Abstract
IMPORTANCE
Data from an autosomal dominant Alzheimer disease (ADAD) kindred were used to track the longitudinal trajectory of cognitive decline associated with preclinical ADAD and explore factors that may modify the rate of cognitive decline.
OBJECTIVES
To evaluate the onset and rate of cognitive decline during preclinical ADAD and the effect of socioeconomic, vascular, and genetic factors on the cognitive decline.
DESIGN, SETTING, AND PARTICIPANTS
We performed a retrospective cohort study from January 1, 1995, through June 31, 2012, of individuals from Antioquia, Colombia, who tested positive for the ADAD-associated PSEN1 E280A mutation. Data analysis was performed from August 20, 2014, through November 30, 2015. A mixed-effects model was used to estimate annual rates of change in cognitive test scores and to mark the onset of cognitive decline.
MAIN OUTCOMES AND MEASURES
Memory, language, praxis, and total scores from the Consortium to Establish a Registry for Alzheimer Disease test battery. Chronologic age was used as a time scale in the models. We explore the effects of sex; educational level; socioeconomic status; residence area; occupation type; marital status; history of hypertension, diabetes mellitus, and dyslipidemia; tobacco and alcohol use; and APOE ε4 on the rates of cognitive decline.
RESULTS
A total of 493 carriers met the inclusion criteria and were analyzed. A total of 256 carriers had 2 or more assessments. At the time of the initial assessment, participants had a mean (SD) age of 33.4 (11.7) years and a mean (SD) educational level of 7.2 (4.2) years. They were predominantly female (270 [54.8%]), married (293 [59.4%]), and of low socioeconomic status (322 [65.3%]). Word list recall scores provided the earliest indicator of preclinical cognitive decline at 32 years of age, 12 and 17 years before the kindred’s respective median ages at mild cognitive impairment and dementia onset. After the change point, carriers had a statistically significant cognitive decline with a loss of 0.24 (95% CI, −0.26 to −0.22) points per year for the word list recall test and 2.13 (95% CI, −2.29 to −1.96) points per year for total scores. Carriers with high educational levels had an increase of approximately 36% in the rate of cognitive decline after the change point when compared with those with low educational levels (−2.89 vs −2.13 points per year, respectively). Onset of cognitive decline was delayed by 3 years in individuals with higher educational levels compared with those with lower educational levels. Those with higher educational level, middle/high socioeconomic status, history of diabetes and hypertension, and tobacco and alcohol use had a steeper cognitive decline after onset.
CONCLUSIONS AND RELEVANCE
Preclinical cognitive decline was evident in PSEN1 E280A mutation carriers 12 years before the onset of clinical impairment. Educational level may be a protective factor against the onset of cognitive impairment.
Research diagnostic criteria have been published for preclinical Alzheimer disease (AD) based on biomarkers of AD-related neurodegeneration.1 The study of carriers of AD-causing mutations provides a unique opportunity to characterize the preclinical changes associated with predisposition to AD.
For more than 25 years, the Neuroscience Group of Antioquia has studied a large kindred with autosomal dominant AD (ADAD) due to a single PSEN1 E280A mutation (OMIM 104311.0009). Carriers from this kindred have an onset of mild cognitive impairment (MCI) at the age of 44 years and an onset of dementia at the age of 49 years.2 Previous studies3–9 have found that carriers have brain Aβ plaque deposition and cerebrospinal fluid (CSF) and plasma biomarker evidence of Aβ overproduction decades before clinical symptoms. Cognitive markers also have been reported during preclinical stages of ADAD.10 However, given the heterogeneity of the cognitive profile of AD, it has been challenging to characterize cognitive changes that precede the onset of clinical impairment and to evaluate factors that may modify the rate and onset of cognitive decline during preclinical AD. Some of these factors, including genetic factors,11 educational level,12 socioeconomic status (SES),13 history of vascular disease (eg, hypertension, diabetes mellitus),14 and tobacco or alcohol use,14 have been related to cognitive decline. Preclinical cognitive markers and early detection of AD are increasingly important as research on new treatments that may slow or halt cognitive decline in AD is under way.15
In this study, we tracked the longitudinal trajectory of cognitive changes for a period of 18 years in preclinical PSEN1 E280A mutation carriers from Antioquia, Colombia. In addition, we estimated the onset and rate of cognitive decline during preclinical AD and the effect of socioeconomic, vascular, and genetic factors on the rate of cognitive decline.
Methods
Study Design and Participants
Participants who tested positive for the ADAD-associated PSEN1 E280A mutation were enrolled from January 1, 1995, through June 31, 2012.2 Data analysis was performed from August 20, 2014, through November 30, 2015. We included retrospective and longitudinal data from those carriers 18 years and older who had a complete neuropsychological assessment using the Consortium to Establish a Registry for Alzheimer Disease (CERAD) test battery. We excluded participants with a history of psychiatric disorders, illiteracy, stroke, epilepsy, traumatic brain injury, kidney failure, human immunodeficiency syndrome or AIDS, or substance abuse. All participants agreed to take part in this study and provided written informed consent before data collection. This study was approved by the ethics research committee of the University of Antioquia, Antioquia, Colombia. During clinical and neuropsychological assessments, physicians were masked to genetic status of the participants with no evident clinical symptoms to minimize the information bias. The genetic status was recorded in a database. Only the database’s coordinator (a system engineer) provided the genetic status for statistical analysis, with the name of the participant presented as a numerical code and not a name.
