Abstract
Research into the neurobiological substrates of psychopathology has been impeded by heterogeneity within diagnostic categories, comorbidity among mental disorders, and the presence of symptoms that transcend diagnostic categories. Solutions to these issues increasingly focus neurobiological research on isolated or narrow groupings of symptoms or functional constructs rather than categorical diagnoses. Here we argue for a more integrative approach that also incorporates the broad hierarchical structure of psychopathological symptoms and their etiological mechanisms. This approach places clinical neuroscience research in the context of a hierarchy of empirically defined factors of symptoms such as internalizing disorders, externalizing disorders, and the general factor of psychopathology. Application of this hierarchical approach has the potential to reveal neural substrates that nonspecifically contribute to multiple forms of psychopathology and their comorbidity, and in doing so, facilitate the study of mechanisms that are specific to single dimensions and subsets of symptoms. Neurobiological research on the hierarchy of dimensions of psychopathology is only just beginning to emerge, but has the potential to radically alter our understanding of the neurobiology of abnormal behavior.
Keywords: Comorbidity, Externalizing, fMRI, Internalizing, Research Domain Criteria, Transdiagnostic
The dramatic growth of cognitive neuroscience and neuroimaging over the last quarter century has produced substantial advances in our ability to examine the functioning of specific neurobehavioral circuits. However, our understanding of the neural substrates of psychopathology has not kept pace with these advances. The NIMH Research Domain Criteria (RDoC) initiative asserts that progress has been slowed by a focus on categorical mental disorder diagnoses (1, 2). At the heart of RDoC's rationale is a concern about the limits of case-control designs in which cases meeting categorical diagnostic criteria for a mental disorder are contrasted with healthy controls. Such designs impose several limitations. First, the heterogeneity of symptoms among cases with the same diagnosis may obscure relations between brain functions and psychopathology since not all cases possess the same characteristics (1, 3). Second, the comorbidity of symptoms (or diagnoses) makes it difficult to ascribe observed relations to a specific target feature of cases (vs. frequently co-occurring nontarget features). Third, because case-control designs select extremely different groups of cases and controls, they create marked ascertainment biases. Fourth, and most important to our present argument, by limiting cases to only one diagnosis, case-control designs limit the range of symptoms that cases can exhibit, making it difficult to identify transdiagnostic mechanisms of psychopathology.
Three alternatives to case-control designs have emerged in clinical neuroscience that vary in how they address the above issues. The narrow symptom approach focuses on single symptoms or small groupings of closely related symptoms instead of diagnoses. In contrast, the broad dimensional approach focuses on over-arching dimensions of psychopathology that cut across diagnoses. Alternatively, the functional constructs approach organizes research around functional processes rather than symptoms or diagnoses. These processes may be related to narrow subsets of symptoms, broader symptom dimensions, or some combination of both. Here we advocate for a hierarchical structural approach that integrates these three strategies in order to elucidate neural correlates at multiple levels of psychopathology's hierarchical structure.
A Hierarchical Structural Model of Psychopathology
A core challenge for clinical neuroscience is determining the “mappings” between neurobehavioral markers and different levels of psychopathology. However, a review of the growing empirical literature on the structure of psychopathological symptoms provides clear guidance on what many of these mappings will look like. Increasingly, this literature indicates that neither a narrow nor a broad dimensional approach in isolation will allow for a full mapping of neurobehavioral systems and psychopathology. Rather, the data suggest that clinical neuroscience would profit from the adoption of a model of psychopathology in which the etiology factors operate simultaneously at multiple levels that range from narrow mechanisms to broad nonspecific influences on mental health.
The hierarchical structural approach to psychopathology places symptoms within an empirically determined hierarchy of dimensions. At least 4 levels can be identified (4). The lowest level reflects individual symptoms. The next level is formed by first-order dimensions (or factors) defined by highly correlated symptoms. Above this, broader second-order dimensions reflect the correlations among subsets of the first-order dimensions. Finally, recent data support the existence of a general factor of psychopathology that reflects the widespread positive correlations among essentially all symptoms of psychopathology (4-6).
Narrow Symptoms
Individual symptoms can be viewed as the lowest level of a symptom hierarchy. Arguably, the simplest strategy for dealing with concerns about the heterogeneity of symptoms within a diagnostic group is to examine the correlates of specific symptoms rather than diagnostic categories. This approach is especially attractive when there is a close conceptual correspondence between a specific symptom and a functional construct with known neural underpinnings. For instance, symptoms of motivational anhedonia can be linked to neurocircuitry involved in facilitating motivated responses (7). Within the context of RDoC, there are multiple examples of correspondence between a given narrow symptom and a proposed functional construct. However, such correspondence is by no means universal as many symptoms and functional constructs defy a one-to-one relationship.
The primary limitation of focusing only at this narrow level is that psychological symptoms rarely occur in isolation (see Figure 1a). When these nontarget symptoms differ from one subject to another they can introduce heterogeneity as severe as that seen for a diagnostic category. This is not necessarily a problem if those nontarget symptoms cancel each other out in analysis. However, the associations between symptoms are often nonrandom, and when co-occurrence is high it becomes difficult to isolate relations between specific symptoms and neurobehavioral circuits or constructs. These co-occurring symptoms are often handled more reliably when aggregated into a first-order dimension.
Fig 1.
