Abstract
Hematopoietic stem cells (HSCs) in the bone marrow (BM) form mature blood cells of all lineages through expansion of lineage-biased progenitors. In a recent issue of Nature, Hérault et al. (2017) uncover a unique spatiotemporal mechanism of granulocyte-macrophage progenitors (GMPs) employed in emergency hematopoiesis that is also hijacked in leukemia.
Hematopoiesis is a well-characterized developmental process originating from hematopoietic stem cells (HSCs) in the bone marrow (BM) that ultimately form mature blood cells of the lymphoid, myeloid, and erythroid lineages. The lineage-biased progeny of the HSCs briefly expand, proliferate, and differentiate to respond to the immediate needs of the body until an appropriate systemic response is achieved. HSCs or progenitors with disrupted cellular responses to growth and self-renewal control mechanisms have the potential to develop leukemic phenotypes, as observed in myeloproliferative neoplasms (MPNs) and acute myeloid leukemias (AMLs). Therein, the implications of understanding the precise regulation and heterogeneity of the HSCs and progenitors are far-reaching. While the transcriptional and environmental cues governing hematopoiesis are better understood, little is known about the spatial organization of hematopoietic progenitors and how they are regulated by niche interactions.
HSCs are reserved in a specialized, perivascular niche where they have direct contact with quiescence- or proliferation-enforcing cytokines released from the BM microenvironment (Acar et al., 2015; Bruns et al., 2014; Ding and Morrison, 2013). These niche interactions protect HSC quiescence, which was considered critical for maintaining lifelong hematopoiesis. However, recent evidence supports significant contribution of progenitors, rather than HSCs, to steady-state hematopoiesis (Sun et al., 2014; Busch et al., 2015). Therefore, the spatiotemporal regulation of progenitors is likely equally critical for proliferation and differentiation during both the steady state and under hematopoietic stress. The frequent turnover of mature neutrophils and macrophages places a heavy regenerative demand on granulocyte-macrophage progenitors (GMPs). Yet, it has remained unclear how corresponding GMP populations are localized and how they respond to BM niche cues in the contexts of emergency myelopoiesis or leukemic states. In a recent issue of Nature, Hérault et al. employ imaging approaches that have unveiled a unique spatial organization of GMPs that is important for tuning their capacity to proliferate, differentiate, and respond to negative feedback in the niche. This spatial orientation is also employed during emergency hematopoiesis and hijacked during leukemia (Hérault et al., 2017).
The authors investigate GMP localization associated with emergency and leukemic myelopoiesis. They broadly define GMPs using the phenotypic markers Lin−cKit+ Sca1−FcRγ+CD34+ and examine their spatial organization and localization in wild-type mice and established models of myeloid malignancies. Following experimentally induced emergency myelopoiesis, they observe that GMPs first organize in loosely collected patches (pGMPs) and then condense to form distinct, compact, and transient clusters of GMPs (cGMPs) tightly surrounded by differentiated myeloid cells. They also identify pGMP and cGMP formation as a gradient of functionally distinct cellular states, as pGMPs maintain a more proliferative progenitor-like phenotype and cGMPs further differentiate down the myeloid lineage. During development of myeloid malignancies, the GMPs organize into pGMPs and then condense to form cGMPs. Interestingly, cGMPs were found in the spleens and the BM of MPN (BCR/ABL and junB-deficient) and myeloid leukemia (MLL-AF9) models, yet were undetected in wild-type mice, suggesting that formation of cGMPs is hijacked in certain myeloid malignancies.
The authors then utilize comprehensive experimental approaches to identify the underlying molecular and environmental factors involved in cGMP formation. They identify groups of gene signatures associated with cell-cycle activation and molecular immaturity that are specifically activated in pGMP formation and completely reverted in the steady-state GMPs and stress-induced cGMPs, reflecting the switch from proliferation to differentiation observed during the transition of pGMP to cGMP. Using single-cell RNA-sequencing to compare GMPs isolated from mice during steady-state hematopoiesis, emergency granulopoiesis, and leukemogenesis, the authors find that the preleukemic and the patch-forming GMPs formed during emergency granulopoiesis share similar molecular profiles and resemble self-renewing GMPs. The preleukemic GMPs also appear to be molecularly primed for cluster formation, displaying cell-cycle activation and myeloid differentiation gene signatures. The balance between self-renewal and differentiation of hematopoietic progenitors is critical to maintain homeostatic levels of mature blood lineages. Irf8, a myeloid transcription factor controlling GMP differentiation, and β-catenin, a self-renewal transcription factor, are functionally antagonistic and implicated in chronic myeloid leukemia (CML) (Scheller et al., 2013). The authors identify Irf8 as a key downregulated gene in the self-renewing GMPs, which coincides with increased nuclear β-catenin. These important gene expression changes correlate with the appearance of key stimulatory cytokines, SCF and G-CSF, prior to pGMP formation and key inhibitory niche factors, TGF-β1 and CXCL4, at the time of cGMP formation. TGF-β1 and CXCL4, released by megakaryocytes, have been well described to restore HSC quiescence, which points to their release as a negative feedback mechanism for cGMPs. These niche factors are found to be downregulated in the leukemia models, likely due to decreased association of megakaryocytes with cGMPs (Figure 1). The authors propose that perturbation of GMP organization by reconstitution or inhibition of niche factors has therapeutic potential in both myelosuppressive disorders and leukemias.