Procedures
All participants underwent comprehensive clinical and neuropsychological assessments. These assessments included an interview and medical and neuropsychological examinations. Medical examinations were performed by a neurologist or a physician trained in dementia assessment. Neuropsychological tests were conducted by psychologists. The neuropsychological protocol included the Mini-Mental State Examination (MMSE)16 and the CERAD test battery, which have been validated in our population.2,17 Clinical Dementia Rating (CDR)18 was used to establish the presence or absence of dementia. A CDR score of 0 indicates cognitive normality; 0.5, very mild dementia; 1, mild dementia; 2, moderate dementia; and 3, severe dementia.
Demographic Information
Socioeconomic status was defined according to the Colombian government classification system as low or middle/high. Our cohort comes from a population with a low educational level; accordingly, we classified educational level into 2 groups based on the median years of formal education: high (≥7 years) and low (<7 years). Marital status was classified in 3 groups: married or cohabitant, single, and divorced or widowed. Occupation was classified into 2 groups based on the nature of the work: manual work (housewives, farmers, domestic service employees, or technician) or nonmanual work (all professionals and managers, higher administrators, or clerical employees). Place of residence was classified as urban or rural.
Cardiovascular Factors
Hypertension was considered to be present if systolic blood pressure was 140 mm Hg or higher or diastolic blood pressure was 90 mm Hg or higher in the clinical assessment or if medications for hypertension were prescribed or self-reported. History of dyslipidemia was defined based on self-reports, the use of cholesterol-lowering agents, or a total cholesterol level greater than 200 mg/dL (to convert to millimoles per liter, multiply by 0.0259) registered in the clinical record. History of diabetes or alcohol or tobacco use was defined based on patient self-report.
Genetic Analyses
Genomic DNA was extracted from blood by standard protocols, and PSEN1 E280A characterization was performed as previously described.19 For exploratory analyses, APOE genotyping was performed in a subsample of 130 mutation carriers following methods previously described.20 Given the low frequency of the ε2/3, ε2/4, and ε4/4 genotypes, data were classified into 2 categories (presence or absence of APOE ε4) for statistical analyses.19
Cognitive Outcomes
Memory, language, and praxis tests from the CERAD test battery were included, and a total score (CERADts) was calculated using the Chandler method21 (range, 0–100) by adding the scores of the CERAD subtests (verbal fluency, 15-item Boston Naming Test, word list memory, word list recall, word list recognition [discrimination], and constructional praxis).
Statistical Analysis
We estimated the statistical power of 90%22 to detect a relative effect size of 0.30 (assuming a relative β coefficient related to time variable or other covariable in the model), with 162 participants and 3 or more assessments per individual assuming an intraclass correlation coefficient of 0.70 and a type I error of 0.05.
A mixed-effects model22 was used to estimate the annual rate of cognitive decline. Previous reports23,24 support the assumption that cognitive decline is not a constant linear process. Instead, the rate of cognitive decline increases after an unknown change point (CP) many years before clinical symptoms.25 Indeed, previous studies have also found differences in cross-sectional analyses between asymptomatic carriers and noncarriers in several biomarkers5 and cognitive markers2,10 at different preclinical stages. Thus, a CP approach provides an indirect estimation of when that cognitive decline could start. The approach taken by Hall et al23 was used to estimate the CP or onset of cognitive decline. The models used allow estimation of the rate of cognitive decline before and after the CP. To give an interpretation of the constant in the model, age was centered at 20 years. Fifty models were adjusted by varying the CP annually between the ages of 18 and 60 years. The likelihood approach was used to compare the models23 based on the goodness-of-fit index as the Akaike information criterion and the Bayesian information criterion.26 The lowest Bayesian information criterion was accepted as appropriate. The effect of sex, SES, educational level, vascular factors, tobacco or alcohol use, and APOE ε4 on cognition was analyzed using the MMSE score, CERAD subtest scores, and CERADts. The interaction coefficient between age (as a time variable) and demographic and vascular factors was analyzed in the model to estimate the annual rate of change in the presence or absence of those factors.
Results
Participants
From 703 PSEN1 E280A mutation carriers, 210 were excluded. A total of 493 carriers met the inclusion criteria and were analyzed (eFigure 1 in the Supplement). There were 256 carriers with 2 or more assessments, the median time between assessments was 2 years (interquartile range, 1–3 years), and the median time of follow-up was 5 years (interquartile range, 3–10 years). At the time of the initial assessment, participants had a mean (SD) age of 33.4 (11.7) years and a mean (SD) educational level of 7.2 (4.2) years. They were predominantly female (270 [54.8%]), married (293 [59.4%]), and of low SES (322 [65.3%]). A total of 432 participants (87.6%) had manual work occupations, 43 (8.7%) had a history of hypertension, 33 (6.7%) had dyslipidemia, and 8 (1.6%) had diabetes. A total of 123 participants (24.9%) had a history of tobacco use, and 58 (11.8%) had a history of alcohol use. The mean MMSE score was 26.5, and 386 (78.3%) were cognitively normal (CDR, 0). A subsample of 130 PSEN1 mutation carriers underwent APOE genotyping; 36 (27.7%) tested positive for at least one ε4 allele (APOE ε4) (Table 1).
Table 1.
