Abstract
To eliminate nitrogen oxides (NOx), a series of highly ordered mesoporous WO3(χ)-CeO2 nanomaterials (χ represents the mole ratio of W/Ce) were synthesized by using KIT-6 as a hard template, which was used for selective catalytic reduction (SCR) to remove NOx with NH3 at low temperatures. Moreover, the nanomaterials were characterized by TEM, XRD, Raman, XPS, BET, H2-TPR, NH3-TPD and in situ DRIFTS. It can be found that all of the prepared mesoporous WO3(χ)-CeO2 (χ = 0, 0.5, 0.75, 1 and 1.25) showed highly ordered mesoporous channels. Furthermore, mesoporous WO3(1)-CeO2 exhibited the best removal efficiency of NOx, and its NOx conversion ratio could reach 100% from 225 ° C to 350 ° C with a gas hourly space velocity of 30 000 h−1, which was due to higher Ce3+ concentrations, abundant active surface oxygen species and Lewis acid sites based on XPS, H2-TPR, NH3-TPD and in situ DRIFTS. In addition, several key performance parameters of mesoporous WO3(1)-CeO2, such as superior water resistance, better alkali metal resistance, higher thermal stability and N2 selectivity, were systematically studied, indicating that the synthesized mesoporous WO3(1)-CeO2 has great potential for industrial applications.
Keywords: NOx removal, Highly-ordered mesoporous, W-Ce oxides, Low temperature SCR
1. Introduction
Recently, release of nitrogen oxides (NOx) into the air from vehicle exhaust or the burning of coal, diesel fuel, oil, and natural gas has resulted in a variety of serious environmental issues, such as ozone depletion, photochemical smog and acid rain [1–3]. Selective catalytic reduction of NOx with NH3 (NH3-SCR) is an effective technology to reduce NOx from stationary sources. Based on the reaction temperature window, the SCR process generally contains three types: high-, medium- and low-temperature SCR reactions. The reaction window from 120 to 320 °C usually belongs to the low-temperature region [4]. In thermal power plants and coal-fired boilers, the commercial V2O5-WO3/TiO2 catalysts have been widely used. However, their working temperature window is too high, which is generally from 300 to 400 °C [5]. Moreover, several inevitable disadvantages of this commercial catalyst restrict its further usages, such as the toxicity of vanadium, weaker sulfur resistance, narrower and higher working temperature window, etc. [6,7]. To overcome these drawbacks, there is an urgent demand to develop novel low-temperature SCR catalysts.
Because of the particular and important structure-activities relationship, mesoporous materials are attracting more attentions recently due to their large specific surface area, highly ordered mesoporous structure and interconnected channels [8,9]. Therefore, there is an increasing trend to apply suitable mesoporous materials in the catalytic field to remove gaseous pollutants such as NOx. For instance, CeOx-based mesoporous silica has been used as a catalyst [10]. However, it is easily poisoned by SO2 or alkali metals, hindering their wider practical application [11–13]. On the other hand, optimal combinations of different metal oxides can usually exhibit better SCR efficiency and thermal stability than single oxides. Li et al. reported that MnOx-CeOx can improve the SCR activity, H2O-resistance and stability by mixing MnOx with CeO2 [14–16]. Besides, they usually exhibit many interesting properties in energy conversion and storage, catalysis and adsorption [17,18]. What’s more, Ce-W oxides in the NH3-SCR reaction were studied. For instance, Shan et al. prepared the CeO2-WO3 and Ce-W/TiO2 catalysts and found that the de-NOx performance and N2 selectivity were greatly improved by doping W species [19,20]. Chen et al. discovered that the strong interaction between Ce and W is helpful to enhance catalytic activities of NO removal [21]. Peng et al. studied CeO2-WO3 bi-metal oxide catalysts, and a mechanism of Ce-W double active center was proposed [22]. However, to the best of our knowledge, mesoporous Ce-W oxides catalysts for SCR have never been reported.
In this paper, a series of highly ordered mesoporous WO3(χ)-CeO2 (χ = 0, 0.5, 0.75, 1 and 1.25) nanomaterials were successfully prepared by using KIT-6 as the hard template. They were further characterized by using TEM, XRD, Raman, BET, H2-TPR, NH3-TPD, XPS and in situ DRIFTS. For investigating its structure-activities relationship, a series of bulk WO3(χ)-CeO2 (χ = 0, 0.5, 0.75, 1 and 1.25) were prepared to remove NOx. Using mesoporous WO3(1)-CeO2 as SCR catalyst, 100% NOx can be cleaned from 225 to 350 °C with a gas hourly space velocity (GHSV) of 30 000 h−1. Besides, several key performance parameters can also be obtained successfully, such as superior water resistance, better alkali metal resistance, higher thermal stability and N2 selectivity, and so on. In addition, how the novel mesoporous WO3(χ)-CeO2 nanocomposites affected the NH3-SCR activities at low temperature were also studied and explained in detail based on the systematic characterization.
2. Experimental
2.1. Preparation of materials
In order to prepare the highly ordered mesoporous WO3(1)-CeO2, 3D mesoporous KIT-6 silica was used as a hard template [23]. Typically, 1.2 g of (NH4)6W7O24·6H2O, 1.2 g of oxalic acid and 1.8 g of Ce(NO3)2·6H2O were dissolved in 30 mL deionized water, and then 1.0 g of KIT-6 was added to the above mixture. The mixture was stirred overnight at room temperature, dried at 80 °C for 4 h and sintered at 500 °C (at a rate of 1 °C/min from room temperature) for 4 h in a muffle furnace. After cooling to room temperature, the obtained powder sample was washed for three times with 2 M NaOH aqueous solution to remove the silica template, followed by washing with deionized water several times until reaching neutral (pH = 7) and then dried at 60 °C in an oven.
By the way, the preparation methods of mesoporous WO3(χ)-CeO2(χ = 0, 0.5, 0.75 and 1.25) were similar with WO3(1)-CeO2 by varying the doped ratio of (NH4)6W7O24·6H2O. What’s more, the preparation processes of bulk WO3(χ)-CeO2 were similar with mesoporous WO3(χ)-CeO2, except that no KIT-6 was added into the mixture during the bulk WO3(χ)-CeO2 preparation. 0.3 K/WO3(1)-CeO2 was prepared by a conventional impregnation method. The fresh catalyst was added to 0.30 wt% potassium nitrate solution. After the mixed solution was stirred to dry at 60 °C, and then calcined at 500 °C for 4 h. The V2O5-WO3/TiO2 catalyst used in the experiment was commercial honeycomb-like V2O5-WO3/TiO2 catalyst, which was used without further purification.
2.2. Characterization
The morphology and structure of mesoporous WO3(χ)-CeO2 were measured by transmission electron microscopy (TEM) with a JEOL Model JEM-2100F at 200 kV. The X-ray diffraction (XRD) patterns were carried out by using an X-ray diffractometer apparatus (Rigaku D/Max 2200PC, λ= 0.15418 nm) with the voltage and electric current of 40 kV and 40 mA. The N2 adsorption-desorption isotherms were measured by using the Quantachrome AutoSorb iQ-MP operated at −196 °C. The specific surface area was measured by using the Brunauer-Emmett-Teller (BET) method, and the pore size distributions of samples were recorded with BJH method. Temperature-programmed reduction of hydrogen (H2-TPR) and temperature-programmed desorption of NH3 (NH3-TPD) were measured by a Micromeritics Autochem 2920 II apparatus with the thermal conductivity detector (TCD) [24]. And in situ Diffuse Reflectance Infrared Fourier Transform spectra (in situ DRIFTS) were acquired using the Nicolet iS50 spectrometer. Prior to each experiment, the samples were heated to 300 °C in an N2 atmosphere for 2 h and then cooled to the desired temperature.