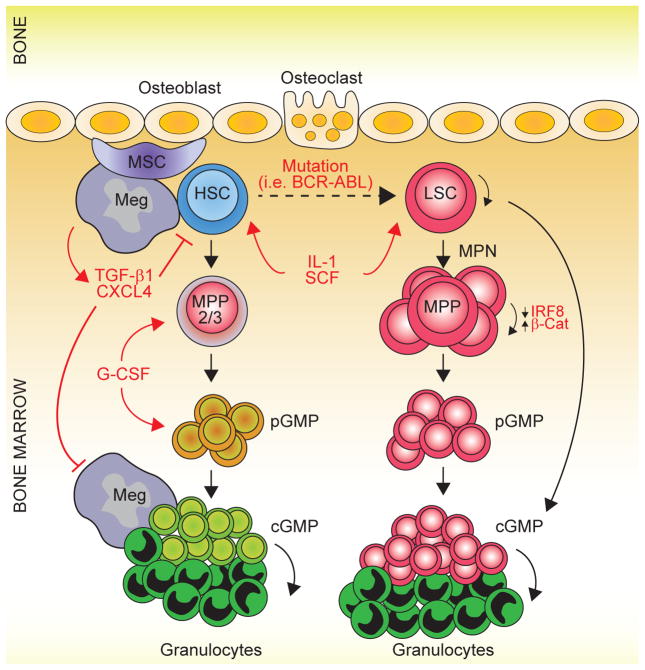
Figure 1. Spatiotemporal Functions of Granulocyte-Macrophage Progenitors in Emergency Hematopoiesis and Myeloid Malignancies.
During emergency hematopoiesis, HSC differentiate into myeloid-biased multipotent progenitors (MPP2/ 3) and then into granulocyte-monocyte progenitors (GMPs). GMPs first organize in loosely collected patches (pGMPs) in the BM and then condense to form distinct, compact, and transient clusters of GMPs (cGMPs) tightly surrounded by differentiated myeloid cells (i.e., granulocytes). These transitions are mediated by stimulatory cytokines, such as IL-1, SCF, and G-CSF, and inhibited by niche factors, such as TGF-β1 and CXCL4. These factors are released by megakaryocytes, which restore HSC quiescence and serve as a negative feedback mechanism for cGMPs. During development of myeloid malignancies, this mechanism is conserved by a lack of TGF-β1 and CXCL4 and an aberrant β-catenin-Irf8 self-renewal network. MSC, mesenchymal stromal cell; Meg, megakaryocyte; HSC, hematopoietic stem cell; MPP, multipotent progenitor; BM, bone marrow.
In summary, Hérault et al. uncover a unique organization of GMPs in the BM that allows them to rapidly, transiently proliferate in patches and then undergo molecular reprogramming resulting in condensed, differentiating clusters. The transition of GMPs from patches to clusters, and their eventual resolution, is largely dependent on cell-intrinsic and -extrinsic cues. Importantly, these spatiotemporal mechanisms are employed in emergency myelopoiesis and are hijacked in leukemia development (Figure 1). The authors find that these cluster-forming GMPs are clonal, originating from the myeloid-biased multipotent progenitors (MPPs). Bar-coding and lineage-tracing systems recently challenged the dogma that lifelong hematopoiesis is driven by long-term HSCs (Sun et al., 2014; Busch et al., 2015). Recent evidence has uncovered a major role for MPPs and lineage-committed progenitors to ongoing blood production in an unstressed state, rather than LT-HSCs, which is further supported by the elaborate work of Hérault et al. The markers of functionally distinct GMPs are being further refined (Olsson et al., 2016; Yáñez et al., 2015). Thus, it will be relevant to determine whether newly discovered, transient-intermediate GMPs identified by single-cell sequencing (Olsson et al., 2016) have functional roles in either patch or cluster formation. Perhaps more importantly, these unique populations that comprise pGMPs and cGMPs can be targeted in leukemia or activated in myelosuppressive disorders by the implementation of novel therapeutic approaches. Collectively, this study advances our understanding of myeloid progenitor biology and of the BM microenvironment, with important therapeutic implications in hematological disease.
References
- Acar M, Kocherlakota KS, Murphy MM, Peyer JG, Oguro H, Inra CN, Jaiyeola C, Zhao Z, Luby-Phelps K, Morrison SJ. Nature. 2015;526:126–130. doi: 10.1038/nature15250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns I, Lucas D, Pinho S, Ahmed J, Lambert MP, Kunisaki Y, Scheiermann C, Schiff L, Poncz M, Bergman A, Frenette PS. Nature Medicine. 2014;20:1315–1320. doi: 10.1038/nm.3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch K, Klapproth K, Barile M, Flossdorf M, Holland-Letz T, Schlenner SM, Reth M, Hofer T, Rodewald HR. Nature. 2015;518:542–546. doi: 10.1038/nature14242. [DOI] [PubMed] [Google Scholar]
- Ding L, Morrison SJ. Nature. 2013;495:231–235. doi: 10.1038/nature11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hérault A, Binnewies M, Leong S, Calero-Nieto FJ, Zhang SY, Kang YA, Wang X, Pietras EM, Chu SH, Barry-Holson K, et al. Nature. 2017;544:53–58. doi: 10.1038/nature21693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson A, Venkatasubramanian M, Chaudhri VK, Aronow BJ, Salomonis N, Singh H, Grimes HL. Nature. 2016;537:698–702. doi: 10.1038/nature19348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller M, Schönheit J, Zimmermann K, Leser U, Rosenbauer F, Leutz A. J Exp Med. 2013;210:2239–2256. doi: 10.1084/jem.20130706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Ramos A, Chapman B, Johnnidis JB, Le L, Ho YJ, Klein A, Hofmann O, Camargo FD. Nature. 2014;514:322–327. doi: 10.1038/nature13824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yáñez A, Ng MY, Hassanzadeh-Kiabi N, Goodridge HS. Blood. 2015;125:1452–1459. doi: 10.1182/blood-2014-09-600833. [DOI] [PubMed] [Google Scholar]