Demographic and Clinical Characteristics of 493 PSEN1 E280A Mutation Carriers
| Characteristic | Finding (N = 493)a |
|---|---|
| Women | 270 (54.8) |
| Age at first assessment, mean (SD), y | 33.4 (11.7) |
| Educational level, mean (SD), y | 7.2 (4.2) |
| Socioeconomic status | |
| Low | 322 (65.3) |
| Middle | 166 (33.7) |
| High | 5 (1.0) |
| Occupation | |
| Nonmanual | 61 (12.4) |
| Manual | 432 (87.6) |
| Residence area | |
| Urban | 354 (71.8) |
| Rural | 139 (28.2) |
| Marital status | |
| Married or cohabitant | 293 (59.4) |
| Single | 164 (33.3) |
| Divorced or widowed | 36 (7.3) |
| Vascular factors | |
| Hypertension | 43 (8.7) |
| Dyslipidemia | 33 (6.7) |
| Diabetes mellitus | 8 (1.6) |
| Tobacco use | 123 (24.9) |
| Alcohol use | 58 (11.8) |
| APOE (n = 130) | |
| ε2/3 | 11 (8.5) |
| ε2/4 | 4 (3.1) |
| ε3/3 | 83 (63.8) |
| ε3/4 | 32 (24.6) |
| MMSE score, mean (SD) | 26.5 (5.5) |
| Clinical Dementia Rating | |
| 0 | 386 (78.3) |
| 0.5 | 51 (10.3) |
| 1 | 43 (8.7) |
| 2 | 13 (2.7) |
Abbreviation: MMSE, Mini-Mental State Examination.
Data are presented as number (percentage) of study participants unless otherwise indicated.
Description and Estimate of Cognitive Decline
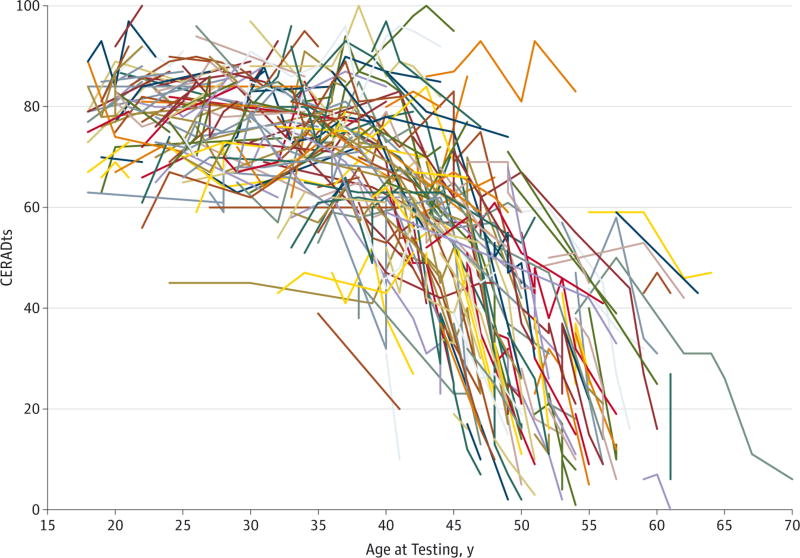
Figure 1 shows the spaghetti plot for CERADts (eFigure 2 in the Supplement illustrates the MMSE and CERAD subtests). The goodness-of-fit indexes suggested a CP for CERADts at the age of 34 years. Word list recall scores provided the earliest indication of cognitive decline at the age of 32 years, followed by word list learning, word list recognition, verbal fluency, and naming at the age of 34 years and constructional praxis at the age of 38 years (eFigure 3 in the Supplement shows the Akaike and Bayesian goodness-of-fit indexes for the models). Table 2 gives the coefficients of the estimated cognitive decline before and after the CP for the CERADts, MMSE, and CERAD subtests. After the CP, the CERADts revealed a statistically significant cognitive decline with a loss of 2.13 (95% CI, −2.29 to −1.96) points per year.
Figure 1.
Spaghetti Plot for Longitudinal Assessment of the Consortium to Establish a Registry for Alzheimer Disease Total Score (CERADts) in PSEN1 E280A Mutation Carriers
Table 2.
