 (7) | 1 (17) | 2 (6) | 2 (13) | 4 (11) | 0 (0) | |||
|
| |||||||||
| CD8 | |||||||||
| 0–5% | 36 (82) | 2 (33) | 0.0179 | 29 (88) | 8 (53) | 0.0219 | 33 (87) | 5 (45) | 0.0088 |
| >5% | 7 (16) | 4 (67) | 4 (12) | 7 (47) | 5 (13) | 6 (55) | |||
|
| |||||||||
| FOX3 | |||||||||
| 0–5% | 16 (36) | 0 (0) | 0.1588 | 15 (45) | 1 (7) | 0.009 | 15 (39) | 1 (9) | 0.0762 |
| >5% | 27 (61) | 6 (100) | 18 (55) | 14 (93) | 23 (61) | 10 (91) | |||
: p <0.05 set as significant
Among the various clinicopathologic factors, there were no significant associations with tumor PD-L1 expression, lymphocytic or macrophage PD-L1 expression and PD-1 expression. There was no identified association between tumor and lymphocyte PD-L1 or PD-1 status and OS. (Table 5)
Table 5.
Association of clinicopathologic features with overall survival
| n | No. events |
Median OS |
5-years OS |
p-valuea | |
|---|---|---|---|---|---|
| Age | 47 | 11 | -- | -- | 0.3523 |
| Primary Tumor Size | 47 | 11 | -- | -- | 0.5842 |
| Gender | |||||
| Female | 17 | 3 | 12.17 | 1 | 0.0209 |
| Male | 30 | 8 | 10.24 | 0.78 | |
| Histology | |||||
| Gastrointestinal Stromal Tumor | 13 | 3 | 10.24 | 0.92 | 0.1806 |
| Leiomyosarcoma | 3 | 1 | 12.17 | 1 | |
| Liposarcoma | 5 | 2 | 7.52 | 1 | |
| Synovial | 3 | 0 | NR | 1 | |
| Chondrosarcoma | 3 | 1 | 5.96 | 1 | |
| Other | 20 | 4 | NR | 0.61 | |
| Original Location | |||||
| Trunk | 5 | 0 | NR | NR | 0.2957 |
| Abdomen/Pelvis/Retroperitoneum | 29 | 8 | 10.24 | 0.91 | |
| Head & Neck | 6 | 3 | 2.78 | 0.5 | |
| Extremities | 7 | 0 | NR | 1 | |
| Grade | |||||
| Low | 5 | 0 | NR | 1 | 0.4201 |
| Intermediate/High | 36 | 10 | 10.24 | 0.85 | |
| Depth | |||||
| Superficial | 3 | 1 | 12.17 | 1 | 0.0534 |
| Deep | 42 | 10 | 10.24 | 0.84 | |
| Margins | |||||
| Negative | 34 | 6 | 11.33 | 0.9 | 0.3846 |
| Positive | 13 | 5 | 8.13 | 0.74 | |
| Tumor PDL1 | |||||
| Neg | 43 | 10 | 10.24 | 0.87 | 0.8678 |
| Pos | 4 | 1 | NR | 0.75 | |
| Lymphocyte PDL1 | |||||
| Neg | 32 | 9 | 10.24 | 0.88 | 0.8764 |
| Pos | 14 | 2 | NR | 0.76 | |
| Macrophage PDL1 | |||||
| Neg | 14 | 3 | 11.33 | 0.92 | 0.3326 |
| Pos | 28 | 7 | 10.24 | 0.79 | |
| Tumor PD1 | |||||
| Neg | 37 | 8 | 11.33 | 0.85 | 0.1202 |
| Pos | 10 | 3 | 8.13 | 0.71 | |
| Macrophage Infiltration | |||||
| No | 3 | 1 | 6.91 | 1 | 0.6489 |
| Yes | 44 | 10 | 11.33 | 0.85 | |
| Lymphocyte Infiltration | |||||
| Yes | 47 | 11 | 10.24 | 0.86 | -- |
| CD3 | |||||
| 0–5% | 27 | 5 | 11.33 | 0.95 | 0.0496 |
| >5% | 20 | 6 | 8.13 | 0.72 | |
| CD4 | |||||
| 0–5% | 43 | 8 | 11.33 | 0.91 | 0.0459 |
| >5% | 4 | 3 | 6.51 | 0.5 | |
| CD8 | |||||
| 0–5% | 38 | 8 | 11.33 | 0.89 | 0.091 |
| >5% | 9 | 3 | 10.24 | 0.71 | |
| FOXP3 | |||||
| 0% | 16 | 4 | 11.33 | 0.92 | 0.2598 |
| 1–5% | 31 | 7 | 10.24 | 0.83 | |
| CD8/FOXP3 Ratio | |||||
| <= 1 | 12 | 2 | 6.91 | 0.9 | 0.707 |
| >1 | 12 | 4 | 10.24 | 0.79 |
Abbreviations: OS overall survival, NR not reached
: p <0.05 set as significant
Discussion
With the advent of new and novel systemic immunotherapeutic agents, exploring the sarcoma tumor microenvironment remains a high priority. We noted infiltration of lymphocytes and macrophages in 98% and 90% of tumor samples, respectively. This finding was interesting since there is limited data demonstrating T cell infiltration in sarcoma tumor specimens. There were high amounts of CD3+, CD4+, and CD8+ cells relative to FOXP3+ cells. GIST appeared to have highest density of FOXP3+ cells. Previous literature has demonstrated FOXP3+ infiltration in GIST tumors [2]. While deep tumors appeared to be more likely to have high FOXP3+ infiltration compared to superficial tumors, it must be noted that there were only 6 superficial tumors in the analysis. In addition, most of the high density FOXP3+ tumors were GIST histology.