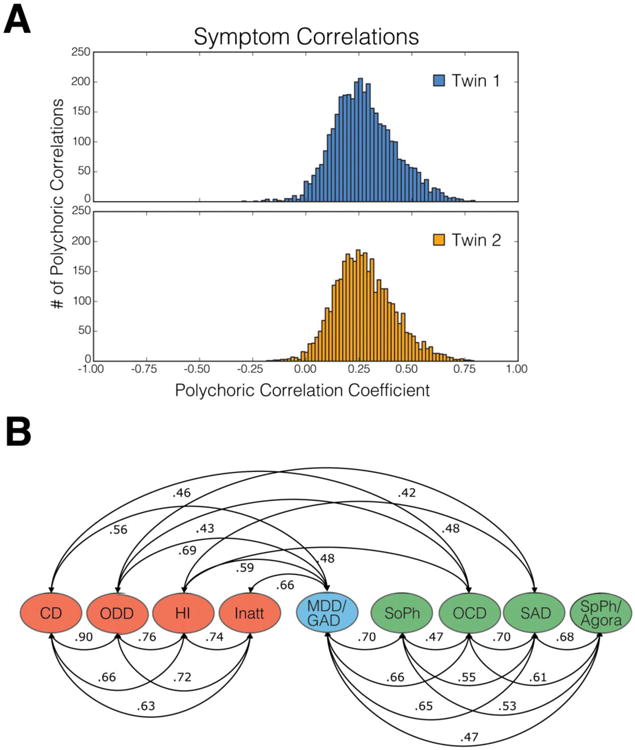
A) Histograms of polychoric correlations among psychopathology symptoms based on caretaker interview with the Child and Adolescent Psychopathology Scale (74) for adolescents in Wave 1 of the Tennessee Twin Study (TTS) (16). Although there is variability in the magnitudes of correlations, most symptoms show at least modest positive correlations with a broad array of other symptoms. Note: a small number of items with correlations +/- than 4 s.d. below the mean were excluded. In each case these involved items with extremely low endorsement rates. B) Correlations among first-order latent dimensions of psychopathology in wave 1 of the TTS based on the same symptoms. The figure is redrawn from Figure 5 page 196 (16). Note: only correlations greater than r = .40 are shown, but all additional correlations are statistically significant. Abbreviations: ODD = oppositional defiant disorder; CD = conduct disorder; HI = hyperactivity-impulsivity; INATT = inattention; MDD = major depressive disorder; GAD = generalized anxiety disorder; social = social phobia; SAD = separation anxiety disorder; spec = specific phobia; agora = agoraphobia.
First-Order Dimensions of Psychopathology
Frequently co-occurring symptoms are often handled by aggregation into first-order dimensions. Factor analysis (FA) studies indicate that these first-order dimensions generally (but not universally) parallel different DSM-IV/V diagnoses (8-13), although there is some circularity in such studies given that the symptoms queried are frequently limited to those that are included in the DSM. Examination of first-order dimensions instead of diagnoses may nevertheless aid in neuroimaging research to the extent that they better capture the dimensional nature of psychopathology and eliminate artificial boundaries between diagnostic groups and clinical vs. subclinical diagnostic distinctions (14).
Correlations Among First-Order Dimensions and Comorbidity
Although studying first-order dimensions provides an empirical refinement over categorical diagnoses, as seen in Figure 1b, these dimensions (and the parallel categorical diagnoses) are far more correlated than orthogonal (15-21). This comorbidity has often been treated as a failure of the current diagnostic system to achieve the Platonic ideal of “carving nature at its joints.” We believe that clinical neuroscience research needs a paradigm shift in conceptualizing the high correlations among dimensions or disorders. Correlations among first-order dimensions of psychopathology should not be viewed as a flaw, but rather as an important source of information about the nature and etiology of psychopathology (15, 18, 22, 23). This shift has already begun to take hold in behavior genetics (23-26). It is arguably time for clinical neuroscience to take similar notice.
Second-Order Dimensions and the General Factor of Psychopathology
FA of the covariance of first-order symptom dimensions generate second-order factors. Two second-order factors, typically labelled externalizing and internalizing disorders, have emerged in many studies of children, adolescents, and adults and explain a substantial part of the variance in first-order dimensions of psychopathology (16, 27-29) (see Supplemental Materials for review of alternative second-order factors, including a thought disorders factor, and division of internalizing disorders into fears and distress factors).
An important, but until recently ignored, observation from FA studies is that the second-order factors are themselves robustly positively correlated rather than being orthogonal (5, 15, 16). These correlations indicate that persons with high levels of internalizing symptoms are likely to exhibit externalizing symptoms and vice versa. Based on such findings we hypothesized that the correlations among second-order factors of psychopathology reflect a general factor of psychopathology on which every first-order dimension loads (5). Using data from NESARC (30), we tested this hypothesis using a bifactor model (31) in which diagnoses of every mental disorder were allowed to load on a general factor of psychopathology (5). Each diagnosis also loaded on either an externalizing factor or two subfactors of internalizing psychopathology (fears and distress). This bifactor model demonstrated better fit than a correlated three-factor (fears distress, externalizing) model lacking the general factor (See Figure 2). Subsequent papers have confirmed the presence of a general factor using bifactor models (6, 32, 33) (see supplemental materials and (4) for review and critique). Caspi and colleagues (6) have proposed labelling the general factor the “p-factor,” suggesting a possible parallel to the g-factor of intelligence.
Fig 2.