Coefficients of the Estimated Cognitive Decline Before and After the CP for the CERADts, MMSE, and CERAD Subtests in 493 PSEN1 E280A Mutation Carriers
| Test | Age at CP, y |
Raw Coefficient (95% CI) | Adjusted Coefficient (95% CI)a | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Mean | Before CP | After CP | Mean | Before CP | After CP | ||
| CERADts | 34 | 75.41 (73.65 to 77.17) | 0.01 (−0.17 to 0.19) | −2.20 (−2.36 to −2.03) | 82.10 (79.18 to 85.02) | 0.10 (−0.08 to 0.28) | −2.13 (−2.29 to −1.96) |
|
| |||||||
| MMSE | 36 | 29.45 (28.93 to 29.97) | 0.02 (−0.03 to 0.07) | −0.71 (−0.77 to −0.65) | 29.96 (29.01 to 30.91) | 0.04 (−0.01 to 0.09) | −0.71 (−0.77 to −0.65) |
|
| |||||||
| WLR | 32 | 6.38 (6.13 to 6.64) | −0.03 (−0.06 to 0.00) | −0.25 (−0.27 to −0.23) | 7.09 (6.61 to 7.58) | −0.01 (−0.05 to 0.02) | −0.24 (−0.26 to −0.22) |
|
| |||||||
| WLM | 34 | 17.65 (17.07 to 18.23) | 0.00 (−0.06 to 0.06) | −0.60 (−0.65 to −0.55) | 19.81 (18.73 to 20.89) | 0.02 (−0.04 to 0.09) | −0.58 (−0.63 to −0.53) |
|
| |||||||
| WLRd | 34 | 9.60 (9.33 to 9.88) | 0.00 (−0.03 to 0.03) | −0.30 (−0.33 to −0.27) | 9.76 (9.23 to 10.28) | 0.01 (−0.02 to 0.04) | −0.29 (−0.32 to −0.27) |
|
| |||||||
| Verbal fluency | 34 | 18.87 (18.18 to 19.56) | 0.05 (−0.02 to 0.12) | −0.49 (−0.55 to −0.43) | 21.52 (20.19 to 22.85) | 0.10 (0.03 to 0.18) | −0.46 (−0.52 to −0.41) |
|
| |||||||
| BNT | 34 | 12.75 (12.44 to 13.06) | 0.01 (−0.03 to 0.04) | −0.20 (−0.23 to −0.18) | 13.63 (13.10 to 14.16) | 0.01 (−0.02 to 0.05) | −0.20 (−0.23 to −0.17) |
|
| |||||||
| Construction praxis | 38 | 9.87 (9.59 to 10.14) | −0.02 (−0.04 to 0.01) | −0.34 (−0.37 to −0.31) | 10.35 (9.85 to 10.85) | 0.01 (−0.02 to 0.03) | −0.34 (−0.37 to −0.30) |
Abbreviations: BNT, 15-item Boston Naming Test; CERADts, Consortium to Establish a Registry for Alzheimer Disease total score; CP, change point; MMSE, Mini-Mental State Examination; WLM, word list memory; WLR, word list recall; WLRd, word list recognition (discrimination).
Adjusted by sex, educational level, socioeconomic status, residence area, occupation type, marital status, hypertension, dyslipidemia, diabetes mellitus, and tobacco and alcohol use.
Onset of cognitive decline on the word list recall test was delayed by 3 years in individuals with a high educational level (minimum Akaike information criterion, 2949) when compared with those with a low educational level (minimum Akaike information criterion, 2115). Mutation carriers with high educational levels had a CP at the age of 37 years for CERADts; at the age of 38 years for the MMSE; at the age of 36 years for word list recall, word list learning, and word list recognition; at the age of 38 years for verbal fluency; and at the age of 39 years for naming and constructional praxis. Mutation carriers with low educational levels had a CP at the age of 35 years for CERADts; at the age of 36 years for the MMSE; at the age of 33 years for word list recall; at the age of 37 years for word list learning, word list recognition, and verbal fluency; and at the age of 38 years for naming and constructional praxis.
Effects of Risk Factors on Cognitive Decline
Table 3 gives the coefficients of the estimated cognitive decline on CERADts in relation to the presence or absence of risk factors. Carriers with high educational levels had an increase of approximately 36% in the rate of cognitive decline after the CP when compared with those with low educational levels (−2.89 vs −2.13 points per year after adjustment). Similar results were found when cognitive decline was estimated in relation to SES, place of residence, diabetes, and tobacco or alcohol use (eTable 1, eTable 2, and eTable 3 in the Supplement estimate the cognitive decline in the MMSE and CERAD subtests). No statistical differences in the rate of cognitive decline were found after the CP in relation to sex, SES, occupation, residence area, marital status, and history of hypertension, diabetes, dyslipidemia, and tobacco and alcohol use.
Table 3.
Cognitive Decline in CERADts by Risk Factors in 493 PSEN1 E280A Mutation Carriers
| Characteristic | Raw Coefficient (95% CI) | Adjusted Coefficient (95% CI)a | ||
|---|---|---|---|---|
|
|
|
|||
| Before 34 Years Old | After 34 Years Old | Before 34 Years Old | After 34 Years Old | |
| Sex | ||||
|