Low CD3+ infiltration p= 0.050 and low CD4+ infiltration p= 0.050 appeared to correlate with better overall survival on univariate analysis. This must be interpreted with caution; the tumor microenvironment may be influenced by other factors not evaluated in this analysis. In addition, the number of events in our sample size was quite small. This finding is contrary to what one would expect. In a large meta-analysis that evaluated the impact of TIL in multiple different tumors, CD3+ and CD8+ infiltration had a positive impact on survival[19]. This apparent discrepancy may warrant further exploration.
We identified a greater number of CD8+ cells in patients that had larger tumors or presented with metastatic disease. Further, those tumors with higher amounts of infiltrating CD8+ or CD3+ cells were more likely to express PD-L1 and PD-1 in their tumors and lymphocytes. This finding suggests that these T cells may be functionally deficient or “exhausted” due to the upregulated inhibitory receptor [20]. T cell exhaustion has been previously described as the clonal deletion of virus specific CD8+ T cells in the setting of high grade infections[21]. In addition, there is data to suggest that this occurs in metastatic lesions in melanoma patients [20]. As T cells become exhausted, they acquire multiple inhibitory molecules and become unable to mount an appropriate immune response. Investigating other inhibitor molecules such as T-cell immunoglobulin and mucin domain-containing protein 3 (TIM-3), CTLA-4 and lymphocyte activated gene-3 (LAG-3) may yield additional findings and identify other potential targets for future clinical studies beyond PD-1.
There has been great interest in exploring checkpoint inhibitors such as those that block the PD-1 pathway. Initially, PD-L1 expression was thought to correlate with benefit to PD-1 blockade [17]. It has since been demonstrated in multiple tumor types that lack of PD-L1 expression does not preclude a response to PD-1 blockade, with overall response rates ranging from 10–15%[18, 22–24]. That is comparable to most cytotoxic chemotherapies used in the metastatic setting for sarcoma[1].
We identified tumor PD-L1 and PD-1 expression in 12% and 22% of samples, respectively. Lymphocytic and macrophagic PD-L1 expression was identified in 30% and 58% of specimens. GIST did have the highest tumor PD-L1 expression, noted in 4/14 (29%) of tumor specimens. The expression patterns were different from the previously published report which noted tumor PD-L1 and PD-1 expression in 65% and 58% of sarcoma specimens [16]. In that analysis; the Santa Cruz antibody was used. The disparity in our findings may support the variability in the PD-L1 assays and lack of standardized approach to PD-L1 testing. The type of antibody, the staining conditions, automated versus manual reading and the definition of a positive result with a specific cutoff contributes to the discordance. The interval between biopsy and treatment and whether the primary disease versus a metastatic site was tested may further impact PD-L1 expression. Evaluating PD-L1 expression at an isolated time point may not represent its true prevalence. Our data represent the first attempt to characterize PD-L1 expression in sarcomas with the same Dako assay and definition of positive staining that has been utilized in prior studies[17, 18]. In addition, our analysis was expanded to additional histological subtypes not tested in the prior published study.
Kim et al. did identify PD-L1 and PD-1 positivity as independent prognostic indicators of overall survival [16]. However we could not replicate those findings. Nor did we identify a relationship between tumor PD-L1 expression and clinicopathological variables. Limitations of our analysis were the heterogeneity of the samples as well as insufficient number of each histological subtype included. There were over 20 histologies represented; and typically only 1–2 tumor specimens within each histological subtype. Further, the patients’ baseline characteristics were diverse. There were some patients with primary disease while others with metastatic disease at various time points in their treatment. The immune milieu likely is different in the setting of advanced cancer. The fact the metastatic cancer has developed is likely reflective of failed immune surveillance and tumor escape. Evaluating more tumor specimens within each histological subtype and with similar presentation status may provide a more accurate estimate of the true prevalence of PD-L1 expression in sarcoma.
Conclusion
Our current analysis has demonstrated that TIL are present in the sarcoma microenvironment. We found low expression of PD-L1 in sarcoma specimens which is different from the previously published literature [16]. This discordance highlights the need for a standardized approach to test for PD-L1 expression. Although tumor PD-L1 expression has prognostic significance in many malignancies, its role beyond that of a prognostic biomarker is unknown [11–14, 16]. Further, lack of PD-L1 expression does not correlate to lack of benefit from PD-1 blockade; therefore, this data should not preclude clinical trials evaluating such agents in sarcoma. Moving forward, identifying alternate biomarkers to predict which patients are most likely to benefit from PD-1 blockade is necessary.