2.3. Catalytic activity test
The NH3-SCR activities were measured in a fixed-bed quartz reactor with 10 mm inner diameter under atmospheric pressure from 100 °C to 400 °C. The typical inlet gas component was 500 ppm NO and 500 ppm NH3, 5 vol% O2, N2 balance, 10 ol% or 15 vol% H2O (when used), 100 ppm SO2 (when needed). The total flow rate of feeding gases was 200 mL/min, with a gas hourly space velocity (GHSV) of 30,000 h−1. Before the test, 0.5 g sample was pressed into blocks, then crushed and sieved with 40–60 meshes. And the component and concentration of outlet gas were measured by KM-940 flue gas analyzer (Kane International Limited, UK). The NOx conversion ratio and N2 selectivity were calculated by the following equations:
Herein NOx included NO and NO2, where the [NOx]in and [NOx]out indicated the inlet and outlet concentration at steady-state, respectively.
3. Results and discussion
3.1. Materials characterization
3.1.1. TEM analysis
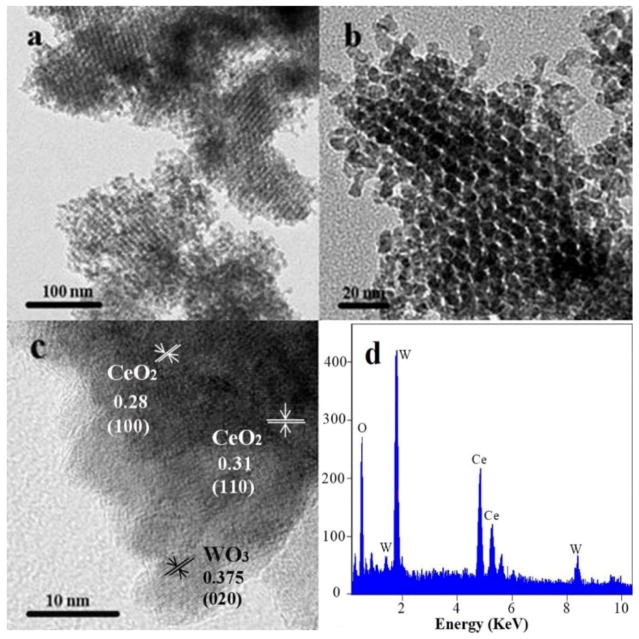
In order to explore microstructure of mesoporous WO3(1)-CeO2, the typical TEM tests were conducted and the results were shown in Fig. 1. From Fig. 1a, it could conclude that the sample was highly ordered mesoporous structure. Moreover, it was found that the average pore diameter of mesoporous WO3(1)-CeO2 was 2.9 nm (Fig. 1b), which was similar with 3.6 nm wall thickness of KIT-6 (Fig. S1e), suggesting that cubic mesoporous WO3(1)-CeO2 was replicated successfully by thermal decomposition of metal precursors within the restricted channels of KIT-6 [25]. In Fig. 1c, lattice fringe (d = 0.28 nm) belonged to the (100) crystallographic planes of CeO2, and the (110) crystallographic planes (d = 0.31 nm) were observed clearly [26,27]. The lattice fringe of WO3 belonging to the (020) crystallographic planes (d = 0.375 nm) was also observed [28], indicating that it was well crystallized. With increasing W-doping ratio, the mesoporous channels became not obvious (Fig. S1). In addition, X-ray energy dispersive spectroscopy (EDS) was performed to investigate the chemical composition of mesoporous WO3(1)-CeO2 and the results confirmed that the sample was composed of Ce, W and O elements (Fig. 1d).
Fig. 1.
(a, b) TEM images, (c) HRTEM and (d) energy spectrum analysis (EDS) images of mesoporous WO3 (1)-CeO2.
3.1.2. BET analysis
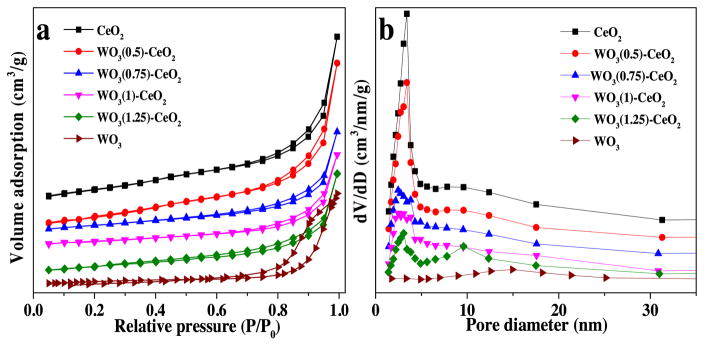
To further investigate the surface areas of mesoporous WO3(χ)-CeO2, we characterized the catalysts using BET analysis. The N2 adsorption-desorption isotherms and the pore diameter distribution of mesoporous CeO2, WO3(0.5)-CeO2, WO3(0.75)-CeO2, WO3(1)-CeO2, WO3(1.25)-CeO2 and bulk WO3 were evaluated and listed in Table 1. Fig. 2a suggested that the adsorption-desorption curves of mesoporous CeO2 and WO3(χ)-CeO2 can be classified as type IV isotherms in the relative pressure from 0.6 to 0.95. All the isotherms were very similar, with a type H1 hysteresis loop [22], indicating that these structures possessed highly ordered mesoporous channels, which were in accordant with TEM results. As depicted in Fig. 2b, the average pore sizes of these mesoporous materials were mainly focused on 3.2 nm, indicating that the pore size distribution of all the samples was uniform relatively. However, there was no typical adsorption-desorption curve for bulk WO3 catalysts, suggesting that the sample had no ordered pore structure. According to Fig. 2b, the average pore size of bulk WO3 was much bigger than other mesoporous catalysts. Considering the low specific area (5.3 m2/g) and small pore volume (0.02 cc/g) of bulk WO3 (Table 1), it can be assumed that the bulk WO3 was made up of sintered particles. The results suggested that the mesoporous structure can effectively inhibit the crystallization of the catalyst.
Table 1.
Summary of textural parameters of the samples.
| Materials | Specific area (m2/g) | Pore volume (cc/g) | Pore diameter (nm) |
|---|---|---|---|
| Mesoporous CeO2 | 163.3 | 0.46 | 3.41 |
| Mesoporous WO3 (0.5)-CeO2 | 91.1 | 0.28 | 2.46 |
| Mesoporous WO3 (0.75)-CeO2 | 105.6 | 0.27 | 2.22 |
| Mesoporous WO3 (1)-CeO2 | 100.2 | 0.26 | 2.02 |
| Mesoporous WO3 (1.25)-CeO2 | 89.4 | 0.25 | 2.11 |
| Bulk WO3 | 5.3 | 0.02 | 15.76 |
Fig. 2.