| ||||
| Female | −0.28 (−0.46 to −0.10) | −2.16 (−2.37 to −1.95) | 0.11 (−0.11 to 0.33) | −2.10 (−2.31 to −1.90) |
|
| ||||
| Male | 0.46 (0.07 to 0.85) | −2.24 (−2.75 to −1.72) | 0.09 (−0.43 to 0.61) | −2.15 (−2.66 to −1.65) |
|
| ||||
| Educational levelb | ||||
|
| ||||
| High (median years for formal education, ≥7) | 0.11 (−0.08 to 0.29) | −2.89 (−3.25 to −2.52) | 0.12 (−0.08 to 0.32) | −2.89 (−3.25 to −2.53) |
|
| ||||
| Low (median years of formal education, <7) | −0.04 (−0.28 to 0.19) | −2.08 (−2.28 to −1.88) | −0.08 (−0.34 to 0.18) | −2.13 (−2.34 to −1.93) |
|
| ||||
| Socioeconomic status | ||||
|
| ||||
| Middle/high | 0.14 (−0.12 to 0.40) | −2.65 (−3.02 to −2.29) | 0.17 (−0.09 to 0.43) | −2.52 (−2.86 to −2.17) |
|
| ||||
| Low | −0.07 (−0.74 to 0.59) | −1.92 (−2.63 to −1.22) | 0.04 (−0.59 to 0.68) | −1.87 (−2.54 to −1.20) |
|
| ||||
| Residence area | ||||
|
| ||||
| Urban | 0.00 (−0.19 to 0.19) | −2.32 (−2.70 to −1.94) | 0.14 (−0.06 to 0.33) | −2.24 (−2.60 to −1.88) |
|
| ||||
| Rural | −0.09 (−0.68 to 0.49) | −1.87 (−2.61 to −1.12) | 0.00 (−0.57 to 0.56) | −1.84 (−2.56 to −1.12) |
|
| ||||
| Occupation type | ||||
|
| ||||
| Nonmanual | 0.07 (−0.86 to 0.99) | −1.47 (−1.97 to −0.97) | 0.02 (−0.88 to 0.93) | −1.48 (−1.95 to −1.02) |
|
| ||||
| Manual | 0.08 (−0.87 to 1.04) | −2.20 (−3.64 to −0.76) | 0.11 (−0.79 to 1.01) | −2.15 (−3.53 to −0.77) |
|
| ||||
| Marital status | ||||
|
| ||||
| Single | 0.08 (−0.55 to 0.72) | −2.49 (−3.12 to −1.86) | 0.33 (−0.32 to 0.99) | −2.13 (−2.77 to −1.49) |
|
| ||||
| Married | 0.14 (−0.10 to 0.38) | −2.15 (−2.33 to −1.97) | 0.39 (0.10 to 0.68) | −1.93 (−2.14 to −1.73) |
|
| ||||
| Hypertension | ||||
|
| ||||
| Present | 0.30 (−1.28 to 1.87) | −2.24 (−2.86 to −1.63) | 0.73 (−0.84 to 2.29) | −2.04 (−2.67 to −1.40) |
|
| ||||
| Absent | 0.00 (−1.39 to 1.39) | −2.20 (−2.63 to −1.77) | 0.37 (−0.94 to 1.68) | −1.94 (−2.36 to −1.53) |
|
| ||||
| Dyslipidemia | ||||
|
| ||||
| Present | 0.16 (−1.08 to 1.41) | −1.74 (−2.39 to −1.10) | 0.30 (−0.91 to 1.51) | −1.61 (−2.27 to −0.94) |
|
| ||||
| Absent | 0.02 (−0.17 to 0.20) | −2.28 (−2.46 to −2.10) | 0.38 (0.12 to 0.64) | −2.02 (−2.23 to −1.81) |
|
| ||||
| Diabetes mellitus | ||||
|
| ||||
| Present | 1.72 (−2.51 to 5.96) | −3.10 (−4.45 to −1.75) | 1.27 (−2.74 to 5.28) | −2.60 (−3.93 to −1.28) |
|
| ||||
| Absent | 0.00 (−0.18 to 0.18) | −2.19 (−2.35 to −2.02) | 0.37 (0.12 to 0.63) | −1.95 (−2.14 to −1.75) |
|
| ||||
| Tobacco use | ||||
|
| ||||
| Present | 0.00 (−0.54 to 0.54) | −2.41 (−2.62 to −2.20) | 0.50 (−0.09 to 1.10) | −2.18 (−2.41 to −1.95) |
|
| ||||
| Absent | 0.01 (−0.19 to 0.21) | −2.05 (−2.26 to −1.85) | 0.35 (0.09 to 0.61) | −1.83 (−2.06 to −1.60) |
|
| ||||
| Alcohol use | ||||
|
| ||||
| Present | −0.14 (−0.94 to 0.66) | −2.57 (−3.19 to −1.94) | 0.53 (−0.29 to 1.35) | −2.44 (−3.08 to −1.81) |
|
| ||||
| Absent | −0.01 (−0.62 to 0.61) | −2.09 (−2.53 to −1.65) | 0.37 (−0.20 to 0.93) | −1.85 (−2.28 to −1.43) |
|
| ||||
| APOE ε4 | ||||
|
| ||||
| Present | −0.66 (−2.19 to 0.87) | −2.16 (−3.00 to −1.33) | −0.26 (−2.01 to 1.49) | −2.31 (−3.33 to −1.29) |
|
| ||||
| Absent | 0.20 (−0.39 to 0.79) | −2.58 (−2.88 to −2.28) | 0.37 (−0.37 to 1.11) | −2.45 (−2.82 to −2.08) |
Abbreviations: CERADts, Consortium to Establish a Registry for Alzheimer’s Disease total score; CP, change point.
Adjusted coefficients by sex, educational level, socioeconomic status, residence area, occupation type, marital status, hypertension, dyslipidemia, diabetes mellitus, and tobacco and alcohol use.
The CP by educational level: high (CP, 37 years old) or low (CP, 35 years old).
Exploratory Analysis With APOE Genotype and Cognitive Decline
The frequency of APOE genotypes was as follows: APOE ε2/3, 11 patients; APOE ε2/4, 4 patients; APOE ε3/3, 83 patients; and APOE ε3/4, 32 patients. No differences in cognitive decline on any measure were found between APOE ε4 carriers and APOE ε4 noncarriers. No statistically significant difference was found in frequency of APOE ε4 status in demographic or vascular factors (sex: P = 0.48; SES: P = 0.66; educational level: P = 0.72; occupation: P = 0.12; residence area: P = 0.61; marital status: P = 0.54; history of hypertension: P = 0.34; diabetes: P = 0.21; dyslipidemia: P = 0.94; tobacco use: P = 0.65; and alcohol use: P = 0.56).