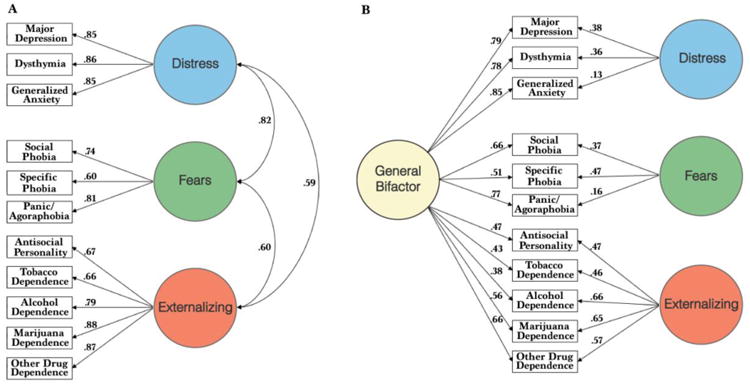
Best fitting models of the correlational structure of 11 categorical mental disorders in confirmatory factor analyses of diagnosis data from wave 1 of the NESARC sample. (A) The 3-factor model in which the second-order internalizing factor splits into distinct distress and fears factors fits the data well, but it includes significant positive correlations among the distress, fears and externalizing second-order factors. (B) The bifactor model with a general psychopathology factor that is associated directly with each disorder further improves the fit. Adapted from Figure 1, page 973 (5).
Correlations among first-order dimensions within a higher-order factor, and between different higher order factors could arise for a number of reasons including overlapping symptoms, one dimension influencing another dimension, and correlated measurement or reporting biases (see (4) for a discussion of these alternative explanations). We focus here on the testable hypothesis that the robust correlations among both first-order dimensions, as well as between higher order factors, are largely due to shared etiological mechanisms. A failure to attend to this shared variance may seriously compromise any endeavor to understand the neural correlates of psychopathology. We recognize that it is not necessarily the case that shared variance implies directly shared causes or mechanisms. Indeed, recent network analyses have raised the, as yet largely untested, hypothesis that some covariance of symptoms arise because some symptoms (or their functional consequences) exert causal influences on other symptoms (34). Nevertheless, it seems reasonable to test the hypothesis that a significant portion of the shared variance in symptoms is due to common causes and mechanisms.
Heritability of Higher Order Factors of Psychopathology
Two critical questions arise regarding the higher order factors of psychopathology: 1) Do higher-order factors reflect causal influences shared across the lower-order dimensions that comprise the higher-order factor, and 2) Is there evidence that some of those shared causal influences are mediated by common biological mechanisms? Increasing data indeed suggest that a substantial portion of the genetic variance of psychopathology is shared at the level of higher-order dimensions rather than being specific to first-order symptom dimensions (35-41). Strikingly, a recent study found that among children and adolescents, at least 2/3rds of the genetic variance of nearly all first-order symptom dimensions examined was accounted for by higher-order genetic factors operating at the level of the internalizing, externalizing, or general psychopathology factors, with a relatively modest proportion of genetic variance being unique to first-order dimensions (23). Similar evidence of shared heritability across diagnoses arises in other studies of adolescents (42) and adults (43). Molecular genetic studies have likewise identified genes that are associated with more than one disorder. (26, 44-46), and significant variance in the general factor appears attributable to the additive effects of common significant single nucleotide polymorphism (47). Taken together, these findings suggest that both dimension-specific and broadly pleiotropic genetic influences operate at varying levels of the structural hierarchy of psychopathology.
Applying a Hierarchical Approach to the Clinical Neuroscience of Psychopathology
The above findings lead to several immediately testable hypotheses for clinical neuroscience research. First, based on the substantial, but incomplete, sharing of genetic influences across dimensions of psychopathology, we hypothesize that the neural substrates of psychopathology operate at multiple levels in the hierarchy of psychopathology dimensions. This ranges from narrow mechanisms that mediate only a small number of closely related symptoms to very broad nonspecific mechanisms that affect the risk for, and the expression of, all prevalent forms of psychopathology. However, the observation that there is greater heritability of higher-order than first-order factors of psychopathology (4, 23) suggest that relations between neurobehavioral systems and psychopathology may be more robust at the higher-order dimensional level than at the narrower levels of symptoms or first-order dimensions. If this is the case, the need to revise our field's approach to conceptualizing and studying the neural substrates of psychopathology becomes even more urgent. There are at least three ways in which this hierarchical view can be integrated into research on the neurobiology of psychopathology.
1. Although inefficient, one way to identify neurobiological systems associated with higher order dimensions of psychopathology is to systematically reanalyze existing case-control studies of different disorders to generate hypotheses regarding neural features that are nonspecifically related to multiple forms of psychopathology. Indeed, case-control neuroimaging studies often converge on the same “usual suspects,” with individual differences in regions such as the amygdala, ventral striatum, and areas of the anterior cingulate and prefrontal cortices repeatedly emerging in contrasts between cases and controls (48-52). Whereas most findings from case-control studies were originally interpreted as disorder-specific effects, many results may actually reflect second-order or general factor associations. Reviews of psychophysiological measures, and a small but growing group of neuroimaging studies, also provide some support for this idea, at least at the level of internalizing or externalizing domains (53-57). For instance, a recent meta-analysis of resting state data observed that altered functional connectivity between the amygdala and pregenual/subgenual cingulate arises across case-control studies of different internalizing disorders and their risk factors (61). The repeated emergence of common neural and psychophysiological correlates is particularly impressive given that case-control studies of individual disorders are suboptimal for identifying transdiagnostic associations.
Meta-analysis of structural differences in psychopathology provides further support for the hypothesis that there are nonspecific neural correlates of psychopathology that arise across case control studies of different diagnostic groups. Using data from 193 voxel-based morphometry studies, comprising 15,892 individuals and including patients with affective, anxiety, substance use and psychotic disorders, Goodkind and colleagues found convergent gray matter loss across all diagnoses in the dorsal anterior cingulate and bilateral insula (58). By contrast, there were only a few diagnosis-specific effects that reached statistical significance. This type of analysis cannot reveal whether the decreased grey matter plays a causal role in the disorders, or is a consequence of the disorder or its treatment. Nonetheless, it is intriguing that the anterior insula is identified in such an analysis given its key position in networks related to saliency, affect, interoception and autonomic regulation (59). Similarly, the anterior cingulate plays a key role in the integration of negative emotion processing and cognitive control (60).