Supplementary Material
Acknowledgments
Mosaic Laboratory, for performing multiplex IHC analysis.
Funding: Supported by Cycle for Survival.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None of the authors have any disclosures to report.
References
- 1.Brennan MFAC, Maki RG. Management of Soft Tissue Sarcoma. New York: Springer; 2012. [Google Scholar]
- 2.Rusakiewicz S, Semeraro M, Sarabi M, et al. Immune infiltrates are prognostic factors in localized gastrointestinal stromal tumors. Cancer Res. 2013;73:3499–3510. doi: 10.1158/0008-5472.CAN-13-0371. [DOI] [PubMed] [Google Scholar]
- 3.Berghuis D, Santos SJ, Baelde HJ, et al. Pro-inflammatory chemokine-chemokine receptor interactions within the Ewing sarcoma microenvironment determine CD8(+) T-lymphocyte infiltration and affect tumour progression. J Pathol. 2011;223:347–357. doi: 10.1002/path.2819. [DOI] [PubMed] [Google Scholar]
- 4.Sorbye SW, Kilvaer T, Valkov A, et al. Prognostic impact of lymphocytes in soft tissue sarcomas. PLoS One. 2011;6:e14611. doi: 10.1371/journal.pone.0014611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang L, Zhao Y. The regulation of Foxp3 expression in regulatory CD4(+)CD25(+)T cells: multiple pathways on the road. J Cell Physiol. 2007;211:590–597. doi: 10.1002/jcp.21001. [DOI] [PubMed] [Google Scholar]
- 6.Balachandran VP, Cavnar MJ, Zeng S, et al. Imatinib potentiates antitumor T cell responses in gastrointestinal stromal tumor through the inhibition of Ido. Nat Med. 2011;17:1094–1100. doi: 10.1038/nm.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 8.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 9.Latchman Y, Wood CR, Chernova T, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 10.Ravetch JV, Lanier LL. Immune inhibitory receptors. Science. 2000;290:84–89. doi: 10.1126/science.290.5489.84. [DOI] [PubMed] [Google Scholar]
- 11.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 12.Konishi J, Yamazaki K, Azuma M, et al. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res. 2004;10:5094–5100. doi: 10.1158/1078-0432.CCR-04-0428. [DOI] [PubMed] [Google Scholar]
- 13.Strome SE, Dong H, Tamura H, et al. B7-H1 blockade augments adoptive T-cell immunotherapy for squamous cell carcinoma. Cancer Res. 2003;63:6501–6505. [PubMed] [Google Scholar]
- 14.Thompson RH, Gillett MD, Cheville JC, et al. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci U S A. 2004;101:17174–17179. doi: 10.1073/pnas.0406351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wintterle S, Schreiner B, Mitsdoerffer M, et al. Expression of the B7-related molecule B7-H1 by glioma cells: a potential mechanism of immune paralysis. Cancer Res. 2003;63:7462–7467. [PubMed] [Google Scholar]
- 16.Kim JR, Moon YJ, Kwon KS, et al. Tumor infiltrating PD1-positive lymphocytes and the expression of PD-L1 predict poor prognosis of soft tissue sarcomas. PLoS One. 2013;8:e82870. doi: 10.1371/journal.pone.0082870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus Ipilimumab in Advanced Melanoma. N Engl J Med. 2013 doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer. 2011;105:93–103. doi: 10.1038/bjc.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baitsch L, Baumgaertner P, Devevre E, et al. Exhaustion of tumor-specific CD8(+) T cells in metastases from melanoma patients. J Clin Invest. 2011;121:2350–2360. doi: 10.1172/JCI46102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moskophidis D, Lechner F, Pircher H, Zinkernagel RM. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 1993;362:758–761. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- 22.Herbst RGM, Gordon M, Fine GD, Sosman JA, Soria JC, Hamid O, Powderly JD, Burris HA, Mokatrin A, Kowanetz M, Leabman M, Anderson M, Chen DS, Hodi SF. A study of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic tumors. Journal of Clinical Oncology. 2013;31(suppl) abstr 3000. [Google Scholar]
- 23.Powderly JD, Koeppen H, Hodi SF, et al. Biomarkers and associations with the clinical activity of PD-L1 blockade in a MPDL3280A study. Journal of Clinical Oncology. 2013;31(suppl) abstr 3001. [Google Scholar]
- 24.Powles TVN, Vogelzang N, Fine GD, Eder JP, Braiteh FS, Loriot Y, Zambrano CC, Bellmunt J, Burris HA, Teng SM, Shen X, Koeppen H, Hegde PS, Chen DS, Petrylak DP. Inhibition of PD-L1 by MPDL3280A and clinical activity in pts with metastatic urothelial bladder cancer (UBC) Journal of Clinical Oncology. 2014;32(suppl):5. abstr 5011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.