(a) N2 adsorption-desorption isotherms, (b) pore diameter distribution.
As shown in Table 1, it was found that the surface area of mesoporous CeO2 was 163.3 m2/g, which was larger comparing to those of mesoporous WO3(0.5)-CeO2 (91.1 m2/g), WO3(1)-CeO2 (100.2 m2/g) and mesoporous WO3(1.25)-CeO2 (89.4 m2/g). Because pure metal oxide was easier to form mesoporous structure, and the regularity property of mesoporous CeO2 was better than mesoporous WO3(χ)-CeO2(χ = 0.5, 0.75, 1, 1.25), which was in agreement with the result of TEM analysis. Also, the pore volume and pore diameter became smaller gradually with the increase of W-doping ratio, which was due to the increased tungsten content damaging the pore channel and making the formation of pore structure less likely.
3.1.3. XRD patterns
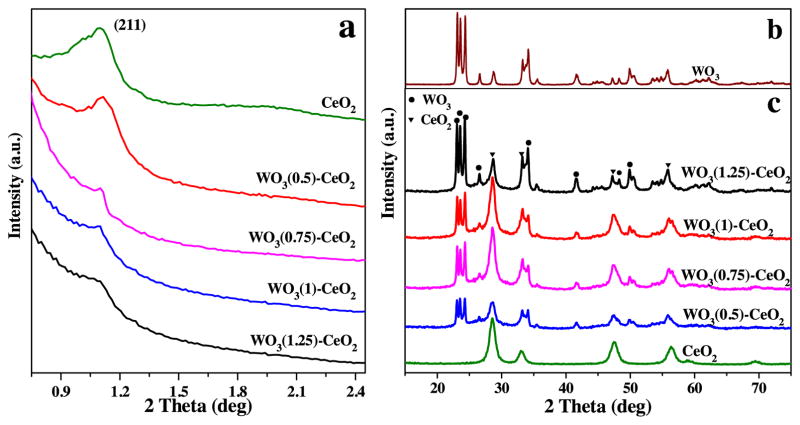
To further study their microstructure, low-angle XRD patterns of mesoporous WO3(χ)-CeO2 were analyzed and the results were shown in Fig. 3a. The stronger diffraction peaks of (211) plane around 1.2° for mesoporous WO3(χ)-CeO2 were observed, suggesting that the ordered mesoporous structure came into being [29]. Furthermore, with increasing W-doping content, the intensity of diffraction peaks became weaker and weaker, and the peak positions also shifted to a higher-angle region, indicating that parts of mesoporous channels collapsed. It was demonstrated that the regularity of mesoporous samples was much poorer than pure mesoporous CeO2, which may be due to the doped tungsten resulting in channel collapse. It can also be verified from BET analysis results [30,31]. Moreover, it was difficult to prepare pure mesoporous WO3, indicating that tungsten element had a devastating impact on the formation of mesoporous channels.
Fig. 3.
(a) Low-angle XRD; (b) wide-angle XRD patterns of bulk WO3 ; (c) wide-angle XRD patterns of mesoporous WO3 (χ)-CeO2.
Furthermore, wide-angle XRD patterns of bulk WO3 and mesoporous WO3(χ)-CeO2 results were depicted in Fig. 3b and c. The spectrum of bulk WO3 was individually presented in Fig. 3b because of its strong intensity that can make weaker peaks unobvious if the spectra of all the catalysts were put together. As shown in Fig. 3b, the diffraction peaks of bulk WO3 were well indexed to WO3 (JCPDS20-1324). In Fig. 3c, compared with pure mesoporous CeO2, the diffraction peaks of WO3 could be verified and the peak intensity became stronger with increasing W-doping ratio. And the diffraction peaks of mesoporous WO3(χ)-CeO2 can be well indexed to CeO2 (JCPDS 81-0792) [15] and WO3 (JCPDS20-1324) [30]. Furthermore, no peaks of other crystal phases were detected, indicating that the prepared mesoporous WO3(χ)-CeO2 catalysts were made up of CeO2 and WO3. In addition, the average particle sizes of WO3 and CeO2 in mesoporous WO3(1)-CeO2 were calculated to be about 8 nm and 7 nm based on the Scherrer formula, which were in agreement with 5.44 nm from TEM.
3.1.4. Raman characterization
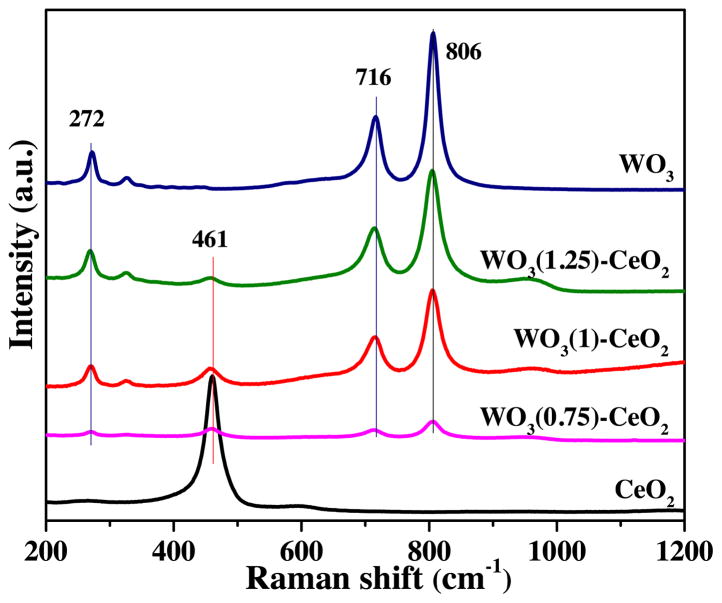
Raman spectrum (Fig. 4) was employed to further characterize the existence states of the tungsten species (WOx) in the mixed Ce-W bi-metal oxides catalysts. The spectrum of CeO2 showed a sharp peak at 461 cm−1, which belonged to the F2g mode of the symmetric breathing mode of oxygen atoms surrounding cerium ions in the cubic fluorite phase CeO2 [32]. As for WO3(χ)-CeO2 catalysts, the peak that belonged to the F2g mode of CeO2 became weaker significantly. At the same time, the peaks at 900–1000 cm−1 enhanced gradually, suggesting that the adding of tungsten species inhibited the crystallization of CeO2. The conclusion was highly consistent with the XRD results. However, It’s worth mentioning that the Ce-O vibration peak shifted to much lower wavenumber, which may be related with the decreased CeO2 particle size [33]. Also, typical WO3 crystallization peaks (272, 716 and 806) occurred on the WO3(χ)-CeO2 and WO3 catalysts [34]. Through the analysis, we could realize that the WO3(χ)-CeO2 catalysts remained the cubic fluorite structure of CeO2 and the tungsten species existed mainly in the state of crystalline WO3.
Fig. 4.
Raman patterns of mesoporous WO3 (χ)-CeO2 and WO3.