2. To date, the most common strategy for studying psychobiological mechanisms that act at the second-order level of psychopathology has been to utilize rating scales of externalizing and/or internalizing behaviors rather than categorical diagnoses. This has been primarily implemented with children and adolescents using the Child Behavior Checklist (CBCL) (62). For example, in a large study of normal development, CBCL externalizing scores were negatively correlated with brain volume in portions of the left orbitofrontal, right retrosplenial, and right medial temporal cortical thickness (63). In a smaller sample of adolescents with externalizing disorders, Bjork et al. (64) observed a positive correlation between ventral striatal activation during the monetary incentive delay task and the CBCL externalizing dimension. Additionally, among high risk children and adolescents, a general factor score derived from a bifactor analysis of the CBCL has been observed to correlate with a measure of default mode network maturity (65).
3. Finally, an as yet underutilized alternative to case-control designs is to model the hierarchical factor structure of psychopathology in representative samples. In neuroimaging studies, this approach involves examining whether individual differences in factor scores at different levels of the symptom hierarchy are correlated with structural or functional imaging measures. Only recently have neuroimaging studies attempted to examine the neural correlates of psychopathology in this way. Using data from the IMAGEN study of adolescents (n=1,778), Castellanos-Ryan et a. (66) demonstrated that scores on a second-order latent externalizing factor were associated with differential BOLD responses during successful stopping on a stop-signal task (with higher activation in the presupplemental motor area and precentral gyrus but reduced substantia nigra and subthalamic nucleus activity). By contrast, other neural associations showed greater specificity, arising only at the level of first-order dimensions. Similarly, using data from the Philadelphia Neurodevelopmental cohort (n= 1,129), Shanmugan et al. (67) report that a general psychopathology bifactor is associated with both abnormal patterns of activation and a failure to activate executive regions within a cingulate-opercular control network, including the frontal pole, and anterior insula during a working memory task. Critically, unlike prior studies that have only treated second-order factors like internalizing and externalizing as correlated factors without accounting for their common variance, this bifactor study revealed neural correlates of the orthogonalized second-order factors after controlling for the general factor (see Supplemental Materials for full discussion). Recent resting state fMRI studies have also revealed differential connectivity in relation to general adaptive-maladaptive psychological-lifestyle features (resembling a general factor) as well as different connectivity related to an externalizing-internalizing division (68, 69). Taken together, these findings elegantly demonstrate the feasibility of integrating a hierarchical structural approach to psychopathology into psychiatric neuroimaging and again suggest the importance of paralimbic regions to higher-order factors of psychopathology.
Causal Factors and Etiological Heterogeneity within a Hierarchical Approach
One reason for advocating that neuroscience research incorporate analyses at more than one level of the structural hierarchy is the likely existence of substantial etiological heterogeneity within first-order dimensions of psychopathology. There are presumably multiple combinations of both narrow and general mechanisms that can increase the risk for, and expression of, specific forms of psychopathology. For example, individuals exhibiting symptoms of depression may be influenced by different combinations of mechanisms operating at the level of the general factor, the internalizing factor, the first-order dimension of depression and at the level of psychobiological constructs closely related to specific depressive symptoms. The relative balance of these nonspecific and more specific mechanisms likely differs between individuals, with the breadth of symptoms being heavily driven by the level at which the etiological mechanism acts within the symptom hierarchy. Only by correctly identifying the hierarchical level at which the etiological factors exert their influence will it become possible to tease apart mechanistic heterogeneity (see Supplemental Materials for additional discussion).
Critically, the neural substrates of psychopathology may similarly be conceptualized in terms of their circuit-specific versus broad network properties. Within this framework, the neural correlates of psychopathology are hypothesized to differ in breadth in direct relation to the level of the structural hierarchy of psychopathology. Narrower neural correlates may be expected when symptoms are limited to a single dimension. By contrast, broad neural features, such as those captured by whole brain metrics (70), will be more saliently associated with higher order factors of psychopathology.
Implications for RDoC
The structure of psychopathology as outlined above has immediate implications for the functional constructs approach as articulated in the RDoC initiative. By emphasizing the elucidation of functional constructs, RDoC takes advantage of the strong conceptual and empirical links between these constructs and neurobehavioral circuits. Indeed, to the extent that function mediates relations between brain and symptoms, these functional constructs may be more closely related to neuroimaging measures than dimensions of psychopathology. However, the hierarchical structural approach raises a critical question – at what level of the hierarchy of psychopathology do these functional constructs map? We suspect that many clinical neuroscientists will focus primarily on exploring mappings at the lower levels of the hierarchy. It will be essential to complement such studies with research designs that attend to the larger meta-structure of psychopathology. As such, we need to consider which functional constructs or combination of constructs exert influences at higher levels of the hierarchy. Constructs showing the broadest patterns of associations such as negative emotionality, are likely to exert influences at higher-order levels of the hierarchy. Alternatively, if higher-order factors represent a simple aggregation of symptoms, identifying the neural substrates of multiple RDoC constructs will be required to understand the neurofunctional substrates of these factors. It seems doubtful that the goals of RDoC can be reached in the absence of designs that facilitate exploration of associations between constructs and both narrow and broad dimensional features of mental illness.
