Summary
Vitamin D has immunomodulating properties. The nuclear receptor for vitamin D is expressed in several immune cells, which convert 25-hydroxyvitamin D (25OHD) to the active form 1,25 hydroxyvitamin D [1,25(OH)2 D]. Under conditions of infection, 1,25(OH)2 D promotes production of cathelicidin (an antimicrobial peptide) in monocytes and activated macrophages. In vitro studies have shown the ability of cathelicidin to inhibit replication of human immunodeficiency virus (HIV-1) in T CD4 lymphocytes and macrophages.
Objective
To evaluate vitamin D levels and their impact on mineral metabolism in HIV infected patients.
Materials and methods
Seventy-four clinical records of HIV/AIDS patients seen at the outpatients clinic were reviewed. The following data were collected: age, sex, time since diagnosis of HIV, HIV-1 viral load, CD4 counts (absolute value and percentage), and mineral metabolism determinations: 25OHD, intact parathormone (iPTH); serum calcium (sCa); serum phosphorus (sP) and serum crosslaps (sCTX). Vitamin D levels were stratified as follows: optimal: ≥30ng/ml; insufficient: 21–29ng/ml; moderately deficient: 20≥ -25OHD- >10 ng/ml and severely deficient ≤10 ng/ml.
Results
Fifty-five clinical records were included; 82% of patients had 25OHD levels below 30ng/ml (insufficient: 23.6%, moderately deficient: 36.4%; and severely deficient: 21.8%). A significantly higher serum PTH levels in the moderately and severely deficient groups than in the optimal and insufficient groups was observed (p<0.05 and p<0.03 respectively). A weak negative correlation was observed between serum 25OHD and PTH levels (r=−0.268; p<0.004).
Conclusion
Sub-optimal vitamin D levels are frequently observed in HIV/AIDS patients on antiretroviral therapy (ART). Systematic assessment of mineral metabolism is considered necessary in HIV/AIDS positive patients.
Keywords: HIV, 25-hydroxyvitamin D, mineral metabolism
Introduction
Since the discovery of vitamin D, our knowledge on this vitamin has evolved throughout the years from being a hormone that regulates mineral metabolism to being a complex factor involved in multiple physiological processes in the body. Classically, vitamin D acts as a regulator of calcium homeostasis, promoting calcium transport and ensuring adequate levels of calcium in the blood to meet specific physiological functions. Vitamin D is synthesized in the skin after exposure to ultraviolet (UV) rays, and then undergoes two successive hydroxylations, first in the liver and then in the kidneys, and is thus converted to its biologically active form known as calcitriol or 1,25 hydroxyvitamin D [1,25(OH) 2D], which has a high affinity for vitamin D receptor (VDR) expressed in different tissues. VDR was first identified in human leukocytes in 1983 (1). Since then, the role of vitamin D in the immune system has been investigated extensively.
Vitamin D is currently considered an immunomodulator affecting several subpopulations of hematopoietic cells such as monocytes, macrophages, and B and T lymphocytes (2). The role of vitamin D in defense mechanisms against different types of infections, including HIV/AIDS, has been studied. One of the most widely known mechanisms is the production of cathelicidin (LL-37), a 1,25 (OH)2 D-dependent antimicrobial peptide synthesized by monocytes and activated macrophages in response to pathogenic agents that cause infections including HIV/AIDS. Recent studies have demonstrated the ability of LL-37 to inhibit HIV-1 replication in T CD+4 lymphocytes (CD4+LT) and macrophages (3, 4). Hence, sub-optimal levels of vitamin D could affect the response of the immune system in patients with HIV/AIDS.
The aim of the present study was to explore vitamin D levels and their impact on mineral metabolism in adult patients with HIV/AIDS seeking outpatient care at a University Hospital in the City of Buenos Aires.
Materials and methods
Study design
A retrospective study was performed by reviewing the clinical records of adult patients diagnosed with HIV/AIDS receiving medical care at the Infectology Division of the Clinical Hospital (Hospital de Clínicas) of the University of Buenos Aires (34ºS latitude). From an average 136 HIV patients seen annually at the Infectology Division of the Hospital, only the clinical records that provided the following data were included in the study: 1) CD4 counts, expressed as an absolute value and as a percentage, as indicator of the immune status of the patient; 2) HIV-1 viral load; and 3) mineral metabolism determinations 25OHD, intact parathormone (iPTH); serum calcium (sCa); serum phosphorus (sP) and serum crosslaps (sCTX).