3.1.5. NH3-TPD
To better analyze the different acidic sites and their relative strength on the surface of the materials, NH3-TPD patterns of mesoporous WO3(χ)-CeO2 with different W-doping ratio were measured (Fig. 5a). Because the thermal stability of the NH4+ restrained in the Brønsted acid sites was lower than the NH3 molecules attributed to the Lewis acid sites, it can be concluded that the desorption peaks at high temperatures originated from the Lewis acid sites, and the peaks below 200 °C belonged to Brønsted acid sites [35]. Moreover, the temperature window of Lewis acid sites was wider than Brønsted acid sites. Additionally, there was a stronger peak around 132 °C for mesoporous CeO2, which belonged to NH4+ ions resulting from Brønsted acid sites. Two obvious peaks resulted from the coordinated NH3 molecules appeared from 250 to 450 °C, which belonged to the Lewis acid sites [36,37]. However, for mesoporous WO3(χ)-CeO2 (χ = 0.5, 0.75, 1, 1.25), their desorption peaks shifted to higher temperatures. And the peak intensity became stronger compared with pure mesoporous CeO2 and pure WO3, which may be due to the synergetic effect between tungsten and cerium oxides. What’s more, the amount of peaks above 300 °C increased, which were associated with coordinated NH3 molecules originating from Lewis acid sites at high temperatures. It can also be proved by the desorption curve of pure WO3, which only owned the obvious peak at 200 °C related to Brønsted acid sites. For mesoporous WO3(0.75)-CeO2, two NH3 desorption peaks at 531 and 590 °C were due to weakly bonded and strongly bonded NH3, respectively, which were related to Lewis acid sites [9]. Meanwhile, it was reported that NH3 adsorbed species could be more easily desorbed due to the addition of tungsten element at higher temperature [37]. Furthermore, the NH3-TPD curve of mesoporous WO3(1)-CeO2 showed larger area than other samples at high temperatures, indicating that there were enough Lewis acid sites on the surface. It was well known that the amount of the Lewis acid sites played a significant role in NH3-SCR activity [22], and more Lewis acid sites on the surface of mesoporous WO3(1)-CeO2 resulted in preferable removal efficiency at the low temperature region.
Fig. 5.
(a) NH3 -TPD and (b) H2 -TPR patterns of mesoporous WO3 (χ)-CeO2 and bulk WO3.
3.1.6. Reducibility (H2-TPR)
As we all know, the surface reduction ability of materials played an important role in NH3-SCR activity. The H2-TPR test of mesoporous WO3(χ)-CeO2 was shown in Fig. 5b, which presented that the reduction potentials of mesoporous WO3(χ)-CeO2 were weaker than mesoporous CeO2 below 500 °C. For mesoporous CeO2, the reduction peak at 450 °C could be attributed to the reduction of surface oxygen species [38]. It was found that the reduction peaks of the bulk oxygen (bulk Ce4+ to Ce3+) and the surface oxygen (surface Ce4+ to Ce3+) of ceria focused on 650 and 508 °C, respectively [39]. Comparing with mesoporous CeO2, the peak of mesoporous WO3(1)-CeO2 at 550 and 740 °C could be attributed to the reduction of surface oxygen species because of the oxygen vacancies in Ce-W composite. For pure WO3, the three peaks at 600, 698, 788 °C can be ascribed to the stepwise reduction of tungsten from W6+ to W0 [15,21]. Comparing with mesoporous CeO2 and pure WO3, the peak of mesoporous WO3(χ)-CeO2 (χ = 0.5, 0.75, 1, 1.25) assigning to the transformation of tungsten shifted to lower temperatures, which would be favorable to improve NH3-SCR activity.
3.1.7. XPS analysis
The XPS spectra of Ce 3d, O 1s and W 4f on mesoporous WO3(χ)-CeO2 were shown in Fig. 6, these kinds of absorbed peaks were calibrated against the C 1s peak standardized at 284.6 eV [40]. As shown in Fig. 6a, Ce 3d, O 1s and W 4f appeared in the survey spectrum XPS spectra for mesoporous WO3(χ)-CeO2. The XPS spectra of chemical states of Ce over different materials were showed in Fig. 6b. The peaks assigned u1 and v1 were the main representatives of the 3d104f1 electronic state of Ce3+ ions, while the peaks assigned u, u2, u3, v, v2 and v3 were the representative of the 3d104f0 state corresponding to Ce4+ [37]. The amount of Ce3+ increased gradually from 15.8% to 22.49% by the W-doping. The increasing amount of Ce3+ was due to the interaction between cerium and the neighboring atoms such as W. The existence of the Ce3+ species could bring about a charge imbalance and unsaturated chemical bonds on the sample surface, thereby resulting in the improvement of chemisorbed oxygen on the surface [24,41]. The increase of chemisorbed oxygen was beneficial to SCR reaction.
Fig. 6.
XPS spectrum of mesoporous WO3 (χ)-CeO2, (a) wide-scan spectra, (b) Ce 3d, (c) O 1s, (d) W 4f.
As presented in Fig. 6c, two kinds of surface oxygen species were identified by performing a peak-fitting deconvolution. The peaks at higher Binding Energy of 531.0–533.0 eV were assigned to the surface chemisorbed oxygen (Oα), and the peaks at lower Binding Energy of 529.0–531.0 eV were attributed to the surface lattice oxygen (Oβ). The ratio of Oα/(Oα+Oβ) in mesoporous CeO2 (27.5%) was much lower than that in mesoporous WO3(1)-CeO2 (39.8%), which may be due to the interaction between the tungsten and cerium oxides with the W-doping. It is well known that the higher rate of Oα would have significant promotion of the SCR activity, which was proved by the following SCR catalytic test results.
The W4f spectra of the tungsten-containing catalysts were shown in Fig. 6d. With increasing tungsten content, the peak position of these five catalysts had no significant change, suggesting that neighboring environment of the W6+ cations stayed the same to a certain extent [22,23]. However, it’s worth noting that peak intensities enhanced gradually with the increasing of WO3 concentration. The enhancement of the peak intensity suggested the increase of the tungsten molar density on the surface. And the above characteristics were in consistent with the analysis results of H2-TPR as well.
3.2. The NH3-SCR activity
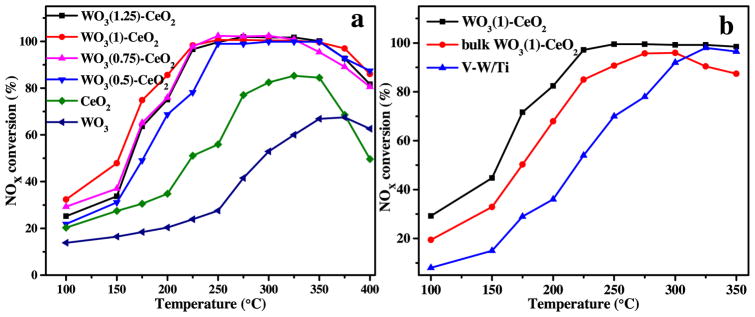
The NH3-SCR activity measurements of mesoporous WO3(χ)-CeO2 and bulk WO3 materials were presented in Fig. 7a. It shows that 100% NOx conversion ratio with mesoporous WO3(1)-CeO2 can be reached from 225 to 350 °C with a GHSV of 30 000 h−1, which may be due to the stronger synergistic effect of WO3 and CeO2 and more adsorbed NOx and NH3 species. The decrement of NOx conversion at high temperature was basically due to the emergence of the NH3 oxidation phenomenon. (see Fig. S4 for details of the NH3 oxidation analysis). However, when an excess amount of W was added, the NOx conversion ratio would decrease, which showed that the optimal doping ratio was 1:1. Furthermore, the NH3-SCR activity of mesoporous WO3(1)-CeO2 was better than other mesoporous WO3(χ)-CeO2(χ = 0.5, 0.75, 1.25) catalysts at lower temperatures from 100 to 225 °C. The NOx conversion ratio of mesoporous WO3(1)-CeO2 was better than bulk (non-mesoporous) WO3(1)-CeO2 and commercial V2O5-WO3/TiO2 catalysts from 100 to 300 °C, which may be related to the unique structure-activity relationship of the mesoporous structure (Fig. 7b). The NOx conversion ratios of bulk WO3(χ)-CeO2 and bulk pure WO3 were presented in Supporting information (Fig. S3).