Discussion and Future Directions
We have asserted that a hierarchical structural approach to psychopathology will be necessary to achieve the goals of clinical neuroscience. Given the context of this review, we have emphasized the implications of the hierarchical structural approach for clinical neuroscience, but the same principles apply to environmental etiological influences. For instance, childhood maltreatment may have its largest impact at a general rather than disorder-specific level (5, 6). That said, we believe that an emphasis on the meta-structure of psychopathology in clinical neuroscience is particularly important because the field has been slower than other areas of psychology and psychiatry to employ experimental designs that utilize hierarchical models. The lack of attention to the structure of psychopathology may arise because of a combination of theoretical orientation, guild, politics, and pragmatic factors, and we are certainly not the first to suggest that researchers should attend to the structure of psychopathology (e.g., (22, 55, 71)). Yet, to date such calls have not been widely attended to within the clinical neuroscience and neuroimaging research community. This situation must change.
In order to facilitate an integrative structural approach to psychiatric neuroimaging and clinical neuroscience we make two general recommendations for future research.
1) Examine multiple dimensions of psychopathology simultaneously
Whenever possible, research should include samples that contain multiple forms of psychopathology. This requires revision of typical inclusion-exclusion criteria. In the desire to have “pure cases” without comorbidity, many studies over-represent “cleaner” prototypical cases at the expense of patients with more diffuse symptoms. Moreover, exclusion of participants due to comorbidity fundamentally limits the ability to retrospectively analyze second-order factors in many existing datasets. In contrast, allowance of multiple types of psychopathology in studies can alleviate biases arising from poor matching between convenience samples of healthy controls and patients. At a minimum, if we use case-control designs, we should include multiple types of cases (see Supplemental Materials for discussion of multi-diagnosis case-control designs).
Indeed, an argument can be made for utilizing recruitment procedures that apply no, or minimal, diagnostic inclusion criteria. This “agnostic” recruitment approach allows for far more demographically representative samples than have been used in most psychiatric neuroimaging research, and provides unbiased measurement of all of the symptoms and dimensions that define the hierarchy of psychopathology. Measurement biases at the symptom level have generally not been attended to in neuroimaging studies, but utilization of methods that minimize biases in correlations among symptoms are necessary to accurately model the neural correlates of each level of the psychopathology hierarchy. The primary limitation of this agnostic recruitment approach is that it is expensive. Nevertheless, it is possible to increase cost-efficiency by oversampling individuals who exhibit any psychopathology.
2) Model specific and non-specific neural correlates of psychopathology simultaneously
In order to successfully disentangle the neural substrates of psychopathology, it will be necessary to model both specific and nonspecific neural correlates in the same samples. This should greatly enhance our ability to appropriately assign influences and mechanisms to different levels of the hierarchy. A parallel approach arises in genetics research where geneticists model both single gene effects on a given phenotype, and pleiotropic effects in which a gene influences the expression of multiple phenotypes. If the neural correlates of a disorder follow a similar pattern as heritability estimates, then the neural characteristics of any first-order dimension of psychopathology will involve both distinct specific and nonspecific features. In an era in which we have started to apply neuroimaging for differential diagnosis, and neuromodulation to target specific neural circuits, it is essential to determine if these neural circuits impact narrow features of psychopathology or produce nonspecific transdiagnostic effects.
Methodological Developments
Several methodological improvements are needed to facilitate assessment within hierarchical dimensional approach. First, we need to consider additional symptoms that are not currently in the DSM5 (72, 73), especially symptoms that might load heavily on higher-order factors but have been previously excluded because they fail to discriminate between disorders. Second, we need new approaches to measuring dimensions of symptoms, particularly in adults. Nearly all existing diagnostic interviews for adults use skip-outs in order to remove the need to ask questions about symptoms after the initial gateway symptoms of a diagnosis are determined to be absent. This approach is economical if only diagnoses are important, but it makes comprehensive dimensional assessment impossible because all symptoms are not queried in each individual.
To date, the assessment instruments that have been used to generate higher order factors in adults have varied in terms of the specific first-order symptom domains covered, which limits comparison of findings across studies. Development and adoption of a common comprehensive measure is especially needed in the context of large multisite studies and data sharing repositories where the ability to combine data depends on utilization of common instruments for operationalizing the hierarchy of psychopathology.
Finally, we note that the appropriate selection of neuroimaging paradigm and analytic techniques may vary depending upon the level of the hierarchy of psychopathology under investigation. Because task-based fMRI paradigms are designed to isolate specific neurobehavioral circuits, this approach may be optimal for identifying narrow constructs acting at lower levels of the symptoms hierarchy. By contrast, techniques assessing functioning across multiple neurobehavioral systems, such as graph theoretical approaches that provide whole brain metrics (70), may prove particularly useful for detecting and characterizing the neural correlates of higher-order factors of psychopathology.
Supplementary Material
Acknowledgments
This work was supported by a grant from the National Institute of Mental Health; R01-MH098098 to DHZ and BBL. We thank Kevin Anderson for assistance with Figure 1.