The following data were also collected from patient records, since they were considered potential confounding factors for low vitamin D levels: age, sex, duration of HIV/AIDS, anti-retroviral therapy (ARV), presence of renal disease (defined as glomerular filtration rate <60ml/minute or known renal disease). Clinical records of patients receiving vitamin D supplementation or vitamin D-containing multivitamin supplements within six months prior to the study, known bone disease or clinical condition, or receiving medication known to affect vitamin D metabolism were excluded.
Fifty-five clinical records of patients diagnosed with HIV/AIDS met the inclusion criteria and were selected for the study.
Biochemical determinations
All values were retrieved from patient files. As shown on patient records, determinations were performed in 8-hr fasting blood samples obtained before 10:30am; the laboratory of choice for sample processing depended on the health care provider of each patient.
CD4 lymphocyte counts (absolute values and percentages) and HIV viral load were determined by flow cytometry and reverse transcription-polymerase chain reaction (RT-PCR) respectively. 25OHD levels were measured by radioimmunoassay (RIA) and electrochemiluminescence immunoassay [ECLIA Elecsys]. iPTH was measured by ECLIA Elecsys [reference values (rv): 6–65pg/ml)]. Serum crosslaps (a marker of bone resorption) levels were determined by ECLIA Elecsys; reference values were as follows: pre-menopausal women 40–450ng/ml; post-menopausal women: 80–590ng/ml; men: 14–450ng/ml. sCa (rv: 8.5–10.4mg/dl) and sP (rv: 2.4–4.5mg/dl) levels were measured using standard techniques.
Statistical analysis
Statistical analysis was performed using SPSS 19.0 for Windows (SPPS, Inc., Chicago, IL, USA). Based on the objective of the study, 25OHD levels were stratified in four categories as follows: optimal ≥ 30ng/ml; insufficient 21–29ng/ml; moderately deficient ≥ 20–25OHD->10ng/ml; and severely deficient ≤ 10ng/ml. Patient characteristics were compared among groups using a non-parametric unpaired t test (Mann-Whitney). Correlation among variables was explored using the Pearson and Spearman test. A value of p lower than 0.05 was considered statistically significant.
Results
Fifty-five clinical records corresponding to HIV/AIDS patients were included in the study. The total group comprised 35 women (63.6%) and 20 men (36.6%), with an average age (X±DS) of 52.6±13 years and 10.4±7 years duration of the disease. Average CD4 lymphocyte count was 558±288 cells/mm3, 26±10%, and 90% of patients had an HIV viral load ≤ 1.7copies/ml. Vitamin D nutritional status of the whole group was 21.4±13ng/ml, as assessed by 25OHD determinations. All patients were on ART.