Fig. 7.
NOx conversion ratio of (a) mesoporous WO3 (χ)-CeO2 and bulk WO3, and (b) different kind of SCR catalysts under 500 ppm NO, 500 ppm NH3, 5% O2, GHSV of 30 000 h−1 and N2 balance gas.
3.3. The effect of H2O, alkali metal poisoning, SO2 resistance and thermal stability
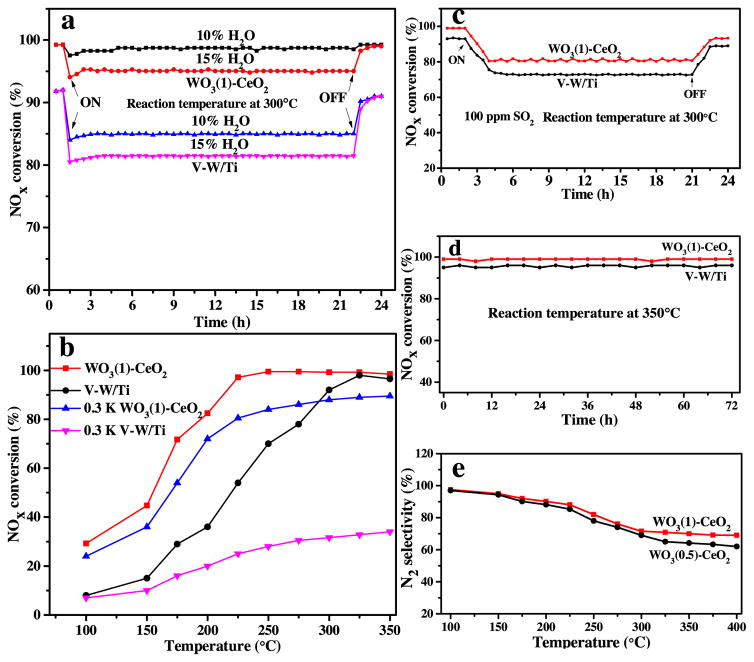
As we all know, water vapor from NH3-SCR reaction process may affect the NOx conversion ratio. Therefore, it is necessary to determine how the water vapor affected their SCR activities. In order to explore the effect of water vapor, 10% or 15% H2O was added into the system with GHSV of 30 000 h−1 at 300 °C (Fig. 8a). It was found that the NOx conversion ratio of mesoporous WO3(1)-CeO2 and commercial V2O5-WO3/TiO2 at 300 °C was 100% and 91%, respectively. Under 10% or 15% H2O, NOx conversion ratio of mesoporous WO3(1)-CeO2 declined to 98% and 95%, respectively. In contrast, the conversion ratio of V2O5-WO3/TiO2 decreased to 84% and 81%, respectively, which was caused by the blocked active sites on the catalyst surface. Furthermore, it was found that the NOx conversion ratio decreased with increasing water content, indicating that the presence of water vapor had an obvious influence on the active sites of samples. The negative effect of H2O was more serious for commercial V2O5-WO3/TiO2 than mesoporous samples. After turning off the H2O entrance, the NOx conversion ratio of mesoporous WO3(1)-CeO2 could recover to 100% soon, but commercial V2O5-WO3/TiO2 could recover only 90%. Therefore, these mesoporous materials have better water tolerance. Meanwhile, it was suggested that the effect of H2O on NH3-SCR activity with mesoporous WO3(1)-CeO2 was reversible.
Fig. 8.
NH3 -SCR activity of the mesoporous WO3 (1)-CeO2 and commercial V2 O5 -WO3/TiO2: (a) H2 O resistance, (b) resistance of alkali metal, (c) SO2 tolerance, (d) stability test and (e) N2 selectivity. Reaction conditions: [NO] = [NH3 ] = 500 ppm, [O2 ] = 5 vol%, [H2 O] = 10% or 15% (when used), [SO2 ] = 100 ppm (when used), N2 balance and GHSV = 30 000 h−1.
The NH3-SCR activity measurements of mesoporous WO3(1)-CeO2, commercial V2O5-WO3/TiO2 catalyst and homologous 0.3 wt% K-doped samples at 100–400 °C were presented in Fig. 8b [15]. For V2O5-WO3/TiO2 catalyst with 0.3 wt% K-doping, the activity decreased significantly, only producing 35% NOx conversion ratio. For 0.3 wt% K-doped mesoporous WO3(1)-CeO2, although the NOx conversion ratio was lower compared to the fresh, it could also reach 76% at 225 °C during the test. Therefore, it was proved that the novel mesoporous WO3(1)-CeO2 can provide much stronger alkali resistance than commercial V2O5-WO3/TiO2 catalyst. According to previous reports, the decreases in the reducibility and the surface acidity should be responsible for the alkali metal poisoning of the catalysts [15,41].
The SO2 tolerance tests for mesoporous WO3(1)-CeO2 and commercial V2O5-WO3/TiO2 were also shown in Fig. 8c. After pumping into SO2, the NOx conversion ratio of mesoporous WO3(1)-CeO2 decreased from 100% to 81%. As for commercial V2O5-WO3/TiO2, there was 73% removal efficient. The sulfur-poisoning of catalysts may be caused by the formation of thermally stable sulfate species, which deposited on catalysts’ surface and blocked catalytic sites [42,43]. However, after cutting off inlet SO2, the NOx conversion ratio of mesoporous WO3(1)-CeO2 recovered to 93% after 3 h, and remained at 93%, showing that the mesoporous WO3(1)-CeO2 had better reversible inhibition effect.
The thermal stability of mesoporous WO3(1)-CeO2 and commercial V2O5-WO3/TiO2 catalyst were presented in Fig. 8d. The NOx conversion ratio of mesoporous WO3(1)-CeO2 and commercial V2O5-WO3/TiO2 maintained at 100% and 91% at 300 °C for 72 h, respectively. It showed that the aging ability of sample was just as good as V2O5-WO3/TiO2 catalyst, which was a very important factor in practical application. Also, the N2 selectivity test was shown in Fig. 8e and our test results showed that the WO3(1)-CeO2 catalyst had a relatively high N2 selectivity under low temperature, while the N2 selectivity became lower under higher temperature, which may be due to the increasing intermediate product (NH4NO3) in the NH3-SCR reaction, resulting in lower N2 selectivity at high temperatures [22]. Moreover, the N2 selectivity of mesoporous WO3(1)-CeO2 was better than mesoporous WO3(0.5)-CeO2, proving that the higher N2 selectivity could be conducive to promote the excellent NH3 oxidation ability.