Sensitivity Analysis
Results of the analysis that included participants with 2 or more assessments (n = 256) compared with the total sample (n = 493) provided similar results in the estimation of CP and the rate of cognitive decline. For all cognitive tests before the CP, coefficients in those carriers with 2 or more assessments were similar to coefficients in the total sample. We found similar results in the estimation of the CP (eFigure 4 in the Supplement), and thereafter there was a median increase of 4.5% in the rate of cognitive decline in all subtests (range, 3.2%–5.7%) (eTable 4 in the Supplement). Moreover, additional analysis in carriers with a CDR of 0 provided similar results in CP estimation (eFigure 5 in the Supplement).
Discussion
This study provides evidence of preclinical cognitive decline in PSEN1 mutation carriers as early as 12 years before the estimated median age at MCI diagnosis and 17 years before dementia diagnosis. A memory test score provided the earliest indicator of cognitive decline. A higher educational level was associated with a delay in onset of cognitive decline and also with more rapid cognitive decline after onset.
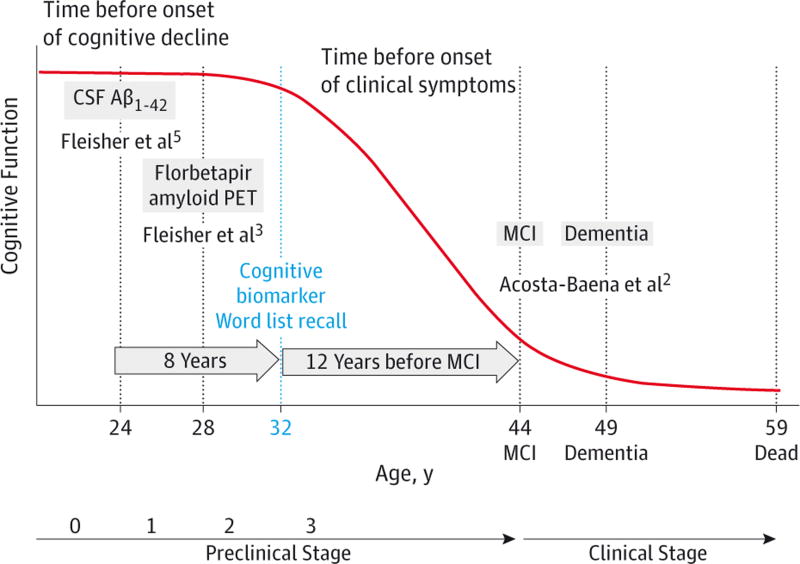
Previous studies in sporadic AD have found that cognitive decline begins at approximately 3 years before MCI25 and between 3 and 9 years before dementia.27 On the basis of previous work at identifying early biomarkers3–5 and the prevailing model of preclinical AD biomarker trajectory,28 we propose a model (Figure 2) of this trajectory, including cognitive changes, from preclinical to clinical stages in ADAD due to the PSEN1 E280A mutation.3 According to this model, the first biomarker changes are evident as CSF Aβ1–42 abnormalities at the age of 24 years (stage 1) followed by increased fibrillar Aβ accumulation at the age of 28 years (stage 2) and onset of cognitive decline at the age of 32 years (stage 3).
Figure 2.
Model for Preclinical and Clinical Stage in PSEN1 E280A Mutation Carriers
CSF indicates cerebrospinal fluid; MCI, mild cognitive impairment; and PET, positron emission tomography.
In addition, high educational level delayed by 3 years the onset of cognitive decline. This finding is consistent with other studies12,29 reported for sporadic AD. The theory of cognitive reserve could explain our findings.30 Cognitive reserve has been conceptualized as a multifactor construct that may include educational level, SES, and occupation, as well as lifestyle factors, which may contribute to cognitive reserve as part of a more dynamic process that changes throughout life.30 Studies12,30 of cognitive reserve have found that older individuals whose life experiences include more daily, challenging cognitive activities may have more compensatory strategies in place before the onset of cognitive impairment. In contrast, an early study performed by Pastor et al19 with PSEN1 E280A mutation carriers found that those carriers with high educational levels had an earlier onset of dementia. Some explanations for these opposite results are the outcome and a potential information bias. Pastor et al19 analyzed as the primary outcome the diagnosis of dementia using time to event models, and in some patients the diagnosis could be delayed because the onset of dementia was defined from information provided by a relative.
The main strength of the present study is the longitudinal follow-up of members from a large ADAD kindred with a single-gene mutation, with characterized ages at the onset of MCI and dementia2 and previously characterized associations of brain imaging and CSF biomarkers in adulthood.3–5 Another strength is the opportunity to evaluate the influence of risk factors on AD-related cognitive decline and clinical onset independent of potentially confounding effects of older age and age-related disorders.
This study also had several limitations. Exposures were measured retrospectively from clinical records, which does not account for the accumulative process of exposure, frequency, duration, and severity. Our analyses did not include other factors associated with the risk of dementia or cognitive decline (eg, obesity, physical activity, diet, cognitive stimulation, or cognitive or behavior-modifying drugs, such as memantine hydrochloride) because these were not systematically assessed and collected during the clinical assessments. In addition, the period of cognitive decline is larger than the time of observations for each participant. In our cohort, the data from carriers with 2 or more assessments had a median of 2 years between assessments in a mean longitudinal length of 5 years. Although mixed models can handle unbalanced design and missing data, the amount of information in our cohort limited the possibilities of more statistical analysis to determine how the CP varied among carriers. Further analysis is necessary, including a new cohort of carriers with a systematic assessment in the preclinical stage, to study how variation in the CP and the rate of cognitive decline between participants and how demographic, vascular, and other factors are related. Other methods using a Bayesian approach31 are necessary to find this variation. CERAD is sensitive to detect early changes associated with MCI.21 However, the floor or ceiling effects of CERAD subtests may affect the sensitivity to evaluate cognitive decline in the preclinical stage. Our cohort comes from a population with a low educational level, which is an important limitation for comparison with other studies. Finally, given the conditions of our sample, it is uncertain to what extent our findings may be generalizable to other AD-causing mutations and late-onset AD.