Footnotes
Financial Disclosures: Dr. Zald and Dr. Lahey each report that they have no financial or other conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, et al. Research domain criteria (RDoC): Toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 2.Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC medicine. 2013;11:126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanislow CA, Pine DS, Quinn KJ, Kozak MJ, Garvey MA, Heinssen RK, et al. Developing constructs for psychopathology research: research domain criteria. J Abnorm Psychol. 2010;119:631–639. doi: 10.1037/a0020909. [DOI] [PubMed] [Google Scholar]
- 4.Lahey BB, Krueger RF, Rathouz PJ, Waldman ID, Zald DH. A hierarchical causal taxonomy of psychopathology across the life span. Psych Bull. doi: 10.1037/bul0000069. in press. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lahey BB, Applegate B, Hakes JK, Zald DH, Hariri AR, Rathouz PJ. Is there a general factor of prevalent psychopathology during adulthood? J Abnorm Psychol. 2012;121:971–977. doi: 10.1037/a0028355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caspi A, Houts RM, Belsky DW, Goldman-Mellor SJ, Harrington H, Israel S, et al. The p factor: one general psychopathology factor in the structure of psychiatric disorders? Clin Psychol Sci. 2014;2:119–137. doi: 10.1177/2167702613497473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev. 2011;35:537–555. doi: 10.1016/j.neubiorev.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bezdjian S, Krueger RF, Derringer J, Malone S, McGue M, Iacono WG. The structure of DSM-IV ADHD, ODD, and CD criteria in adolescent boys: a hierarchical approach. Psychiatry Res. 2011;188:411–421. doi: 10.1016/j.psychres.2011.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spence SH. Structure of anxiety symptoms among children: A confirmatory factor-analytic study. J Abnorm Psychol. 1997;106:280–297. doi: 10.1037//0021-843x.106.2.280. [DOI] [PubMed] [Google Scholar]
- 10.Trosper SE, Whitton SW, Brown TA, Pincus DB. Understanding the latent structure of the emotional disorders in children and adolescents. J Abnorm Child Psych. 2012;40:621–632. doi: 10.1007/s10802-011-9582-7. [DOI] [PubMed] [Google Scholar]
- 11.Markon KE. Modeling psychopathology structure: a symptom-level analysis of Axis I and II disorders. Psychol Med. 2010;40:273–288. doi: 10.1017/S0033291709990183. [DOI] [PubMed] [Google Scholar]
- 12.Sheeran T, Zimmerman M. Factor structure of the Psychiatric Diagnostic Screening Questionnaire (PDSQ), a screening questionnaire for DSM-IV axis I disorders. J Behav Ther Exp Psychiatry. 2004;35:49–55. doi: 10.1016/j.jbtep.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Lahey BB, Rathouz PJ, Applegate B, Van Hulle C, Garriock HA, Urbano RC, et al. Testing structural models of DSM-IV symptoms of common forms of child and adolescent psychopathology. J Abnorm Child Psychol. 2008;36:187–206. doi: 10.1007/s10802-007-9169-5. [DOI] [PubMed] [Google Scholar]
- 14.Widiger TA, Samuel DB. Diagnostic categories or dimensions? A question for the Diagnostic and statistical manual of mental disorders. J Abnorm Psych. 2005;114:494. doi: 10.1037/0021-843X.114.4.494. [DOI] [PubMed] [Google Scholar]
- 15.Krueger RF, Markon KE. Reinterpreting comorbidity: A model-based approach to understanding and classifying psychopathology. Ann Rev of Clin Psychol. 2006:111–133. doi: 10.1146/annurev.clinpsy.2.022305.095213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lahey BB, Rathouz PJ, Applegate B, Hulle CV, Garriock HA, Urbano RC, et al. Testing structural models of DSM-IV symptoms of common forms of child and adolescent psychopathology. J Abnorm Child Psych. 2008;36:187–206. doi: 10.1007/s10802-007-9169-5. [DOI] [PubMed] [Google Scholar]
- 17.Kessler RC, Chiu WT, Demler O, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Angold A, Costello EJ, Erkanli A. Comorbidity. J Child Psychol Psychiatry. 1999;40:57–87. [PubMed] [Google Scholar]
- 19.Caron C, Rutter M. Comorbidity in child psychopathology: Concepts, issues and research strategies. J Child Psychol Psychiatry. 1991;32:1063–1080. doi: 10.1111/j.1469-7610.1991.tb00350.x. [DOI] [PubMed] [Google Scholar]
- 20.Kessler RC. The National Comorbidity Survey: Preliminary Results and Future Directions. Int J Methods Psychiatr Res. 1995;5:139–151. [Google Scholar]
- 21.Jacobi F, Wittchen HU, Holting C, Hofler M, Pfister H, Muller N, et al. Prevalence, co-morbidity and correlates of mental disorders in the general population: results from the German Health Interview and Examination Survey (GHS) Psychol Med. 2004;34:597–611. doi: 10.1017/S0033291703001399. [DOI] [PubMed] [Google Scholar]
- 22.Kendler KS, Aggen SH, Knudsen GU, Roysamb E, Neale MC, Reichborn-Kjennerud T. The structure of genetic and environmental risk factors for syndromal and subsyndromal common DSM-IV axis I and axis II disorders. Am J Psychiatry. 2011;168:29–39. doi: 10.1176/appi.ajp.2010.10030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lahey BB, Van Hulle CA, Singh AL, Waldman ID, Rathouz PJ. Higher-order genetic and environmental structure of prevalent forms of child and adolescentpsychopathology. Arch Gen Psychiatry. 2011;68:181–189. doi: 10.1001/archgenpsychiatry.2010.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rhee SH, Lahey BB, Waldman ID. Comorbidity among dimensions of childhood psychopathology: Converging evidence from behavior genetics. Child Devel Perspect. 2015;9:26–31. doi: 10.1111/cdep.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee S, Ripke S, Neale B, Faraone S, Purcell S. Cross-Disorder Group of the Psychiatric Genomics Consortium Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Genet. 