3.4. In situ DRIFTS
3.4.1. NH3 adsorption
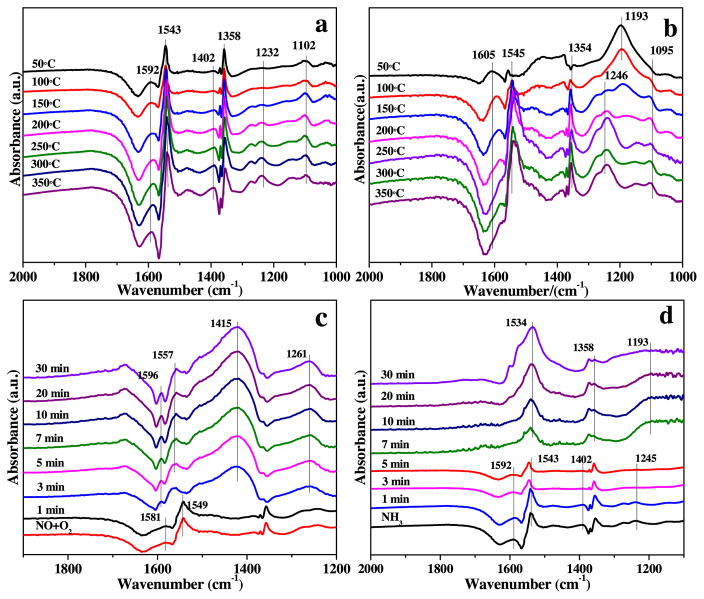
The in situ DRIFT spectra were carried out and the results were shown in Fig. 9. Prior to the experiment of gas adsorption, the mesoporous CeO2, WO3(1)-CeO2 and bulk WO3(1)-CeO2 were firstly treated with N2 for 2 h at 300 °C to blow away the CO2 and H2O in air, and then measured the background spectra under the same conditions. When cooling to the goal temperature of 50 °C, 500 ppm NH3 was pumped into the system for 30 min, and then the in situ DRIFT spectra were measured with the increasing temperature. The recorded results were presented in Figs. 9a and S6 (a, c). For mesoporous WO3(1)-CeO2, the peak at 1402 cm−1 was correlated to NH4+ ions, and the peak at 1592 cm−1 was assigned to the coordinated NH3 linked to Lewis acid sites [44]. The peaks at 1232 and 1543 cm−1 may be resulted from the metabolic species of adsorbed ammonia species from Lewis acid sites [44,45]. The peak at 1102 cm−1 was assigned to symmetric deformation of harmonious NH3. A new peak at 1358 cm−1 for mesoporous WO3(1)-CeO2 was quite different from adsorbed NH3 and NOx species, which may be attributed to the formed intermediate species during NH3-SCR reaction. It was obvious that Brønsted acid sites and Lewis acid sites coexisted on the samples surface, suggesting that NH3 could be adsorbed on different active sites. Compared with Fig. S6 (a, c), Fig. 9a showed that both the addition of tungsten oxides to CeO2 and the mesoporous structure would result in more NH3 adsorption sites, which was significantly beneficial to NH3-SCR reaction. It was in good consistent with the result of NH3-TPD analysis.
Fig. 9.
In situ DRIFT spectra of (a) NH3 adsorption after the catalyst was exposed to a flow of 500 ppm of NH3 for 30 min and (b) NO + O2 adsorption after the catalyst was exposed to a flow of 500 ppm of NO + 5% O2 for 30 min on mesoporous WO3 (1)-CeO2 as a function of temperature; in situ DRIFT spectra of (c) passing NH3 over NO + O2 preadsorbed mesoporous WO3 (1)-CeO2, and (d) passing NO + O2 over NH3 preadsorbed mesoporous WO3 (1)-CeO2 at 200 ° C.
3.4.2. NO + O2 adsorption
The in situ DRIFT spectra of NO + O2 adsorption were presented in Figs. 9b and S6(b, d). Several peaks in the range of 1000–2000 cm−1 were detected. The peaks of mesoporous WO3(1)-CeO2 were stronger than mesoporous CeO2 (Fig. S6b), which may connect with the synergetic effect between the tungsten and cerium species. The peaks at 1095 and 1193 cm−1 belonged to asymmetric and symmetric NO2 vibration of monodentate nitrite, respectively [44]. The peaks at 1545, and 1246 cm−1 were due to asymmetric and symmetric NO2 vibration of bidentate nitrate, respectively [46]. The band at 1605 cm−1 was assigned to bridging nitrate, which originated from the adsorbed NO2 on the oxide surface. For mesoporous WO3(1)-CeO2, the peak at 1193 cm−1 moved to 1246 cm−1 with temperature increasing, suggesting that the surface species transformed from monodentate nitrite to bidentate nitrate. Compared with Fig. S6 (b, d), Fig. 9b indicated that the mesoporous structure and the synergetic effect between cerium oxide and tungsten oxide species were beneficial to the formation of monodentate nitrate, bidentate nitrate and bridging nitrate species. More monodentate nitrite species, bidentate nitrate and bridging nitrate species on the surface of mesoporous WO3(1)-CeO2 were necessary to improve the NH3-SCR activity.
3.4.3. Reactions between NH3 and adsorbed NO + O2 species
In order to explore the reactivity of absorbed NOx species in the SCR reaction on mesoporous WO3(1)-CeO2 surface, in situ DRIFTS of the reaction between preadsorbed NOx and NH3 at 200 °C was tested and the results were shown in Fig. 9c. After the adsorption of NO + O2, the surface of the material was mainly covered by bridging nitrate (at 1581 cm−1) and bidentate nitrate (at 1549 cm−1) [47]. After the further introduction of NH3, the bands of bridging nitrate and bidentate nitrate decreased and disappeared in 3 min. At the same time, the bands at 1415 cm−1 attributed to ionic NH4+ and coordinated NH3 (at 1261 cm−1) appeared after 3 min, indicating that both bridging nitrate and bidentate nitrate could react with NH3 [48,52]. Based on Figs. 9b and S6d, the amount of nitrate species on mesoporous WO3(1)-CeO2 surface was higher than that on bulk WO3(1)-CeO2 surface, suggesting that mesoporous WO3(1)-CeO2 should be an excellent material to carry out the NH3-SCR reaction. Compared mesoporous WO3(1)-CeO2 (Fig. 9b) with mesoporous CeO2 (Fig. S6b), it can be found that doping W brought in more acid sites, which was beneficial for the adsorption of NH3 species, and thus enhanced the low-temperature activity [15]. Therefore, it can be concluded that enough adsorption amount of nitrate species and NH3 species was conducive to the higher NH3-SCR activity of mesoporous WO3(1)-CeO2.