Conclusions
The systematic assessment of ADAD mutation carriers for more than 20 years is a unique opportunity to characterize the trajectory of some of the earliest preclinical changes and will guide future preventive interventions. Our findings suggest that cognitive decline could be detected by neuropsychological tests in preclinical AD more than a decade before the onset of clinical symptoms. Furthermore, our findings also support the idea that high educational level may be a protective effect against onset of cognitive decline.
Supplementary Material
Key Points.
Question
When is the onset of cognitive decline in the PSEN1 E280A carriers with autosomal dominant Alzheimer disease (ADAD)?
Findings
In this retrospective cohort study, which included 493 PSEN1 E280A carriers, a word list test provided the earliest indicator of preclinical cognitive decline at 32 years of age, which is 12 and 17 years before the kindred’s respective median ages at mild cognitive impairment and dementia onset.
Meaning
The systematic assessment of ADAD carriers for more than 20 years is a unique opportunity to characterize the trajectory of some of the earliest preclinical changes, which might guide future preventive interventions.
Acknowledgments
Funding/Support: This study was supported by the Banner Alzheimer Institute; grant 528 from the Departamento Administrativo de Ciencia, Tecnologia e Innovación, Colciencias, Republic of Colombia (Dr Aguirre-Acevedo); grant 609 from Comité para el desarrollo de la investigación CODI-Mediana cuantía; grants P30 AG019610 and R01 AG031581 from the National Institute on Aging (Dr Reiman); and grant DP5OD019833 from the National Institute of Health Office (Dr Quiroz).
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
Additional Contributions: We thank the PSEN1 E280A mutation carriers who participated in this study for contributing their valuable time and effort.
Footnotes
Author Contributions: Dr Aguirre-Acevedo had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Aguirre-Acevedo, Lopera, Bangdiwala, Langbaum, Jaimes.
Acquisition, analysis, or interpretation of data: Aguirre-Acevedo, Lopera, Henao, Tirado, Muñoz, Giraldo, Bangdiwala, Reiman, Tariot, Quiroz, Jaimes.
Drafting of the manuscript: Aguirre-Acevedo, Quiroz.
Critical revision of the manuscript for important intellectual content: Aguirre-Acevedo, Lopera, Henao, Tirado, Muñoz, Giraldo, Reiman, Tariot, Langbaum, Quiroz, Jaimes.
Statistical analysis: Aguirre-Acevedo, Bangdiwala, Jaimes.
Obtaining funding: Aguirre-Acevedo, Lopera, Reiman, Jaimes.
Administrative, technical, or material support: Aguirre-Acevedo, Lopera, Tariot, Langbaum, Jaimes.
Study supervision: Lopera, Bangdiwala, Jaimes.
Conflict of Interest Disclosures: None reported.
References
- 1.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Acosta-Baena N, Sepulveda-Falla D, Lopera-Gómez CM, et al. Pre-dementia clinical stages in presenilin 1 E280A familial early-onset Alzheimer’s disease: a retrospective cohort study. Lancet Neurol. 2011;10(3):213–220. doi: 10.1016/S1474-4422(10)70323-9. [DOI] [PubMed] [Google Scholar]
- 3.Fleisher AS, Chen K, Quiroz YT, et al. Florbetapir PET analysis of amyloid-β deposition in the presenilin 1 E280A autosomal dominant Alzheimer’s disease kindred: a cross-sectional study. Lancet Neurol. 2012;11(12):1057–1065. doi: 10.1016/S1474-4422(12)70227-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reiman EM, Quiroz YT, Fleisher AS, et al. Brain imaging and fluid biomarker analysis in young adults at genetic risk for autosomal dominant Alzheimer’s disease in the presenilin 1 E280A kindred: a case-control study. Lancet Neurol. 2012;11(12):1048–1056. doi: 10.1016/S1474-4422(12)70228-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fleisher AS, Chen K, Quiroz YT, et al. Associations between biomarkers and age in the presenilin 1 E280A autosomal dominant Alzheimer disease kindred: a cross-sectional study. JAMA Neurol. 2015;72(3):316–324. doi: 10.1001/jamaneurol.2014.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quiroz YT, Budson AE, Celone K, et al. Hippocampal hyperactivation in presymptomatic familial Alzheimer’s disease. Ann Neurol. 2010;68(6):865–875. doi: 10.1002/ana.22105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quiroz YT, Stern CE, Reiman EM, et al. Cortical atrophy in presymptomatic Alzheimer’s disease presenilin 1 mutation carriers. J Neurol Neurosurg Psychiatry. 2013;84(5):556–561. doi: 10.1136/jnnp-2012-303299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodriguez R, Lopera F, Alvarez A, et al. Spectral analysis of EEG in familial Alzheimer’s disease with E280A presenilin-1 mutation gene. Int J Alzheimers Dis. 2014;2014:180741. doi: 10.1155/2014/180741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fagan AM, Xiong C, Jasielec MS, et al. Dominantly Inherited Alzheimer Network. Longitudinal change in CSF biomarkers in autosomal-dominant Alzheimer’s disease. Sci Transl Med. 2014;6(226):226ra30. doi: 10.1126/scitranslmed.3007901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tirado V, Motta M, Aguirre-Acevedo DC, Pineda DA, Lopera F. Analysis of intrusive errors in a memory test as possible pre-clinical marker of familial Alzheimer disease, in E280A presenilin-1 mutation carrier [in Spanish] Rev Neurol. 2008;47(6):290–294. [PubMed] [Google Scholar]