2013;45:984–994. doi: 10.1038/ng.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Consortium C-DGotPG. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. The Lancet. 2013;381:1371–1379. doi: 10.1016/S0140-6736(12)62129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kessler RC, Petukhova M, Zaslavsky AM. The role of latent internalizing and externalizing predispositions in accounting for the development of comorbidity among common mental disorders. Curr Opin Psychiatry. 2011;24:307–312. doi: 10.1097/YCO.0b013e3283477b22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blanco C, Wall MM, He JP, Krueger RF, Olfson M, Jin CJ, et al. The space of common psychiatric disorders in adolescents: comorbidity structure and individual latent liabilities. J Am Acad Child Adolesc Psychiatry. 2015;54:45–52. doi: 10.1016/j.jaac.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slade T, Watson D. The structure of common DSM-IV and ICD-10 mental disorders in the Australian general population. Psychol Med. 2006;36:1593–1600. doi: 10.1017/S0033291706008452. [DOI] [PubMed] [Google Scholar]
- 30.Grant BF, Moore T, Shepard J, Kaplan K. Source and accuracy statement: Wave 1 national epidemiologic survey on alcohol and related conditions (NESARC) Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism; 2003. p. 52. [Google Scholar]
- 31.Holzinger KJ, Swineford F. The bi-factor method. Psychometrika. 1937;2:41–54. [Google Scholar]
- 32.Lahey BB, Rathouz PJ, Keenan K, Stepp SD, Loeber R, Hipwell AE. Criterion validity of the general factor of psychopathology in a prospective study of girls. J Child Psychol Psychiatry. 2015;56:415–422. doi: 10.1111/jcpp.12300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laceulle OM, Vollebergh WA, Ormel J. The Structure of Psychopathology in Adolescence Replication of a General Psychopathology Factor in the TRAILS Study. Clin Psychol Sci. 2015;3:850–860. [Google Scholar]
- 34.Borsboom D, Cramer AO. Network analysis: an integrative approach to the structure of psychopathology. Ann Rev of Clin Psychol. 2013;9:91–121. doi: 10.1146/annurev-clinpsy-050212-185608. [DOI] [PubMed] [Google Scholar]
- 35.Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- 36.Tuvblad C, Zheng M, Raine A, Baker LA. A common genetic factor explains the covariation among ADHD ODD and CD symptoms in 9-10 year oldboys and girls. J Abnorm Child Psych. 2009;37:153–167. doi: 10.1007/s10802-008-9278-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waldman ID, Rhee SH, Levy F, Hay DA. Causes of the overlap among symptoms of ADHD, oppositional defiant disorder, and conduct disorder. In: Levy F, Hay DA, editors. Attention, genes, and ADHD. New York: Brunner-Routledge; 2001. pp. 115–138. [Google Scholar]
- 38.Korhonen T, Latvala A, Dick DM, Pulkkinen L, Rose RJ, Kaprio J, et al. Genetic and environmental influences underlying externalizing behaviors, cigarette smoking and illicit drug use across adolescence. Behav Genetics. 2012;42:614–625. doi: 10.1007/s10519-012-9528-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Middeldorp CM, Cath DC, Van Dyck R, Boomsma DI. The co-morbidity of anxiety and depression in the perspective of genetic epidemiology. A review of twin and family studies. Psychol Med. 2005;35:611–624. doi: 10.1017/s003329170400412x. [DOI] [PubMed] [Google Scholar]
- 40.Hettema JM. What is the genetic relationship between anxiety and depression? Am J Medical Genetics. 2008;148C:140–146. doi: 10.1002/ajmg.c.30171. [DOI] [PubMed] [Google Scholar]
- 41.Hicks BM, Krueger RF, Iacono WG, McGue M, Patrick CJ. Family transmission and heritability of externalizing disorders: a twin-family study. Arch Gen Psychiatry. 2004;61:922–928. doi: 10.1001/archpsyc.61.9.922. [DOI] [PubMed] [Google Scholar]
- 42.Cosgrove VE, Rhee SH, Gelhorn HL, Boeldt D, Corley RC, Ehringer MA, et al. Structure and etiology of co-occurring internalizing and externalizing disorders in adolescents. J Abnorm Child Psych. 2011;39:109–123. doi: 10.1007/s10802-010-9444-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kendler KS. “A gene for…”: the nature of gene action in psychiatric disorders. Am J Psychiatry. 2005;162:1243–1252. doi: 10.1176/appi.ajp.162.7.1243. [DOI] [PubMed] [Google Scholar]
- 44.Gatt JM, Burton KL, Williams LM, Schofield PR. Specific and common genes implicated across major mental disorders: a review of meta-analysis studies. J Psychiatr Res. 2015;60:1–13. doi: 10.1016/j.jpsychires.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 45.Salvatore JE, Aliev F, Bucholz K, Agrawal A, Hesselbrock V, Hesselbrock M, et al. Polygenic risk for externalizing disorders gene-by-development and gene-by-environment effects in adolescents and young adults. Clin Psychol Sci. 2014;5:330–346. doi: 10.1177/2167702614534211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hettema J, Chen X, Sun C, Brown T. Direct, indirect and pleiotropic effects of candidate genes on internalizing disorder psychopathology. Psychol Med. 2015;45:2227–2236. doi: 10.1017/S0033291715000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neumann A, Pappa I, Lahey BB, Verhulst FC, Medina-Gomez C, Jaddoe VW, et al. Single nucleotide polymorphism heritability of a general psychopathology factor in children. J Am Acad Child Adolesc Psychiatry. 2016;55:1038–1045. e1034. doi: 10.1016/j.jaac.2016.09.498. [DOI] [PubMed] [Google Scholar]