3.4.4. Reactions between NO + O2 and adsorbed NH3 species
The reactivity of preadsorbed NH3 with NO + O2 species was also studied on mesoporous WO3(1)-CeO2 by use of in situ DRIFTS at 200 °C, which was measured as a function of time (Fig. 9d). After introducing NO + O2, the bands at 1402 cm−1 attributed to ionic NH4+, 1592, 1543 and 1245 cm−1 ascribed to coordinated NH3 decreased obviously in intensity [49,52]. Moreover, all of the bands were substituted by nitrate species after 7 min, suggesting that both coordinated NH3 and ionic NH4+ on mesoporous WO3(1)-CeO2 surface could decrease the amount of NOx. Although coordinated NH3 on mesoporous WO3(1)-CeO2 surface was important for high SCR activity, ionic NH4+ could also participate in the SCR reaction [51]. Therefore, it indicated that the addition of WO3 to mesoporous CeO2 could produce more coordinated NH3 and ionic NH4+, both of which could play a significant role in the NH3-SCR reaction.
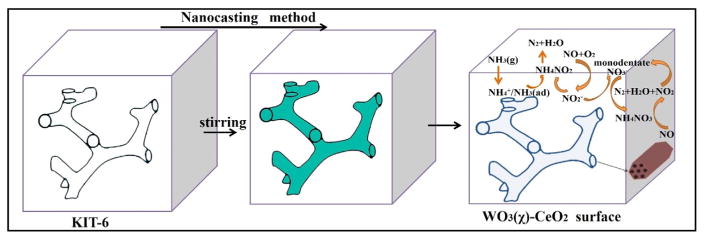
4. Reaction mechanism
The above analysis results and in situ DRIFT spectra study demonstrated that the reaction of adsorbed monodentate NO3− with adsorbed NH3 was attributed to the Langmuir-Hinshelwood mechanism at low temperatures on the surface of mesoporous WO3(1)-CeO2 (Fig. 10). The NH3-SCR reaction mechanism was proposed that NH3(g) was adsorbed on the surface of Lewis acid sites and Brønsted acid sites in the shape of NH4+ ions and gaseous NH3. Besides, the adsorption of NO could exist in the form of gaseous or oxide ions NO2− on the surface of mesoporous WO3(1)-CeO2 according to the DRIFT spectra. Compared to the adsorption of NO + O2, the adsorption of NH3 was stronger. The adsorbed NH3 species could react with NO2− species easily to produce NH4NO2 via the following reaction:
Fig. 10.
The NH3 -SCR reaction mechanism on WO3 (χ)-CeO2 surface at low temperature.
The NH4NO2 was extremely unstable and rapidly decomposed into the innocuous N2 and H2O according to the reaction:
On the other hand, the adsorbed NO2− could be oxidized to monodentate NO3− to proceed in the subsequent reaction. And then NH4+ ions reacted with monodentate NO3− on active sites to generate NH4NO3.
The NH4NO3 formation has been observed in many other SCR catalyst systems, which was reported relatively stable at low temperature. Therefore, it may block the active sites of the SCR reaction. If the temperature was higher than 200 °C, NH4NO3 could decompose to N2O and H2O. N2O was considered as an intermediate product during the process of SCR reaction, which can be activated into N-NO or NN-O [47,50]. Noteworthily, comparing mesoporous CeO2 and pure WO3 with mesoporous WO3(1)-CeO2, the amount of N2O on the surface of mesoporous WO3(1)-CeO2 decreased, indicating that most of NH4NO3 reacted with NO on mesoporous WO3(1)-CeO2 surface by the following reaction:
The reaction was considered to be decisive in the process of NH3-SCR reaction since it could remove surface NH4NO3 and continuously transform NO into N2. The NO2 that simultaneously formed could be adsorbed again and reacted with NH3 [51,52]. Besides, only a negligible amount of N2O was detected under NH3-SCR reaction conditions, indicating that the NH4NO3 decomposition reaction was not significant. That is to say, once NH4NO3 species formed under NH3-SCR conditions, it would decrease quickly due to the reaction with excess NO. Therefore, it was not observed on the surface of mesoporous WO3(1)-CeO2 through in situ DRIFTS.
5. Conclusions
In this work, highly ordered mesoporous WO3(χ)-CeO2 nano-materials have been synthesized by using KIT-6 as the hard template and applied in the NH3-SCR reaction to remove NOx. The NOx conversion efficiency reached 100% at a wider temperature window from 225 to 350 °C. In order to explore the reaction mechanism, mesoporous CeO2, bulk WO3(χ)-CeO2 and commercial V2O5-WO3/TiO2 were compared with mesoporous WO3(χ)-CeO2. It can be found that mesoporous WO3(1)-CeO2 exhibited the best NH3-SCR activity in wider operating temperature window, superior water-resistance effect, better alkali metal poisoning resistance, higher stability and a relatively high N2 selectivity at low temperature, which was due to its ordered mesoporous structure, the higher Ce3+/Ce4+ mole ratio, enough surface chemisorbed oxygen and the much more amount of Lewis acid sites. Therefore, mesoporous WO3(1)-CeO2 catalyst has great potential for industrial applications in controlling NOx emissions.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the financially support by Natural Science Foundation of China (21377061, 81270041), and Key Technologies R&D Program of Tianjin (15JCYBJC48400, 14ZCZDSF00001, 15JCZDJC41200 and 16YFZCSF00300). Financial support from NIH (R21AI107415 and SC2GM105584), UT STARS Award, BBRC Pilot and IDR2 Award from UTEP is also gratefully acknowledged.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.apcatb.2016.10.010.