- 11.Haan MN, Shemanski L, Jagust WJ, Manolio TA, Kuller L. The role of APOE ε4 in modulating effects of other risk factors for cognitive decline in elderly persons. JAMA. 1999;282(1):40–46. doi: 10.1001/jama.282.1.40. [DOI] [PubMed] [Google Scholar]
- 12.Amieva H, Mokri H, Le Goff M, et al. Compensatory mechanisms in higher-educated subjects with Alzheimer’s disease: a study of 20 years of cognitive decline. Brain. 2014;137(pt 4):1167–1175. doi: 10.1093/brain/awu035. [DOI] [PubMed] [Google Scholar]
- 13.Osler M, Avlund K, Mortensen EL. Socio-economic position early in life, cognitive development and cognitive change from young adulthood to middle age. Eur J Public Health. 2013;23(6):974–980. doi: 10.1093/eurpub/cks140. [DOI] [PubMed] [Google Scholar]
- 14.Panza F, Capurso C, D’Introno A, et al. Vascular risk factors, alcohol intake, and cognitive decline. J Nutr Health Aging. 2008;12(6):376–381. doi: 10.1007/BF02982669. [DOI] [PubMed] [Google Scholar]
- 15.Reiman EM, Langbaum JB, Fleisher AS, et al. Alzheimer’s Prevention Initiative: a plan to accelerate the evaluation of presymptomatic treatments. J Alzheimers Dis. 2011;26(suppl 3):321–329. doi: 10.3233/JAD-2011-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 17.Aguirre-Acevedo DC, Gómez RD, Moreno S, et al. Validity and reliability of the CERAD-Col neuropsychological battery [in Spanish] Rev Neurol. 2007;45(11):655–660. [PubMed] [Google Scholar]
- 18.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 19.Pastor P, Roe CM, Villegas A, et al. Apolipoprotein ε4 modifies Alzheimer’s disease onset in an E280A PS1 kindred. Ann Neurol. 2003;54(2):163–169. doi: 10.1002/ana.10636. [DOI] [PubMed] [Google Scholar]
- 20.Lendon CL, Martinez A, Behrens IM, et al. E280A PS-1 mutation causes Alzheimer’s disease but age of onset is not modified by ApoE alleles. Hum Mutat. 1997;10(3):186–195. doi: 10.1002/(SICI)1098-1004(1997)10:3<186::AID-HUMU2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 21.Chandler MJ, Lacritz LH, Hynan LS, et al. A total score for the CERAD neuropsychological battery. Neurology. 2005;65(1):102–106. doi: 10.1212/01.wnl.0000167607.63000.38. [DOI] [PubMed] [Google Scholar]
- 22.Diggle P. Analysis of Longitudinal Data. Oxford, England: Oxford University Press; 2002. [Google Scholar]
- 23.Hall CB, Lipton RB, Sliwinski M, Stewart WF. A change point model for estimating the onset of cognitive decline in preclinical Alzheimer’s disease. Stat Med. 2000;19(11–12):1555–1566. doi: 10.1002/(sici)1097-0258(20000615/30)19:11/12<1555::aid-sim445>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 24.Salthouse TA. When does age-related cognitive decline begin? Neurobiol Aging. 2009;30(4):507–514. doi: 10.1016/j.neurobiolaging.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howieson DB, Carlson NE, Moore MM, et al. Trajectory of mild cognitive impairment onset. J Int Neuropsychol Soc. 2008;14(2):192–198. doi: 10.1017/S1355617708080375. [DOI] [PubMed] [Google Scholar]
- 26.van den Hout A, Muniz-Terrera G, Matthews FE. Change point models for cognitive tests using semi-parametric maximum likelihood. Comput Stat Data Anal. 2013;57(1):684–698. doi: 10.1016/j.csda.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu L, Boyle P, Wilson RS, et al. A random change point model for cognitive decline in Alzheimer’s disease and mild cognitive impairment. Neuroepidemiology. 2012;39(2):73–83. doi: 10.1159/000339365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jack CR, Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9(1):119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hall CB, Derby C, LeValley A, Katz MJ, Verghese J, Lipton RB. Education delays accelerated decline on a memory test in persons who develop dementia. Neurology. 2007;69(17):1657–1664. doi: 10.1212/01.wnl.0000278163.82636.30. [DOI] [PubMed] [Google Scholar]
- 30.Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012;11(11):1006–1012. doi: 10.1016/S1474-4422(12)70191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hall CB, Ying J, Kuo L, Lipton RB. Bayesian and profile likelihood change point methods for modeling cognitive function over time. Comput Stat Data Anal. 2003;42(1):91–109. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.