Table 1 shows the baseline characteristics of patients according to 25OHD category. No significant differences in age, duration of the disease, CD4 counts (absolute value and percentage), and HIV-1 viral load were observed among groups. 25OHD levels did not differ significantly between seasons (winter-spring: 18.0±8ng/ml and summer-autumn: 23.0±14ng/ml).
Table 1.
Patient characteristics according to vitamin D levels.
| 25OHD levels (ng/ml)
| ||||
|---|---|---|---|---|
|
| ||||
| Characteristics | Optimal (≥ 30) (n=10) | Insufficient (21–29) (n=13) | Moderately Deficient (≤ 20->10) (n=20) | Severely Deficient (≤ 10) (n=12) |
| Age (years) | 48.0±14 | 52.7±13 | 54.0±14 | 58.0±14 |
| Sex: F/M (n) | 7/3 | 7/6 | 14/6 | 7/5 |
| Season: WS/SA (n) | 8/2 | 9/4 | 13/7 | 8/4 |
| Duration of HIV(years) | 9.6±6 | 10.9±5 | 11.5±8 | 7.0±5 |
| CD4 (cells/ml) | 636.90±381 | 565.38±235 | 512.81±254 | 616.91±285 |
| CD4 (%) | 27.2±11 | 27.6±8 | 25.3±10 | 27.8±10 |
| HIV viral load, log10 (n) | 0 | 3 | 2 | 1 |
| 25OHD (ng/ml) | 43.8±11* | 24.8±2 | 12.4±4 | 8.3±2 |
Results are expressed as mean± standard deviation (X±SD). F: Female; M: Male
p<0.000 Optimal vs insufficient, moderately deficient, and severely deficient
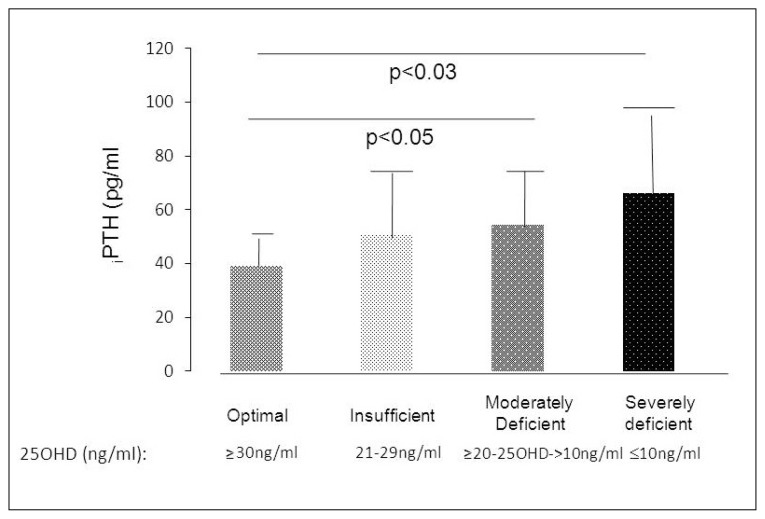
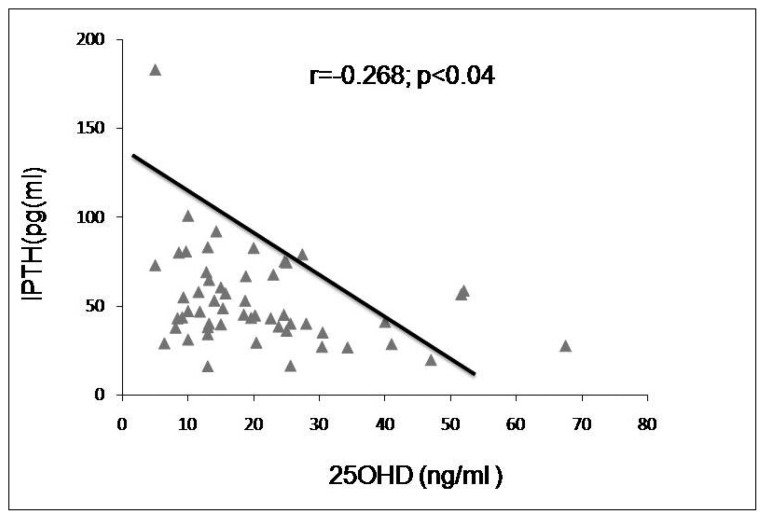
Analysis of biochemical determinations of mineral metabolism showed significantly higher serum PTH levels in the moderately and severely deficient groups than in the optimal and insufficient groups (Table 2 and Figure 1). Four cases of secondary hyperparathyroidism were found in the moderately deficient group [iPTH (X±DS):73.78±8pg/ml], and 5 cases were observed in the severely deficient group [iPTH (X±DS):103.5±45 pg/ml]. A weak negative correlation was observed between serum 25OHD and PTH levels (r=−0.268; p< 0.04) (Figure 2).
Table 2.
Results of mineral metabolism biochemical determinations according of 25OHD levels.
| 25OHD levels (ng/ml) | ||||
|---|---|---|---|---|
|
| ||||
| Optimal (≥ 30) (n=10) | Insufficient (21–29) (n=13) | Moderately Deficient (≤20->10) (n=20) | Severely Deficient (≤10) (n=12) | |
| sCa (rv: 8.9–10.4mg%) |
9.6±0.4 | 9.4±0.5 | 9.2±0.4*** | 9.2±0.4 |
| sP (rv: 2.6–4.4mg%) |
2.5±1.0 | 2.4±1.4 | 2.4±1.2 | 2.5±1.4 |
| sCTX (rv: see □) |
386±179 | 429±213 | 467±141 | 483±47 |
| 25OHD (rv:>30ng/ml) |
43.8±11* | 24.8±2 | 12.4±4 | 8.3±2 |
| iPTH (rv: 6–65pg/ml) |
39.13±17 | 50.43±20 | 54.52±18** | 66.45±43△ |
Results are expressed as mean± standard deviation (X±SD). rv: reference values.
p<0.000 Optimal vs. Insufficient, moderately deficient and severely deficient.
p<0.05 Optimal vs. Insufficient.
p< 0.02 Optimal vs. Moderately Deficient;
p< 0.03 Optimal vs Severely Deficient.
□ rv: Female: Pre-Menopausal: 40–450ng/ml; Post-Menopausal 80–590ng/ml; Male: 14–450ng/ml.
Figure 1.
PTH levels according to levels of 25OHD.
Figure 2.
Correlation between PTH and 25OHD levels.