The other related figures and data about the WO3(χ)-CeO2 materials, such as N2 adsorption-desorption isotherms and pore diameter distribution, XPS spectrum, SO2 tolerance and in situ DRIFT spectra. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Zhi B, Ding H, Wang D, Cao Y, Zhang Y, Wang X, Liu Y, Huo Q. J Mater Chem A. 2014;2:2374–2382. [Google Scholar]
- 2.Chang H, Jong M, Wang C, Qu R, Du Y, Li J, Hao J. Environ Sci Technol. 2013;47:11692–11699. doi: 10.1021/es4022014. [DOI] [PubMed] [Google Scholar]
- 3.Xie X, Li Y, Liu Z, Haruta M, Shen W. Nature. 2009;458:746–749. doi: 10.1038/nature07877. [DOI] [PubMed] [Google Scholar]
- 4.Li J, Chang H, Ma L, Hao J, Yang R. Catal Today. 2011;175:147–156. [Google Scholar]
- 5.Peng Y, Li J, Shi W, Xu J, Hao J. Environ Sci Technol. 2012;46:12623–12629. doi: 10.1021/es302857a. [DOI] [PubMed] [Google Scholar]
- 6.Shen B, Wang Y, Wang F, Liu T. Chem Eng J. 2014;236:171–180. [Google Scholar]
- 7.Chang H, Chen X, Li J, Ma L, Wang C, Liu C, Johannes WS, Hao J. Environ Sci Technol. 2013;47:5294–5301. doi: 10.1021/es304732h. [DOI] [PubMed] [Google Scholar]
- 8.Li J, Li S. J Phys Chem C. 2008;112:16938–16944. [Google Scholar]
- 9.Liu J, Wu X, Zou S, Dai Y, Xiao L, Gong X, Fan J. J Phys Chem C. 2014;118:24950–24958. [Google Scholar]
- 10.Strunk J, Vining WC, Bell AT. J Phys Chem C. 2011;115:4114–4126. [Google Scholar]
- 11.Kwon D, Nam K, Hong S. Appl Catal B. 2015;166–167:37–44. [Google Scholar]
- 12.Wijayanti K, Kumar A, Joshi S, Kamasamudram K, Currier NW, Yezerets A, Olsson L. Appl Catal B. 2015;163:382–392. [Google Scholar]
- 13.Auvray X, Partridge W, Choi J, Pihl J, Coehlo F, Yezerets A, Kamasamudram K, Currier N, Olsson L. Appl Catal B. 2015;163:393–403. [Google Scholar]
- 14.Yang S, Liao Y, Xiong S, Qi F, Dang H, Xiao X, Li J. J Phys Chem C. 2014;118:21500–21508. [Google Scholar]
- 15.Peng Y, Li J, Chen L, Chen JH, Han J, Zhang H, Han W. Environ Sci Technol. 2012;46:2864–2869. doi: 10.1021/es203619w. [DOI] [PubMed] [Google Scholar]
- 16.Shwana S, Jansson J, Olsson L, Skoglundh M. Appl Catal B. 2015;166–167:277–286. [Google Scholar]
- 17.Casanova M, Nodari L, Sagar A, Schermanz K, Trovarelli A. Appl Catal B. 2015;176–177:699–708. [Google Scholar]
- 18.Yu J, Si Z, Chen L, Wu X, Weng D. Appl Catal B. 2015;163:223–232. [Google Scholar]
- 19.Shan W, Liu F, He H, Shi X, Zhang C. Chem Commun. 2011;47:8046–8048. doi: 10.1039/c1cc12168e. [DOI] [PubMed] [Google Scholar]
- 20.Shan W, Liu F, He H, Shi X, Zhang C. Appl Catal B. 2012;115–116:100–106. [Google Scholar]
- 21.Chen L, Li J, Ablikim W, Wang J, Chang H, Ma L, Xu J, Ge M, Arandiyan H. Catal Lett. 2011;141:1859–1864. [Google Scholar]
- 22.Peng Y, Li K, Li J. Appl Catal B. 2013;140–141:483–492. [Google Scholar]
- 23.Hu B, Liu H, Tao K, Xiong C, Zhou S. J Phys Chem C. 2013;117:26385–26395. [Google Scholar]
- 24.Liu C, Chen L, Li J, Ma L, Arandiyan H, Du Y, Xu J, Hao J. Environ Sci Technol. 2012;46:6182–6189. doi: 10.1021/es3001773. [DOI] [PubMed] [Google Scholar]
- 25.Luo J, Zhang J, Xia Y. Chem Mater. 2006;18:5618–5623. [Google Scholar]
- 26.Ji P, Zhang J, Chen F, Anpo M. J Phys Chem C. 2008;112:17809–17813. [Google Scholar]
- 27.Ho C, Yu J, Kwong T, Mak AC, Lai S. Chem Mater. 2005;17:4514–4522. [Google Scholar]
- 28.Serra RM, Aspromonte SG, Miró EE, Boix AV. Appl Catal B. 2015;166–167:592–602. [Google Scholar]
- 29.Gao C, Lin Y, Li Y, Evans DG, Li D. Ind Eng Chem Res. 2009;48:6544–6549. [Google Scholar]
- 30.Chang Y, Yu K, Zhang C, Li R, Zhao P, Lou L, Liu S. Appl Catal B. 2015;176:363–373. [Google Scholar]
- 31.Ma Z, Wu X, Feng Y, Si Z, Weng D. Catal Commun. 2015;69:188–192. [Google Scholar]
- 32.Qu R, Gao X, Cen K, Li J. Appl Catal B. 2013;142–143:290–297. [Google Scholar]
- 33.Xu J, Li P, Song X, He C, Yu J, Han Y. J Phys Chem Lett. 2010;1:1648–1654. [Google Scholar]
- 34.Mamede A, Payen E, Grange P, Poncelet G, Ion A, Alifanti M, Parvulescu V. J Catal. 2004;223:1–12. [Google Scholar]
- 35.Deng J, Zhang L, Dai H, Xia Y, Jiang H, Zhang H, He H. J Phys Chem C. 2010;114:2694–2700. [Google Scholar]
- 36.Hahn C, Endischa M, Schottb FJ, Kuretia S. Appl Catal B. 2015;168–169:429–440. [Google Scholar]
- 37.Li P, Xin Y, Li Q, Wang Z, Zhang Z, Zheng L. Environ Sci Technol. 2012;46:9600–9605. doi: 10.1021/es301661r. [DOI] [PubMed] [Google Scholar]
- 38.Wang P, Wang H, Chen X, Liu Y, Weng X, Wu Z. J Mater Chem A. 2015;3:680–690. [Google Scholar]
- 39.Liu Z, Zhu J, Li J, Ma L, Woo S. ACS Appl Mater Interfaces. 2014;6:14500–14508. doi: 10.1021/am5038164. [DOI] [PubMed] [Google Scholar]
- 40.Maitarad P, Han J, Zhang D, Shi L, Namuangruk S, Rungrotmongkol T. J Phys Chem C. 2014;118:9612–9620. [Google Scholar]
- 41.Chen L, Li J, Ge M. Chem Eng J. 2011;170:531–537. [Google Scholar]
- 42.Huang Z, Li H, Gao J, Gu X, Zheng L, Hu P, Xin Y, Chen J, Chen Y, Zhang Z, Chen J, Tang X. Environ Sci Technol. 2015;49:14460–14465. doi: 10.1021/acs.est.5b03972. [DOI] [PubMed] [Google Scholar]
- 43.Shi Y, Shu H, Zhang Y, Fan H, Zhang Y, Yang L. Fuel Process Technol. 2016;150:141–147. [Google Scholar]
- 44.Chen L, Li J, Ge M. Environ Sci Technol. 2010;44:9590–9596. doi: 10.1021/es102692b. [DOI] [PubMed] [Google Scholar]
- 45.Gao R, Zhang D, Maitarad P, Shi L, Rungrotmongkol T, Li H, Zhang J, Cao W. J Phys Chem C. 2013;117:10502–10511. [Google Scholar]
- 46.Liu Y, Gu T, Weng X, Wang Y, Wu Z, Wang H. J Phys Chem C. 2012;116:16582–16592. [Google Scholar]
- 47.Hu H, Cai S, Li H, Huang L, Shi L, Zhang D. ACS Catal. 2015;5:6069–6077. [Google Scholar]
- 48.Liu Y, Xu J, Li H, Cai S, Hu H, Fang C, Shi L, Zhang D. J Mater Chem A. 2015;3:11543–11553. [Google Scholar]
- 49.Zhang L, Shi L, Huang L, Zhang J, Gao R, Zhang D. ACS Catal. 2014;4:1753–1763. [Google Scholar]
- 50.Zhang X, Shen Q, He C, Ma C, Cheng J, Li L, Hao Z. ACS Catal. 2012;2:512–520. [Google Scholar]
- 51.Wang D, Zhang L, Kamasamudram K, Epling WS. ACS Catal. 2013;3:871–881. [Google Scholar]
- 52.Ding S, Liu F, Shi X, Liu K, Lian Z, Xie L, He H. ACS Appl Mater Interfaces. 2015;7:9497–9506. doi: 10.1021/acsami.5b00636. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.