- 48.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fitzgerald PB, Laird AR, Maller J, Daskalakis ZJ. A meta-analytic study of changes in brain activation in depression. Hum Brain Mapping. 2008;29:683–695. doi: 10.1002/hbm.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, Gotlib IH. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of base line activation and neural response data. Am J Psychiatry. 2012;169:693–703. doi: 10.1176/appi.ajp.2012.11071105. [DOI] [PubMed] [Google Scholar]
- 51.Ersche KD, Williams GB, Robbins TW, Bullmore ET. Meta-analysis of structural brain abnormalities associated with stimulant drug dependence and neuroimaging of addiction vulnerability and resilience. Curr Opin Neurobiol. 2013;23:615–624. doi: 10.1016/j.conb.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 52.Cortese S, Kelly C, Chabernaud C, Proal E, Di Martino A, Milham MP, et al. Toward systems neuroscience of ADHD: a meta-analysis of 55 fMRI studies. Am J Psychiatry. 2012;169:1038–1055. doi: 10.1176/appi.ajp.2012.11101521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dichter GS, Damiano CA, Allen JA. Reward circuitry dysfunction in psychiatric and neurodevelopmental disorders and genetic syndromes: animal models and clinical findings. J Neurodev Disord. 2012;4:19. doi: 10.1186/1866-1955-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Iacono WG, Malone SM, McGue M. Substance use disorders, externalizing psychopathology, and P300 event-related potential amplitude. Int J Psychophysiology. 2003;48:147–178. doi: 10.1016/s0167-8760(03)00052-7. [DOI] [PubMed] [Google Scholar]
- 55.Vaidyanathan U, Patrick CJ, Cuthbert BN. Linking dimensional models of internalizing psychopathology to neurobiological systems: affect-modulated startle as an indicator of fear and distress disorders and affiliated traits. Psychol Bull. 2009;135:909. doi: 10.1037/a0017222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vaidyanathan U, Nelson LD, Patrick CJ. Clarifying domains of internalizing psychopathology using neurophysiology. Psychol Med. 2012;42:447–459. doi: 10.1017/S0033291711001528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Olvet DM, Hajcak G. The error-related negativity (ERN) and psychopathology: toward an endophenotype. Clin Psychol Rev. 2008;28:1343–1354. doi: 10.1016/j.cpr.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goodkind M, Eickhoff SB, Oathes DJ, Jiang Y, Chang A, Jones-Hagata LB, et al. Identification of a Common Neurobiological Substrate for Mental Illness. JAMA Psychiatry. 2015 doi: 10.1001/jamapsychiatry.2014.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Structure Function. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci. 2011;12:154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marusak H, Thomason M, Peters C, Zundel C, Elrahal F, Rabinak C. You say ‘prefrontal cortex’and I say ‘anterior cingulate’: meta-analysis of spatial overlap in amygdala-to-prefrontal connectivity and internalizing symptomology. Transl Psychiatry. 2016;6:e944. doi: 10.1038/tp.2016.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Achenbach TM. Manual for the Child Behavior Checklist/4-18 and 1991 profile. Department of Psychiatry, University of Vermont; Burlington, VT: 1991. [Google Scholar]
- 63.Ameis SH, Ducharme S, Albaugh MD, Hudziak JJ, Botteron KN, Lepage C, et al. Cortical thickness, cortico-amygdalar networks, and externalizing behaviors in healthy children. Biol Psychiatry. 2014;75:65–72. doi: 10.1016/j.biopsych.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 64.Bjork JM, Chen G, Smith AR, Hommer DW. Incentive-elicited mesolimbic activation and externalizing symptomatology in adolescents. J Child Psychol Psychiatry. 2010;51:827–837. doi: 10.1111/j.1469-7610.2009.02201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sato JR, Salum GA, Gadelha A, Crossley N, Vieira G, Manfro GG, et al. Default mode network maturation and psychopathology in children and adolescents. J Child Psychol Psychiatry. 2016;57:55–64. doi: 10.1111/jcpp.12444. [DOI] [PubMed] [Google Scholar]
- 66.Castellanos-Ryan N, Struve M, Whelan R, Banaschewski T, Barker GJ, Bokde AL, et al. Neural and Cognitive Correlates of the Common and Specific Variance Across Externalizing Problems in Young Adolescence. Am J Psychiatry. 2014 doi: 10.1176/appi.ajp.2014.13111499. [DOI] [PubMed] [Google Scholar]
- 67.Shanmugan S, Wolf DH, Calkins ME, Moore TM, Ruparel K, Hopson RD, et al. Common and dissociable mechanisms of executive system dysfunction across psychiatric disorders in youth. Am J Psychiatry. 2016;173:517–526. doi: 10.1176/appi.ajp.2015.15060725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Smith SM, Nichols TE, Vidaurre D, Winkler AM, Behrens TE, Glasser MF, et al. A positive-negative mode of population covariation links brain connectivity, demographics and behavior. Nat Neurosci. 2015;18:1565–1567. doi: 10.1038/nn.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Van Dam N, O'Connor D, Marcelle ET, Ho EJ, Craddock RC, Tobe RH, et al. Data-driven phenotypic categorization for neurobiological analyses: Beyond DSM-5 labels. Biol Psychiatry. doi: 10.1016/j.biopsych.2016.06.027. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- 71.Krueger RF. The structure of common mental disorders. Arch Gen Psychiatry. 1999;56:921–926. doi: 10.1001/archpsyc.56.10.921. [DOI] [PubMed] [Google Scholar]
- 72.Kendler KS. The phenomenology of major depression and the representativeness and nature of DSM criteria. Am J Psychiatry. 2016 doi: 10.1176/appi.ajp.2016.15121509. [DOI] [PubMed] [Google Scholar]
- 73.Lahey BB, Applegate B, Waldman ID, Loft JD, Hankin BL, Rick J. The structure of child and adolescent psychopathology: Generating new hypotheses. J Abnorm Psychol. 2004;113:358–385. doi: 10.1037/0021-843X.113.3.358. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