In addition, serum calcium levels were lower in the moderately and severely deficient vitamin D groups, and were significantly lower in the moderately deficient group as compared to the optimal group (p<0.02) (Table 2). No statistically significant differences in serum CTX levels were observed among groups; nevertheless, values tended to be higher, though within normal range, in the moderately (CTXs: 467±141ng/ml) and severely (CTXs: 483±47ng/ml) deficient groups. Serum phosphorus levels were in the lower normal range.
Evaluation of immune status and disease status according to serum 250HD levels showed no correlation between serum 25OHD levels and CD4 counts (absolute value and percentage) and HIV-1 viral load in the optimal, insufficient, and moderately deficient groups. Serum 25OHD levels and HIV-1 viral load (log10) were found to correlate negatively (r=−0.568; p<0.05) in the severely deficient group only.
Discussion
The present study sought to evaluate the relation between 25OHD levels and immune and viral status of adult patients diagnosed with HIV/AIDS and receiving ART, seen at a university hospital in the City of Buenos Aires. Our results showed that 82% of the studied sample had vitamin D levels below adequate: 23.6% had insufficient levels, 36.4% showed moderately deficient levels, and 21.8% exhibited severely deficient levels. Only 16.3% had optimal vitamin D levels. No season-related changes in vitamin D levels were observed; values were similar to the winter 25OHD levels of healthy elderly and young adults in the city of Buenos Aires [25OHD (X±DS):17.2±9 ng/ml], but lower than 25OHD levels observed in these age-groups in summer [elderly: 28.6±10ng/ml and young adults: 32.5±12.8ng/ml] (5).
The prevalence of hypovitaminosis D (defined as 25OHD levels below 30ng/ml) in the studied population was similar to that reported by other research groups. It is estimated to be in the order of 60 to 90%, varying among the reported series (6–8). The low levels of vitamin D observed in HIV/AIDS patients can be due to a number of causes. The infection per sé leads to activation of toll-like receptors (TLR) expressed by cells of the immune system, activating 1α-hydroxylase (CYP27B1), decreasing 25OHD levels as a result of an increase in hydroxylation to 1,25(OH) 2D, and triggering induction of LL-37 synthesis at the genomic level.
Another factor associated with low 25OHD levels is the use of ART. The use of efavirenz may induce cytochrome P450 3A4 (CYP3A4) and 24-hydroxylase (CYP24), reducing 25-hydroxylase (CYP2R1) function and thus decreasing 25OHD levels. Tenofovir has been associated with hyperparathyroidism, resulting in an increase in 25OHD consumption. Protease inhibitors interfere with vitamin D metabolism in vitro, via the inhibition of cytochrome P450 (CYP450), although this mechanism has not been demonstrated in clinical trials (9). In addition, the chronicity of HIV/AIDS may also contribute to risk of sub-optimal vitamin D levels in these patients. As a result of the advent of new, more effective drugs in terms of decreasing mortality and controlling opportunistic infections, longer survival rates are observed in HIV/AIDS patients, who are thus also exposed to the effects of ageing. Sub-optimal vitamin D levels may be associated with high PTH levels. Nine of the patients studied here had hyperparathyroidism: 4 were in the group with moderately deficient and 5 were in the group with severely deficient levels of vitamin D. Serum calcium levels were lower in the moderately and severely deficient groups, but were only significantly lower in the moderately deficient group, likely due to the size of the study sample.
Hyperparathyroidism secondary to sub-optimal vitamin D levels contributes to an increase in bone remodeling, loss of bone mass, and osteoporosis. Levels of CTXs, a specific and sensitive marker of bone resorption, were higher in the moderately and severely deficient vitamin D groups, though the difference among groups did not reach statistical significance (10). These results confirm that bone remodeling increases in conditions of vitamin D deficiency.
As regards the relation between HIV/AIDS infection and vitamin D levels, the results obtained in the sample studied here showed a negative correlation between vitamin D status and HIV viral load in the group with severely deficient vitamin D levels only. This could imply that at lower vitamin D levels, i.e. in the severely deficient range, the level of HIV viral load is higher, possibly because the mechanisms of vitamin D that arrest progression of HIV-1 infection could not function. These mechanisms include autophagia and LL-37 production. The former is induced by the concentration of 1,25 (OH)2 D; 1,25 (OH)2 D-mediated autophagia has been shown to inhibit replication of HIV in macrophages (11). Furthermore, LL-37 expression in humans depends on 25OHD concentration, and LL-37 has been found to inhibit HIV replication in CD4+LT (4, 12). However, a high viral load in patients under ART may be more associated with poor adherence and resistance mutations rather than with low vitamin D levels and the impact of the latter on viral load may be only marginal.
Deficient vitamin D levels not only contribute to progression of the disease but also to mortality in HIV/AIDS patients. The EuroSIDA study showed that patients with vitamin D levels <12ng/ml were at greater risk of clinical progression during follow-up.
The sequence of events regarding HIV/AIDS infection, 25OHD levels, and disease progression remains unknown. The question is whether sub-optimal levels of 25OHD, a substrate for 1,25 (OH)2 D conversion in immune cells, would interfere with viral replication inhibition mechanisms in immune cells, and thus lead to progression of the disease, or whether it is the high HIV viral load that promotes conversion of 25OHD to 1,25 (OH)2 D with the aim to inhibit viral replication, and ultimately leads to severely deficient vitamin D levels and disease progression in these patients.
The present work has limitations that must be pointed out. Determinations of risk factors for sub-optimal vitamin D levels, such as body mass index, were not available on the patient records. Also, 25OHD determinations were not performed using the same method or the same assay. Lastly, this is a retrospective study (review of clinical records) and the sample size is small, which limits the robustness of the findings and, hence, the generalizability of the conclusions.
Further prospective studies must be conducted to evaluate the results presented here, and to evaluate the status of vitamin D on mineral metabolism in HIV/AIDS patients with suboptimal vitamin D levels, in order to contribute to a better understanding of the role of vitamin D in HIV/AIDS infection.
Footnotes
Conflict of interests
Dr. Beatriz Oliveri received honoraria for lectures from Company Glaxo SmithKline, Shire Human Genetic Therapies and TRB Pharma. All other Authors have no conflict of interest. This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
References
- 1.Provvedini DM, Tsoukas CD, Deftos LJ, Manolagas SC. 1,25-Dihydroxyvitamin D3 receptors in human leukocytes. Science. 1983;221:1181–1183. doi: 10.1126/science.6310748. [DOI] [PubMed] [Google Scholar]
- 2.Mora JR, Iwata M, von Andrian UH. Vitamin effect on the immune system: vitamins A and D take centre stage. Nat Rev Immunol. 2008;8:685–698. doi: 10.1038/nri2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beard JA, Bearden A, Striker R. Vitamin D and the anti-viral state. J Clin Virol. 2011;50:194–200. doi: 10.1016/j.jcv.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergman P, Walter-Jalow L, Broliden K, Agerberth B, Soderlund J. The antimicrobial peptide LL-37 inhibits HIV-1 replication. Curr HIV Res. 2007;5:410–415. doi: 10.2174/157016207781023947. [DOI] [PubMed] [Google Scholar]
- 5.Fassi J, Russo Picasso MF, Furci A, Sorroche P, Jauregui R, Plantalech L. Variaciones estacionales de 25-hidroxivitamina D en jóvenes y ancianos de la Ciudad de Buenos Aires. MEDICINA (Buenos Aires) 2003;63:215–220. [PubMed] [Google Scholar]
- 6.Mueller NJ, Fux CA, Ledergerber B, et al. High prevalence of severe vitamin D deficiency in combined antiretroviral therapy-naive and successfully treated Swiss HIV patients. AIDS. 2010;24:1127–1134. doi: 10.1097/QAD.0b013e328337b161. [DOI] [PubMed] [Google Scholar]
- 7.Dao CN, Pate IP, Overton ET, et al. Low vitamin D among HIV-infected adults: prevalence of and risk factors for low vitamin D levels in a cohort of HIV-infected adults and comparison to prevalence among adults in the US general population. Clin Infec Dis. 2011;52:396–405. doi: 10.1093/cid/ciq158. [DOI] [PubMed] [Google Scholar]
- 8.Virad JP, Souberbielle JC, Kirk O, et al. Vitamin D and clinical disease progression in HIV infection: results from the EuroSIDA study. AIDS. 2011;25:1305–1315. doi: 10.1097/QAD.0b013e328347f6f7. [DOI] [PubMed] [Google Scholar]
- 9.Panayiotopoulos A, Bhat N, Bhangoo A. Bone and vitamin D metabolism in HIV. Rev Endocr Metab Disord. 2013;14:119–125. doi: 10.1007/s11154-013-9246-8. [DOI] [PubMed] [Google Scholar]
- 10.Vasikaran S, Eastell R, Bruyère O, et al. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos Int. 2011;22:391–420. doi: 10.1007/s00198-010-1501-1. [DOI] [PubMed] [Google Scholar]
- 11.Campbell GR, Spector SA. Hormonally active vitamin D3 (1α,25-dihydroxycholecalciferol) triggers autophagy in human macrophages that inhibits HIV-1infection. J Biol Chem. 2011;286:18890–18902. doi: 10.1074/jbc.M110.206110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang TT, Nestel JS, Bourdeau V, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173:2909–2912. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]